Copper-based coordination polymers from thiophene and furan dicarboxylates with high isosteric heats of hydrogen adsorption†
Abstract
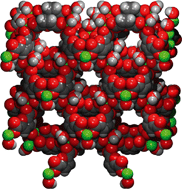
Self-assembled Cu-based coordination polymers derived from thiophene-2,5-dicarboxylic acid (Cu-TDC) and furan-2,5-dicarboxylic acid (Cu-FDC) were synthesized via a solvothermal method and their H2 adsorption behaviour was investigated and contrasted with isophthalic acid (Cu-m-BDC) and terephthalic acid (Cu-BDC) derivatives. Both heterocyclic-based coordination polymers exhibit low surface areas (<300 m2 g−1) upon activation but unusually high isosteric heats of hydrogen adsorption (7.5–9.2 kJ mol−1). Hydrogen uptake values of 0.64–0.75 wt% (77 K and 1 bar) were recorded and these high uptake values are attributed to the optimal pore size (5.4–8 Å) and the polarizability of the 5-membered heterocycles.

 Please wait while we load your content...
Please wait while we load your content...