Novel N-sulfonamide trans-platinum complexes: synthesis, reactivity and in vitro evaluation†
José
Alemán
*a,
Virginia
del Solar
b,
Amparo
Alvarez-Valdés
b,
Carla
Ríos-Luci
c,
José M.
Padrón
c and
Carmen
Navarro-Ranninger
*b
aDepartamento de Química Orgánica (C-1), Universidad Autónoma de Madrid, Cantoblanco, 28049, Madrid, Spain
bDepartamento de Química Inorgánica (C-7), Universidad Autónoma de Madrid, Cantoblanco, 28049, Madrid, Spain. Fax: +34 914974833
cInstituto Universitario de Bio-Orgánica “Antonio González” (IUBO-AG), Universidad de La Laguna, C/Astrofísico Francisco Sánchez 2, 38206, La Laguna, Spain. E-mail: jose.aleman@uam.es; carmen.navarro@uam.es
First published on 30th June 2011
Abstract
In this work, we described for the first time the synthesis of trans-N-sulfonamide platinum complexes. The antiproliferative activity (GI50, μM) of these new compounds in human solid tumors cells was compared to cisplatin.
Introduction
Among the traditional antitumour metal compounds,1cisplatin (CDDP)2 remains the most successful drug and has been used in nearly 50% of all tumor therapies. However, many different adverse effects, such as dose-limiting, nephrotoxicity, peripheral neuropathy, tinnitus and hearing loss, are known.3 Thousands of platinum complexes have been evaluated during the last half-century.4 Continuous searching for new metallodrugs has provided novel platinum complexes with different reactivities,5 including some rule-breakers,6etc. leading medicinal chemists to find new platinum complexes with improved biological activities and enhanced pharmacological profiles.N-Sulfonamides have been utilized extensively in medicinal chemistry.7 Since they are original antibacterial agents (i.e., the sulfa drugs), many other activities have also been described; anticonvulsant (Sultiame),7 as inhibitors of the carbonic anhydrase,8,9inhibitors of histone deacetylases,10inhibitors of microtubule polymerization,11 non-peptide luteinizing hormone-releasing hormone antagonists,12 and PET agents for imaging of tubulin polymerization in cancer.13
Interestingly, the inclusion of these N-sulfonamides in platinum complexes has received relatively little attention. Despite the fact that together platinum and sulfonamide structures could have a synergistic or complementary effect in the treatment of cancer processes, only few platinum complexes with cis-geometry have been synthesized and none of them have been evaluated in biological assays.14–16 In 1999, Cagné et al. described the synthesis of chiral platinum bisulfonamides, obtaining a number of interesting complexes, however they were not biologically evaluated (left, Fig. 1).14 Later, Henderson et al.15 synthesized similar bisulfonamides, with small structural changes (middle-left, Fig. 1). Interestingly, and more recently, Marzilli et al.16 reported the synthesis of polyamines with a fluorophore unit as a part of the N-sulfonamide (middle-right, Fig. 1). Although their interactions with various biomolecules were studied, no biological data concerning their antitumor effects were described. More recently, Goldberg and coworkers showed the synthesis of platinum(IV) sulfonamides for mechanistic studies.17 In all of these cases, only cis-platinum complexes and their derivatives were synthesized and not the analogous trans-platinum complexes.
 | ||
| Fig. 1 Reported and proposed platinum sulfonamide complexes. | ||
As part of our program dealing with trans-platinum complexes,18 our research group has found that these complexes, with several and different aliphatic amines in trans geometry, display in some cases higher activity than cisplatin. Cytotoxicity data obtained from these complexes are very promising, because in general, they do not exhibit cross-resistance with cisplatin.19 It should be emphasized that some of these trans complexes, such as for example trans-[PtCl2(dimethylamine)(isopropylamine)], are able to kill cisplatin resistant cell lines through apoptosis and that, moreover, these complexes are also active “in vivo”.20
Based on these precedents from our own research and from the literature, we envisioned the use of mono-sulfonamide complexes is an interesting topic for various reasons: (i) to the best of our knowledge, no examples of trans-platinum complexes with sulfonamide ligands have been described (right, Fig. 1), (ii) the use of sulfonamides represents a versatile strategy because they would allow for the synthesis of biologically interesting complexes by selection of the appropriate R group (e.g. fluorophore units, DNA intercalators, etc., right, Fig. 1), (iii) finally, the biological evaluation of these compounds would provide information on the activities of combinations of the N-sulfonamide and platinum complexes against cancer cells which until now has not been available.
