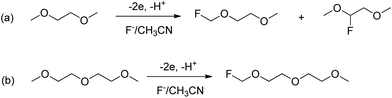
Glymes as versatile solvents for chemical reactions and processes: from the laboratory to industry
Shaokun Tang
a and
Hua Zhao
*b
aKey Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering & Technology, Tianjin University, Tianjin 300072, China
bDepartment of Chemistry and Forensic Science, Savannah State University, Savannah, GA 31404, USA. E-mail: huazhao98@gmail.com; zhaoh@savannahstate.edu
First published on 18th December 2013
Abstract
Glymes, also known as glycol diethers, are saturated non-cyclic polyethers containing no other functional groups. Most glymes are usually less volatile and less toxic than common laboratory organic solvents; in this context, they are more environmentally benign solvents. However, it is also important to point out that some glymes could cause long-term reproductive and developmental damage despite their low acute toxicities. Glymes have both hydrophilic and hydrophobic characteristics that common organic solvents lack. In addition, they are usually thermally and chemically stable, and can even form complexes with ions. Therefore, glymes are found in a broad range of laboratory applications including organic synthesis, electrochemistry, biocatalysis, materials, Chemical Vapor Deposition (CVD), etc. In addition, glymes are used in numerous industrial applications, such as cleaning products, inks, adhesives and coatings, batteries and electronics, absorption refrigeration and heat pumps, as well as pharmaceutical formulations, etc. However, there is a lack of a comprehensive and critical review on this attractive subject. This review aims to accomplish this task by providing an in-depth understanding of glymes' physicochemical properties, toxicity and major applications.
Shaokun Tang was born in 1974, and graduated from Tianjin University (China) with BS, MS and PhD degrees in Chemical Engineering. She was a research fellow at the National University of Singapore from 2007 to 2008. She is currently an Associate Professor at Tianjin University. She was awarded “Excellent Teacher” of Tianjin University. Her major research interests focus on green chemistry (catalytic preparation of biofuels, supercritical fluid technology for natural compounds extraction, and ionic liquids for renewable energy), as well as functional materials & nanotechnology (controlled synthesis and functionalization of nanostructured materials). |
Hua Zhao studied chemistry (BS) and chemical engineering (MS) at Tianjin University (China) before he earned his PhD degree in 2002 from New Jersey Institute of Technology (NJIT) and completed a post-doctoral training at Rutgers University. He is currently an Associate Professor of Chemistry at Savannah State University. He became fascinated with new solvents at graduate school while working on organic synthesis and enzymatic resolution of amino acids in aqueous ionic liquids. His current research interests include biocatalysis in ionic liquids and glymes, preparation of biofuels using ionic liquids and glymes, synthesis of medicinal molecules with anti-cancer and anti-HIV properties, and microwave-assisted enzymatic reactions. |
1. Introduction
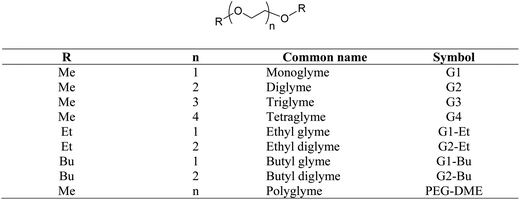
Glymes, i.e. glycol diethers, are saturated polyethers containing no other functional groups. When compared with glycols (such as polyethylene glycols, PEGs), glymes do not carry free hydroxyl groups and thus are aprotic polar and chemically inert compounds. There are two major types of glymes: ethylene oxide (EO)-based glymes (also known as PEG-based) and propylene oxide-based glymes (i.e. polypropylene glycol (PPG)-based). The general structure of PEG-based glymes and their common names are illustrated in Scheme 1.Most glymes are completely miscible with both water and hydrocarbon solvents, and could solvate alkali cations. In addition, glymes have many other favorable properties including being liquid at a wide range of temperatures (typically >200 °C, except monoglyme), low viscosity, high chemical and thermal stability, relatively low vapor pressure and low toxicity. Due to these excellent solvent properties, glymes have been widely used in many laboratory and commercial applications including reaction media (i.e. organometallic reactions, polymerization, and reactions involving alkali metals, oxidations and reductions), extraction solvents (for metals and organics), gas purification, absorption refrigeration, formulation of adhesives and coatings, textiles, solvents for electronic industry, pharmaceutical formulation, batteries, cleaning solutions, etc.
Despite numerous studies on glymes, there is a lack of a comprehensive and critical review on this subject to meet the continuing interest in glymes; in recent years, there has been a strong focus on their applications in electrochemistry, catalysts, Chemical Vapor Deposition (CVD), nanomaterials, and the dissolution of CO2. This review aims to provide a comprehensive set of physicochemical properties of glymes and a systematic overview of their applications, as well as a critical analysis of their structure–property–application relationships. To be focused on the subject, glycol monoethers and glycol ether esters are not discussed herein as their physical properties, toxicity and applications have been reviewed elsewhere.1–3
2. Preparations of glymes
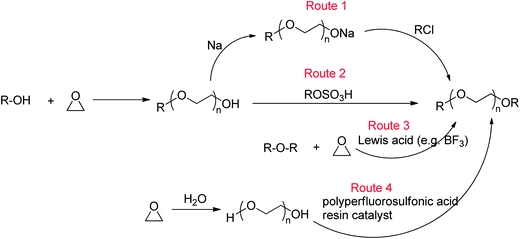
Ethylene oxide (EO)-based glymes can be prepared by several common methods on large scales using ethylene epoxide (Scheme 2): Route 1, ethylene oxide reacts with an alcohol to produce glycol monoether, which is further converted to glyme following the Williamson synthesis (i.e. glycol ether reacts with sodium to yield sodium alkoxide, which then reacts with alkyl halide to produce the glyme); Route 2, methylation of glycol ether with methyl sulfate; Route 3, Lewis acid-catalyzed cleavage of ethylene oxide by ether, typically resulting in glyme mixtures; Route 4, the reaction of ethylene glycol with alcohol catalyzed polyperfluorosulfonic acid resin at high temperature and pressure. Similarly, propylene oxide-based glymes can be prepared by replacing ethylene oxide with propylene oxide in the above methods.In addition, Haymore et al.4 described a two-step synthesis of pentaglyme (Scheme 3): the reaction of 2-methoxyethanol with sodium metal to yield an alkoxide followed by a nucleophilic substitution of dichloride. Adcock and Lagow5 prepared perfluoroglyme and perfluorodiglyme as potential high stability fluids and solvents through a direct fluorination method (fluorine gas).
3. Toxicity of glymes
In general, glymes exhibit low to moderate acute toxicity (see toxicity data in Tables 1 and 2) when compared with common organic solvents (such as toluene, THF and chloroform). Ethylene glycol dimethyl ether (monoglyme) triggered maternal deaths of pregnant Sprague-Dawley rats at 1000 mg per kg per day and was fetolethal at doses ranging from 120 to 1000 mg per kg per day; a dose of 60 mg per kg per day caused a 7% weight decrease and severe edema in pups surviving to birth.6 When rats were exposed to 200 ppm diglyme vapor for an extended period of time (15 × 6 h), no toxic effect was observed in terms of normal blood and urine tests and normal organs by autopsy; however, at a higher vapor concentration (600 ppm) for the same period of time, irregular weight gain was observed and autopsy suggested atrophied thymus and congested adrenals although the blood and urine tests were normal.7| Solvent | Oral rat LD50 (mg kg−1) | Bioaccumulation factor | Developmental toxicity | Mutagenicity |
|---|---|---|---|---|
| a Toxicity was estimated with the consensus method using Toxicity Estimation Software Tool (TEST) (http://www.epa.gov/nrmrl/std/qsar/qsar.html); disclaimer: these estimated values are for research and evaluation purposes, not for guiding clinical or production applications.b Experimental values from the ChemidPlus database (http://chem.sis.nlm.nih.gov/chemidplus/).c Experimental values from the CAESAR database (http://www.caesar-project.eu/index.php?page=results%26section=endpoint%26ne=5).d Experimental values from the Toxicity Benchmark database (http://doc.ml.tu-berlin.de/toxbenchmark/).e ‘+’ means mutagenicity positive, ‘−’ means mutagenicity negative. | ||||
| Ethanol | (7055b) | 1.28 | (1.00,c Toxicant) | −0.10 (+)e |
| Toluene | 1083 (636b) | 48.88 | 0.29 (non) | −0.01 (−) |
| Benzene | 2030 (931b) | 30.78 | 0.29 | 0.03 (+) |
| THF | 932 (1652b) | 2.67 | 0.48 | 0.05 (−) |
| Chloroform | 1794 (695b) | 5.07 (6.30 (ref. 19)) | 0.67 (Toxicant) | 0.45 (−) |
| Monoglyme | 2997 | 1.66 | 0.59 (Toxicant17) | 0.59 (1.00d) (+) |
| Diglyme | 3779 (5404b) | 2.04 | 0.43 (Toxicant17) | 0.38 |
| Triglyme | 6496 | 1.73 | 0.36 | 0.18 (1.00d) (+) |
| Tetraglyme | 5146 (5140b) | 6.97 | −0.10 | 0.23 |
| Pentaglyme | 5604 | 2.85 | −0.10 | 0.33 |
| Hexaglyme | 6112 | 6.02 | −0.06 | 0.26 |
| Ethyl glyme | 3949 (3619b) | 4.60 | 0.32 (Toxicant17) | 0.20 |
| Butyl glyme | 4427 (3253b) | 24.33 | 0.47 | 0.05 |
| Ethyl diglyme | 6818 (4968b) | 5.82 | 0.26 | 0.16 |
| Butyl diglyme | 3378 (3901b) | 15.57 | 0.05 | 0.23 |
| Glyme | Freezing point (°C) | Boiling point (°C) | Dynamic viscosity (mPa s) at 20 °C | p (mm Hg)d at 20 °C | Density (g mL−1)v at 25 °C | Flash pointe (°C) | LD50f (mg kg−1) | Solubility (20 °C) |
|---|---|---|---|---|---|---|---|---|
| a Ref. 40.b Production specifications from Novolyte Technologies (now a part of BASF).c Production specifications from Clariant (viscosity is kinematic viscosity).d Vapor pressure.e Closed cup.f Acute toxicity.g Ref. 41.h Ref. 42.i Ref. 43.j Ref. 44.k Ref. 45 (this reference also reported other thermodynamic properties of diglyme at 25 °C such as dipole moment μ = 1.87 D, heat capacity Cp = 276.9 J mol−1 K−1, etc.).l Ref. 46.m Ref. 47.n Ref. 48.o Ref. 49 (densities and viscosities of monoglyme at 308.15 K and 318.15 K were also reported).p Ref. 50.q Ref. 51.r Ref. 52 (this reference also reported a dielectric constant of 7.62 as well as refractive index and molar refraction for triglyme).s Ref. 53.t Ref. 54.u Ref. 55.v Density data for monoglyme and diglyme were reported at temperatures between 293.15 K and 353.15 K and up to 60 MPa.56w Ref. 57.x Ref. 58.y Ref. 59 (density data at 288.18 and 308.15 K were also reported).z Ref. 60.aa Ref. 61.bb Ref. 62 (densities and viscosities were reported for several glymes from 288.15 K to 343.15 K).cc Ref. 63.dd Ref. 64.ee Ref. 65.ff Ref. 66.gg Ref. 67 (densities, kinematic viscosities and heat capacities were reported for several glymes including pentaethylene glycol dimethyl ether from 283.15 to 423.15 K).hh Ref 68 (data on density, isentropic compressibility and isothermal compressibility of triglyme and tetraglyme were reported at 293.15–353.15 K and 0.1–100 MPa).ii Ref. 69.jj Ref. 70.kk Ref. 71.ll Ref. 72.mm Ref. 73 (densities, refractive indexes, and boiling points of other 1,2-disubstituted ethylene glycol derivatives such as propyl glyme were also reported).nn Ref. 74. | ||||||||
| Monoglyme (G1) | −69.0b,c,h | 85.2,b,h 85,c 84.5mm | 0.4236,o 0.432,q 0.417,bb 0.420,c 0.455,ll 1.1 (20 °C)b,h | 54b,h | 0.86124,j 0.86207,n 0.86132,o 0.8605,q 0.8613,s 0.86114,t,u 0.864,w 0.86260,y 0.86155,z 0.8615,bb 0.8626,cc,dd 0.8612,ii 0.859,ll 0.86370,mm, 0.8683 (20 °C),b,h 0.867 (20 °C),c 0.86765 (20 °C),gg | −6b,c,h | 5370b | Miscible with H2O |
| Ethyl glyme (G1-Et) | −74.0b,h | 121,b 121.4,h 121.2mm | 0.593,bb 0.7 (20 °C),b 0.65 (20 °C)h | 9,b 9.4h | 0.83607,z 0.8362,aa 0.8360,bb 0.83510,mm 0.8417 (20 °C),b,h | 27b | 4400b | 20.4% in H2O, 3.3% H2O inb |
| Butyl glyme (G1-Bu) | −69.1h | 203.6,h 206 (dec)mm | 0.09h | 0.83189,mm 0.8374 (20 °C)h | 85h (open cup) | 0.2 wt% in H2O, 0.6 wt% H2O inh | ||
| Diglyme (G2) | −64.0,a,c −70,g −68h | 162a,c,g | 0.981,m 1.06,x 0.989,bb 0.985,cc 0.991,jj 0.976,nn 2.0 (20 °C),b,h 1.14(20 °C)x | 2,b 3.0 (100 °C)h | 0.93873,j 0.93882,k 0.93924,l 0.9394,m 0.93892,n 0.93871,t 0.93935,u 0.938,w 0.93897,y 0.93875,z 0.9385,bb 0.9389,cc 0.9399,jj 0.9397,kk 0.93961,nn, 0.9434 (20 °C),a 0.944 (20 °C),c 0.945 (20 °C),g,h 0.94511 (20 °C)gg | 57,b 51c | 4670b | Miscible with H2O, ethanol, diethyl ether |
| Ethyl diglyme (G2-Et) | −44.3b | 189b | 1.238,bb 1.241,cc 1.4 (20 °C)b | 0.5b | 0.9082 (20 °C),b 0.9028,z 0.9021,bb 0.9035cc | 90b | 5000b | Miscible with H2O |
| Butyl diglyme (G2-Bu) | −60.2,b −60c | 256b,c | 2.122,cc 2.4 (20 °C)b | <0.01b | 0.8814 (20 °C),b 0.884 (20 °C),c 0.87830,z 0.8781cc | 118,b 120c | 3900b | 0.3% in H2O, 1.4% H2O inb,c |
| Triglyme (G3) | −43.8,a −40,c −45.0h | 218,a 220,c 216h | 1.96,nn,r 2.16,x 1.950,cc 3.8 (20 °C),b,h 2.41 (20 °C)x | 0.02b,h | 0.98001,i 0.98067,j 0.98117,l 0.9795,r 0.98058,t,u 0.981,w 0.98071,y 0.9807,cc 0.98042,ee 0.97981,ff 0.98023,nn, 0.986 (20 °C),a,h 0.987 (20 °C),c 0.98569 (20 °C),gg 0.98541 (20 °C)hh | 111,b,h 113c | 5000b | Very soluble in H2O, benzene |
| Tetraglyme (G4) | −29.7,b,h −30c | 275b,c,h | 3.295,p 3.67,x 3.40,nn 4.1 (20 °C),b,h 4.18 (20 °C)x | <0.01b,h | 1.00662,i 1.00564,j 1.00627,l 1.0047,p 1.00666,t 1.00668,u 1.00628,y 1.00620,ee 1.00743,nn, 1.0132 (20 °C),b,h 1.0 (20 °C),c 1.0116 (20 °C),gg 1.01115 (20 °C)hh | 141b,c,h | 5100b | Miscible with H2O |
| Proglyme (P2) | −71b, −80c | 175b,c | 1.1 (20 °C)b | 0.55b | 0.900 (20 °C)b,c | 65b,c | — | 35% in H2O, 4.5% H2O inb,c |
| PEG-DME 200 | −37c | >300c | 4.3 mm2 s−1 (20 °C)c | — | 1.01–1.02 (20 °C)c | 154c | — | |
| PEG-DME 250 | −23c | >300c | 7 mm2 s−1 (20 °C),c 7.215 mm2 s−1 (20 °C),gg | — | 1.02–1.04 (20 °C),c 1.0355 (20 °C)gg | 137c | — | |
| Polyglyme (MW = 236)b | −28b | 275b | 12 (20 °C)b | 0.01b | 1.03(20 °C)b | 135b | — | |
| Polyglyme (MW = 275)b | −23b | 275b | 12 (20 °C)b | <0.01b | 1.04 (20 °C)b | >130b | — | Miscible with H2O |
| Higlyme (MW > 400)b | -5–10b | >300b | 34 (20 °C)b | 0.1b | 0.975 (20 °C)b | 140b | — | Miscible with H2O |
| PEG-DME 500 | 13c | >300c | 25 mm2 s−1 (20 °C)c | — | 1.05 (50 °C)c | 220c | — | |
| PEG-DME 1000 | 36c | >300c | 11 mm2 s−1 (100 °C)c | — | 1.10 (50 °C)c | 260c | — | |
| PEG-DME 2000 | 50c | >300c | 30 mm2 s−1 (100 °C)c | — | 1.08 (60 °C)c | 254c | — | |
However, there is a rising concern that glymes may cause reproductive and developmental harm to exposed workers and consumers using paint, carpet cleaners, inkjet cartridges and other products. McGregor et al.8 studied the exposure of male rats to 250 or 1000 ppm diglyme, and found that diglyme was a reproductive toxicant causing increased sperm abnormalities. Schuler et al.9 examined fifteen glycol ethers for their adverse reproductive toxic effects using an in vivo mouse screening bioassay; this group found that all mice exposed to glycol ethers with terminal methyl groups, i.e., ethylene glycol monomethyl ether, monoglyme, diethylene glycol monomethyl ether, diglyme and triglyme produced few viable litters (0, 0, 16, 0, and 0%, respectively); similar results were also observed for ethylene glycol monoether ether and ethyl monoglyme (0 and 11% viable litters, respectively). However, two other ethyl ethers (diethylene glycol monoethyl ether and ethyl diglyme), three butyl ethers (ethylene glycol monobutyl ether, diethylene glycol monobutyl ether, butyl diglyme), and three glycol ethers with terminal hydroxyl groups (ethylene glycol, diethylene glycol and triethylene glycol) failed to show this kind of fetotoxicity. They also suggested that: (1) the appending of an alkyl group considerably increased the maternal toxicity of glycols. For example, ethyl glycol monobutyl ether appeared to be more toxic than ethylene glycol monomethyl ether, which was more toxic than ethylene glycol monoethyl ether; but all three showed greater toxicity than ethylene glycol. The diethylene glycol mono-alkyl ethers and (alkyl) diglymes were more toxic than diethylene glycol, and triglyme was more toxic than triethylene glycol. (2) Methyl ethers usually seem more toxic than ethyl or butyl ethers, except for ethylene glycol monobutyl ether. Similarly, Johnson et al.10 found that butyl diglyme was more toxic than diethylene glycol, but did not induce significant developmental toxicity to the hydra.
A review11 on the genetic toxicology of glycol ethers suggested that diglyme lacks genotoxic potential in some mutagenicity tests, but it was a reproductive toxicant in the mouse sperm test and male rat dominant lethal test. Repeated daily oral doses of diglyme at 684 mg kg−1 in a subchronic study of Sprague-Dawley rats suggested the onset of testicular pathology, which was similar to the pathology of equal molar doses of 2-methoxyethanol or 2-ethoxyethanol.12 Furthermore, it was confirmed that there were two major metabolites of the testicular toxin (e.g. diglyme), (2-methoxyethoxy)acetic acid (MEAA) (ca. 70%) and methoxyacetic acid (MAA) (ca. 6%), along with several other unidentified metabolites, and two major metabolism pathways; however, only MAA is accounted for in the reproductive toxicity of diglyme.13–15 Hardin and Eisenmann16 gave time-mated CD-1 mice oral doses of glycol ethers at a dose of 4 mmol kg−1 on gestation day 11, and then examined fetuses on gestation day 18 for weight and gross external malformations. All four glycol ethers (ethylene glycol monomethyl ether, monoglyme, diglyme and triglyme) exhibited no treatment-related maternal toxicity and no impact on the intrauterine survival; in addition, no treatment-related gross external malformations other than paw defects were reported. Except for triglyme, the other three glycol ethers produced different degrees of paw defects: 87.5% of ethylene glycol monomethyl ether-treated litters (68.5% of fetuses), 86.7% of monoglyme-treated litters (33.8% of fetuses), and 77.8% of diglyme-treated litters (39.7% of fetuses).
In conclusion, although glymes show low to moderate acute toxicity, they have raised concerns regarding chronic exposure and reproductive effects. Therefore, glymes are recommended as benign solvents for industrial applications, not consumer products. The U.S. Environmental Protection Agency (EPA) announced in July 2011 that three glymes pose “high concern to workers, consumers and children” because they may have reproductive or developmental effects: monoglyme and diglyme caused reproductive and developmental damage in rodent studies, and animal studies on ethyl glyme exhibited developmental toxicity and the potential for gene mutation; EPA also plans to restrict new uses of 11 other glymes in the U.S. marketplace.17 Since lower molecular weight glymes have shown reproductive toxicity in rats and mice, only higher molecular weight glymes should be used in pharmaceutical applications. In the European Union, products containing monoglyme or diglyme have been regulated; their labels must indicate “may impair fertility” or “may cause harm to the unborn child.” On the contrary, dipropylene glycol dimethyl ether is known as a very versatile and environmentally friendly solvent. It is not listed as a hazardous air pollutant (HAP), and it has been shown that propylene-based glycol ethers are less toxic than those ethylene-based glycol ethers.18
Although toxicity and biodegradability data are not readily available for many glymes, models based on Quantitative Structure Activity Relationships (QSARs) can be used to predict measures of toxicity from physical characteristics of chemical structures. As shown in Table 1, although there are differences between estimated and experimental values, the estimated toxicity may be used as an empirical evaluation of glymes. In general, the estimated toxicity data in Table 1 are consistent with earlier discussions based on experimental data: glymes have low acute toxicity (high oral rat LD50 values when compared with common organic solvents), however, there are concerns of their developmental toxicity and mutagenicity. Except for butyl glymes, methyl- and ethyl glymes seems to have relatively low bioaccumulation factors.