Synthesis
Our first approach to the synthesis of the sulfonamide platinum complexes was the direct reaction of simple commercially available N-sulfonamide 1 with different platinum species, such as K2PtCl4 (4) or PtCl2(DMSO)2 (3). However, no reaction was observed in either case, probably due to the low nucleophilicity of the nitrogen of the N-sulfonamide 1 (Scheme 1). | ||
| Scheme 1 First trials for the synthesis of platinum N-sulfonamide complexes. | ||
With these initial data in hand, we hypothesized that having a more reactive nucleophile would allow for formation of the desired complexes. Thus, we attempted the aminesulfonamides 6 which, as primary amines, should undergo rapid addition to the electrophilic platinum (Scheme 2).
 | ||
| Scheme 2 Strategy for the synthesis of mono-N-sulfonamide platinum complexes. | ||
Along these lines, we selected for our efforts propane-1,3-diamine and the bulkier diaminerac-trans-1,2-cyclohexanediamine. We were pleased to find that upon addition of the propane-1,3-diamine to a dilute solution of the commercially available 2,4,6-trimethylphenylsulfonyl chloride 7a in CH2Cl2 at room temperature, bis-sulfonylation was not observed and the mono-sulfonamide 6a was isolated in good yield (eqn (a), Scheme 3). In order to obtain the bulkier ligand 6b, we carried out the same reaction with the rac-trans-1,2-cyclohexanediamine and the sulfonyl chloride 7a. A similar result was obtained when p-toluene-sulfonyl chloride 7b (instead of 2,4-6-trimethylbenzene sulfonyl chloride 7a) was used (eqn (b), Scheme 3). In addition, we were able to incorporate 5-(dimethylamino)naphthalene-1-sulfonyl chloride (dansyl chloride) 7c which can act as fluorophore unit21 and could be useful for in vivo evaluation (eqn (c), Scheme 3). These results indicate that this is a general methodology for the synthesis of mono-sulfonamide ligand 6.
 | ||
| Scheme 3 Synthesis of mono-N-sulfonamide platinum complexes 8a–d. | ||
With ligands 6a–d in hand, we accomplished the synthesis of their corresponding platinum complexes 8a–d by the addition of the N-sulfonamides 6a–d to a solution of cis-PtCl2(DMSO)2 in methanol at room temperature. After 48–64 hours, a yellow precipitate was obtained. The reaction mixture was filtered and the resulting solid was washed with cold methanol (see the ESI† for more details) to provide pure compounds without further purification and in good yields (right, Scheme 3). The trans-configuration of the complex 8a was confirmed by X-ray analysis (Fig. 2)22 and 8a–c were assumed to have the same configuration based on similarity in the NMR data (see the ESI†).