4. Physicochemical and metal complexing properties of glymes
4.1. General properties
Glymes are dipolar aprotic solvents with high chemical stability. They are usually stable under neutral and basic conditions, and only undergo pyrolysis under acidic conditions to form methanol and oxycarbonium ions.20 Some of their common physical and thermodynamic properties are systematically compiled in Table 2. Typically, glymes have high boiling points and a wide range of temperature at which they are liquid (>200 °C, or even >300 °C) except monoglyme. In contrast to some volatile organic solvents, most glymes have very low vapor pressures (<0.5 mmHg at 20 °C) except mono- and diglyme. Many simple glymes have low viscosities in the range 1–4 mPa s at 20 °C. Most glymes are completely miscible with both water and organic solvents (such as ethanol, acetone, benzene, and octane), and tend to solvate cations in the same way as crown ethers. They are also outstanding solvents for many organic materials (including triglycerides21,22). Different polarity measures of several glymes are listed in Table 3; dipole moments and dielectric constants increase with the increase in ethylene oxide chain length. The solvatochromic polarity scales in Table 3 also follow the same trend and confirm that glymes are generally less polar than methanol (ENT = 0.762) and acetone (0.355) but more polar than THF (0.207).23| Glyme | Dipole moment (D) at 25 °C | Dielectric constant at 25 °C | Solvatochromic polarity23 | Heat capacity at 25 °C (J mol−1 K−1) | |
|---|---|---|---|---|---|
| ET(30), kcal mol−1 | ENT | ||||
| Monoglyme | 1.62,75 1.61,57 1.59,76 1.71 (in benzene)76 | 7.18,77 7.20,72 7.5578 | 38.2 | 0.231 | 191.1479 |
| Diglyme | 1.91,75 1.92,57,76 1.87,45 1.97 (in benzene)76 | 7.480 | 38.6 | 0.244 | 277.76,79 276.9,45 279.0581 |
| Triglyme | 2.16,57,76 2.22 (in benzene)76 | 7.6252 | 38.9 | 0.253 | 367.78,79 367.3066 |
| Tetraglyme | 2.44,76 2.4555,76 | — | — | — | 457.1079 |
Proton affinity (PA) is a direct measure of gas-phase basicities of organic molecules. Meot-Ner24 calculated the proton affinities (in kcal mol−1) from measurements by pulsed high-pressure mass spectrometry as: monoglyme (205), diglyme (219), triglyme (226), 12-crown-4 (220) and 18-crown-6 (221). Sharma et al.25 determined the proton affinities (in kcal mol−1) of several glymes and crown ethers by measuring proton-transfer equilibria with a pulsed electron beam high ion source pressure mass spectrometer: Me2O (191), monoglyme (204), diglyme (218), triglyme (224), tetraglyme (227), 12-crown-4 (221), 15-crown-5 (223) and 18-crown-6 (230) [based on PA(NH3) = 204 kcal mol−1]. The ab initio calculations of 12-crown-4, 15-crown-5, 18-crown-6, glymes and protonated species suggest that protonated crown ethers share similar moieties with protonated diglyme; the calculated proton affinities (in kcal mol−1) are: diglyme (222), 12-crown-4 (221), 15-crown-5 (225), and 18-crown-6 (227).26 Using the B3LYP density functional method, Adötoledo et al.27 found that protonated monoglyme Gl·H+ and protonated monoglyme dimer (Gl)2H+, as well as protonated 12-crown-4-ether (12c4H+), form two internal hydrogen bonds with NH3, CH3OH, CH3NH2, and (CH3)2NH with the exception of (Gl)2H+·NH3 that bears four O⋯H bonds. The insertion energy of NH3, CH3OH and amines into 12c4H+ or (Gl)2H+ increases with increasing proton affinity of the base, while the association energy of CH3OH2+, NH4+, etc., with 12c4 or (Gl)2 decreases with increasing proton affinity (of NH3, CH3OH, etc.). However, in terms of protonation equilibrium constants (Kp), there are considerable differences between macrocyclic polyethers and linear glymes. For example, the Kp constant for the protonation of dicyclohexyl-18-crown-6 (DCC) by HBr at 25 °C is about 106 M−1, while the Kp values for diglyme and triglyme are only 0.17 and 0.20 M−1, respectively.28
Physical properties of aqueous glymes are important for the understanding of solvent properties of glymes. Meot-Ner et al.29 examined the complexing of H+ from the formation of intramolecular or intermolecular hydrogen bonds with glymes (monoglyme, diglyme or triglyme) and 0–3 water molecules by pulsed high-pressure mass spectrometry; they found that the bonding of H3O+ typically involves two or three hydrogen bonds, and complexes of H+ and H3O+ can be stabilized by interactions with bond dipoles of free ether groups of glymes and crown ethers whilst such a stabilization is enhanced by the decreasing constraints on the geometries of polar groups. Anomalous conformational behaviors of short glymes [CH3(OCH2CH2)mOCH3 with m = 2–6] in water were studied by FT-IR30 and Raman spectroscopy;31 both methods indicate that poly(ethylene oxide) chains progressively prefer the gauche (g) conformation for the OCH2–CH2O segment in the first stage, but this direction of the conformational preference is reversed for concentrations lower than a particular solution composition. Such an anomaly can be attributed to specific interactions and/or structures relevant to the glyme–water system. Furthermore, molecular dynamics simulations of monoglyme and diglyme in aqueous solutions32 confirmed that the g population of the O–C–C–O dihedral increases with the increase in water content; a further examination of the composition dependence of diglyme conformational populations implies that the decrease in the O–C–C–O g population in extremely dilute solutions can be attributed to a decrease in the tgt population of the C–O–C–C–O–C conformational triad. An atomistic force field for simulations of monoglyme reveals that the binding of monoglyme to water is comparable to water–water binding in water dimers, suggesting strong hydrogen bonding between monoglyme and water.33 Bedrov and Smith34 found that the properties of monoglyme–water solutions in a water model depend on the solution composition; in dilute solutions, structural and conformational properties are almost independent of the water model. However, short glymes [CH3(OCH2CH2)mOCH3 with m = 1–4] in formamide showed a different conformational behavior based on a Raman spectroscopic study:35 for the solutions with solute mole fractions between 0.5 and 0.01, the population of the gauche conformation for the OCH2–CH2O segment increases progressively with increasing solvent fraction. Monte Carlo simulations of monoglyme in water at 298 and 398 K indicate that the anti–anti–anti conformer is the only conformer that increases its probability with temperature; the anti–anti–gauche conformer is the most populated one among all the types.36 The Bernal group37,38 investigated the apparent molar volumes and adiabatic compressibilities of glymes (e.g. triglyme and tetraglyme) and crown ethers (e.g. 18-crown-6, 15-crown-5 and 12-crown-4) in H2O and D2O; their results suggest that the hydration of crown ethers increases with their size and is predominated by the hydrophobic hydration of –CH2– groups; they also indicate that there is a subtle difference between the hydration of the –CH2CH2O– group in crown and straight-chain compounds, and the compressibility data reveal a more negative compressibility due to the addition of a –CH2CH2O– group to crown ethers compared to a similar addition to a straight-chain glyme. Douhéret et al.39 measured the densities and ultrasound speeds of aqueous solutions of di-, tri- and tetra-glyme, and determined the excess molar volumes and the excess molar isentropic compressions; the excess molar properties are generally negative values. The standard Gibbs energy changes associated with aggregate formation are negative, implying the aggregation is moderate in aqueous glymes (aggregation number N = 4–6), although these aggregates are less stable than those formed from self-assembled species containing hydroxyl groups.
Other experimental or theoretical studies of the physical properties of glymes or glyme-containing mixtures can be found in the literature: optical anisotropies of monoglyme, diglyme, triglyme and tetraglyme,82 excess heat capacities of liquid mixtures of triglyme and tetraglyme with cyclohexane as well as tetraglyme with n-heptane at 288.15, 298.15 and 308.15 K and at atmospheric pressure,83 weak self-association of glymes based on the evaluation of excess isobaric thermal expansion of glyme and alkane mixtures by an associated mixture model with equation of state contribution,84 excess molar volumes and excess molar isobaric heat capacities of glymes and ethyl acetate,44 excess molar volumes and viscosities of glyme and acetonitrile,85 excess thermodynamic and equilibrium properties of glyme–n-alkane mixtures,45,65,66,71,81 isobaric vapor–liquid equilibrium for the binary systems of monoglymes and alcohols,53 excess molar volumes of binary mixtures of glymes and 1-propanol,59,60,63 a calorimetric study of interactions between glyme and alcohol,61 dynamic viscosities of mixtures of refrigerant (HFC-134a) + glyme at different temperatures and pressures,86 excess molar enthalpies of mixtures of methanol or trifluoroethanol + glyme,87,88 static relative permittivities of the ternary system of 2-methoxyethanol + 1,2-dimethoxyethane + water from −10–80 °C,78 vapor–liquid equilibrium of binary systems consisting of monoglyme with toluene, methylcyclohexane, or (trifluoromethyl)benzene,69 solubility of HFC-134a refrigerant in glymes,89 densities, viscosities, and refractive indices of diglyme + cyclohexane or + 1,2,3,4-tetrahydronaphthalene,70 excess molar heat capacities of mixtures of glymes and various alkanes,79 and excess heat capacities of glyme–dimethylsiloxane systems at 25 °C,90 etc. López et al.91 estimated the densities, isothermal compressibilities, and isobaric thermal expansion coefficients of glymes in the temperature range 293.15 K–353.15 K at pressures up to 100 MPa from the PcT data (c is the speed of sound in glyme), and found that the indirect predictions matched the direct experimental values.
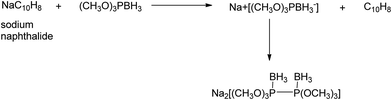
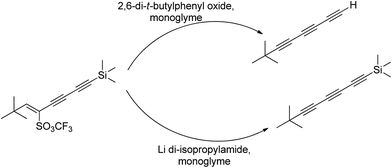
4.2. Ion complexing properties
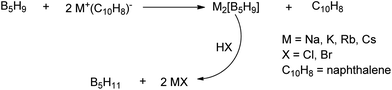
A unique feature of glymes is that they contain multiple ether-type oxygen atoms (similar to crown ethers) and flexible alkoxy chains. Therefore, they often behave like crown ethers in terms of solvating metal ions through oxygen–ion complexation (chelating) properties, which leads to many valuable applications. The chain flexibility was demonstrated by the spin–lattice relaxation of methoxy protons in glymes: the experimental T1−l values of glymes are significantly lower than those calculated for a rigid molecule although higher than those for completely free motion of methyl groups.92 This section places special emphasis on the ion chelation properties of glymes while their applications in electrochemistry and chemical reactions are discussed in later sections.A vivid illustration of the ion–glyme complexation is demonstrated in Fig. 1, where a lithium cation is chelated by a tridentate diglyme molecule. Matsui and Takeyama93 confirmed that Li+ is coordinated with six oxygen atoms in either monoglyme or diglyme; however, monoglyme is a bidentate ligand and diglyme is a tridentate ligand. The trans–cis isomerization of diglyme is necessary for the coordination to a single cation although it typically has a trans–trans conformation of minimum energy.94 Carvajal et al.72 studied the solvation behaviors of [Cs+][BPh4−], [Na+][BPh4−] and [Bu(i-Am)3+][BPh4−] in monoglyme and tetrahydrofuran (THF), and indicated that: (a) free Cs+ coordinates with monoglyme but not with THF, (b) in both monoglyme and THF, [Na+][BPh4−] primarily yields solvent-separated pairs which dissociate into solvent-coordinated Na+ ions, (c) however, [Bu(i-Am)3+][BPh4−] forms contact ion pairs in both media and dissociates into free Bu(i-Am)3+ and BPh4− ions not coordinated with the solvent. Using Electron Spin Resonance (ESR) spectroscopy, Hoefelmann et al.95 determined the equilibrium constant of sodium naphthalene and tetraglyme forming a loose ion pair to be 200–300 M−1 at 27 °C. Collins et al.96 determined the formation constants of tetraglyme separated ion pairs of bolaform electrolytes Na+, −Fl(CH2)nFl−, Na+ (Fl− is a fluorenyl carbanion; n = 2, 3, 4, or 6) in THF and tetrahydropyran (THP) at 25 °C. They suggested that it is easier to separate the ion-pair with tetraglyme in THP than in THF. For both monoglyme and diglyme, the lithium-ion solvation-shell coordination number is 4 at room temperature and 5 at a lower temperature based on the matrix-solvation FT-IR data.97 A molecular dynamics simulation study98,99 on different concentrations of NaI dissolved in dimethyl ether and monoglyme suggests that in diluted solutions, free ions exist as the most common ionic species, followed by ion pairs; with a further increase in salt concentration, ion pairs become the predominant species and many of them are in the form of clusters of 3–6 ions or more ions. Replacing dimethyl ether by monoglyme substantially reduces the ion clustering due to the chelating effect of glyme oxygens with the cation (Na+). At the highest concentration studied in monoglyme (i.e. an oxygen/cation ratio of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), free ions constitute ∼50% of the total ion concentration and neutral pairs constitute about 20%. For Na+–glyme systems, the electric current is mainly due to the movement of free ions and the relative movement of ions within loosely bound ion pairs. Based on the ab initio calculations of metal ion–glyme complexes,100,101 the Lindgren group suggested that the high chain flexibility of glymes enables many stable structures within a narrow energy range with very different geometrical arrangements of the ether oxygens, for example, at least 4 structures of different geometries for M-glyme complexes (M = Li+, Na+, K+, Mg2+ and Ca2+)100 and 11 structures of different geometries for the Li+–tetraglyme systems.101 The coordination numbers of lithium range from 4 to 6 for 1
1), free ions constitute ∼50% of the total ion concentration and neutral pairs constitute about 20%. For Na+–glyme systems, the electric current is mainly due to the movement of free ions and the relative movement of ions within loosely bound ion pairs. Based on the ab initio calculations of metal ion–glyme complexes,100,101 the Lindgren group suggested that the high chain flexibility of glymes enables many stable structures within a narrow energy range with very different geometrical arrangements of the ether oxygens, for example, at least 4 structures of different geometries for M-glyme complexes (M = Li+, Na+, K+, Mg2+ and Ca2+)100 and 11 structures of different geometries for the Li+–tetraglyme systems.101 The coordination numbers of lithium range from 4 to 6 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of Li+ with tetra-, penta- and hexaglyme, and the total binding energy increases with the glyme length; the coordination figures mainly consist of the quadratic pyramid, trigonal bipyramid and the trigonal prism type of geometries.101 Johansson et al.102 performed the ab initio calculations of 1
1 complexes of Li+ with tetra-, penta- and hexaglyme, and the total binding energy increases with the glyme length; the coordination figures mainly consist of the quadratic pyramid, trigonal bipyramid and the trigonal prism type of geometries.101 Johansson et al.102 performed the ab initio calculations of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (cation
1 (cation![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) molecule) complexes of lithium ions with linear oligomers, CH3X(CH2CH2X)nCH3, (n = 0–5; X = O, NH or S); their results suggest that the total binding energy increases with the increase in glyme chain length and follows the order NH > O > S. Shen et al.103 studied the complexing behavior between alkaline earth cations and crown ethers/triglyme in the gas phase, forming mainly sandwich complexes of doubly charged cations (12-crown-4)2M2+, (12-crown-4)(triglyme)M2+, and (triglyme)2M2+; it was also found that triglyme could be easily replaced by 18-crown-6 than 12-crown-4 possibly due to the rate-limiting step disrupting less cation–ligand interactions for the flexible ligand than for the rigid one. Henderson et al.104 indicated that both [Li2(CF3SO3)2(diglyme)] and [Li3(CF3CO2)3(diglyme)] contain five-coordinate Li+ cations coordinated by a tridentate diglyme molecule and two O atoms (each from separate anions) (see Fig. 2). Henderson et al.105 also suggested that the (monoglyme)2:LiClO4 crystals consist of contact ion pairs where the anions have bidentate coordination to the Li+ cations, whilst the (diglyme)2:LiClO4 crystals consist of fully solvated Li+ cations where the cations and anions do not directly interact. Grondin et al.106 further studied the Raman spectra of crystalline complexes of (monoglyme)2:LiClO4, (diglyme)2:LiClO4 and (triglyme)1:LiClO4, and established the vibrational assignment for ClO4− involved in the solvent-separated ion pair, contact ion pair and aggregate solvates. Dhuaml and Gejji107 employed ab initio Hartree–Fock and density functional calculations to study the electronic structure, charge distribution and vibrational characteristics of CH3O(CH2CH2O)nCH3 (n = 3–7). They found that the trans- conformation around C–C and C–O bonds of the backbone of tri- to hexaglymes leads to the lowest energy conformers; however, for heptaglyme (n = 7), the gauche-conformation around the C–C bonds affords a higher stability to the conformer.
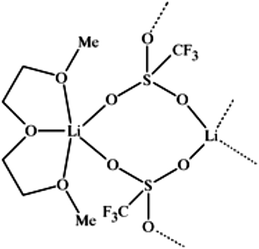
molecule) complexes of lithium ions with linear oligomers, CH3X(CH2CH2X)nCH3, (n = 0–5; X = O, NH or S); their results suggest that the total binding energy increases with the increase in glyme chain length and follows the order NH > O > S. Shen et al.103 studied the complexing behavior between alkaline earth cations and crown ethers/triglyme in the gas phase, forming mainly sandwich complexes of doubly charged cations (12-crown-4)2M2+, (12-crown-4)(triglyme)M2+, and (triglyme)2M2+; it was also found that triglyme could be easily replaced by 18-crown-6 than 12-crown-4 possibly due to the rate-limiting step disrupting less cation–ligand interactions for the flexible ligand than for the rigid one. Henderson et al.104 indicated that both [Li2(CF3SO3)2(diglyme)] and [Li3(CF3CO2)3(diglyme)] contain five-coordinate Li+ cations coordinated by a tridentate diglyme molecule and two O atoms (each from separate anions) (see Fig. 2). Henderson et al.105 also suggested that the (monoglyme)2:LiClO4 crystals consist of contact ion pairs where the anions have bidentate coordination to the Li+ cations, whilst the (diglyme)2:LiClO4 crystals consist of fully solvated Li+ cations where the cations and anions do not directly interact. Grondin et al.106 further studied the Raman spectra of crystalline complexes of (monoglyme)2:LiClO4, (diglyme)2:LiClO4 and (triglyme)1:LiClO4, and established the vibrational assignment for ClO4− involved in the solvent-separated ion pair, contact ion pair and aggregate solvates. Dhuaml and Gejji107 employed ab initio Hartree–Fock and density functional calculations to study the electronic structure, charge distribution and vibrational characteristics of CH3O(CH2CH2O)nCH3 (n = 3–7). They found that the trans- conformation around C–C and C–O bonds of the backbone of tri- to hexaglymes leads to the lowest energy conformers; however, for heptaglyme (n = 7), the gauche-conformation around the C–C bonds affords a higher stability to the conformer.
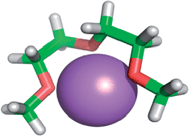 | ||
| Fig. 1 Lithium cation chelated by a tridentate diglyme molecule (molecular geometry obtained via a PM3 semi-empirical method in ArgusLab by Richard Terrett at the Australian National University). | ||
The Watanabe group108 prepared an equimolar complex [Li(glyme)1][Tf2N] (Tf2N− also known as TFSA− = bis(trifluoromethane)sulfonimide), which maintains a stable liquid state over a wide temperature range and exhibits a high thermal stability and Li+ ionic conductivity, behaving like a room-temperature ionic liquid (IL). The physicochemical properties (e.g. melting point and viscosity) of the glyme–Li salt complex can be manipulated by the glyme structure. The same group109 further observed a higher oxidative stability of glyme molecules when complexing with Li+ cations. They found that the electrochemical oxidation of [Li(glyme)1][TFSA] occurred at the electrode potential of ∼5 V vs. Li/Li+, while the oxidation of solutions with excess glyme molecules ([Li(glyme)x][TFSA], x > 1) occurred at a lower potential of ∼4 V vs. Li/Li+. Further ab initio molecular orbital calculations explained that the increased oxidative stability is due to the donation of lone pairs of ether oxygen atoms to the Li+ cation, lowering the highest occupied molecular orbital (HOMO) energy level of glyme molecules. The thermal stability of bis(dipivaloylmethanato)strontium [i.e. Sr(dpm)2] compounds containing glyme adducts [i.e. Sr(dpm)2–triglyme and Sr(dpm)2–tetraglyme] was examined by Cho et al.110 using thermogravimetric analysis, mass spectrometry, and FT-IR spectroscopy. They observed that glyme adducts are decomposed below 200 °C, while the Sr–O and the C–C(CH3)3 bonds are dissociated at higher temperatures, and the C–O and the C–C bonds are stable up to 400 °C. Glyme molecules in ion complexes weaken the Sr–O bond between the Sr atom and the dpm ligand, leading to the dissociation of the Sr–O bond in Sr(dpm)2–glymes at lower temperatures than the bond in Sr(dpm)2. However, Sr(dpm)2–glymes are less thermally degradable than Sr(dpm)2, resulting in fewer residues at elevated temperatures.
The stability constants (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K, i.e., equilibrium constants for complexing) for 18-crown-6 with Na+, K+, and Cs+ are 3–6 in methanol, and are 1.5–2.2 for pentaglyme.4,112 The stability constants (log
K, i.e., equilibrium constants for complexing) for 18-crown-6 with Na+, K+, and Cs+ are 3–6 in methanol, and are 1.5–2.2 for pentaglyme.4,112 The stability constants (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K) of 1
K) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of alkali cations M+ (Na+, K+, or Cs+) with glymes CH3O(CH2CH2O)nCH3 in methanol increase with n, which is different from the complexes between M+ and crown-ethers (–CH2–CH2–O–)n: their stability constants reached the maximum at n = 6 (Na+, K+) and 6, 7 (Cs+) (see Table 4).111,113,114 The stability constants of glymes are slightly lower than those of corresponding PEGs, but both of them are considerably lower than those of crown ethers.115 The higher stability of the complexes of crown-ethers compared to those of acyclic analogues (such as glymes) can be attributed to the so called “macrocyclic effect” (ME), which is a function of the polyether's topology and cation's size.111,112 The macrocyclic effect is not only observed in solution, but also in the gas phase formation of alkali metal cation-bound dimers of crown ethers or glymes: the rates for crown ethers were about an order of magnitude higher than those for glymes.116 Varnek et al.111 demonstrated that the Substructural Molecular Fragments method can be used to evaluate the stability constants of these complexes. The Smid group investigated the relative ligand affinities of glymes, crown ethers, polyamines and other cation-binding ligands toward lithium and sodium picrate in toluene117 and dioxane.118 The affinity of glymes in binding lithium and sodium picrate dramatically increases with increasing ether chain length up to tetraglyme, and a further increase in the affinity for longer glymes is primarily due to the increase in the number of binding sites. However, crown ethers generally have much higher binding equilibrium constants toward Li+ and Na+, which could be over 100 times higher than most glymes. Davidson and Kebarle119 indicated that monoglyme forms a stronger complex with K+ than ethylene diamine. Plewa-Marczewska et al.120 determined the formation constants (Ka) of ionic pairs between Li[CF3SO3] (or Li[BF4]) and glymes (monoglyme, diglyme or triglyme) using the anion state sensitive 19F NMR and cation 7Li NMR techniques, and found that the Ka values (log
1 complexes of alkali cations M+ (Na+, K+, or Cs+) with glymes CH3O(CH2CH2O)nCH3 in methanol increase with n, which is different from the complexes between M+ and crown-ethers (–CH2–CH2–O–)n: their stability constants reached the maximum at n = 6 (Na+, K+) and 6, 7 (Cs+) (see Table 4).111,113,114 The stability constants of glymes are slightly lower than those of corresponding PEGs, but both of them are considerably lower than those of crown ethers.115 The higher stability of the complexes of crown-ethers compared to those of acyclic analogues (such as glymes) can be attributed to the so called “macrocyclic effect” (ME), which is a function of the polyether's topology and cation's size.111,112 The macrocyclic effect is not only observed in solution, but also in the gas phase formation of alkali metal cation-bound dimers of crown ethers or glymes: the rates for crown ethers were about an order of magnitude higher than those for glymes.116 Varnek et al.111 demonstrated that the Substructural Molecular Fragments method can be used to evaluate the stability constants of these complexes. The Smid group investigated the relative ligand affinities of glymes, crown ethers, polyamines and other cation-binding ligands toward lithium and sodium picrate in toluene117 and dioxane.118 The affinity of glymes in binding lithium and sodium picrate dramatically increases with increasing ether chain length up to tetraglyme, and a further increase in the affinity for longer glymes is primarily due to the increase in the number of binding sites. However, crown ethers generally have much higher binding equilibrium constants toward Li+ and Na+, which could be over 100 times higher than most glymes. Davidson and Kebarle119 indicated that monoglyme forms a stronger complex with K+ than ethylene diamine. Plewa-Marczewska et al.120 determined the formation constants (Ka) of ionic pairs between Li[CF3SO3] (or Li[BF4]) and glymes (monoglyme, diglyme or triglyme) using the anion state sensitive 19F NMR and cation 7Li NMR techniques, and found that the Ka values (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = 3–6) are dependent on the salt concentration range used for calculation and are generally a few orders of magnitude lower than those estimated from the conductivity data.
Ka = 3–6) are dependent on the salt concentration range used for calculation and are generally a few orders of magnitude lower than those estimated from the conductivity data.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K) of glymes [CH3O(CH2CH2O)nCH3] and crown ethers with alkali ions in methanol at 25 °C111
K) of glymes [CH3O(CH2CH2O)nCH3] and crown ethers with alkali ions in methanol at 25 °C111
| Glyme, n = | Na+ | K+ | Cs+ | Crown ether | Na+c | K+c | Cs+c |
|---|---|---|---|---|---|---|---|
| a Calculated value.b Experimental value.c Selected value from the THECOMAC database. | |||||||
| 3 | 1.18a | 1.38a | 1.17a | 12c4 | 1.41 | 1.58 | 1.6 |
| 4 | 1.28b | 1.72b | 1.45b | 15c5 | 3.30 | 3.35 | 3.58 |
| 5 | 1.47b | 2.20b | 1.85b | 18c6 | 4.36 | 6.07 | 4.79 |
| 6 | 1.60b | 2.55b | 2.17b | 21c7 | 2.54 | 4.41 | 5.01 |
| 7 | 1.67b | 2.87b | 2.41b | 24c8 | 2.35 | 3.53 | 4.15 |
| 8 | 1.83a | 3.28a | 2.77a | 27c9 | 2.14 | 3.47 | 3.95 |
| 9 | 1.96a | 3.66a | 3.09a | 30c10 | 2.14 | 3.98 | 4.15 |
Many studies focus on the ion complexation of glymes in solution. Chan et al.121–123 studied the coordination of fluorenyllithium, -sodium, and -potassium (carbanion pairs) with various glymes: CH3O(CH2CH2O)nCH3 (1 ≤ n ≤ 6) in dioxane, THF, or tetrahydropyran (THP) using optical and NMR spectroscopy. In the case of fluorenylsodium, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 coordination complexes are formed for glyme-separated ion pairs with glyme-5 (n = 4), glyme-6 (n = 5) and glyme-7 (n = 6), but the separated ion pair contains two glyme molecules with glyme-4 and probably glyme-3. In the case of potassium salt, glyme-separated 1
1 coordination complexes are formed for glyme-separated ion pairs with glyme-5 (n = 4), glyme-6 (n = 5) and glyme-7 (n = 6), but the separated ion pair contains two glyme molecules with glyme-4 and probably glyme-3. In the case of potassium salt, glyme-separated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes are observed for glyme-6 and glyme-7, but glyme-4 and glyme-5 yield mainly glymated contact ion pairs and a second glyme molecule is required to convert them to separated ion pairs. Therefore, depending on the cation size and glyme chain length, the chelation of glyme with contact ion pairs results in either glymated contact ion pairs or glyme-separated ion pairs or a mixture of both. A further study on temperature dependence suggests that the reason for glymes being effective complexing agents of alkali ions is mainly due to a small loss in entropy as compared to solvent separated ion-pair formation in THF. Takaki and Smid124 titrated difluorenylbarium with glymes and crown ethers in THF at 25 °C under vacuum to examine the formation of ion pair–glyme and –crown ether complexes, and found that 1
1 complexes are observed for glyme-6 and glyme-7, but glyme-4 and glyme-5 yield mainly glymated contact ion pairs and a second glyme molecule is required to convert them to separated ion pairs. Therefore, depending on the cation size and glyme chain length, the chelation of glyme with contact ion pairs results in either glymated contact ion pairs or glyme-separated ion pairs or a mixture of both. A further study on temperature dependence suggests that the reason for glymes being effective complexing agents of alkali ions is mainly due to a small loss in entropy as compared to solvent separated ion-pair formation in THF. Takaki and Smid124 titrated difluorenylbarium with glymes and crown ethers in THF at 25 °C under vacuum to examine the formation of ion pair–glyme and –crown ether complexes, and found that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes are formed for difluorenylbarium with mono- and dibenzo-18-crown-6 as well as with glyme-7 and glyme-9 while a 2
1 complexes are formed for difluorenylbarium with mono- and dibenzo-18-crown-6 as well as with glyme-7 and glyme-9 while a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) l crown–ion pair complex is formed for monobenzo-15-crown-5. Canters58 analyzed the shift and line widths in 23Na NMR spectra of glyme solutions of NaBPh4 and NaBH4 as a function of temperature; it was found that the transition from solvent separated to contact ion pairs and the type of anions show a considerable impact on the position of alkali NMR signals, while the line width is typically a linear function of the viscosity of pure solvent divided by absolute temperature. Detellier and Laszlo125 observed the tetracoordination of Na+ when studying the 23Na NMR chemical shifts for NaClO4 in binary mixtures of glyme and tetrahydrofurfuryl alcohol. Although monoglyme tends to form monocyclic intermediates, diglyme and triglyme form bicyclic intermediates. Gilkerson and Jackson126 noticed that lithium picrate could be dissolved up to 1 mM in a solvent containing 1 equiv. of either triphenylphosphine oxide (TPPO), hexamethylphosphoramide (HMPA), triglyme, or tetraglyme; however, to dissolve 1 mM sodium picrate, at least a tenfold excess of either TPPO, HMPA, or tetraglyme is needed; furthermore, through the conductance and spectrophotometric measurements, they observed a very small extent of dissociation in 1
l crown–ion pair complex is formed for monobenzo-15-crown-5. Canters58 analyzed the shift and line widths in 23Na NMR spectra of glyme solutions of NaBPh4 and NaBH4 as a function of temperature; it was found that the transition from solvent separated to contact ion pairs and the type of anions show a considerable impact on the position of alkali NMR signals, while the line width is typically a linear function of the viscosity of pure solvent divided by absolute temperature. Detellier and Laszlo125 observed the tetracoordination of Na+ when studying the 23Na NMR chemical shifts for NaClO4 in binary mixtures of glyme and tetrahydrofurfuryl alcohol. Although monoglyme tends to form monocyclic intermediates, diglyme and triglyme form bicyclic intermediates. Gilkerson and Jackson126 noticed that lithium picrate could be dissolved up to 1 mM in a solvent containing 1 equiv. of either triphenylphosphine oxide (TPPO), hexamethylphosphoramide (HMPA), triglyme, or tetraglyme; however, to dissolve 1 mM sodium picrate, at least a tenfold excess of either TPPO, HMPA, or tetraglyme is needed; furthermore, through the conductance and spectrophotometric measurements, they observed a very small extent of dissociation in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand–metal picrate complexes and the formation of 2
1 ligand–metal picrate complexes and the formation of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand–cation complexes in the presence of additional ligand. Plewa et al.127 employed 19F NMR spectroscopy and conductivity data to study the ion pair formation of mixtures of salts (LiBF4 and LiCF3SO3) with 1,4-dioxane and glyme (or water). They suggested that mixtures containing monoglyme or diglyme have similar coordinating properties (in terms of donor and acceptor numbers) to liquid PEG dimethyl ether and solid PEG.
1 ligand–cation complexes in the presence of additional ligand. Plewa et al.127 employed 19F NMR spectroscopy and conductivity data to study the ion pair formation of mixtures of salts (LiBF4 and LiCF3SO3) with 1,4-dioxane and glyme (or water). They suggested that mixtures containing monoglyme or diglyme have similar coordinating properties (in terms of donor and acceptor numbers) to liquid PEG dimethyl ether and solid PEG.
The formation of glyme and salt into a crystal solid has been used to probe the ion complexing properties of glymes. Smid and Grotens128 reported the crystalline 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) l complexes of NaBPh4 with glyme-5, glyme-6 and glyme-7, as well as a 2
l complexes of NaBPh4 with glyme-5, glyme-6 and glyme-7, as well as a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) l complex with glyme-4. de Boer et al.129 studied alkali biphenyls (NaBp, KBp and RbBp) in triglyme or tetraglyme solutions by NMR and the crystal structures of NaBp·(2 triglyme), KBp·(2 tetraglyme) and RbBp·(2 tetraglyme) by X-ray diffraction; these crystals consist of solvent-separated ion pairs in the solid state. Magnetic experiments were performed for single crystals of these three systems, and the susceptibility measurements suggested a ferromagnetic coupling in NaBp·(2 triglyme) and KBp·(2 tetraglyme) and an antiferromagnetic coupling in RbBp·(2 tetraglyme). The Frech group130 analyzed the crystal structure of diglyme:LiCF3SO3 and found that the local environment of Li+ ions and the torsional angle sequence of bonds in ethylene oxide units are very similar to those in the high molecular weight poly(ethylene oxide)3:LiCF3SO3. On the other hand, their spectroscopic data of diglyme-LiCF3SO3 solutions suggest that Li+ is coordinated by only three oxygen atoms from diglyme and one oxygen atom from a CF3SO3− anion (i.e. five-fold coordinated) while triflate anions are two-fold coordinated. The same group131 further examined the crystal structures of monoglyme:(LiCF3SO3)2 and triglyme:(LiCF3SO3)2, and indicated in the case of monoglyme:(LiCF3SO3)2, that triflate anions are three-fold coordinated and lithium ions are four-fold coordinated whilst in the case of triglyme:(LiCF3SO3)2, triflate anions are three-fold coordinated and lithium ions are both four-fold and five-fold coordinated. They also observed the formation of trans–gauche–trans conformations for the bond order –O–C–C–O– in adjacent ethylene oxide sequences interacting with a five-coordinate lithium ion. Furthermore, this group132 characterized the crystalline phases of glyme:NaCF3SO3 using DSC, X-ray diffraction, and vibrational spectroscopy, and suggested that Na+ coordinates with 5 to 7 ether and triflate oxygens and more ethylene oxide units lead to higher coordination numbers. They also emphasized that these structures are the result of a convoluted effect of ion–ion interactions, ion-chain heteroatom interactions, and packing constraints from the organic chains. Henderson et al.133 characterized the crystalline phases of (triglyme)1:LiX (X = CF3SO3−, BF4−, ClO4− and AsF6−), and found that the phases are isostructural and are different from that of (triglyme)1:LiBPh4. The same group134 also studied the crystal structures of lithium salt complexes with tetraglyme including (tetraglyme)1:LiAsF6, (tetraglyme)1/2:LiBF4, and (tetraglyme)2/5:LiCF3CO2; and found a novel form of six-coordinate Li+ cation coordination by glyme oxygen atoms resembling double-helix dimers. The ionic association strength of LiX salts was investigated in a variety of aprotic solvents including glymes (see a short review in the Supporting Information of ref. 135), and is correlated with the crystallization kinetics of glyme–LiX and PEO–LiX mixtures.136,137 The approximate ionic association strength in aprotic solvents is shown below in an increasing order:135,136
l complex with glyme-4. de Boer et al.129 studied alkali biphenyls (NaBp, KBp and RbBp) in triglyme or tetraglyme solutions by NMR and the crystal structures of NaBp·(2 triglyme), KBp·(2 tetraglyme) and RbBp·(2 tetraglyme) by X-ray diffraction; these crystals consist of solvent-separated ion pairs in the solid state. Magnetic experiments were performed for single crystals of these three systems, and the susceptibility measurements suggested a ferromagnetic coupling in NaBp·(2 triglyme) and KBp·(2 tetraglyme) and an antiferromagnetic coupling in RbBp·(2 tetraglyme). The Frech group130 analyzed the crystal structure of diglyme:LiCF3SO3 and found that the local environment of Li+ ions and the torsional angle sequence of bonds in ethylene oxide units are very similar to those in the high molecular weight poly(ethylene oxide)3:LiCF3SO3. On the other hand, their spectroscopic data of diglyme-LiCF3SO3 solutions suggest that Li+ is coordinated by only three oxygen atoms from diglyme and one oxygen atom from a CF3SO3− anion (i.e. five-fold coordinated) while triflate anions are two-fold coordinated. The same group131 further examined the crystal structures of monoglyme:(LiCF3SO3)2 and triglyme:(LiCF3SO3)2, and indicated in the case of monoglyme:(LiCF3SO3)2, that triflate anions are three-fold coordinated and lithium ions are four-fold coordinated whilst in the case of triglyme:(LiCF3SO3)2, triflate anions are three-fold coordinated and lithium ions are both four-fold and five-fold coordinated. They also observed the formation of trans–gauche–trans conformations for the bond order –O–C–C–O– in adjacent ethylene oxide sequences interacting with a five-coordinate lithium ion. Furthermore, this group132 characterized the crystalline phases of glyme:NaCF3SO3 using DSC, X-ray diffraction, and vibrational spectroscopy, and suggested that Na+ coordinates with 5 to 7 ether and triflate oxygens and more ethylene oxide units lead to higher coordination numbers. They also emphasized that these structures are the result of a convoluted effect of ion–ion interactions, ion-chain heteroatom interactions, and packing constraints from the organic chains. Henderson et al.133 characterized the crystalline phases of (triglyme)1:LiX (X = CF3SO3−, BF4−, ClO4− and AsF6−), and found that the phases are isostructural and are different from that of (triglyme)1:LiBPh4. The same group134 also studied the crystal structures of lithium salt complexes with tetraglyme including (tetraglyme)1:LiAsF6, (tetraglyme)1/2:LiBF4, and (tetraglyme)2/5:LiCF3CO2; and found a novel form of six-coordinate Li+ cation coordination by glyme oxygen atoms resembling double-helix dimers. The ionic association strength of LiX salts was investigated in a variety of aprotic solvents including glymes (see a short review in the Supporting Information of ref. 135), and is correlated with the crystallization kinetics of glyme–LiX and PEO–LiX mixtures.136,137 The approximate ionic association strength in aprotic solvents is shown below in an increasing order:135,136
| beti−, Tf2N− < PF6− < ClO4−, I− < SCN− < BF4− < CF3SO3− < Br− < NO3− < CF3COO− < Cl− |
In addition to alkali metals, the complexing properties of glymes with other metal and organic cations, or even non-ionic molecules have also been studied. Timko et al.138 compared the complexation of t-butylammonium thiocyanate with pentaglyme and 18-crown-6 in chloroform at 24 °C, and found a macrocyclic effect of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700. The Bartsch group139 studied the complexing effect of p-tert-butylbenzenediazonium tetrafluoroborate with glymes in 1,2-dichloroethane at 50 °C, and suggested a macrocyclic effect of ∼30 when comparing the complexation constants (K) for pentaglyme and 18-crown-6 with aryldiazolium ions. They also observed that the K value is basically constant (∼2) for diglyme, triglyme and tetraglyme, followed by a steady increase for pentaglyme (4.78), hexaglyme (7.71), and heptaglyme (11.8) due to an increasing ability of the glyme to assume a pseudo cyclic structure; however, there is a drastic drop in K value for octaglyme (3.81) followed by a gradual increase for nonaglyme (6.32) and decaglyme (13.6). The same group140 further investigated the complexation of PEG and their dimethyl ethers with p-tert-butylbenzenediazonium tetrafluoroborate, and found that complexation constants for PEG 1000 and 1500 and their dimethyl ethers are 12–18% of that for 18-crwon-6. Relying on 1H NMR spectroscopy, Otera et al.141 suggested the formation of 1
700. The Bartsch group139 studied the complexing effect of p-tert-butylbenzenediazonium tetrafluoroborate with glymes in 1,2-dichloroethane at 50 °C, and suggested a macrocyclic effect of ∼30 when comparing the complexation constants (K) for pentaglyme and 18-crown-6 with aryldiazolium ions. They also observed that the K value is basically constant (∼2) for diglyme, triglyme and tetraglyme, followed by a steady increase for pentaglyme (4.78), hexaglyme (7.71), and heptaglyme (11.8) due to an increasing ability of the glyme to assume a pseudo cyclic structure; however, there is a drastic drop in K value for octaglyme (3.81) followed by a gradual increase for nonaglyme (6.32) and decaglyme (13.6). The same group140 further investigated the complexation of PEG and their dimethyl ethers with p-tert-butylbenzenediazonium tetrafluoroborate, and found that complexation constants for PEG 1000 and 1500 and their dimethyl ethers are 12–18% of that for 18-crwon-6. Relying on 1H NMR spectroscopy, Otera et al.141 suggested the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes between glymes, CH3O(CH2CH2O)nCH3 (n = 2, 3, 4), and dimethyltin dichloride (DMTC) in benzene, as well as both 1
1 complexes between glymes, CH3O(CH2CH2O)nCH3 (n = 2, 3, 4), and dimethyltin dichloride (DMTC) in benzene, as well as both 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 glyme–DMTC complexes for glymes (n = 5, 6) in benzene and with all glymes studied in toluene and 1-chloronaphthalene. Hirashima et al.142 suggested the formation of 1
2 glyme–DMTC complexes for glymes (n = 5, 6) in benzene and with all glymes studied in toluene and 1-chloronaphthalene. Hirashima et al.142 suggested the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes between lanthanoid chlorides and triethylene glycol, tetraethylene glycol, pentaethylene glycol, tetraglyme or pentaglyme. The same group143 found that lanthanoid nitrates [i.e. Ln(NO3)3] form solid complexes with polyethylene glycols and glymes at different compositions: 1
1 complexes between lanthanoid chlorides and triethylene glycol, tetraethylene glycol, pentaethylene glycol, tetraglyme or pentaglyme. The same group143 found that lanthanoid nitrates [i.e. Ln(NO3)3] form solid complexes with polyethylene glycols and glymes at different compositions: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Ln
1 (Ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand) for triethylene glycol, tetraethylene glycol, pentaethylene glycol and tetraglyme, 1
ligand) for triethylene glycol, tetraethylene glycol, pentaethylene glycol and tetraglyme, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Ln
2 (Ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand) for diethylene glycol, 2
ligand) for diethylene glycol, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for heptaethylene glycol, and 4
1 for heptaethylene glycol, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for pentaglyme and hexaglyme. Inoue and Hakushi144 found the enthalpy–entropy compensation effect for the complexation of cations with glymes/podands, crown ethers, cryptands, and macrocyclic antibiotics in different solvents (such as water and methanol, etc.). Guerra et al.145 observed that bis(trifluorosilyl)cadmium was marginally stable at 20 °C but became very stable in monoglyme by forming alkyl–cadmium–glyme complexes. Markies et al.146 studied the complexation of bis(p-tert-butylphenyl)magnesium with 1,3-xylylene crown ethers and glymes, and indicated that in the complex with diglyme, magnesium is pentacoordinated, whilst in the complex with tetraglyme, magnesium is pentacoordinated to three adjacent oxygens of the five available (including the one from the methoxy group). In both cases, coordinative saturation has been reached within the limits of steric restraints. Simple ethers are usually associated with a tetrahedrally coordinated magnesium, but polyethers induce higher coordination states, which could result in improved reactivity of the organomagnesium reagent. Meot-Ner et al.147 determined the binding energies of NH4+ to glymes using pulsed high-pressure mass spectrometry, and suggested that binding energies in these complexes increase with the ligand size and the number of available oxygen groups. The ab initio calculations of complexing with monoglyme result in the following order of binding energies of ligands: H3O+ > Na+ > NH4+ ≈ K+. To prepare new inorganic polymers as precursors for thin layer deposition, two different dimensional compounds [Ca(monoglyme)n(H2O)m]I2·(monoglyme)x (1: n = 3, m = 3, x = 1; 2: n = 2, m = 4, x = 0) and [Ca(triglyme)(H2O)4]I2 were formed through metal ion complexation and hydrogen bonding.148 Mishra et al.149 prepared hydroxo-bridged, centrosymmetric dimeric yttrium complexes with glymes: [Y2L2(μ-OH)2(H2O)x(ROH)y]I4 [1: L = triglyme, x = 2, y = 2, R = iPr; 2: L = tetraglyme, x = 2, y = 0; 3·2EtOH: L = diglyme, x = 2, y = 4, R = Et; 4·2EtOH: L = triglyme, x = 4, y = 0; 5: L = tetraglyme, x = 2, y = 2, R = Et]; these ionic derivatives are potential sources of yttrium oxide in high Tc superconductors. Chantooni et al.150 observed two polymorphic forms (i.e. triclinic polymorph I and orthorhombic polymorph II) of crystals of the 1
3 for pentaglyme and hexaglyme. Inoue and Hakushi144 found the enthalpy–entropy compensation effect for the complexation of cations with glymes/podands, crown ethers, cryptands, and macrocyclic antibiotics in different solvents (such as water and methanol, etc.). Guerra et al.145 observed that bis(trifluorosilyl)cadmium was marginally stable at 20 °C but became very stable in monoglyme by forming alkyl–cadmium–glyme complexes. Markies et al.146 studied the complexation of bis(p-tert-butylphenyl)magnesium with 1,3-xylylene crown ethers and glymes, and indicated that in the complex with diglyme, magnesium is pentacoordinated, whilst in the complex with tetraglyme, magnesium is pentacoordinated to three adjacent oxygens of the five available (including the one from the methoxy group). In both cases, coordinative saturation has been reached within the limits of steric restraints. Simple ethers are usually associated with a tetrahedrally coordinated magnesium, but polyethers induce higher coordination states, which could result in improved reactivity of the organomagnesium reagent. Meot-Ner et al.147 determined the binding energies of NH4+ to glymes using pulsed high-pressure mass spectrometry, and suggested that binding energies in these complexes increase with the ligand size and the number of available oxygen groups. The ab initio calculations of complexing with monoglyme result in the following order of binding energies of ligands: H3O+ > Na+ > NH4+ ≈ K+. To prepare new inorganic polymers as precursors for thin layer deposition, two different dimensional compounds [Ca(monoglyme)n(H2O)m]I2·(monoglyme)x (1: n = 3, m = 3, x = 1; 2: n = 2, m = 4, x = 0) and [Ca(triglyme)(H2O)4]I2 were formed through metal ion complexation and hydrogen bonding.148 Mishra et al.149 prepared hydroxo-bridged, centrosymmetric dimeric yttrium complexes with glymes: [Y2L2(μ-OH)2(H2O)x(ROH)y]I4 [1: L = triglyme, x = 2, y = 2, R = iPr; 2: L = tetraglyme, x = 2, y = 0; 3·2EtOH: L = diglyme, x = 2, y = 4, R = Et; 4·2EtOH: L = triglyme, x = 4, y = 0; 5: L = tetraglyme, x = 2, y = 2, R = Et]; these ionic derivatives are potential sources of yttrium oxide in high Tc superconductors. Chantooni et al.150 observed two polymorphic forms (i.e. triclinic polymorph I and orthorhombic polymorph II) of crystals of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 pentaglyme
2 pentaglyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloropicric acid
dichloropicric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water adduct complex. The IR spectra of the 1
water adduct complex. The IR spectra of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 tri-, tetra-, and pentaglyme
2 tri-, tetra-, and pentaglyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dichloropicric acid
dichloropicric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water complexes indicate that the hydrogen-bonding in the triglyme complex is as strong as or stronger than that in the pentaglyme complex, but the hydrogen-bonding in the tetraglyme complex is weaker than in the pentaglyme complex.
water complexes indicate that the hydrogen-bonding in the triglyme complex is as strong as or stronger than that in the pentaglyme complex, but the hydrogen-bonding in the tetraglyme complex is weaker than in the pentaglyme complex.