 | ||
| Fig. 2 X-Ray analysis ORTEP of compound 8a. Ellipsoids displayed at 30% probability. | ||
Interaction experiments and biological evaluation
Before attempting more advanced biological studies, we performed some simple experiments in order to better understand the possible interaction between these new complexes 8a–d and DNA. One model system with which to perform these interaction experiments was the guanosine monophosphate (GMP). NMR experiments were not possible due to their broad signals and the difficulty of clearly identifying the interaction of the complex with GMP. Therefore, we incubated the sulfonamide complex 8d (the most UV active complex due to the fluorophore unit21) with the commercially available guanosine monophosphate (GMP), and studied their interaction by UV-visible spectrophotometry which is outlined in Fig. 3.23 Two characteristic absorption peaks were observed for the complex 8d at t = 0 hours (240 nm and 330 nm). After 24 h incubation at 37 °C, the absorption band at 240 nm became more intense, and a new absorption at 296 nm appeared, suggesting that an interaction between GMP and the platinum complex 8d had taken place. These data could be consistent with the antiproliferative test which is usually carried out at 24–48 h (see below).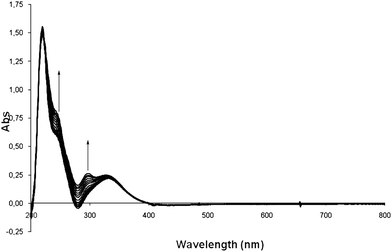 | ||
| Fig. 3 Interaction of compound 8d with GMP at different times. | ||
Following these initial trials, and in order to confirm the interaction between complexes 8 and GMP, various mass spectrometry experiments were carried out. Thus, we found that incubation of complex 8d with GMP, analyzed by electrospray ionization-mass spectrometry (ESI-MS),24 provided three main signals (Table 1 and ESI†). The first peak at 362 m/z matched with the free GMP. The two additional major peaks (585 and 902 m/z) displayed the typical isotope patterns for platinum compounds, indicating that complexation of the platinum atom with GMP has occurred. According to the proposed complexation mechanisms,25 an aquation of one chloride ligand of complex 8d should take place, and the corresponding mono-aqua-complex of 8d could then interact with GMP. This extent was confirmed by the peak observed at mass 902 m/z (entry 3, Table 1). Further evidence is offered by the substitution of the sulfonamide ligand with MeOH, which gives the peak at 585 m/z, corresponding to the GMP/Pt complex without the sulfonamide group.
| Entry | m/z | Adduct |
|---|---|---|
| 1 | 362 | (Free GMP) |
| 2 | 585 | Pt(GMP) + MeOH |
| 3 | 902 | Pt(GMP)(L) |
The antiproliferative activity of 8a–d was evaluated against a panel of representative human tumor cell lines including HBL-100 (breast), HeLa (cervix), Ishikawa (endometrial), SW1573 (non-small cell lung) and WiDr (colon), using the SRB assay.26 The experimental GI50 values are summarized in Table 2 and compared to those of CDDP after 48 h of treatment. Notably, for most of the selected cell lines, complex 8a was found to be less active than cisplatin, except for WiDr (colon) which was similar to CDDP (entry 1, Table 2). In the same context, several drugs based on platinum chemistry have the trans-1,2-cyclohexanediamine group in their structure (see Fig. 4). Thus, e.g.Oxaliplatin (Eloxatin™) is a recent world-wide approved drug by FDA in combination with 5-fluorouracil and leucovorin for patients with colorectal cancers (top-left, Fig. 4). Other relevant examples with the cyclohexanediamine moiety are Aroplatin, Ormaplatin (also known as tetraplatin and NSC 363812) and TRK-710 which have been in recent clinical trials and with different results (Fig. 4). For these reasons we chose the 1,2-trans-cyclohexanediamine, and we synthesized complexes 8b–d (Scheme 3).
|
|
||||||
|---|---|---|---|---|---|---|
| Entry | Complex | HBL-100 | HeLa | Ishikawa | SW1573 | WiDr |
| a Data were collected after 48 h of exposure to the drugs. Values are given in μM (between brackets are the standard deviation results and are the means of 3–5 experiments). | ||||||
| 1 | 8a | 28 (±5.4) | 23 (±5.2) | 17 (±1.6) | 27 (±2.9) | 25 (±3.0) |
| 2 | 8b | 2.2 (±0.22) | 2.2 (±0.06) | 1.8 (±0.28) | 3.4 (±0.31) | 2.6 (±0.69) |
| 3 | 8c | 1.7 (±0.28) | 2.3 (±0.34) | 2.6 (±0.91) | 2.5 (±0.51) | 3.5 (±1.0) |
| 4 | 8d | 1.7 (±0.65) | 1.8 (±0.71) | 2.3 (±0.12) | 2.6 (±0.62) | 3.0 (±0.34) |
| 5 | CDDP | 1.9 (±0.16) | 2.0 (±0.32) | 8.9 (±1.3) | 3.0 (±0.37) | 26 (±5.3) |
 | ||
| Fig. 4 Different platinum complexes with the 1,2-cyclohexanediamine. | ||
Interestingly, the change of a linear alkyl chain (8a) to a cyclic hexyldiamine (8b) increased the biological activity against all the tested cell lines. Compound 8b was 10 fold more potent than the n-propyl derivative 8a, and more interestingly, it was also more active than CDDP against WiDr and Ishikawa cell lines (entries 2 and 5, Table 2). The substitution of the 2,4,6-trimethylbenzene (8b) by a tolyl group (8c) resulted in only minor differences, and has also a slight change for some cell lines (see entries 3 and 4).
Finally, for practical reasons, we synthesized the fluorophore derivative 8d, in order to follow the compound due to its fluorescent properties.21 We found the best activities for this compound, which was active against all cell lines, with a similar activity against HBL-100, HeLa, SW1573 and four-eight times higher in WiDr and Ishikawa cell lines when compared to CDDP. These results indicated again that a bulkier group at the sulfonamide moiety has a positive effect in the antiproliferative activity.
Conclusions
In this work, a comparison of the antiproliferative activities between novel trans-N-sulfonamide platinum complexes and CDDP was described. To the best of our knowledge, this is the first report of the antiproliferative activity of sulfonamide platinum complexes. We provide important and relevant data for the synthesis of future new platinum complexes with sulfonamide ligands. The synthesis of these novel platinum complexes is easy and rapidly accessible in only two synthetic steps from commercially available starting materials. We are currently running in vivo studies in order to study the maximum tolerated dose of these novel drugs and the results will be reported elsewhere.Acknowledgements
We acknowledge grants co-financed by the European Social Fund (FEDER) from the Spanish MEC (SAF2009-09431), the Spanish MICINN (CTQ2008-06806-C02-01/BQU), the Spanish MSC (RTICC RD06/0020/1046), the Canary Islands ACIISI (PI2007/021), and the Canary Islands FUNCIS (PI 35/06 and 43/09). J.A. and J.M.P. thank the Spanish MICINN for Ramón y Cajal contracts. V.S. thanks the Spanish MICINN for a pre-doctoral fellowship. We acknowledge the technical assistance of Cesar Pastor and SIdI (UAM) for the X-ray analysis.Notes and references
- (a) For recent reviews in the use of metal compounds in medicinal chemistry, see: M. A. Jakupec, M. Galanski, V. B. Arion, C. G. Hartinger and B. K. Keppler, Dalton Trans., 2008, 183 RSC; (b) T. W. Hambley, Dalton Trans., 2007, 4929 RSC; (c) R. W.-Y. Sun, D.-L. Ma, E. L.-M. Wong and C. M. Che, Dalton Trans., 2007, 4884 Search PubMed; (d) S. P. Fricker, Dalton Trans., 2007, 4903 RSC; (e) G. Gasser, I. Ott and N. Metzler-Nolte, J. Med. Chem., 2011, 54, 3 CrossRef CAS; (f) A. M. Pizarro, A. Habtemariam and P. J. Sadler, Top. Organomet. Chem., 2010, 32, 21 CrossRef CAS; (g) S. H. Rijt and P. J. Sadler, Drug Discovery Today, 2009, 14, 1089 CrossRef; (h) P. C. A. Bruijnincx and P. J. Sadler, Adv. Inorg. Chem., 2009, 61, 1 CrossRef CAS; (i) A. M. Pizarro and P. J. Sadler, Nucleic Acid–Metal Ion Interactions, ed. N. V. Hud, 2009, pp. 350–416 Search PubMed.
- B. Rosenberg, Interdiscip. Sci. Rev., 1978, 3, 134 CAS.
- P. J. O'Dwyer, J. P. Stevenson and S. W. Johnson, Drugs, 2000, 59 (suppl. 4), 19 CrossRef CAS.
- (a) For recent reviews in platinum anticancer complexes, see: M. Bruyninx and P. J. Sadler, Curr. Opin. Chem. Biol., 2008, 12, 197 CrossRef; (b) L. Kelland, Expert Opin. Invest. Drugs, 2007, 16, 1009 CrossRef CAS.