The complexing properties of some glyme analogues have been reported in the literature. Jaycox et al.151 examined the insoluble complexes formed in acidic aqueous media upon mixing poly(acrylic acid) (PAA) and poly(vinylbenzo-18-crown-6) (P18C6) or polyvinylbenzoglymes. The complex formation is a direct result of the hydrogen bonding between carboxyl groups and crown ether- or glyme–oxygen atoms and also from hydrophobic interactions; such a precipitation is pH dependent. Saraswathi and Miller152 studied the interaction of protons and alkali metal ions with dinucleotide analogs of acyclic polyethers using fast-atom bombardment (FAB) mass spectrometry, and suggested the following order of chelation of alkali metal ions, acyclic glymes < dinucleotide analogs (acyclic glymes substituted with nitrogen bases) < crown ethers.
5. Overview of industrial applications
Glymes have a broad range of industrial applications, such as in cleaning products, inks, adhesives and coatings, batteries and electronics, absorption refrigeration and heat pumps, pharmaceutical formulations, etc. For example, mixtures of methanol or trifluoroethanol + PEG-DME 250 or tetraglyme can be used as working fluids for absorption refrigeration machines;87,88 triglyme and tetraglyme are lubricants for the automotive air-conditioning (A/C) compressor when mixed with refrigerants (such as HFC-134a).68,86,89 Table 5 summarizes some representative commercial applications of common glymes.| Glyme | Solvent features | Representative industrial applications |
|---|---|---|
| a Polyglymes are polyethylene glycol dimethyl ethers with different molecular weights ranging from 200–2000. | ||
| Monoglyme (G1) | • Low b.p. (85.2 °C) | Production of active ingredients, metal–organics, electrolyte solvent for sealed lithium batteries, entrainer, chromium electroplating, cyanoacrylate based adhesives, etching of printed circuit boards, treating aluminum surfaces |
| • High stability | ||
| Ethyl glyme (G1-Et) | Solvent in paints, adhesives, coatings, shellacs, resins, detergents, dyes and polycarbonate products | |
| Diglyme (G2) | • High solubility for Na/K alloys | API production (such as anti-AIDS drug Nevirapine), reaction solvents for organometallic reagents, entrainer for azeotropic distillation, battery electrolyte, conducting thermoplastic paste, solvent for Teflon etchants, a suspension of sodium and naphthalene in G2 used for destruction of polychlorinated biphenyls (PCBs) in transformer oil, solvent in a formulation to improve the bonding of tire cord to rubber, solvent in printing and inkjet inks and inkjet cartridges, brake fluid, paints and other coatings, plastics, adhesives and sealants |
| • Good solvent for Grignard reagents, LiAlH4 and NaBH4 | ||
| • Chelate ligand for cations | ||
| • Excellent stability even at high pH values | ||
| Butyl diglyme (G2-Bu) | • Hydrophobic | Selective extraction of gold from hydrochloric solutions containing other metals, used in compositions for production of printed circuits and diode fabrication, |
| • High b.p. (256 °C) | ||
| Triglyme (G3) | • High b.p. (218 °C) | Solvent for Teflon etching, high boiling and inert solvent for organic reactions, a solvent in consumer adhesives and paints, a component of consumer brake fluids and paint/graffiti removers |
| • Chemically inert | ||
| Tetraglyme (G4) | • High b.p. (275 °C) | Flue gas cleaning systems, solvent for production of binders for paints, coalescing agent in paint formulations, adhesives production, electrodeposition, manufacture of soldering fluxes/solder pastes, adsorption liquid and gas scrubbing, formulations of paint strippers and adhesive removers, extraction of volatile organic compounds from solid wastes, inert additive for the fixation of methylated methylolmelamine resins in durable-press cotton and cellulosic fabrics, an HFC/CFC lubricant |
| • High stability | ||
| • High solubility of inorganic salts | ||
| Proglyme (P2) | • Environmentally friendly | Excellent replacement for NMP (N-methyl pyrrolidone) in many applications, polyurethane dispersion (PUD) formulation and two-pack PU coatings, waterborne coatings and high solid coatings, paint strippers, coatings- and graffiti removers, cleaners for degreasing, electrodeposition coatings, co-solvent in aluminum paste formulations, replacement of xylene for the production of alkyd and polyester resins |
| • High chemical stability | ||
| • High b.p. (175 °C) | ||
| • Replacement for NMP | ||
| • Replacement for xylene | ||
| Polyglymes (MW 236 or 275) | • Dissolution of gases such as CO2 and H2S | Gas purification by removing CO2, H2S, COS and water from natural gas or ammonia synthesis gas feed stocks, solvent for manufacturing polyester fibers with improved moisture retention and multiporous hygroscopic fibers, formulation in dyes to dye polyester-cotton textiles, consumer paint strippers |
| • High b.p. | ||
| Higlyme (MW > 400) | • High b.p. (>300 °C) | Formulations of coatings (urethanes and inks) and agro-chemicals |
| • High flash point (140 °C) | ||
| PEG-DME 200 | • High b.p. (>300 °C) | Special solvent, flue gas cleaning systems, delacquing |
| PEG-DME 250 | • High b.p. (>300 °C) | PTC, special solvent, absorption solvent for H2S, COS, mercaptans and CO2, |
| PEG-DME 500 | • High b.p. (>300 °C) | PTC, high boiling solvent, electroplating |
| PEG-DME 1000 | • High b.p. (>300 °C) | PTC, high boiling solvent, electrodeposition |
| PEG-DME 2000 | • High b.p. (>300 °C) | PTC, high boiling solvent, electroplating, depolymerization reactions |
In particular, dipropylene glycol dimethyl ether, known as proglyme (P2), is considered an environmentally friendly solvent and is not listed as a hazardous air pollutant (HAP). Therefore, P2 has versatile applications including the replacement of N-methyl pyrrolidone (NMP). NMP is known to have reproductive toxicity whilst much less toxic P2 has similar physical properties including similar solubility behaviors. Thus, P2 is an excellent solvent for coatings (such as waterborne coatings and high solid coatings) and paints (such as electrodeposition coatings, paint strippers, coating- and graffiti-removers), as well as for industrial cleaners for degreasing (such as formulating hard surface cleaners containing bleach).
6. Electrochemical applications
Due to the unique ion complexing properties of glymes (as discussed in 4.2), glymes have been extensively investigated in electrochemistry as electrolyte solvents. Slates and Szwarc153 suggested that when sodium biphenyl coordinates with diglyme or triglyme in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio, diglyme coordinates with the periphery of the ion pair while two isomeric ion pairs are formed in triglyme (glyme attaching to the periphery of the pair, and glyme separating the ions). Canters et al.154 prepared single crystals of the alkali radical ion pairs of [Li+][biphenyl−] in tetrahydropyran (THP), [Na+][biphenyl−] in triglyme, and [K+][biphenyl−] and [Rb+][biphenyl−] in tetraglyme. They found the presence of solvent molecules in stoichiometric quantities in the crystals, acting as the chelating agents of alkali ions. In particular, Na2[biphenyl]2[triglyme]5 has shown a strong paramagnetism and electron exchange interaction, and the electron correlation time is in the order of 10−l1 to 10−l2 s. Smyrl et al.155 suggested that a spontaneous discharge process for doped polyacetylene in a LiI-monoglyme solution could restrict the reversibility of this and other conductive polymers when used as electrode materials. Foos et al.156 examined the conductivity of LiBr dissolved in glymes (mono- and di-) and their mixtures with other ethers, and found that solution conductivity increases with the salt concentration up to a maximum. Interestingly, they also discovered that the conductivity of 1.0 M LiBr in a 1
1 stoichiometric ratio, diglyme coordinates with the periphery of the ion pair while two isomeric ion pairs are formed in triglyme (glyme attaching to the periphery of the pair, and glyme separating the ions). Canters et al.154 prepared single crystals of the alkali radical ion pairs of [Li+][biphenyl−] in tetrahydropyran (THP), [Na+][biphenyl−] in triglyme, and [K+][biphenyl−] and [Rb+][biphenyl−] in tetraglyme. They found the presence of solvent molecules in stoichiometric quantities in the crystals, acting as the chelating agents of alkali ions. In particular, Na2[biphenyl]2[triglyme]5 has shown a strong paramagnetism and electron exchange interaction, and the electron correlation time is in the order of 10−l1 to 10−l2 s. Smyrl et al.155 suggested that a spontaneous discharge process for doped polyacetylene in a LiI-monoglyme solution could restrict the reversibility of this and other conductive polymers when used as electrode materials. Foos et al.156 examined the conductivity of LiBr dissolved in glymes (mono- and di-) and their mixtures with other ethers, and found that solution conductivity increases with the salt concentration up to a maximum. Interestingly, they also discovered that the conductivity of 1.0 M LiBr in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture of monoglyme and dioxolane increases with the decrease in temperature; this is particularly valuable for battery electrolytes with maximized conductivities at low temperatures.
1 (v/v) mixture of monoglyme and dioxolane increases with the decrease in temperature; this is particularly valuable for battery electrolytes with maximized conductivities at low temperatures.
Sharma and Bhagwat157 measured the conductivities of alkali metal picrates dissolved in tetraglyme, 18-crown-6 and tetraethylene glycol diquinoline ether at 25 °C in a methanol–water mixture. The formation constants (KML) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of alkali metal ions with these ligands in 70% methanol were found to be dependent on the anion; the log
1 complexes of alkali metal ions with these ligands in 70% methanol were found to be dependent on the anion; the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KML values of alkali metal ions with tetraglyme, 18-crown-6 and quinoline ether are in a decreasing order of K+ > Na+ > Rb+ ≈ Cs+ > Li+, K+ ≫ Na+ > Rb+ > Cs+, and Na+ > K+ > Rb > CS+ > Li+, respectively. They also observed that the cyclic ligand gives a more stable complex and a higher selectivity than non-cyclic ligands. Pyati and Murray158 found that the microelectrode voltammetry-derived heterogeneous electron transfer kinetic rates kET for the redox couple cobalt tris(bipyridine) [Co(bpy)3]2+/3+ in a series of glyme solvents are inversely proportional to the solvent’s longitudinal relaxation time and viscosity but directly proportional to the diffusion coefficient of the metal complex. Teeters et al.159 measured the surface tension of a tetraglyme–lithium triflate system, and observed a preferential distribution of the triflate ion between the interface and the bulk. They indicated that the surface concentration increases with the concentration of lithium triflate until a high ‘ion aggregation’ concentration was reached. These results further stimulated the infrared study of poly(ethylene oxide)–lithium triflate films, and enabled a more mechanistic understanding of polymer electrolyte materials. To provide an aprotic environment for lithium batteries, Choquette et al.160 studied the phase diagrams, potential windows, conductivities and the lithium interfacial resistance of Li[Tf2N] dissolved in sulfamides and glymes. They suggested that glymes or their mixtures with sulfamides could be useful for batteries whose cathode is not a 2D layer structure. Plewa et al.161 constructed composite electrolytes comprising polyglyme (Mw = 500), LiX (X = I−, BF4− and CF3SO3−) and a calix[6]pyrrole derivative (C6P), and focused on the role of C6P as an anion complexing agent. To probe the potential use of tin as an alternative anode material for rechargeable lithium batteries, Katayama et al.162 examined the charge–discharge properties of a tin thin film electrode in an ionic liquid, 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide containing 0.1 M Li[Tf2N]. To reduce the interfacial resistance in the ionic liquid electrolyte, a small amount (0.2 M) of glymes (mono-, di-, tri- and tetra-) were added to coordinate with the Li+ ions.
KML values of alkali metal ions with tetraglyme, 18-crown-6 and quinoline ether are in a decreasing order of K+ > Na+ > Rb+ ≈ Cs+ > Li+, K+ ≫ Na+ > Rb+ > Cs+, and Na+ > K+ > Rb > CS+ > Li+, respectively. They also observed that the cyclic ligand gives a more stable complex and a higher selectivity than non-cyclic ligands. Pyati and Murray158 found that the microelectrode voltammetry-derived heterogeneous electron transfer kinetic rates kET for the redox couple cobalt tris(bipyridine) [Co(bpy)3]2+/3+ in a series of glyme solvents are inversely proportional to the solvent’s longitudinal relaxation time and viscosity but directly proportional to the diffusion coefficient of the metal complex. Teeters et al.159 measured the surface tension of a tetraglyme–lithium triflate system, and observed a preferential distribution of the triflate ion between the interface and the bulk. They indicated that the surface concentration increases with the concentration of lithium triflate until a high ‘ion aggregation’ concentration was reached. These results further stimulated the infrared study of poly(ethylene oxide)–lithium triflate films, and enabled a more mechanistic understanding of polymer electrolyte materials. To provide an aprotic environment for lithium batteries, Choquette et al.160 studied the phase diagrams, potential windows, conductivities and the lithium interfacial resistance of Li[Tf2N] dissolved in sulfamides and glymes. They suggested that glymes or their mixtures with sulfamides could be useful for batteries whose cathode is not a 2D layer structure. Plewa et al.161 constructed composite electrolytes comprising polyglyme (Mw = 500), LiX (X = I−, BF4− and CF3SO3−) and a calix[6]pyrrole derivative (C6P), and focused on the role of C6P as an anion complexing agent. To probe the potential use of tin as an alternative anode material for rechargeable lithium batteries, Katayama et al.162 examined the charge–discharge properties of a tin thin film electrode in an ionic liquid, 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide containing 0.1 M Li[Tf2N]. To reduce the interfacial resistance in the ionic liquid electrolyte, a small amount (0.2 M) of glymes (mono-, di-, tri- and tetra-) were added to coordinate with the Li+ ions.
As discussed in detail in Section 4.2, glymes dissolve alkali salts and form complexes with them; such unique properties have significant electrochemical implications. Izutsu et al.163 studied the complexing of lithium ions in propylene carbonate (PC) with glymes (mono-, di-, tri- and tetra-) using a univalent cation-sensitive glass electrode. An early study by Aurbach and Granot164 suggested that electrolytes consisting of various lithium salts and glymes (monoglyme, diglyme and ethyl glyme) might not be suitable for rechargeable Li battery systems using Li metal anodes due to a rough morphology of Li upon deposition–dissolution cycling causing a low Li cycling efficiency. However, Brouillette et al.165 indicated that glymes (mono-, di-, tri- and tetra-) are electrochemically stable, possess a good redox window, and are analogs of PEOs used in polymer-electrolyte batteries. Therefore, this group measured the conductance and apparent molar volume and heat capacity of Li[Tf2N] at various concentrations in glymes; from these data, they concluded that: (a) at low concentrations, Li[Tf2N] is strongly associated in glymes; (b) at intermediate concentrations, there is a stable solvate of Li[Tf2N] in glymes in the solution state; (c) at high concentrations, the thermodynamic properties of the lithium salt resemble those of molten salts. Hayamizu et al.166 measured the self-diffusion coefficients of Li+, Tf2N−, and the solvent (including glymes) in Li[Tf2N]–solvent systems using a pulse-gradient spin-echo (PGSE) NMR method. They observed that the ionic conductivity and the diffusion coefficients increase with the increase in glyme chain length (monoglyme → diglyme → triglyme), and the degree of dissociation at 30 °C is in the range of 31–38%. To further understand the role of PEO in electrolyte systems, the same group167 employed pulsed-field gradient spin-echo (PGSE) 1H, 19F, and 7Li NMR to study electrolytes of glymes CH3O(CH2CH2O)nCH3 (n = 3–50) mixed with Li[Tf2N]. They suggested that the segmental motions of the PEO moiety in glymes induce high chain flexibility and enable high solubility and transport of doped Li+ ions; the rate of segmental motion decreases with the increase in PEO chain length. To probe the molecular interactions of lithium salts in glymes used for solid polymer and liquid electrolytes, Henderson et al.168 determined the phase behavior and solvate structures of glyme complexes with Li[Tf2N] and Li[beti] (beti = bis(perfluoroethanesulfonyl)imide) to be (monoglyme)1:Li[beti], (diglyme)2:Li[Tf2N], (diglyme)1/2:Li[Tf2N], and low-temperature (triglyme)1:Li[beti]; most solvates undergo order–disorder solid phase transitions. Kolosnitsyn et al.169,170 studied the cycling of a sulfur electrode in a mixture of 3-methoxysulfolane and sulfolane with glymes (mono-, di- and tetra-) and lithium triflate (Li[OTf]) as the supporting electrolyte. They found a decrease in the electrode capacity with the increase in PEO units of glymes and in the number of donor centers in sulfone molecules, which is due to the variation of the form taken by lithium polysulfides in solutions and the increase in electrolyte viscosity. Tobishima et al.171 investigated the conductivity, lithium ion solvation state and charge–discharge cycling efficiency of lithium metal anodes in glyme-based electrolytes for rechargeable lithium cells; ethylene carbonate (EC) and methylethylcarbonate (MEC) were added to glymes in order to dissolve 1.0 M LiPF6. They found that the conductivity increases with the decrease in PEO chain length and the solution viscosity; they also indicated that di- and tri-glyme exhibit a better performance than mono- and tetra-glyme in terms of conductivities at low temperature (below 0 °C) and the charge–discharge cycling at a high current. The same group172 further demonstrated the use of such tertiary electrolyte systems (mixing glymes with 1 M LiPF6–EC/MEC) in rechargeable lithium cells with Si-based anodes: for both Li/Si + C and Li/Si–C + C cells, the discharge capacity appears to be larger than the system without glyme, and the cycling life of the latter cells is also improved. Kaulgud et al.173 carried out ab initio Hartree–Fock calculations to determine the electronic structure and vibrational frequencies of CH3(OCH2CH2)nOCH3–M+–OTf− (n = 2–4, M = Li, Na, and K) complexes. They suggested that the metal ion has various coordinations from 5 to 7 in these complexes, and Li+ ions bind to one of the oxygen atoms of OTf− in tetraglyme–lithium triflate while Na+ or K+ ions show bidentate coordination. In addition, the metal ion tends to bind more strongly to ether oxygens of tetraglyme than its di- or triglyme analogues. The Watanabe group174 determined the physicochemical properties of triglyme and tetraglyme solutions of Li[Tf2N] and observed the formation of complexes ([Li(glyme)][Tf2N]) in concentrated solutions. The ionic conductivity is concentration-dependent and reaches its maximum at ∼1 M. The viscosity increases with the salt concentration while the self-diffusion coefficient of each species in the solutions decreases with the salt concentration. [Li(glyme)][Tf2N] may be considered as a quasi-ionic liquid in terms of similar ionicity. Orita et al.175 observed that a higher amount of tetraglyme in tetraglyme–Li[Tf2N] complexes decreases the viscosity and increases the ionic conductivity of the mixture; in addition, the mixture has a higher thermal stability than conventional organic electrolytes when the molar ratio of tetraglyme is more than 40 mol%. They further demonstrated the potential of [Li(tetraglyme)][Tf2N] as a replacement of organic electrolytes in lithium ion batteries with appropriate electrode-active materials. Tamura et al.176 prepared glyme–cyclic imide lithium salt (Li[CTFSI]) complexes as thermally stable electrolytes for lithium batteries, i.e. [Li(G3)][CTFSI] (solid) and [Li(G4)][CTFSI] (liquid). The latter complex shows a much higher thermal stability than pure G4, and a high ionic conductivity of 0.8 mS cm−1 at 30 °C despite its high viscosity. They also observed a stable charge–discharge cycling behavior of a [LiCoO2|[Li(G4)][CTFSI]|Li metal] cell during 50 cycles, implying the applicability of the [Li(G4)][CTFSI] complex in a 4 V class lithium secondary battery. The Watanabe group177 investigated the physicochemical and electrochemical properties of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexing mixture of triglyme and Li[N(SO2F)2] (lithium bis(fluorosulfonyl)amide) as a safe lithium-ion secondary battery electrolyte. This new electrolyte has a relatively high thermal stability, and enabled a stable charge–discharge of Li+ ions with both LiFePO4 positive electrode and graphite negative electrode leading to high coulombic efficiency and long cycle. In addition, they achieved 82% of capacity retention after 100 cycles of charge–discharge operations of a [LiFePO4 positive electrode|G3-LiFSI electrolyte|graphite negative electrode] cell. The same group178 further examined the use of a 1
1 complexing mixture of triglyme and Li[N(SO2F)2] (lithium bis(fluorosulfonyl)amide) as a safe lithium-ion secondary battery electrolyte. This new electrolyte has a relatively high thermal stability, and enabled a stable charge–discharge of Li+ ions with both LiFePO4 positive electrode and graphite negative electrode leading to high coulombic efficiency and long cycle. In addition, they achieved 82% of capacity retention after 100 cycles of charge–discharge operations of a [LiFePO4 positive electrode|G3-LiFSI electrolyte|graphite negative electrode] cell. The same group178 further examined the use of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equimolar complex electrolyte of triglyme:Li[Tf2N] in lithium secondary batteries using lithium metal and two positive electrode materials (LiFePO4 and LiNi1/3Mn1/3Co1/3O2). They observed a relatively favorable surface formed at the electrolyte/lithium metal electrode interface; and they also found an excellent capacity retention with low degradation for both 3V-class [LiFePO4|Li metal] and 4V-class [LiNi1/3Mn1/3Co1/3O2|Li metal] cells.
1 equimolar complex electrolyte of triglyme:Li[Tf2N] in lithium secondary batteries using lithium metal and two positive electrode materials (LiFePO4 and LiNi1/3Mn1/3Co1/3O2). They observed a relatively favorable surface formed at the electrolyte/lithium metal electrode interface; and they also found an excellent capacity retention with low degradation for both 3V-class [LiFePO4|Li metal] and 4V-class [LiNi1/3Mn1/3Co1/3O2|Li metal] cells.
The lithium/sulfur (Li/S) battery has become a promising electrochemical system because of its high theoretical capacity of 1675 mA h g−1.179 However, the present Li/S system has a number of obstacles to overcome, such as its poor active material conductivity, active material dissolution, and the highly reactive lithium metal electrode. The Watanabe group180 examined the redox reaction of sulfur supported on inverse opal carbon (IOC) in a [Li(tetraglyme)][Tf2N] molten complex electrolyte. They found that the Li|[Li(tetraglyme)][Tf2N]|sulfur/100 nm IOC cell maintained a large discharge capacity of ca. 800 mA h g−1-sulfur and a high coulombic efficiency of >97% after 50 cycles. They suggested that the [Li(tetraglyme)][Tf2N] molten salt could be a promising electrolyte enabling a high coulombic efficiency of charge–discharge in Li–S batteries. Barchasz et al.181 found that conventional carbonate-based electrolytes cannot be used in Li/S cells and then suggested that Li/S cell electrolytes require solvents with high solvation power such as tetraglyme and ethyl diglyme, but not those solvents (e.g. monoglyme, butyl diglyme and 1,3-dioxolane) with low polysulfide solubility. Polyglyme (average Mn = 250) plays a vital role in Li/S electrolytes, and could prevent fast electrode passivation and extend the length of a second discharge plateau, resulting in a discharge capacity of about 1100 mA h g−1 for the first discharge and over 550 mA h g−1 remaining after 10 cycles.