- (a) J. Alemán, V. del Solar and C. Navarro-Ranninger, Chem. Commun., 2010, 46, 454 RSC; (b) J. Alemán, V. del Solar, L. Cubo, A. G. Quiroga and C. Navarro-Ranninger, Dalton Trans., 2010, 39, 10601 RSC.
- See e.g.: L. Messori, A. Casini, C. Gabbiani, E. Milchelucci, L. Cubo, C. Ríos-Lucí, J. M. Padrón, C. Navarro-Ranninger and A. G. Quiroga, ACS Med. Chem. Lett., 2010, 1, 381 CrossRef CAS.
- (a) N. Anand, Sulfonamides and Sulfones, in Burger's Medicinal Chemistry and Drug Discovery, ed. M. E. Wolff, Therapeutic Agents, J. Wiley & Sons, New York, 5th edn, 1996, Vol. 2, pp. 527–544 Search PubMed; (b) C. T. Supuran and A. Scozzafava, Expert Opin. Ther. Pat., 2002, 12, 217 CrossRef CAS; (c) A. Scozzafava, T. Owa, A. Mastrotorenzo and C. T. Supuran, Curr. Med. Chem., 2003, 10, 925 CrossRef CAS.
- The Carbonic Anhydrases New Horizonts, ed. W. R. Chegwidden, N. D. Carter and Y. H. Edwards, Birkhauser, Basel, 2000 Search PubMed.
- (a) M. F. Sugrue, Pharmacol. Ther., 1989, 43, 91 CrossRef CAS; (b) M. F. Sugrue, J. Med. Chem., 1997, 40, 2793 CrossRef CAS; (c) C. T. Supuran and A. Scozzafava, Curr. Med. Chem.: Immunol., Endocr. Metab. Agents, 2001, 1, 61 CrossRef CAS.
- M. Fournel, M.-C. Trachy-Bourget, P. T. Yan, A. Kalita, C. Bonfils, C. Beaulieu, S. Frechette, S. Leit, E. Abou-Khalil, S.-H. Woo, D. Delorme, A. R. MacLeod, J. M. Besterman and Z. Li, Cancer Res., 2002, 62, 4325 CAS.
- R. Mohan, M. Banerjee, A. Ray, T. Manna, L. Wilson, T. Owa, B. Bhattacharyya and D. Panda, Biochemistry, 2006, 45, 5440 CrossRef CAS.
- K. Hashimoto, M. Tatsuta, M. Kataoka, K. Yasoshima, Y. Shogase, M. Shimzaki, T. Yura, Y. Li, N. Yamamoto, J. B. Gupta and K. Urbanhs, Bioorg. Med. Chem. Lett., 2005, 15, 799 CrossRef CAS.
- M. Wang, M. Gao, K. D. Miller, G. W. Sledge, G. D. Hutchins and Q.-H. Zheng, J. Labelled Compd. Radiopharm., 2008, 51, 6 CrossRef CAS.
- J. J. Becker, P. S. White and M. R. Cagné, Inorg. Chem., 1999, 38, 798 CrossRef CAS.
- C. Evans, W. Henderson and B. K. Nicholson, Inorg. Chim. Acta, 2001, 314, 42 CrossRef CAS.
- A. M. Christoforou, P. A. Marzilli and L. G. Marzilli, Inorg. Chem., 2006, 45, 6771 CrossRef CAS.
- A. V. Pawlikowski, A. D. Getty and K. I. Goldberg, J. Am. Chem. Soc., 2007, 129, 10382 CrossRef CAS.