In addition to the alkaline battery system, other metal battery systems have also been explored using glyme-based electrolytes. Aurbach et al.182 prepared new electrolyte solutions based on glymes (such as mono-, di- and tetra-) and magnesium aluminates, giving an electrochemical window of 2.5 V and >99% efficiency of Mg deposition–dissolution cycles. They further developed new rechargeable Mg batteries (1–1.5 V) using these electrolyte solutions and cathodes of the MgxMoSy type (Chevrel phase) for delivering over 1000 charge–discharge cycles. This group183 further constructed rechargeable Mg battery systems by using Mg(AX4−nRn)2 complexes (A = Al, B, Sb, P, As, Fe, and Ta; X = Cl, Br, and F; and R = butyl, ethyl, phenyl, and benzyl) in several ether solvents and glymes (mono-, di- and tetra-). They found some of these Mg complexes in THF or glymes gave a wide electrochemical window (>2 V) and allowed the reversible deposition of magnesium. This group184 further developed new magnesium batteries comprising Mg metal anodes, an electrolyte with a general structure of Mg(AlX3−nRnR′)2 (R, R′ = alkyl groups, X = halide) in ethereal solutions (such as THF and tetraglyme), and Chevrel phases of MgMo3S4 stoichiometry as highly reversible cathodes. The magnesium battery systems could be cycled thousands of times with little loss in capacity; in addition, they are environmentally benign alternatives to lead–acid and nickel–cadmium batteries and are constructed from abundant, inexpensive, and nonpoisonous materials.
Solid-state electrolytes consisting of poly(ethylene oxide) (PEO) doped with salts have promising applications in batteries, capacitors and fuel cells because these electrolytes exhibit high ionic conductivity. In addition, they are soft solids, and if small glyme molecules are used, they are monodispersed and do not entangle like polymers. Rao and Klemann185 investigated the behavior of Li/TiS2 cells in a low-temperature melt comprising LiI:glyme solvate, and suggested the cells could be discharged at high rates in the low temperature melt and could be stored in solid electrolyte long-term. Zhang et al.186 measured the ionic conductivities of several solid electrolytes comprising lithium salts with glymes, and indicated that the (tetraglyme)0.5:LiBF4 electrolyte has the highest conductivity among those small-molecule electrolytes with a lithium transport number of 0.65. The same group187 further observed an increase in conductivity (from around 10−7 to 10−4 S cm¬1) by substituting PEO molecules in conducting crystalline complexes PEO6:LiXF6 (X = P or As) with glymes (tri- and tetra-). Since the stoichiometric compositions of glyme–salt complexes are lower than 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (4
1 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for G3
1 for G3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiXF6 and 5
LiXF6 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for G4
1 for G4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiXF6), there are three phases present, the 6
LiXF6), there are three phases present, the 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 phase, the glyme complex and a liquid containing some salt; and it is believed that the liquid is primarily responsible for the conductivity. This group188 also evaluated two glyme-based solid electrolytes: G3
1 phase, the glyme complex and a liquid containing some salt; and it is believed that the liquid is primarily responsible for the conductivity. This group188 also evaluated two glyme-based solid electrolytes: G3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiAsP6 and G4
LiAsP6 and G4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiAsP6, and reported that they have quite different transport numbers (t+ = 0.8 and 0.1 respectively). This is due to two factors: (a) there are tunnels in the crystal structure of the G3 complex for Li+ migration, but not in the G4 complex; (b) there is a weaker binding of AsF6− in the structure of G4 than in G3. The Bruce group189 further determined the crystal structures of complexes [CH3O(CH2CH2O)nCH3]:LiAsF6 (n = 8–12), and indicated that Li+ ions are six coordinated if only ether oxygens are involved in coordination and the coordination number becomes five if a fluorine from the AsF6− anion is involved in coordination. They also observed low lithium transport numbers (t+ < 0.3) and lower conductivities in these complexes compared with complexes formed with lower glymes (n = 3, 4). Xia and Smid190 conducted the differential thermal analysis of solid polymer electrolyte complexes of lithium (or sodium) triflate and homopolymers derived from three methacrylate monomers CH2
LiAsP6, and reported that they have quite different transport numbers (t+ = 0.8 and 0.1 respectively). This is due to two factors: (a) there are tunnels in the crystal structure of the G3 complex for Li+ migration, but not in the G4 complex; (b) there is a weaker binding of AsF6− in the structure of G4 than in G3. The Bruce group189 further determined the crystal structures of complexes [CH3O(CH2CH2O)nCH3]:LiAsF6 (n = 8–12), and indicated that Li+ ions are six coordinated if only ether oxygens are involved in coordination and the coordination number becomes five if a fluorine from the AsF6− anion is involved in coordination. They also observed low lithium transport numbers (t+ < 0.3) and lower conductivities in these complexes compared with complexes formed with lower glymes (n = 3, 4). Xia and Smid190 conducted the differential thermal analysis of solid polymer electrolyte complexes of lithium (or sodium) triflate and homopolymers derived from three methacrylate monomers CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)COO(CH2CH2O)nCH3 (n = 4, 8 or 22 on average), and found that the conductivities of these complexes were comparable to those known for alkali complexes of poly(ethylene oxide). Holzer et al.191 described a different type of solid electrolyte containing a glyme motif, poly[1,4-(2,5-bis(1,4,7,10-tetraoxaundecyl))phenylene vinylene] (BTEM-PPV, see Scheme 4), for constructing red-orange light-emitting electrochemical cells (LECs). The high electronic conductivity and ionic conductivity of BTEM-PPV could be attributed to its conjugated backbone and glyme-containing side chains. LECs based on this polymer are relatively bright light emitting devices with low response times. Because of the covalent linkage of PEO chains to the PPV backbone, an ionochromic effect was also observed in both absorption spectrum and electroluminescence spectrum when BTEM–PPV is complexed with metal ions; this indicates its potential application in chemical sensors.
C(CH3)COO(CH2CH2O)nCH3 (n = 4, 8 or 22 on average), and found that the conductivities of these complexes were comparable to those known for alkali complexes of poly(ethylene oxide). Holzer et al.191 described a different type of solid electrolyte containing a glyme motif, poly[1,4-(2,5-bis(1,4,7,10-tetraoxaundecyl))phenylene vinylene] (BTEM-PPV, see Scheme 4), for constructing red-orange light-emitting electrochemical cells (LECs). The high electronic conductivity and ionic conductivity of BTEM-PPV could be attributed to its conjugated backbone and glyme-containing side chains. LECs based on this polymer are relatively bright light emitting devices with low response times. Because of the covalent linkage of PEO chains to the PPV backbone, an ionochromic effect was also observed in both absorption spectrum and electroluminescence spectrum when BTEM–PPV is complexed with metal ions; this indicates its potential application in chemical sensors.
7. Uses In organic reactions
Glymes play various vital roles in organic synthesis. The most common role is acting as the reaction media (solvent role). Other important roles include reaction additives/metal chelators, catalysts and reagents. The following sections discuss some representative examples in each of these categories.7.1. Reaction solvents
Glymes are liquid over a wide temperature range (Table 2) and are suitable for reactions at low temperatures (low freezing points). For example, monoglyme has a freezing point of −69 °C and has been widely used in low-temperature reactions, but it can also be removed easily during the workup (boiling point 85 °C). In addition, glymes have a strong solvating power and can dissolve a variety of compounds, particularly chelating with metal ions. For example, the solvating power of the ether-type solvents increases in the order 2-methyltetrahydrofuran < THF < monoglyme < diglyme < triglyme < tetraglyme; in particular, triglyme and tetraglyme are strong chelating agents for Na+ ions.58 Therefore, glymes (especially monoglyme) have been explored in numerous organic reactions since the 1960s, such as reduction, oxidation, substitution, C–C coupling, borane chemistry, and other reactions.The benzocyclobutadiene radical anion was produced by the addition of trans-1,2-dibromobenzocyclobutene to an excess of solvated electrons in a mixture of monoglyme and diglyme at −60 °C (Scheme 6).193 Similarly, treating cyclooctatetraene with potassium mirror in monoglyme at −90 °C (or −80 °C) yielded an anion radical.194,195 Miller et al.196 treated dibenzonorcaradiene with different electron-transfer reducing agents in anhydrous monoglyme to form a dibenzonorcaradiene anion radical, whose cyclopropane isomerization led to various reduction products; for example, the Na-reduction after 12 h at r.t. produced 9-methylphenanthrene (25.4%), 9-methyl-9,10-dihydrophenanthrene (47.9%), and 6,7-dihydro-5H-dibenzo[a,c]cycloheptene (26.6%).
Walborsky et al.197 studied the reductive cleavage of 1,1-biphenylene-2-methylcyclopropane using sodium and lithium in liquid ammonia, sodium in monoglyme, sodium naphthalide in monoglyme, and by controlled potential electrolysis in acetonitrile at a mercury cathode (Scheme 7). The isomer ratio of two products 9-propylfluorene and 9-isopropylfluorene ranged from 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 81
4 to 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19. However, the use of monoglyme as the solvent gave a lower product ratio. In the synthesis of asymmetric phosphine macrocycles, Wei et al.198 eliminated the protecting tosyl groups at −78 °C by sodium naphthalenide in monoglyme containing t-butyl alcohol (as a proton source); this method was used by the same group in an earlier detosylation study.199
19. However, the use of monoglyme as the solvent gave a lower product ratio. In the synthesis of asymmetric phosphine macrocycles, Wei et al.198 eliminated the protecting tosyl groups at −78 °C by sodium naphthalenide in monoglyme containing t-butyl alcohol (as a proton source); this method was used by the same group in an earlier detosylation study.199
Partial reduction of a lactam to produce the indole alkaloid roxburghine D was achieved by using di-isobutyl aluminium hydride in monoglyme at −70 °C.200 Dahl et al.201 prepared CH3Ge(PH2)2H and CH3Ge(PH2)3 by the reaction of CH3GeCl3 with LiAl(PH2)4 in triglyme at −23 °C. Saavedra202 carried out the reduction of nitrosoamides to alcohols using NaBH4 in dry monoglyme at room temperature for 2–8 h, achieving 50–82% yields (Scheme 8). Ohsawa et al.203 found that the potassium metal–crown ether–diglyme system could be effective for the reductive removal of sulfonyl group from O-sulfonates or sulfonamides. The Rieke group204,205 prepared highly reactive metal powders of Fe, Co, Ni, Pd and Pt from the reduction of anhydrous metal halides in monoglyme or THF by lithium in the presence of a small amount of naphthalene. Inaba et al.206 prepared highly reactive metallic nickel by reducing nickel halides with lithium in monoglyme using naphthalene as an electron carrier, and further investigated it as a reductive homocoupling reagent for benzylic mono- and polyhalides (Scheme 9) to produce 1,2-diarylethanes. Specifically, the coupling of benzylic monohalides at room temperature yielded the corresponding ethane derivatives, and the coupling of benzylic di- and trihalides gave mixtures of cis and trans isomers of substituted ethenes. Carbonyl-substituted closo polyhedral boranes with B10 and B12 cages are important derivatives that can be converted to other functional groups such as cyanide, amide, keto, ester, amine, etc. The same group207 further demonstrated that metallic nickel could be a versatile coupling reagent for ketone preparation by the reaction of benzylic, allylic, vinylic, and pentafluorophenyl halides with acid halides at 85 °C in monoglyme. Reduction of the B12 1,12-dicarbonyl to 1,12-bis(hydroxymethyl)decahydrododecaborate salts was achieved by using LiAlH4 in anhydrous monoglyme at room temperature.208
Bianconi et al.209 described a general method for the reductive coupling of carbonyl ligands in [M(CO)2(dmpe)2Cl] complexes, M = Nb or Ta and dmpe = 1,2-bis(dimethylphosphino)ethane: the reaction of [M(CO)2(dmpe)2Cl] with 40% sodium amalgam in THF or monoglyme, after the filtration, the addition of Me3SiY, Y = Cl or CF3SO3 (triflate), and the recrystallization from pentane results in [M(Me3SiOC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) COSiMe3)(dmpe)2Y] with 40–70% isolated yields. The Walton group210,211 developed a general method for synthesizing dirhenium octahydrides Re2H8(PR3)4 [PR3 = PMe3, PEt3, P-n-Pr3, PMe2Ph, PEt2Ph, PMePh2, Ph2PCH2PPh2 (dppm), and Ph2PCH2CH2PPh2 (dppe)], via the reduction of triply bonded complexes Re2Cl4(PR3)4 using LiAlH4 in monoglyme and the subsequent hydrolysis step. Taking the advantage of glymes as high-boiling inert solvents, Yang and Pittman212 studied the dechlorination of 4-chlorobiphenyl with NaBH4 in glymes at 120–310 °C (Scheme 10). At comparable reaction conditions, the dechlorination rates decreased in the order of tetraglyme > triglyme > diglyme, and a complete dechlorination was observed in NaBH4/tetraglyme at 310 °C in 1 h. The addition of LiCl to NaBH4 could enhance the dechlorination rate in di-, tri-, and tetraglyme respectively at 120–135 °C. On the other hand, the dechlorination of 4-chlorobiphenyl was not successful in the NaBH4/diphenyl ether system. Based on the same methodology, this group213 also accomplished the dechlorination of pentachlorophenol and 1,2,4-trichlorobenzene using NaBH4 in tetraglyme at 290–315 °C or by NaBH4/LiCl at 125–135 °C in diglyme, triglyme or tetraglyme after premixing at room temperature. Furthermore, this group214 achieved a quantitative dechlorination of a polychlorinated biphenyl (PCB) mixture (Aroclor 1016) using NaBH4 in tetraglyme at 290–310 °C in 2 h in a sealed tube, or using NaBH4/LiCl/glyme solvents (di-, tri-, or tetraglyme) at 125–135 °C.
COSiMe3)(dmpe)2Y] with 40–70% isolated yields. The Walton group210,211 developed a general method for synthesizing dirhenium octahydrides Re2H8(PR3)4 [PR3 = PMe3, PEt3, P-n-Pr3, PMe2Ph, PEt2Ph, PMePh2, Ph2PCH2PPh2 (dppm), and Ph2PCH2CH2PPh2 (dppe)], via the reduction of triply bonded complexes Re2Cl4(PR3)4 using LiAlH4 in monoglyme and the subsequent hydrolysis step. Taking the advantage of glymes as high-boiling inert solvents, Yang and Pittman212 studied the dechlorination of 4-chlorobiphenyl with NaBH4 in glymes at 120–310 °C (Scheme 10). At comparable reaction conditions, the dechlorination rates decreased in the order of tetraglyme > triglyme > diglyme, and a complete dechlorination was observed in NaBH4/tetraglyme at 310 °C in 1 h. The addition of LiCl to NaBH4 could enhance the dechlorination rate in di-, tri-, and tetraglyme respectively at 120–135 °C. On the other hand, the dechlorination of 4-chlorobiphenyl was not successful in the NaBH4/diphenyl ether system. Based on the same methodology, this group213 also accomplished the dechlorination of pentachlorophenol and 1,2,4-trichlorobenzene using NaBH4 in tetraglyme at 290–315 °C or by NaBH4/LiCl at 125–135 °C in diglyme, triglyme or tetraglyme after premixing at room temperature. Furthermore, this group214 achieved a quantitative dechlorination of a polychlorinated biphenyl (PCB) mixture (Aroclor 1016) using NaBH4 in tetraglyme at 290–310 °C in 2 h in a sealed tube, or using NaBH4/LiCl/glyme solvents (di-, tri-, or tetraglyme) at 125–135 °C.
Kanth and Brown215 noted that NaBH4 and NaBF4 have reasonably high solubilities in glymes (particularly triglyme and tetraglyme). As illustrated in Scheme 11, they developed an improved procedure for the generation of diborane (B2H6) by the reaction of NaBH4 in triglyme or tetraglyme, followed by the generation of diborane through a reaction of NaBF4 with NaBH4 in triglyme (or tetraglyme) in the presence of Lewis acids such as AlCl3 and BCl3. Triglyme (or tetraglyme) could be easily recovered and recycled.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) monoglyme–water, and found that the product distribution is dependent on the ratio of phenylcyclopropane to Na2PdCl4 (Scheme 12). At a low ratio of phenylcyclopropane/Pd(II), propiophenone is the major oxidation product; however, at a high ratio, an isomerization to trans-propenylbenzene occurs, which is further oxidized to phenylacetone.
1 (v/v) monoglyme–water, and found that the product distribution is dependent on the ratio of phenylcyclopropane to Na2PdCl4 (Scheme 12). At a low ratio of phenylcyclopropane/Pd(II), propiophenone is the major oxidation product; however, at a high ratio, an isomerization to trans-propenylbenzene occurs, which is further oxidized to phenylacetone.
Ochiai and Fujita217,218 synthesized allylic nitrates via the reaction of ent-16-kaurene or ent-15-kaurene with thallium(III) trinitrate in monoglyme. McKillop et al.219 found that thallium(III) nitrate is capable of oxidizing chalcones, deoxybenzoins and benzoins in aqueous glyme–perchloric acid; the same group220 also oxidized diarylacetylenes to benzils in good yields in aqueous acidic glyme or in methanol.
Kice and Kasperek225 examined the hydrolysis of aryl α-disulfones in various Et3N–Et3NH+ buffers in 60% dioxane and 60% monoglyme as solvents; they found that the triethylamine-catalyzed reaction is a general base catalysis (by triethylamine) rather than nucleophilic catalysis (Scheme 15a). The same group226 further investigated the hydrolysis of p-nitrophenyl p-toluenesulfonate in Et3N–Et3NH+ buffer in both 20% acetonitrile and 60% aqueous monoglyme, and found no significant catalysis by triethylamine in this case. However, they observed that N-ethylpyrrolidine (NEP) could catalyze the hydrolysis in 60% monoglyme, which is shown to be via nucleophilic catalysis (Scheme 15b).
The nucleophilic aromatic substitution of haloaryl sulfones with alkali phenoxides was studied in glymes at 160 °C (Scheme 16).227 The reaction was found to be first order in halo sulfone and of fractional (∼0.5) order in phenoxide ion. The longer glyme chain length promoted a faster reaction, and when n ≈ 20 the reaction rate was about 25 times faster than when n = 2 (diglyme). The likely reason for the rate enhancement is that polyglyme is a better cation solvating solvent than diglyme. Pastor and Hessell228 studied the aromatic substitution of hexa-, tetra-, tri-, di-, and monochlorobenzenes with sodium alkanethiolates in several glymes, and found that the product yield decreased in the order: tetraglyme > triglyme > diglyme > monoglyme. It was explained that tetraglyme is more effective in solvating the sodium cation, generating a more nucleophilic unsolvated thiolate anion.
Bis(h5-cyclopentadienyl)tungsten dichloromethide hydride, Cp2WH(CHCl2), was synthesized as the insertion product from the reaction of bis(h5-cyclopentadieny1)tungsten dihydride and sodium trichloroacetate in monoglyme (Scheme 17); however, in a chlorobenzene–diglyme mixture, the thermal decomposition of sodium chlorodifluoroacetate in the presence of bis(h5-cyclopentadieny1)tungsten dihydride yielded the substitution product, bis(h5-cyclopentadienyl)tungsten bis(chlorodifluoroacetate), Cp2W(O2C2ClF2)2.229
White and McGillivray230 reported an effective method for azetidine preparation (Scheme 18) via reductive detosylation using sodium naphthalenide in diglyme, which afforded improved yields and simple procedures (superior to monoglyme and THF). The azetidine–diglyme solution could be further converted to N-aroylazetidines by adding different aroyl chlorides. 6-(Arylalkylamino)uracils and 6-anilinouracils, potent inhibitors of Bacillus subtilis DNA polymerase III, were prepared by the reactions between 6-chlorouracil and appropriate amines in monoglyme instead of aqueous solutions, which considerably reduced the reaction time.231 Izumi and Miller232 examined the nucleophilic attack of carbanions (R3C−) to displace the chloride ion from phenylchloroacetylene (Scheme 19); they suggested that reactions in DMSO–KOH are mainly those of ions, however, in aprotic monoglyme, reactions of R3C–Na+ are those of its aggregates. The DMSO–KOH medium is only suitable for relatively strong carbon acids, while the Na–glyme conditions have broader applications.
Banitt et al.233 carried out the nucleophilic acyl substitution of 2,2,2-trifluoroethyl 2,5-bis(2,2,2-trifluoroethoxy)benzoate with 2-aminomethylpiperidine in monoglyme (Scheme 20), achieving 76.5% isolated yield. Okamoto et al.234 investigated the reaction of 5-dimethylamino-1-naphthalenesulfonyl chloride with butylamine in chloroform with glymes as oligomer co-solvents, and determined the second-order rate constants by fluorometry. The addition of glymes imposed an acceleration effect especially when their concentrations were lower than 20% (v/v), and such an effect became more pronounced for glymes with longer chain length (tetraglyme > triglyme > diglyme > monoglyme). In addition, the rate acceleration seems to correlate with the volume fraction of the co-solvent, which can be attributed to the polymer effects of glymes and is explained by the thermodynamics of polymer solutions.
Reactions of Li+, Na+, and K+ salts of 2,4,6-trimethyl-s-triazine with 2-halomethyl-4,6-dimethyl-s-triazine (X = Cl, Br) in monoglyme yielded 1,2-bis(4,6-dimethyl-s-triazin-2-yl)ethane (Scheme 21), and other compounds including 1,2-bis(4,6-dimethyl-s-triazin-2-yl)ethene, 1,2,3-tris(4,6-dimethyl-s-triazin-2-yl)cyclopropane, 1,2,3-tris(4,6-dimethyl-s-triazin-2-yl)propane and 1,2,3,4-tetrakis(4,6-dimethyl-s-triazin-2-yl)butane.235 It is suggested that 1,2-bis(4,6-dimethyl-s-triazin-2-yl)ethane is produced through an SN2 mechanism, while other products are formed through carbenoid reactions.
Kim et al.237 indicated that Ni nanoparticles (5.7 ± 3.8 nm) exhibited a moderate catalytic activity in the oxidative addition reaction of benzylchloride and bromoacetonitrile in monoglyme under reflux conditions to prepare 3-arylpropanenitrile (Scheme 23), whereas larger Ni particles (3 μm and 100 mesh) showed no activity.
Smith and Fu238 developed a stereoconvergent method for the catalytic asymmetric Negishi cross-coupling of various racemic secondary propargylic halides with arylzinc reagents in monoglyme catalyzed by a chiral Ni/pybox complex (Scheme 24). In most cases, they achieved 70–80% yields and ee (enantiomeric excess) near or above 90%. It is important to point out that the catalyst components (NiCl2·glyme and pybox ligand) are commercially available.238,239
Organometallic compounds are common reagents for C–C coupling reactions. However, Fitt and Gschwend240 pointed out that monoglyme (and perhaps other glymes) should not be used in metalation reactions including those using t-butyllithium (t-BuLi). In the presence of t-BuLi, monoglyme undergoes deprotonation and β-elimination steps even at −70 °C to form lithium methoxide (Scheme 25), which is characteristic of 1,2-diheterosubstituted ethanes. Thus, there is no complex formed between monoglyme and t-BuLi. The reaction rates of different butyllithiums with monoglyme decrease in the order of t-BuLi > sec-BuLi ≫ n-BuLi.
The Delia group241,242 reported the synthesis of mono-, di- and tri-substituted phenylpyrimidines via Suzuki coupling reactions in various solvents (Scheme 26). They found that the monoglyme–water mixture, t-butanol and polar aprotic solvents (such as acetonitrile, acetone, THF, CHCl3 and CH2Cl2) gave the best results; polar protic solvents (such as methanol and ethanol) induced ether byproducts under basic conditions while nonpolar solvents (such as hexane) gave more byproducts and a 2-substituted isomer.
Pelter et al.246 prepared a thioester by the reaction of trisethylthioborane and benzoic acid in refluxing monoglyme for 7 h to give 78% isolated yield. Peacock and Geanangel247 conducted the reduction of trimethyl phosphite-borane by sodium naphthalide (which was prepared in monoglyme) to a new type of diphosphine derivative (Scheme 27). Leyden et al.248 carried out the reaction of [(η5-C5H5)2Ni] with nido-(B11H13)2−, (B10H13)−, or (B9H12)− catalyzed by Na/Hg amalgam in monoglyme to produce closo-[(η5-C5H5Ni)B11H11]−, nido-[(η5-C5H5Ni)B10H12]−, as well as isomeric closo-1- and [2-(η5-C5H5Ni)B9H9]− anions, respectively; they also performed the reaction of [(η5-C5H5)2Ni] or [(η5-C5H5NiCO)2] with closo-(B11H11)2−, (B10H10)2−, or (B9H9)2− in monoglyme to yield closo-[(η5-C5H5Ni)B11H11]−, [(η5-C5H5Ni)2B10H10], and isomeric 1- and [2-(η5-C5H5Ni)B9H9]−, respectively. Wermer and Shore249 suggested that pentaborane (B5H9) could be reduced by alkali metal naphthalide in THF or monoglyme to produce the nonahydropentaborate(2−) dianion [B5H9]2−, which could be further protonated to form B5H11 with yields up to 38% (Scheme 28).
Getman et al.250 synthesized arachno-[B9H13]2− as K+ and Na+ salts via the deprotonation of K[B9H14] by KH in monoglyme and the deprotonation of Na[B9H14] by NaNH2 in liquid ammonia respectively. Kang et al.251 suggested that monoglyme was the best refluxing solvent for converting arachno-S2B7H8− to hypho-S2B7H10−; they further carried out the synthesis of new metalladithiaborane clusters, derived from hypho-S2B7H10− in refluxing monoglyme, such as the treatment of hypho-S2B7H10− with Cp(CO)2FeCl to form of C5H5FeS2B7H8, with (CO)5MnBr to form hypho-l-(CO)4Mn-2,5-S2B6H9, and with [Cp*RhCl2]2 to form arachno-7-Cp*Rh-6,8-S2B6H8. Holub et al.252 carried out the reaction between Na2[nido-6,9-C2B8H10] and PCl3 in monoglyme at room temperature for 24 h to prepare phosphadicarbaborane nido-7,8,11-PC2B8H11 (35%), which was converted to a [7,8,11-nido-PC2B8H10]− anion by deprotonation; following the same strategy by using PhPCl2 as the phosphorus source, they also synthesized the isomeric compound 7-Ph-7,8,10-nido-PC2B8H10 (64%), and nido-7,8,11-PC2B8H11 (14%) (from an accompanying dephenylation reaction).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of potassium t-butoxide and water in aprotic solvents including DMSO, monoglyme, hexamethylphosphoramide, hexane, or diethyl ether. A 90% yield was obtained after 4 h at 30 °C in the cleavage of benzophenone to give benzoic acid using potassium t-butoxide–water–monoglyme.
3 ratio of potassium t-butoxide and water in aprotic solvents including DMSO, monoglyme, hexamethylphosphoramide, hexane, or diethyl ether. A 90% yield was obtained after 4 h at 30 °C in the cleavage of benzophenone to give benzoic acid using potassium t-butoxide–water–monoglyme.Finucane and Thomson255 oxidized triterpene 12-enes with various substituents in ring A (e.g. taraxeryl acetate and cholesteryl acetate) to their corresponding αβ-unsaturated ketones by treating them with N-bromosuccinimide in moist solvents (dioxane, THF, monoglyme, or diglyme) under the irradiation of visible light. Lateef et al.256 suggested that a series of meta- and para-substituted phenyl carbanilates undergo a rapid reversible dissociation in monoglyme (Scheme 29). They also indicated that the reaction followed the Hammett equation with positive ρ values of 1.49–1.66. As a precursor for preparing 2,5-dihydroxy-3,6-diphenyl-5,6-dihydropyrazine-1,4-dioxide, phenylglyoxal 2-oxime was synthesized by the acid hydrolysis of an α-oximino acetal (Scheme 30) in a mixture of monoglyme and pH 3.5 buffer.257 The oxime substrate is insoluble in the buffer alone or in aqueous methanol, but is soluble in a mixture of glyme and buffer. Pittman et al.258 dissolved polystyrene in a mixture of monoglyme and diglyme (2/1, v/v) for a reaction with Cr(CO)6 to prepare styrenetricarbonylchromiumstyrene copolymers.
Monoglyme was used as the solvent in the multi-step synthesis of D,L-muscone from cyclododecanone:259 cyclotetradecenone and triethylsilane were refluxed in monoglyme catalyzed by chloroplatinic acid to afford 1-triethylsiloxycyclotetradecene, which was converted to 2-chloro-2-cyclopentadecenone after adding dichlorocarbene in refluxing glyme-tetrachloroethylene (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); the final product was obtained by a conjugate addition of dimethylcopper lithium in ether, followed by a workup using saturated NH4Cl and then chromium(II) perchlorate reduction of α-chloro ketone in dimethyl formamide. Wilt and Rasmussen260 carried out the Favorskii reaction of bromo ketones in methanol and monoglyme (sodium methoxide as the base) to prepare epimeric methyl benzonorbornene-2-carboxylate, and found that more polar methanol led to more exo ester (exo
4); the final product was obtained by a conjugate addition of dimethylcopper lithium in ether, followed by a workup using saturated NH4Cl and then chromium(II) perchlorate reduction of α-chloro ketone in dimethyl formamide. Wilt and Rasmussen260 carried out the Favorskii reaction of bromo ketones in methanol and monoglyme (sodium methoxide as the base) to prepare epimeric methyl benzonorbornene-2-carboxylate, and found that more polar methanol led to more exo ester (exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo 80
endo 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) while the less polar solvent monoglyme increased the endo ester (exo
20) while the less polar solvent monoglyme increased the endo ester (exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo 58.5
endo 58.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41.5). In addition, higher ester yields were reported in monoglyme than in methanol. Stang and Mangum261 prepared alkyl methylenecyclopropenes by the addition of unsaturated carbenes to alkynes in monoglyme with t-BuOK at −55 °C. The same group262 also synthesized 2-indazoles by the reaction of triflate (CH3)2C
41.5). In addition, higher ester yields were reported in monoglyme than in methanol. Stang and Mangum261 prepared alkyl methylenecyclopropenes by the addition of unsaturated carbenes to alkynes in monoglyme with t-BuOK at −55 °C. The same group262 also synthesized 2-indazoles by the reaction of triflate (CH3)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHOTf with azobenzene and tert-butylazobenzene in monoglyme with t-BuOK at −20 °C via unsaturated carbene additions to azo compounds. To encapsulate chalcogen atoms by transition-metal carbonyl clusters, Vidal et al.263 synthesized the [PhCH2N(C2H5)3]3[Rh17(S)2(CO)32] complex through reacting Rh(CO)2acac and alkali carboxylates in tetraglyme, with H2S or SO2 under ∼300 atm of CO and H2 at 140–160 °C. Similarly, as an example of the encapsulation of arsenic by transition-metal carbonyl clusters, [PhCH2N(C2H5)3]3[Rh10As(CO)22]·C4H8O was prepared by the reaction of Rh(CO)2acac and alkali carboxylates in tetraglyme with Ph3As under ca. 300 atm of CO and H2.264
CHOTf with azobenzene and tert-butylazobenzene in monoglyme with t-BuOK at −20 °C via unsaturated carbene additions to azo compounds. To encapsulate chalcogen atoms by transition-metal carbonyl clusters, Vidal et al.263 synthesized the [PhCH2N(C2H5)3]3[Rh17(S)2(CO)32] complex through reacting Rh(CO)2acac and alkali carboxylates in tetraglyme, with H2S or SO2 under ∼300 atm of CO and H2 at 140–160 °C. Similarly, as an example of the encapsulation of arsenic by transition-metal carbonyl clusters, [PhCH2N(C2H5)3]3[Rh10As(CO)22]·C4H8O was prepared by the reaction of Rh(CO)2acac and alkali carboxylates in tetraglyme with Ph3As under ca. 300 atm of CO and H2.264
Collins et al.265 demonstrated that the repeated action of a sodium–potassium alloy in glyme–triglyme and the subsequent quenching with CH3I or with water could be an efficient low-temperature method to degrade coal by the cleavage of aliphatic and aromatic–aliphatic carbon–carbon bonds. Ladika and Stang266 carried out the elimination of trifluoromethane-sulfonic aid (CF3SO3H) from RCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OSO2CF3)C
C(OSO2CF3)C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CSiMe3 promoted by different bases in monoglyme to yield unsymmetrical trialkynes R[C
CSiMe3 promoted by different bases in monoglyme to yield unsymmetrical trialkynes R[C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]3SiMe3 or R[C
C]3SiMe3 or R[C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]3H (Scheme 31).
C]3H (Scheme 31).
Paxson et al.267 performed cobalt carbonyl-catalyzed reactions of syn gas by using a CO–H2 mixture at about 200–250 °C and 200 bar in glymes: in diglyme, the product selectivity for ethanol was 68% while in ethyl diglyme the selectivity for n-propanol increased to 40% (ethanol 28%). It is suggested that the solvent cleavage occurs and the terminal methoxy moieties supply the methyl group of ethanol. van Tamelen and Seeley268 carried out the catalytic N2-fixation by electrolytic and chemical reduction using monoglyme. Pez et al.269 found that the reaction of a titanium metallocene complex, μ-(η1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η5-cyclopentadienyl)-tris(η-cyclopentadienyl)dititanium, with N2 (∼10 atm) in monoglyme formed a complex giving a characteristic ν(N–N) peak at 1222 cm−1; it was further treated with THF/monoglyme and diglyme to yield a crystalline N2 complex with ν(N–N) = 1282 cm−1. These complex systems could have potential applications in N2-fixation. Budt et al.270 achieved the regioselective NBS-epoxidation of farnesate attached to helical peptides in monoglyme–water (5
η5-cyclopentadienyl)-tris(η-cyclopentadienyl)dititanium, with N2 (∼10 atm) in monoglyme formed a complex giving a characteristic ν(N–N) peak at 1222 cm−1; it was further treated with THF/monoglyme and diglyme to yield a crystalline N2 complex with ν(N–N) = 1282 cm−1. These complex systems could have potential applications in N2-fixation. Budt et al.270 achieved the regioselective NBS-epoxidation of farnesate attached to helical peptides in monoglyme–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture at 0 °C. Hayward and Shapley271 reacted Re2(CO)10 with sodium dispersion in glymes (diglyme and triglyme) to prepare the rhenium carbonyl clusters Re4(CO)162−, H2Re6C(CO)182−, Re7C(CO)213−, and Re8C(CO)242−, as Et4N+ and (PPh3)2N+ salts. Tee and Enos272 examined the kinetics of hydrolysis of six p-nitrophenyl alkanoates in basic aqueous solutions containing up to 80% (v/v) of the co-solvents: ethylene glycol, 2-methoxyethanol, monoglyme, diglyme, or DMSO; they suggested that the ether-type solvents (2-methoxyethanol, monoglyme and diglyme) are more effective than ethylene glycol or DMSO in reducing and eliminating the hydrophobic aggregation and coiling of longer chain alkanoates. Briggs273 carried out the trimerization of ethylene to hex-1-ene using a homogeneous three-component catalyst of chromium, hydrolyzed alkylaluminium and monoglyme with 74% selectivity; the replacements (such as diglyme, triglyme, THF, and o-dimethoxybenzene) for monoglyme gave less desirable results. A tricyclic ring/cage system of RC6H5·5BNR′2 products were formed by reactions between dehalogenation products of F2BN(i-Pr)2 with monoalkylbenzenes using monoglyme as the co-solvent.274 Hung et al.275 prepared linear and cyclic perfluorinated polyethers through the ionic polymerization of a trifluorovinyl ether alcohol (CF2
1) mixture at 0 °C. Hayward and Shapley271 reacted Re2(CO)10 with sodium dispersion in glymes (diglyme and triglyme) to prepare the rhenium carbonyl clusters Re4(CO)162−, H2Re6C(CO)182−, Re7C(CO)213−, and Re8C(CO)242−, as Et4N+ and (PPh3)2N+ salts. Tee and Enos272 examined the kinetics of hydrolysis of six p-nitrophenyl alkanoates in basic aqueous solutions containing up to 80% (v/v) of the co-solvents: ethylene glycol, 2-methoxyethanol, monoglyme, diglyme, or DMSO; they suggested that the ether-type solvents (2-methoxyethanol, monoglyme and diglyme) are more effective than ethylene glycol or DMSO in reducing and eliminating the hydrophobic aggregation and coiling of longer chain alkanoates. Briggs273 carried out the trimerization of ethylene to hex-1-ene using a homogeneous three-component catalyst of chromium, hydrolyzed alkylaluminium and monoglyme with 74% selectivity; the replacements (such as diglyme, triglyme, THF, and o-dimethoxybenzene) for monoglyme gave less desirable results. A tricyclic ring/cage system of RC6H5·5BNR′2 products were formed by reactions between dehalogenation products of F2BN(i-Pr)2 with monoalkylbenzenes using monoglyme as the co-solvent.274 Hung et al.275 prepared linear and cyclic perfluorinated polyethers through the ionic polymerization of a trifluorovinyl ether alcohol (CF2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFORfCH2OH, Rf = CF2CF(CF3)OCF2CF2) and the subsequent fluorination of the polyfluorinated polyethers (Scheme 32). They found that without a solvent, linear polymers with Mn values up to 29
CFORfCH2OH, Rf = CF2CF(CF3)OCF2CF2) and the subsequent fluorination of the polyfluorinated polyethers (Scheme 32). They found that without a solvent, linear polymers with Mn values up to 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 were produced, while in glyme solution, cyclic oligomers were the major products and a cyclic dimer was obtained in yields up to 60%. The reaction of arylcalcium iodides and nitrous oxide (N2O) in monoglyme favored the formation of azobenzenes due to the insertion with N2O into diphenylcalcium.276
000 were produced, while in glyme solution, cyclic oligomers were the major products and a cyclic dimer was obtained in yields up to 60%. The reaction of arylcalcium iodides and nitrous oxide (N2O) in monoglyme favored the formation of azobenzenes due to the insertion with N2O into diphenylcalcium.276
Kirij et al.277 prepared [(CH3)4N][Te(CF3)3] and [(CH3)4N][I(CF3)2] by the reactions of (CH3)3SiCF3/[(CH3)4N]F with Te(CF3)2 and CF3I respectively in THF or monoglyme at −60 °C. Yagupolskii et al.278 carried out an aza Curtius rearrangement by reacting N-(trifluoromethylsulfonyl)carboximidoyl chlorides with sodium azide in monoglyme or acetonitrile at −5 to +10 °C to form carbodiimides (RN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NSO2CF3). Israelsohn et al.279 reported that a PtCl4–CO catalyst could promote the hydration of internal and terminal alkynes in aqueous monoglyme or diglyme to afford aldehyde-free ketones between 80 and 120 °C. The reaction was found to be strongly dependent on the electronic and steric nature of the alkynes. Monoglyme or THF was used to dissolve elemental sulfur, Me3SiCF3, and fluoride salts for the preparation of trifluoromethanethiolates, [NMe4]SCF3, CsSCF3 and [(benzo-15-crown-5)2Cs]SCF3 (Scheme 33).280 It is known that SCF3 salts are versatile nucleophilic reagents for synthesizing a variety of organic, organometallic and inorganic molecules. Tyrra et al.281 prepared tetramethylammonium trifluoromethyltellurate(0), [NMe4]TeCF3, with 60% yield from Me3SiCF3, elemental tellurium and [NMe4]F in monoglyme; they further carried out the cation exchange of [NMe4]TeCF3 with [PNP]Br ([PNP] = bis(triphenylphosphoranylidene)ammonium) and [(dibenzo-18-crown-6)K]Br and demonstrated the high nucleophilicity of TeCF3− when coupled with these low coordinating cations.
NSO2CF3). Israelsohn et al.279 reported that a PtCl4–CO catalyst could promote the hydration of internal and terminal alkynes in aqueous monoglyme or diglyme to afford aldehyde-free ketones between 80 and 120 °C. The reaction was found to be strongly dependent on the electronic and steric nature of the alkynes. Monoglyme or THF was used to dissolve elemental sulfur, Me3SiCF3, and fluoride salts for the preparation of trifluoromethanethiolates, [NMe4]SCF3, CsSCF3 and [(benzo-15-crown-5)2Cs]SCF3 (Scheme 33).280 It is known that SCF3 salts are versatile nucleophilic reagents for synthesizing a variety of organic, organometallic and inorganic molecules. Tyrra et al.281 prepared tetramethylammonium trifluoromethyltellurate(0), [NMe4]TeCF3, with 60% yield from Me3SiCF3, elemental tellurium and [NMe4]F in monoglyme; they further carried out the cation exchange of [NMe4]TeCF3 with [PNP]Br ([PNP] = bis(triphenylphosphoranylidene)ammonium) and [(dibenzo-18-crown-6)K]Br and demonstrated the high nucleophilicity of TeCF3− when coupled with these low coordinating cations.
It is also important to know that glymes may be inferior to other organic solvents in some reactions. For example, the alkylation rates of alkali enolates in DMSO were found to be 1000-fold faster than that in glymes (mono- and di-) although the same reaction was even slower in diethyl ether.282
7.2. Reaction additives/metal chelators
The second important role of glymes in reactions is acting as additives or metal chelators. Shinohara et al.283 observed highly reactive agent-separated ion pairs formed via complexing sodium polystyryl ion pairs with tri- or tetraglyme; by adding glyme to solutions of sodium polystyryl in THF at 25 °C, they found propagation constants of the polymerization of living polymers increasing with the glyme concentration. Trifluorosilyl substituted dialkyl compounds including trans-Pt(SiF3)2(PMe3)2, Pd(SiF3)2(PMe3)2, and Ni(SiF3)2(PMe3)3 were synthesized by reacting an excess amount of Cd(SiF3)2·monoglyme with trimethylphosphine metal dibromides of platinum, palladium, and nickel (Scheme 34).284Liang and Ying285 studied the kinetics and mechanism of the anionic equilibrium polymerization of α-methylstyrene in cyclohexane with butyllithium as the initiator and monoglyme or diglyme as the polar additive; kinetic equations of the equilibrium polymerization is dependent on the mole ratio of glyme/butyllithium; the stability of complexes formed by poly(α-methylstyryl)lithium (PnLi) and the ether additive is in a decreasing order of [PnLi (diglyme)] > [PnLi (monoglyme)] > [PnLi (THF)]. Wang et al.286 employed 7Li and/or l3C NMR spectroscopy to investigate the aggregation equilibrium and electronic structure of methyl α-lithioisobutyrate (MIBLi) in THF with the co-existence of various Li+-binding ligands; they found that the addition of ligands to coexisting tetrameric and dimeric MIBLi in THF increases the dimeric population in the order monoglyme < diglyme < 12-crown-4 < hexamethylphosphoric triamide, which is in agreement with the increasing strength of complexation between MIBLi (lithium cation) and the ligands.
Bis(trifluoromethy1)cadmium·glyme [(CF3)2Cd·monoglyme] is a convenient reagent used in the preparation of trifluoromethyl substituted metal compounds.287 For example, (CF3)2Cd·monoglyme exchanges ligands with GeI4, SnI4, or PI3 to form (CF3)4Ge, (CF3)4Sn, or (CF3)3P respectively at room temperature. The reaction of (CF3)2Cd·monoglyme with acyl halides such as CH3C(O)Br formed the acyl fluorides CH3C(O)F in 95% yield at −25 °C. Krause and Morrison288 prepared Lewis base adducts of bis(trifluoromethyl)cadmium (CF3)2Cd by mixing (CF3)2Hg with dimethylcadmium in THF, monoglyme, diglyme, or pyridine; Lewis base exchange is achieved when the glyme adduct is dissolved in a base, for example, (CF3)2Cd·monoglyme in pyridine. They suggested that (CF3)2Cd·base species are more reactive than (CF3)2Hg: (CF3)4Sn (66% yield) and (CF3)4Ge (43% yield) were synthesized by ligand exchanges between SnBr4 and GeI4 with (CF3)2Cd·monoglyme respectively at room temperature; the reaction of acyl halides with (CF3)2Cd·monoglyme at subambient temperature produced the acyl fluoride with ∼90% yield, and stereospecific difluorocarbene cis-2-butene at −30 °C. This group289 used the same method to prepare monosubstituted compounds (CF3)BrNi(PEt3)2, (CF3)BrPd(PEt3)2, and (CF3)IPt(PBun3)2 in 60–70% yields by the reaction of (CF3)2Cd·monoglyme with bis(trialkylphosphine) group 8B dihalides such as Br2Ni(PEt3)2 within 0.5–5 h; however, when an excess amount of (CF3)2Cd·monoglyme was present, disubstituted compounds (CF3)2M(PEt3)2 (M = Ni, Pd, or Pt) were found over an extended reaction period. The Morrison group290 also conducted the synthesis of (η5-C5H5)Co(CO)(CF3)2 (63% yield) via the reaction of (CF3)2Cd·monoglyme with (η5-C5H5)Co(CO)I2. This group291 further investigated the ligand exchange reactions of Cd(CF3)2·monoglyme with various aryl-containing Pb, Sn, and Ge halides, acetates, and thioethers in THF or CHCl3; they found that when an excess of trifluoromethylating agent was used, these new compounds PbPh(CF3)3 (51%), PbPh2(CF3)2 (61%), PbPh3CF3 (81%), SnPh2(CF3)2 (55%), SnPh3CF3 (80%), and GePh3CF3 (72%) were obtained, and when the trifluoromethylating agent was the limiting reactant, partially substituted compounds PbPh2(Cl)CF3 (78%), PbPh2(O2CCH3)CF3 (89%), PbPh(O2CCH3)2CF3 (63%), and PbPh(O2CCH3)(CF3)2 (64%) were isolated. Murray et al.292 performed the reaction of [Au(CH2)2PPh2]2Br2 with Cd(CF3)2(monoglyme) in CH2Cl2 to synthesize a dialkyl Au(II) phosphorus ylide dimer [Au(CH2)2PPh2]2(CF3)2, whose X-ray crystal structure was also determined. Nair and Morrison293 prepared TlPh(CF3)2 with 87% yield by reacting Cd(CF3)2·monoglyme with TlPhCl2 after 72 h, and Tl(CF3)2OAc with 46% yield by reacting Cd(CF3)2·monoglyme with Tl(OAc)3 after 45 min. Loizou et al.294 prepared cyclopentadienyldinitrosyl(trifluoromethyl)chromium(0) CpCr(NO)2CF3 (71%), and cyclopentadienyldinitrosyl(trifluoromethyl)molybdenum(0) CpMo(NO)2CF3 (44%) via the reaction of Cd(CF3)2·monoglyme with the corresponding chlorides at 65 °C. Ludovici et al.295 achieved nearly quantitative yields of CnF2n+1NO (n = 1, 2, 3, 6) by the reaction of NOCl with Cd(CnF2n+1)2·monoglyme. Daniele et al.296 synthesized La(OTf)(OC6H3-2,6-Me2)2(glyme) [glyme = triglyme or tetraglyme] derivatives through reactions between lanthanum triflate adducts La(OTf)3(glyme) and 2 equiv. LiOAr (Ar![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6H3-2,6-Me2) in THF. The grafting of La(OTf)(OC6H3-2,6-Me2)2(triglyme) onto silica produced a hybrid material, which was used as a catalyst for the activation of formaldehyde in water for hydroxymethylation of silyl enol ether in mild conditions. The Fu group at MIT has demonstrated that the NiCl2·glyme/pybox ligand is an effective chiral catalyst for the asymmetric Negishi cross-coupling of racemic secondary propargylic halides with alkylzinc239 or arylzinc reagents.238
C6H3-2,6-Me2) in THF. The grafting of La(OTf)(OC6H3-2,6-Me2)2(triglyme) onto silica produced a hybrid material, which was used as a catalyst for the activation of formaldehyde in water for hydroxymethylation of silyl enol ether in mild conditions. The Fu group at MIT has demonstrated that the NiCl2·glyme/pybox ligand is an effective chiral catalyst for the asymmetric Negishi cross-coupling of racemic secondary propargylic halides with alkylzinc239 or arylzinc reagents.238
7.3. Catalysts
Glymes contain multiple ethylene oxide units, which define many glymes as amphiphiles (with both hydrophilic and lipophilic properties). Ideally, these glymes can be used as direct phase-transfer catalysts. The second case for glymes acting as catalysts is that glymes can form complexes with alkali cations like crown ethers. The third category is that glyme oxygens can form hydrogen-bonds with reaction intermediates and thus promote the reaction by stabilizing these intermediates. The fourth category is where glymes are ligands of metal salt catalysts.Kudryavtsev and Zakharov306 investigated the phosphorylation of heptafluorobutanol with phosphorus(V) oxychloride (Scheme 36) catalyzed by complex catalysts based on Li+, Na+, K+, or Cs+ chlorides and polydentate ligands (such as dibenzo-18-crown-6, mono-, di-, and tetraglymes, and PEG-600 and PEG-1000); the addition of polydentate ligands improved the solubility of inorganic salts and increased the reaction rates by a factor of 1.3–2.8. Although the LiCl and PEG systems were shown to be most efficient, the use of glymes with LiCl led to 91–92% yields in 1.9–2.7 h (vs. 94% yield in 3.6 h without the addition of any ligand). Kochergina and Anufriev307 carried out the nucleophilic substitution of 6,7-dichloro-3-ethyl-2-ethoxynaphthazarine to prepare echinochrome trimethyl ether (Scheme 37). The reaction was accelerated by the use of monoglyme and diglyme as both catalysts and solvents; in particular, the reaction in diglyme produced up to 72% yield vs. 52% without adding any glyme. It was explained that glymes effectively solvate the potassium cation to form ion pairs.