- See, e.g.: (a) A. G. Quiroga, J. M. Pérez, C. Alonso, C. Navarro-Ranninger and N. Farrell, J. Med. Chem., 2006, 49, 224 CrossRef CAS; (b) F. J. Ramos-Lima, A. G. Quiroga, B. García-Serrelde, F. Blanco, A. Carnero and C. Navarro-Ranninger, J. Med. Chem., 2007, 50, 2194 CrossRef CAS; (c) L. Cubo, T. W. Hambley, P. Sanz Miguel, A. Carnero, C. Navarro-Ranninger and A. Quiroga, Dalton Trans., 2011, 40, 344 RSC; (d) L. Cubo, M. Groessl, P. Dyson, A. Quiroga, C. Navarro-Ranninger and A. Casini, ChemMedChem, 2010, 5, 1335 CrossRef CAS; (e) L. Cubo, A. M. Pizarro, A. Quiroga, L. Salassa, C. Navarro-Ranninger and P. J. Sadler, J. Inorg. Biochem., 2010, 104, 909 CrossRef CAS; (f) F. Ramos-Lima, V. Moneo, A. G. Quiroga, A. Carnero and C. Navarro-Ranninger, Eur. J. Med. Chem., 2010, 45, 134 CrossRef CAS.
- For a review in cisplatin resistance, see: B. Köberle, M. T. Tomicic, S. Usanova and B. Kaina, Biochim. Biophys. Acta, Gen. Subj., 2010, 1806, 172 Search PubMed.
- J. M. Perez, L. R. Kelland, E. Montero, F. E. Boxall, M. A. Fuertes, C. Alonso and C. Navarro-Ranninger, Mol. Pharmacol., 2003, 63, 933 CrossRef CAS.
- See e.g. (a) J. Rohacova, M. L. Marin, A. Martínez-Romero, J. E. O'Connor, M. J. Gomez-Lechon, M. T. Donato, J. V. Castell and M. A. Miranda, Org. Biomol. Chem., 2009, 7, 4973 RSC; (b) A. Misra, S. Mishra and K. Misra, Bioconjugate Chem., 2004, 15, 638 CrossRef CAS; (c) N. Wanichacheva, S. Watpathomsub, V. S. Lee and K. Grudpan, Molecules, 2010, 15, 1798 CrossRef CAS.
- CCDC 805611 (8a) contains the supplementary crystallographic data†.
- The platinum complex 8d was not completed soluble in H2O and the GMP was not soluble in MeOH. For this reason the experiment was carried out in a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture water/MeOH.
1 mixture water/MeOH. - (a) C. G. Hartinger, W. H. Ang, A. Casini, L. Messori, B. Keppler and P. J. J. Dyson, J. Anal. At. Spectrom., 2007, 22, 960 RSC; (b) A. Casini, C. Gabbiani, E. Michelucci, G. Pieraccini, G. Moneti, P. J. Dyson and L. Messori, JBIC, J. Biol. Inorg. Chem., 2009, 14, 761 CrossRef CAS; (c) A. Casini, A. Guerri, C. Gabbiani and L. Messori, J. Inorg. Biochem., 2008, 102, 995 CrossRef CAS; (d) A. Casini, C. Gabbiani, G. Mastrobuoni, R. Z. Pellicani, F. P. Intini, F. Arnesano, G. Natile, G. Moneti, S. Francese and L. Messori, Biochemistry, 2007, 46, 12220 CrossRef CAS; (e) A. Casini, C. Gabbiani, G. Mastrobuoni, L. Messori, G. Moneti and G. Pieraccini, ChemMedChem, 2006, 1, 413 CrossRef CAS.
- (a) S. J. Berners-Price, T. A. Frenkiel, U. Frey, J. D. Ranford and P. J. Sadler, J. Chem. Soc., Chem. Commun., 1992, 789 RSC; (b) S. J. Berners-Price, T. A. Frenkiel, U. Frey, J. D. Ranford and P. J. Sadler, J. Chem. Soc., Chem. Commun., 1992, 789 RSC; (c) For a recent publication, see: L. Cubo, A. G. Quiroga, J. Zhang, D. S. Thomas, A. Carnero, C. Navarro-Ranninger and S. J. Berners-Price, Dalton Trans., 2009, 3457 RSC , and references cited therein.
- P. O Miranda, J. M. Padrón, J. I. Padrón, J. Villar and V. S. Martín, ChemMedChem, 2006, 1, 323 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC reference number 805611. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1md00070e |
| This journal is © The Royal Society of Chemistry 2011 |