 | ||
| Scheme 38 Binding interaction between glyme and intermediate during aminolysis of thiophenyl 4-nitrobenzoate with 4-chlorobenzylamine. | ||
Hogan and Gandour309 suggested that glymes are more effective catalysts than crown ethers (an inverse macrocyclic effect) in the butylaminolysis of p-nitrophenyl acetate in chlorobenzene; this group310 further observed a break in a plot of the catalytic rate constant vs. chain length of catalyst, indicating triglyme gives the optimum catalysis. The same group311 further examined the transition structure complexes for the glyme- and α,ω-dimethoxyalkane-catalyzed butylaminolysis of 4-nitrophenyl acetate in chlorobenzene. They confirmed that the kcat/Oxy values increase with oligomer length up to triglyme and then plateau. The ester aminolysis in aprotic solvents involves a rate-limiting breakdown of a zwitterionic tetrahedral intermediate, which forms a complex with a glyme molecule (Scheme 39). They further concluded that the number of polyether oxygens required for optimum catalysis and the best spacing (in α,ω-dimethoxyalkanes) among these oxygens are strongly dependent on the number of ammonium protons in the transition structure. More recently, Basilio et al.312,313 studied the butylaminolysis of p-nitrophenyl acetate in chlorobenzene catalyzed by crown ethers or glymes as phase-transfer catalysts, and proposed a reaction pathway to reflect a 1st-order dependence on the catalyst concentration and a 2nd-order dependence on butylamine concentration (Scheme 40). The new pathway (red arrows) involves the complexing of ether-TI (ether-tetrahedral intermediate) with an amine molecule, which is an addition to the mechanisms (green and blue arrows) traditionally accepted for the catalysis by phase-transfer agents of aminolysis reactions in aprotic solvents. The green arrows indictate butylamine attacking the ester to form the tetrahedral intermediate (TI), and the amine complexing with the ether catalyst before binding to TI; the blue arrows indicate butylamine attacking the ester to form the TI and a second butylamine forming hydrogen bonds with the TI followed by glyme binding to both butylamine components. The same group314 further examined a more complex reaction system of butylaminolysis of 4-nitrophenylcaprate in water–AOT–chlorobenzene microemulsions catalyzed by triglyme (Scheme 41), and discussed four simultaneous reaction pathways.
It is also interesting to note in some cases that glymes are inactive or less active than PEGs as phase-transfer catalysts, i.e., reactions of aryldiazonium salts with CBrCl3 or CH3I initiated by potassium acetate,316 dehydrohalogenation of 2-bromooctane with aqueous KOH.317
7.4. Reagents
Typically, glymes are chemically inert; however, they could become reactive under certain conditions. Newman and Liang318 observed that when 3-nitroso-5-methyl-5-tert-butyl-2-oxazolidone was treated with sodium phenoxide, the stereospecific cleavage of monoglyme occurred to form 2-methoxyethyl trans-2,2,3-trimethyl-l-butenyl ether in 46% yield. Ishii et al.319 studied the anodic fluorination of monoglyme and diglyme (Scheme 43) in acetonitrile using a fluoride salt as a supporting electrolyte and a fluoride ion source with an undivided cell; they observed that corresponding monofluoromethyl ethers were obtained as the main products in satisfactory yields. However, the anodic fluorination of crown ethers caused C–C bond cleavage, producing selective α,ω-difluoro products with high yields.Grabovskiy et al.320 studied the oxidation of a series of ethers including monoglyme by dimethyldioxirane (DMDO), and suggested a second-order reaction kinetics r = k[DMDO][ether]. They also determined the rate constants at 5–50 °C, as well as the activation parameters of the reaction. The main oxidation products of monoglyme with DMDO (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) in acetone at 25 °C include methanol (32%), 2-methoxyethanal (4%) and methoxyacetic acid (3%). Boron-based compounds including sodium borohydride (NaBH4) and ammonia borane (NH3BH3) have been studied as chemical hydrogen storage materials. Yoon et al.321 developed a safe and efficient method for preparing NH3BH3: iodine oxidation of Bu4N+B3H8- in monoglyme solution yielded (glyme)B3H7, which was converted to NH3BH3 by displacement of the coordinated glyme with anhydrous ammonia.
1 molar ratio) in acetone at 25 °C include methanol (32%), 2-methoxyethanal (4%) and methoxyacetic acid (3%). Boron-based compounds including sodium borohydride (NaBH4) and ammonia borane (NH3BH3) have been studied as chemical hydrogen storage materials. Yoon et al.321 developed a safe and efficient method for preparing NH3BH3: iodine oxidation of Bu4N+B3H8- in monoglyme solution yielded (glyme)B3H7, which was converted to NH3BH3 by displacement of the coordinated glyme with anhydrous ammonia.
In addition, there are some reactive glymes (such as polyglycol allyl methyl ether, polyglycol diallyl ether and PEG methyl ether methacrylate) commercially available. As a typical application, the allyl-containing glymes can react with siloxanes through a Pt-catalyzed hydrosilylation reaction to produce polyether modified silicones, which have applications as silicone surfactants and polyurethane foam stabilizers.
8. (Co-)solvents for biocatalysis
Surprisingly, glymes and their aqueous solutions are not commonly used as solvents for enzymatic processes. There have been some conflicting results on the enzyme activity and stability in aqueous solutions of glymes. Some studies suggest the high enzyme activity and/or stability in aqueous glymes. Yoshpe-Besancon et al.322 observed that a 55% triglyme solution could shift the equilibrium of aminopeptidase A from amide bond hydrolysis to peptide bond formation, which allowed a selective α-amino protection of derivatives of many amino acids (except glycine and proline) by the malyl group. Rosell et al.323 found that 50% (v/v) aqueous solutions of diglyme (G2) or tetraglyme (G4) depressed the hydrolytic activity of penicillin acylase by roughly 65%, but boosted its synthetic activity by ∼4.8 times. In addition, these two solutions showed negligible impact on the enzyme stability. Berkowitz et al.324 suggested that 10% triglyme in aqueous buffer solution enabled the optimal efficiency for a PPL-catalyzed hydrolysis of a drug intermediate at both lower enzyme loading and higher temperature. Schroën et al.325 studied the enzymatic synthesis of antibiotic cephalexin catalyzed by penicillin G acylase (Scheme 44), and found the enzyme retained 90% activity after incubation in 30–36% (v/v) glymes (mono-, di- and tri-) at 30 °C for 24 h; compared with the direct synthesis in water, the addition of methanol and triglyme could increase the equilibrium concentration of cephalexin by a factor of 2–3. However, Illanes and Fajardo326 found that in 50/50 (v/v) mixtures of organic solvents and aqueous buffer, Escherichia coli penicillin acylase maintained a high stability in polyols (such as ethylene glycol and glycerol), a low stability in glyme and diglyme, and no activity after 24 h in methanol, DMF and DMSO.On the other hand, there are even limited studies on the enzymatic reactions in neat glymes although PEGs327–329 and PEG-based aqueous biphasic systems (ABS)330 are known media for enzymatic reactions. The Sheldon group331 indicated that cross-linked enzyme aggregates (CLEAs) of penicillin G acylase was not quite active in a mixture of glyme–water (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v), resulting in low conversions [10% in monoglyme (log
5, v/v), resulting in low conversions [10% in monoglyme (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = −0.8) and 5% in triglyme (log
P = −0.8) and 5% in triglyme (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = −1.8) after 1 h] for the synthesis of ampicillin. The Zhao group21 found that long-chain glymes are highly compatible with immobilized Candida antarctica lipase B (Novozym 435), resulting in higher enzyme activities and stabilities than t-butanol and some ionic liquids. Furthermore, this group noticed that soybean oil is fully miscible with glymes, which enables a homogeneous reaction mixture for enzymatic preparation of biodiesel; in the presence of glymes, the immobilized lipase showed a very high tolerance to high methanol concentrations (up to 60–70% v/v), and nearly quantitative triglyceride conversions could be obtained under mild reaction conditions.
P = −1.8) after 1 h] for the synthesis of ampicillin. The Zhao group21 found that long-chain glymes are highly compatible with immobilized Candida antarctica lipase B (Novozym 435), resulting in higher enzyme activities and stabilities than t-butanol and some ionic liquids. Furthermore, this group noticed that soybean oil is fully miscible with glymes, which enables a homogeneous reaction mixture for enzymatic preparation of biodiesel; in the presence of glymes, the immobilized lipase showed a very high tolerance to high methanol concentrations (up to 60–70% v/v), and nearly quantitative triglyceride conversions could be obtained under mild reaction conditions.
9. Materials
This section focuses on the applications of glymes as materials or as media to prepare new materials.9.1. Nanomaterials
A series of metal or nonmetal nanoparticles with functionalized surfaces were synthesized in glyme solvents by the Kauzlarich group. They demonstrated a straightforward and versatile technique to control both crystal size and surface termination of the nanomaterials. Methyl-terminated Ge nanocrystals with an average particle size of around 3.5 nm were produced by the metathesis reaction between the Zintl salt NaGe and GeCl4 in degassed monoglyme or diglyme.332 The Kauzlarich and Taylor group333,334 further extended this method to prepare alkyl-terminated crystalline Ge nanoparticles by the reactions between GeCl4 and NaGe, KGe, or Mg2Ge followed by surface termination with alkyl Li and Grignard reagents in glymes. It was observed that diglyme and triglyme seemed to support the reaction better than monoglyme and the reactions in triglyme were much faster than in diglyme. Moreover, the largest particles (8–10 nm) of Ge nanoparticles were produced in monoglyme while the smallest particles of 4.5 nm mean size were synthesized in triglyme. Furthermore, alkyl-terminated silicon nanoclusters were also prepared by the reaction of SiCl4 with Mg2Si in monoglyme and surface-terminated with various alkyl groups, R-n-Si (R = methyl, ethyl, n-butyl, and n-octyl).335 Based on the same technique, this group336 further synthesized the surface-capped and organic-functionalized tin nanoparticles of Sn/R, Sn/Si–R (R = n-C4H9), and Sn/SiO2 core–shell particles via the reaction of Mg2Sn with SnCl4 or SiCl4 in monoglyme.336Plasma-deposited PEG-like films have become emerging materials as ‘non-stick’ surfaces toward protein and bacteria. Ratner and co-workers337 designed PEG-like coatings via radio-frequency plasma deposition of short-chain oligoglymes, dioxane, and crown ethers onto glass cover slips; the films were characterized by X-ray photoelectron spectroscopy (XPS), time-of-flight secondary ion mass spectrometry (TOF-SIMS), dynamic contact angle goniometry, and radiolabeled fibrinogen adsorption. The Brétagnol group338,339 constructed new microstructured surfaces through a spatial arrangement of different functional domains by a combination of the plasma polymerization of diglyme (leading to coatings with a high concentration of ethylene oxide groups (>70%)) and photolithography. The high stability of these films in acetone suggests that these coatings could be used in classical lift-off processes involving washing in acetone for designing patterned surfaces.339 The same group340 further developed a straightforward nanoscale writing technique, enabling the fabrication of bio-adhesive patterns directly in a non-bio-adhesive matrix. This method involves two major steps: (a) plasma polymerization of diglyme to form a protein-repelling PEO-like coating, (b) direct electron beam lithography inside the matrix by tuning the ether bond concentration of the coating to produce nanoscale bio-adhesive patterns.
The Stucky group341 developed a time evolution kinetic-dependent crystal growth model to examine the nanocrystal growth of CdS from cadmium acetate and sodium sulfide in different solvents (e.g. ethylene glycol, monoglyme, diglyme, and trioctylphosphine), using trialkylphosphine oxide (alkyl = ethyl or octyl) as a surfactant. They observed that the size of nanoparticles is controllable by the reaction time and temperature; the nanoparticle sizes are suitable for spectroscopic analysis of electron quantum confinement. The same group342 also prepared CdS nanorods through the reaction of cadmium acetate and sodium sulfide at low temperature (25–65 °C) in an aqueous phase using nonionic pluronic amphiphilic triblock copolymers, (EO)x(PO)y(EO)x, as structure-directing agents. However, when the same reaction is refluxed in ethylene glycol and monoglyme without surfactant, a new morphology of microrods with flat ends, dumbbell-shaped microrods, and cotton-ball-like microparticles is produced. Chiu and Kauzlarich343 synthesized crystalline germanium nanoparticles by the reduction of GeCl4 with sodium naphthalide in monoglyme within 10 min. Pickering et al.344 prepared octyloxy-capped boron nanoparticles through a reduction of BBr3 with sodium naphthalenide in dry monoglyme followed by the addition of excess octanol at room temperature. The size distribution of the nanoparticles can be controlled by tuning the reaction conditions, such as concentration and reducing agent. Similarly, Cho345 performed the reaction of SnCl4 and GeCl4 with sodium naphthalide in monoglyme and RLi (R = butyl, ethyl, methyl) to prepare Sn70Ge30@carbon core–shell nanoparticles. The core sizes and shell thicknesses of these nanoparticles are dependent on the alkyl terminator. Electrochemical studies suggest that nanoparticles synthesized with butyl terminators exhibit the highest capacity retention after 40 cycles (95%) and a first charge capacity of 1040 mA h g−1. Shirahata and Sakka346 synthesized highly luminescent Si nanoparticles (NPs) terminated with alkoxy monolayers through the reduction SiCl4 by sodium biphenylide in a toluene–monoglyme mixture using an inverse micelle method; they also observed the size-dependent UV photoluminescence (PL) properties for non-oxidized Si NPs at room temperature, as well as a high quantum efficiency of the UV fluorescence. Mishra et al.347 prepared heterometal–organic complexes including NaY(TFA)4(diglyme), [Na(triglyme)2][Y2(TFA)7(THF)2], Na2Y(TFA)5(tetraglyme), NaLn(TFA)4(diglyme), [Ln = Er, Tm, or Yb], and Na2Ln(TFA)5(tetraglyme) [Ln = Er, or Yb] (TFA = trifluoroacetate), and used them as precursors for up-converting NaY(Ln)F4 (Ln = Yb, Er, Tm) nanocrystals and thin films, which have potential applications as lanthanide-doped up-conversion (UC) emission materials.
9.2. Polymeric materials
Shirai et al.348 carried out radical polymerization to prepare polymers carrying glyme units as alkali cation binding sites and photodimerizable cinnamoyl units. They found that the photodimerization of the cinnamoyl groups with relatively short glyme chains could improve their cation binding ability, and the addition of alkali metal cations as templates enforced the effect of photodimerization on the cation binding properties. Morgado et al.349 found that an orange-emitting PPV-based statistical copolymer with glyme-like side groups (Scheme 45) has a photoluminescence efficiency of ∼17%. The presence of glyme units enables the solvation of salts and the ion mobility under the applied electric field, thus this copolymer carries both ion-coordinating and luminescence moieties.Moore et al.350 dissolved poly(enaminonitrile) (PEAN) or miscible blends of PEAN with poly(ethylene oxide) in a number of glymes, and observed the cloud points of these solutions when the temperature increases. The Sneddon and Remsen groups351,352 found that polyborazylene could be dissolved in monoglyme or THF, and could be further precipitated by the addition of pentane. Monoglyme was used as a solvent for dissolving and mixing two polymeric precursors, namely allylhydridopolycarbosilane (AHPCS) ([Si(CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)2CH2]0.05[SiH2CH2]0.95) and polyborazylene (PBz) [B3N3H4−x]n, which serve as the sources for the SiC and BN phases respectively.353 Following the removal of solvent, the co-pyrolysis of these two precursors at 1000 °C affords a two-phase SiC–BN ceramic composite whose highly unusual microstructure resembles that of certain polymer blends.
CH2)2CH2]0.05[SiH2CH2]0.95) and polyborazylene (PBz) [B3N3H4−x]n, which serve as the sources for the SiC and BN phases respectively.353 Following the removal of solvent, the co-pyrolysis of these two precursors at 1000 °C affords a two-phase SiC–BN ceramic composite whose highly unusual microstructure resembles that of certain polymer blends.
López and Ratner354 reported weakly ionized, radio-frequency and glow-discharge plasmas from the vapor of glyme precursors (mono-, di- and tri-), and then used them to deposit organic thin films on polytetrafluoroethylene (PTFE). Sandner et al.355 performed the free-radical photopolymerization of an oligo(ethylene glycol) dimethacrylate ((EG)23DMA) in two glymes ((EG)3DME and (EG)11DME) as plasticizers in the presence of LiCF3SO3, which was analyzed by differential scanning calorimetry (DSC), FT-Raman spectroscopy and sol–gel analysis. The addition of LiCF3SO3 increased the polymerization rate.
9.3. Inorganic materials
Baker et al.356 prepared homoleptic dicyclohexylphosphide (PCy2) complexes of early transition metals using monoglyme (G1) as the complexing agent for Li+; these new complexes include [Li(G1)][Zr(PCy2)5], [Li(G1)][Hf(PCy2)5], [Li(G1)][Ti(PCy2)4], [Li(G1)][V(PCy2)4], [Li(G1)][Re(PCy2)4], [Li(G1)2][Nb(PCy2)4], Mo(PCy2)4, [Li(G1)3][Cr2(PCy2)5], [Li(G1)3][W2(PCy2)5] and [Li(G1)][Mn2(PCy2)5]. Baxter et al.357 synthesized the adducts of [Gd-(tmhd)3] (tmhd-H = 2,2,6,6-tetramethylheptane-3,5-dione) with a series of glyme ligands from monoglyme to heptaglyme. These complexes were evaluated as precursors for Atmospheric Pressure Chemical Vapor Deposition (APCVD) coatings of Ce0.9Gd0.1O1.95 thick electrolyte films for solid oxide fuel cells (SOFCs). The advantages of using these complexes for metal organic chemical vapor deposition (MOCVD) include: (1) readily available in crystalline solids of fixed stoichiometry; (2) soluble in hydrocarbons (both aliphatic or aromatic) and air stable; and (3) better mass transport properties of more stable complexes can be prepared from longer chain glymes. Drake et al.358 synthesized the monomeric materials [M(β-diketonate)2(L–L)] by reacting oligomeric alkaline earth metal β-diketonate complexes, [M(β-diketonate)2] with a glyme (tri- and tetra-) ligand (L). The same group359 further prepared eight-coordinate triglyme-bridged dimeric complexes, [(Ln(tmhd)3)2L1] (Ln = Eu or Tb, L1 = triglyme, and tmhd = ButCOCHCOBut) through the reaction of hydrated β-diketonate complexes [Ln(tmhd)3(H2O)] with triglyme in hexane. They also prepared the nine-coordinate monomeric compound [La(tmhd)3L2] via the reaction of [La(tmhd)3(H2O)] with tetraglyme (L2) in hexane. These complexes are stable in air with moisture and also have a good volatility and thermal stability. Arunasalam et al.360 used carbonate and hydroxide compounds to prepare a number of Group 2 β-diketonate complexes supported by multidentate glyme ligands (tri-, tetra- or heptaglyme), including a single-crystal structure of a calcium complex of [(Ca(hfpd)2)2(heptaglyme)] where [H-hfpd = 1,1,1,5,5,5-hexafluoropentane-2,4-dione]. These β-diketonate and carboxylate compounds have potential applications as CVD precursors for both MO and MF2 thin films. Arnáiz et al.361 synthesized outer-sphere addition compounds of MoO2Br2(H2O)2 with diethyl ether, dioxane, glyme, diglyme, triglyme and tetraglyme by crystallizing diethyl ether extracts of a solution of sodium molybdate in concentrated hydrobromic acid and the respective ether. They also suggested that the polyether interacts with the MoO2Br2(H2O)2 units through hydrogen bonds based on the X-ray structure analysis of diglyme and tetraglyme adducts. Crochet and Fromm362 prepared and characterized crystalline cobalt, nickel, zinc, and mercury halide adducts with polyethers as ligands including [Co(μ-Cl)2CoCl2(monoglyme)2], cis-[CoI2(H2O)2(monoglyme)2]2+[CoI4]2–, [NiI2(monoglyme)2], [ZnI2(monoglyme)], [HgCl2(monoglyme)], [CoI2(diglyme)], [ZnI2(diglyme)], [HgI2(diglyme)], [CoCl(μ-Cl)(diglyme)]2, [NiI(μ-I)(diglyme)]2, [Co(μ-Cl)(triglyme)]22+[CoCl2(μ-Cl)]22−, and cis-[(NiI2)(triglyme)]n. Some of these adducts exhibit unusual coordination numbers and arrangements.10. Other applications
10.1. Chemical Vapor Deposition (CVD)
CVD is an important method for producing new materials. When preparing f-element oxides by the CVD procedure, the reproducibility becomes a challenge because regular precursors often self-associate, form a hydrate and undergo hydrolysis or cleavage of the ligands on storage. As an effort to design new lanthanide complexes with suitable mass transport properties for metal–organic chemical vapor deposition (MOCVD) applications, the Fragalà group363,364 have developed new adducts La(hfac)·monoglyme·H2O, La(hfac)·diglyme and La(hfac)·triglyme (hfac = CF3COCHCOCF3), which possess better volatility and thermal stability than conventional lanthanum CVD precursors. Pollard et al.365 found that monoglyme and diglyme formed neutral complexes of [Y(hfac)3(glyme)], whilst triglyme and tetraglyme produced ionic complexes [Y(hfac)2(glyme)]+ [Y(hfac)4]−; upon CVD at 250–350 °C using oxygen as a carrier gas, these complexes yielded mixed yttrium oxide–fluoride ceramics. The Fragalà group366 prepared and characterized [Y(hfac)3·monoglyme], [Y(hfac)3·diglyme], [Y(hfac)3·(H2O)2·triglyme] and [Y(hfac)2·tetraglyme]+[Y(hfa)4]−; they found these adducts were suitable for MOCVD applications due to their high volatility and high thermal stabilities with a residue lower than 2–4%. In particular, a YBaCuO HTc superconductor was produced from the low-pressure MOCVD process of a [Y(hfa)3·monoglyme] complex using a multimetal molten single source. Kang et al.367 synthesized thermally stable Ln(hfac)3·monoglyme (Ln = Ho or Y) complexes and indicated their high potential as MOCVD precursors. Pollard et al.368 designed new glyme-adduct precursors of [Ce(hfac)3(glyme)] (glyme = mono-, di-, or tri-) and [(Ce(hfac)3)2(μ-tetraglyme)] and used them in the CVD formation of films of cerium oxides on substrates Si, Pt, and TiN. The Fragalà group369 also prepared Ce(hfac)3·diglyme, Ce(hfac)3·diethyldiglyme and Ce(hfac)3·dibutyldiglyme, and found that these complexes are ideal for CeO2 film deposition due to their high volatility and high thermal stability with low residue. Malandrino et al.370 synthesized novel complex precursors Eu(hfac)3·glyme (glyme = mono- or di-) and found that both complexes are thermally stable and could be evaporated with <4% residue. Another study by this group371 suggested that La(hfac)3·diglyme could be evaporated from the melt up to 130 °C without side decomposition processes. The Malandrino group372 further prepared and characterized Nd(hfac)3·monoglyme·H2O and Nd(hfac)3·diglyme, which exhibit high volatility and good thermal stability with a remaining residue lower than 3%. In particular, Nd(hfa)3·diglyme was used in a low-pressure MOCVD preparation of NdBa2Cu3O7−δ thin films.In addition to these lanthanide adducts using glymes, other metal complexes have also been investigated. In an attempt to prepare precursors for Supercritical Fluid Transport (SFT) CVD, Blake et al.373 synthesized new S-donor tetraphenyldithioimidodiphosphinate compounds [Na(Ph2P(S)NP(S)Ph2)(L)] (where L = triglyme or tetraglyme); however, these complexes are not quite soluble in supercritical CO2. The Winter group374 prepared calcium complexes with η2-pyrazolato ligands including those adducts containing glymes [e.g. Ca(tBu2pz)2(triglyme), and Ca(tBu2pz)2(tetraglyme) Ca(Me2pz)2(triglyme), and Ca(Me2pz)2(tetraglyme)], resulting in volatile, thermally stable precursors for CVD applications.
10.2. NMR solvents
Deuterated monoglyme can be used as a co-solvent for low-temperature reactions monitored by a direct NMR analysis to probe the underlying reaction mechanism, as pioneered by the Buncel group. This group375 performed low-temperature (−40 °C) NMR studies using a new solvent mixture of CD3CN–monoglyme-d10 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the medium for a reaction of 2,4,6-trinitroanisole (TNA) with phenoxide; their results suggest the 1,1 adduct is the only species produced and no 1,3 O-adduct of phenoxide formed either prior to the 1,1 species or later. A further study of this reaction system by this group376 revealed both O- and C-bonded phenoxide σ-complex adducts, implying the formation of the former via kinetic control and of the latter through thermodynamic control. This group377 also used the same solvent system at −40 °C to room temperature to investigate the regioselectivity in Meisenheimer complexation of the reaction of 2,4,6-trimethylphenoxide ion (MesO−) with 2,4,6-trinitroanisole (TNA) via 1H and 13C NMR, and suggest a kinetic preference for C-1 attachment and the σ-adduct from C-3 attack being more thermodynamically stable. The same deuterated solvent system was also employed by this group to examine the reactivity of 4-nitrobenzofuroxan (NBF) with several aryloxide nucleophiles; they observed the formation of a C-7 O-adduct with the ambident (O– and C–) nucleophile phenoxide ion at −40 °C.378
1, v/v) as the medium for a reaction of 2,4,6-trinitroanisole (TNA) with phenoxide; their results suggest the 1,1 adduct is the only species produced and no 1,3 O-adduct of phenoxide formed either prior to the 1,1 species or later. A further study of this reaction system by this group376 revealed both O- and C-bonded phenoxide σ-complex adducts, implying the formation of the former via kinetic control and of the latter through thermodynamic control. This group377 also used the same solvent system at −40 °C to room temperature to investigate the regioselectivity in Meisenheimer complexation of the reaction of 2,4,6-trimethylphenoxide ion (MesO−) with 2,4,6-trinitroanisole (TNA) via 1H and 13C NMR, and suggest a kinetic preference for C-1 attachment and the σ-adduct from C-3 attack being more thermodynamically stable. The same deuterated solvent system was also employed by this group to examine the reactivity of 4-nitrobenzofuroxan (NBF) with several aryloxide nucleophiles; they observed the formation of a C-7 O-adduct with the ambident (O– and C–) nucleophile phenoxide ion at −40 °C.378
10.3. Chromatography
Rouse et al.379 synthesized new glyme-substituted polysiloxane (Scheme 46) and 18-crown-6-substituted polysiloxane and applied them as the stationary phase for gas chromatography. The glyme polysiloxane has an operational temperature range of 20–280 °C and a selectivity comparable to that of Carbowax 20 M. Schuetz et al.380 found that an eluent of hexane/monoglyme/formic acid (85%) (150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) could be used to purify p-biphenyl-n-hexanoic acid in silica-based column chromatography, achieving 98% purity.
3) could be used to purify p-biphenyl-n-hexanoic acid in silica-based column chromatography, achieving 98% purity.
10.4. Dissolution of CO2 and other gases
Sciamanna and Lynn381 determined the gas solubilities of H2S, SO2, CO2, propane and n-butane in glymes (di-, tri- and tetra-) and PEG monoethers at the partial pressure of gas solute between 3 and 100 kPa. The temperature dependence of gas solubility was described by Henry's law coefficients. The presence of a small amount of water (<6 wt %) decreases the gas solubility in glymes; the hydrogen-bonding properties of PEG monoethers also reduces the gas solubility. Henni et al.382 reported the solubilities of CO2 in 14 solvents including glymes, PEG monoethers, selexol® and sulfolane at 25, 40 and 60 °C; they suggested that glymes (particularly diglyme, triglyme and tetraglyme) are outstanding solvents for CO2 removal. Kodama et al.74 measured the solubilities and saturated densities of CO2 in glymes (di-, tri- and tetra-) at 313.15 K as a function of pressure (at high pressures, CO2 mole fraction is 0.857 at 7.126 MPa in diglyme, 0.827 at 7.202 MPa in triglyme and 0.822 at 7.316 MPa in tetraglyme). These data of CO2 solubilities and saturated densities were further correlated with a three parameter pseudo-cubic equation of state.10.5. Other applications
Monoglyme was used in aqueous solutions to improve the solubility of oxiranes in order to determine their nanomole quantities spectrophotometrically.383 Interestingly, Dasilva-Carbalhal et al.384 studied the effect of glymes on the conductance percolation of AOT/isooctane/water microemulsions. The addition of glymes (mono-, di-, tri-, and tetra-) to the microemulsion led to a decrease in the percolation threshold. This modification promotes the exchange of matter between droplets in the AOT film. More recently, various glymes were used to dissolve triglycerides, and thus were investigated as co-solvents for the CaO-catalyzed transesterification of soybean oil (>50% v/v loading) into biodiesel; under the optimum conditions, a >98% conversion of triglycerides could be achieved in 4 h using dipropylene glycol dimethyl ether (P2) as the co-solvent.2211. Perspectives
Recent active research on glymes seems to focus on their applications in electrochemistry, catalysis, CVD, nanomaterials, and the dissolution of CO2. However, there could be renewed interest in exploring their solvent role in organic reactions, biocatalysis and biofuel production. Glymes can also be derived to contain functional groups such as double bonds, and thus become precursors for other applications. Many glymes are less volatile and less toxic than some common laboratory organic solvents; however, there is still lack of a systematic database on their acute and long-term toxicity as well as biodegradability. In addition, despite their versatile roles in industrial and consumer products, glymes have not been well studied in terms of their physicochemical properties such as dielectric constant, polarity, hydrophobicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P), heat capacity, phase equilibrium, etc.
P), heat capacity, phase equilibrium, etc.
In addition to glymes, other solvents carrying polyether or polyol moieties have also been extensively studied; these solvents include glycol monoethers and glycol ether esters,1–3 polyethylene glycol (PEG) and aqueous solutions,330 and glycerol and its derivatives.385–387 New solvents can also be developed to carry the functionality of these moieties, for example, ionic liquids can be functionalized with various glycol groups to become tailored-made solvents.388 As another example, a new family of glycerol derivatives including 1,3-dialkoxy-2-propanols and 1,2,3-trialkoxypropanes were prepared by García et al.;389 these new solvents share some structural features with glymes and have a wide range of polarity properties, implying their high potential for solvent substitution.
Acknowledgements
HZ acknowledges the supports by the Henry Dreyfus Teacher-Scholar Award (2012), NIH MBRS-RISE grant (1R25GM096956), NIH NIBIB contract award (HHSN268201200011C), and the National Natural Science Foundation of China (21328601).References
- S. T. Cragg, in Patty’s Toxicology, ed. E. Bingham and B. Cohrssen, John Wiley & Sons, Hoboken, NJ, 6th edn, 2012, vol. 4, pp. 641–787 Search PubMed.
- S. T. Cragg, in Patty's Toxicology, ed. E. Bingham and B. Cohrssen, John Wiley & Sons, Hoboken, NJ, 6th edn, 2012, vol. 4 Search PubMed.
- R. L. Smith, Environ. Health Perspect., 1984, 57, 1–4 CrossRef CAS PubMed.
- B. L. Haymore, J. D. Lamb, R. M. Izatt and J. J. Christensen, Inorg. Chem., 1982, 21, 1598–1602 CrossRef CAS.
- J. L. Adcock and R. J. Lagow, J. Org. Chem., 1973, 38, 3617–3618 CrossRef CAS.
- D. E. Leonhardt, L. W. Coleman and W. S. Bradshaw, Reprod. Toxicol., 1991, 5, 157–162 CrossRef CAS PubMed.
- J. C. Gage, Br. J. Ind. Med., 1970, 27, 1–18 CAS.
- D. B. McGregor, M. J. Willins, P. McDonald, M. Holmström, D. McDonald and R. W. Niemeier, Toxicol. Appl. Pharmacol., 1983, 70, 303–316 CrossRef CAS PubMed.
- R. L. Schuler, B. D. Hardin, R. W. Niemeier, G. Booth, K. Hazelden, V. Piccirillo and K. Smith, Environ. Health Perspect., 1984, 57, 141–146 CrossRef CAS PubMed.
- E. M. Johnson, B. E. Gabel and J. Larson, Environ. Health Perspect., 1984, 57, 135–139 CrossRef CAS PubMed.
- D. B. McGregor, Environ. Health Perspect., 1984, 57, 97–103 CrossRef CAS PubMed.
- K. L. Cheever, W. W. Weigel, D. E. Richards, J. B. Lal and H. B. Plotnick, Toxicologist, 1985, 5, 140 Search PubMed.
- K. L. Cheever, D. E. Richards, W. W. Weigel, J. B. Lal, A. M. Dinsmore and F. B. Daniel, Toxicologist, 1986, 6, 32 Search PubMed.
- K. L. Cheever, D. E. Richards, W. W. Weigel, J. B. Lal, A. M. Dinsmore and F. B. Daniel, Toxicol. Appl. Pharmacol., 1988, 94, 150–159 CrossRef CAS PubMed.
- K. P. Lee, L. A. Kinney and R. Valentine, Toxicology, 1989, 59, 239–258 CrossRef CAS PubMed.
- B. D. Hardin and C. J. Eisenmann, Teratology, 1987, 35, 321–328 CrossRef CAS PubMed.
- http://www.environmentalhealthnews.org/ehs/news/2011/epa-takes-on-glymes.
- P. J. Spencer, Toxicol. Lett., 2005, 156, 181–188 CrossRef CAS PubMed.
- J. A. Arnot and F. A. P. C. Gobas, Environ. Rev., 2006, 14, 257–297 CrossRef CAS.
- L. W. Sieck and M. Meot-Ner, J. Phys. Chem., 1984, 88, 5324–5327 CrossRef CAS.
- S. Tang, C. L. Jones and H. Zhao, Bioresour. Technol., 2013, 129, 667–671 CrossRef CAS PubMed.
- S. Tang, H. Zhao, Z. Song and O. Olubajo, Bioresour. Technol., 2013, 139, 107–112 CrossRef CAS PubMed.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- M. Meot-Ner, J. Am. Chem. Soc., 1983, 105, 4906–4911 CrossRef CAS.
- R. B. Sharma, A. T. Blades and P. Kebarle, J. Am. Chem. Soc., 1984, 106, 510–516 CrossRef CAS.
- H. Wasada, Y. Tsutsui and S. Yamabe, J. Phys. Chem., 1996, 100, 7367–7371 CrossRef CAS.
- D. Adötoledo, V. Aviyente, J. M. L. Martin and C. Lifshitz, J. Phys. Chem. A, 1998, 102, 6357–6365 CrossRef.
- E. Shchori and J. Jagur-Grodzinski, J. Am. Chem. Soc., 1972, 94, 7957–7962 CrossRef CAS.
- M. Meot-Ner, L. W. Sieck, S. Scheiner and X. Duan, J. Am. Chem. Soc., 1994, 116, 7848–7856 CrossRef CAS.
- H. Matsuura and T. Sagawa, J. Mol. Liq., 1995, 65–66, 313–316 CrossRef.
- S. Masatoki, M. Takamura, H. Matsuura, K. Kamogawa and T. Kitagawa, Chem. Lett., 1995, 24, 991–992 CrossRef.
- D. Bedrov and G. D. Smith, J. Chem. Phys., 1998, 109, 8118 CrossRef CAS.
- D. Bedrov, M. Pekny and G. D. Smith, J. Phys. Chem. B, 1998, 102, 996–1001 CrossRef CAS.
- D. Bedrov and G. D. Smith, J. Phys. Chem. B, 1999, 103, 3791–3796 CrossRef CAS.
- R. Begum, S. Masatoki and H. Matsuura, J. Mol. Struct., 1996, 384, 115–120 CrossRef CAS.
- O. Engkvist and G. Karlström, J. Chem. Phys., 1997, 106, 2411–2417 CrossRef CAS.
- P. Bernal, A. Bunn, J. Logan and J. McCluan, J. Solution Chem., 2000, 29, 651–665 CrossRef CAS.
- P. Bernal and J. McCluan, J. Solution Chem., 2001, 30, 119–131 CrossRef CAS.
- G. Douhéret, J. C. R. Reis, M. I. Davis, I. J. Fjellanger and H. Høiland, Phys. Chem. Chem. Phys., 2004, 6, 784–792 RSC.
- CRC Handbook of Chemistry and Physics, ed. W. M. Haynes, CRC Press, Boca Raton, 2012 Search PubMed.
- N. P. Cheremisinoff, Industrial Solvents Handbook, Marcel Dekker, Inc., New York, 2003 Search PubMed.
- Industrial solvents handbook, ed. E. W. Flick, Noyes Data Corporation, Westwood, NJ, 1998 Search PubMed.
- T. Treszczanowicz, G. C. Benson and B. C.-Y. Lu, Thermochim. Acta, 1990, 168, 95–102 CrossRef CAS.
- C. A. Tovar, E. Carballo, C. A. Cerdeiriña and L. Romanı, J. Chem. Eng. Data, 1997, 42, 1085–1089 CrossRef CAS.
- H. Nakai, H. Soejima, K. Tamura, H. Ogawa, S. Murakami and Y. Toshiyasu, Thermochim. Acta, 1991, 183, 15–27 CrossRef CAS.
- C. Dethlefsen and A. Hvidt, J. Chem. Thermodyn., 1985, 17, 193–199 CrossRef CAS.
- M. E. de Ruiz Holgado, C. R. de Schaefer, E. L. Arancibia and M. Katz, Fluid Phase Equilib., 1994, 95, 299–312 CrossRef CAS.
- A. Spanedda, L. Lepori and E. Matteoli, Fluid Phase Equilib., 1991, 69, 209–222 CrossRef CAS.
- P. K. Muhuri and D. K. Hazra, J. Chem. Eng. Data, 1994, 39, 375–377 CrossRef CAS.
- R. L. McGee, W. J. Wallace and R. D. Rataiczak, J. Chem. Eng. Data, 1983, 28, 305–307 CrossRef CAS.
- W. J. Wallace and A. L. Mathews, J. Chem. Eng. Data, 1963, 8, 496–498 CrossRef CAS.
- W. J. Wallace, C. S. Shephard and C. Underwood, J. Chem. Eng. Data, 1968, 13, 11–13 CrossRef CAS.
- J. L. Cabezas, S. Beltran and J. Coca, J. Chem. Eng. Data, 1991, 36, 184–188 CrossRef CAS.
- J. C. R. Reis and T. P. Iglesias, Phys. Chem. Chem. Phys., 2011, 13, 10670–10680 RSC.
- C. F. Riadigos, R. Iglesias, M. A. Rivas and T. P. Iglesias, J. Chem. Thermodyn., 2011, 43, 275–283 CrossRef CAS.
- M. J. P. Comuñas, A. Baylaucq, C. Boned and J. Fernández, J. Chem. Eng. Data, 2003, 48, 1044–1049 CrossRef.
- J.-L. M. Abboud and R. Notari, Pure Appl. Chem., 1999, 71, 645–718 CrossRef CAS.
- G. W. Canters, J. Am. Chem. Soc., 1972, 94, 5230–5235 CrossRef CAS.
- A. Pal and A. Kumar, Int. J. Thermophys., 2003, 24, 1073–1087 CrossRef CAS.
- A. Serna, I. García de la Fuente, J. A. González and J. C. Cobos, Fluid Phase Equilib., 1997, 133, 187–192 CrossRef CAS.
- M. A. Villamañan, C. Casanova, A. H. Roux and J.-P. E. Grolier, J. Chem. Thermodyn., 1982, 14, 251–258 CrossRef.
- H.-C. Ku and C.-H. Tu, J. Chem. Eng. Data, 2000, 45, 391–394 CrossRef CAS.
- A. Pal and S. Sharma, J. Chem. Eng. Data, 1998, 43, 532–536 CrossRef CAS.
- A. Pal and S. Sharma, J. Chem. Eng. Data, 1999, 44, 212–215 CrossRef CAS.
- T. Treszczanowicz and D. Cieślak, J. Chem. Thermodyn., 1993, 25, 661–665 CrossRef CAS.
- G. C. Benson, M. K. Kumaran, T. Treszczanowicz, P. J. D'arcy and C. J. Halpin, Thermochim. Acta, 1985, 95, 59–66 CrossRef CAS.
- A. Conesa, S. Shen and A. Coronas, Int. J. Thermophys., 1998, 19, 1343–1358 CrossRef CAS.
- E. R. López, J. L. Daridon, A. Baylaucq and J. Fernández, J. Chem. Eng. Data, 2003, 48, 1208–1213 CrossRef.
- K. Aizawa and M. Kato, J. Chem. Eng. Data, 1991, 36, 159–161 CrossRef CAS.
- T. M. Aminabhavi and B. Gopalakrishna, J. Chem. Eng. Data, 1995, 40, 462–467 CrossRef CAS.
- A. J. Treszczanowicz, C. J. Halpin and G. C. Benson, J. Chem. Eng. Data, 1982, 27, 321–324 CrossRef CAS.
- C. Carvajal, K. J. Tölle, J. Smid and M. Szwarc, J. Am. Chem. Soc., 1965, 87, 5548–5553 CrossRef CAS.
- K. Kusano, J. Chem. Eng. Data, 1978, 23, 141–143 CrossRef CAS.
- D. Kodama, M. Kanakubo, M. Kokubo, S. Hashimoto, H. Nanjo and M. Kato, Fluid Phase Equilib., 2011, 302, 103–108 CrossRef CAS.
- A. Lago, M. A. Rivas, J. Legido and T. P. Iglesias, J. Chem. Thermodyn., 2009, 41, 257–264 CrossRef CAS.
- K. Kimura and R. Fujishiro, Bull. Chem. Soc. Jpn., 1966, 39, 608–610 CrossRef CAS.
- J.-F. Côté, D. Brouillette, J. E. Desnoyers, J.-F. Rouleau, J.-M. St-Arnaud and G. Perron, J. Solution Chem., 1996, 25, 1163–1173 CrossRef.
- M. Cocchi, P. De Benedetti, A. Marchetti, M. C. Menziani, R. Seeber, L. Tassi and A. Ulrici, J. Solution Chem., 2001, 30, 149–169 CrossRef CAS.
- L. M. Trejo, M. Costas and D. Patterson, J. Chem. Soc., Faraday Trans., 1991, 87, 3001–3008 RSC.
- G. T. Hefter and M. Salomon, J. Solution Chem., 1994, 23, 579–593 CrossRef CAS.
- F. Kimura, P. J. D'arcy, M. E. Sugamori and G. C. Benson, Thermochim. Acta, 1983, 64, 149–154 CrossRef CAS.
- G. D. Patterson and P. J. Flory, J. Chem. Soc., Faraday Trans. 2, 1972, 68, 1111–1116 RSC.
- C. A. Tovar, E. Carballo, C. A. Cerdeiriña, M. I. Paz Andrade and L. Romaní, J. Chem. Soc., Faraday Trans., 1997, 93, 3505–3509 RSC.
- A. J. Treszczanowicz and T. Treszczanowicz, Fluid Phase Equilib., 1998, 148, 209–220 CrossRef CAS.
- A. Pal and H. Kumar, J. Chem. Eng. Data, 1999, 44, 1330–1334 CrossRef CAS.
- M. J. P. Comuñas, A. Baylaucq, C. Boned and J. Fernández, Ind. Eng. Chem. Res., 2004, 43, 804–814 CrossRef.
- E. R. López, J. García, A. Coronas and J. Fernández, Fluid Phase Equilib., 1997, 133, 229–238 CrossRef.
- E. R. López, J.-Y. Coxam, J. Fernández and J.-P. E. Grolier, J. Chem. Eng. Data, 1999, 44, 1409–1413 CrossRef.
- S. I. Tseregounis and M. J. Riley, AIChE J., 1994, 40, 726–737 CrossRef CAS.
- J. Smith, L. Andreoli-Ball and D. Patterson, J. Chem. Soc., Faraday Trans., 1992, 88, 2875–2881 RSC.
- E. R. López, J. L. Daridon, F. Plantier, C. Boned and J. Fernández, Int. J. Thermophys., 2006, 27, 1354–1372 CrossRef.
- T. Nogrady and A. S. V. Burgen, J. Am. Chem. Soc., 1969, 91, 3890–3893 CrossRef CAS PubMed.
- T. Matsui and K. Takeyama, Electrochim. Acta, 1998, 43, 1355–1360 CrossRef CAS.
- M. Andersson and G. Karlstrom, J. Phys. Chem., 1985, 89, 4957–4962 CrossRef CAS.
- K. Hoefelmann, J. Jagur-Grodzinski and M. Szwarc, J. Am. Chem. Soc., 1969, 91, 4645–4651 CrossRef CAS.
- G. L. Collins, T. E. H. Esch and J. Smid, J. Solution Chem., 1978, 7, 9–18 CrossRef CAS.
- G. Ritzhaupt and J. P. Devlin, J. Phys. Chem., 1986, 90, 1143–1147 CrossRef CAS.
- V. A. Payne, J.-H. Xu, M. Forsyth, M. A. Ratner, D. F. Shriver and S. W. de Leeuw, Electrochim. Acta, 1995, 40, 2087–2091 CrossRef CAS.
- V. A. Payne, J. Xu, M. Forsyth, M. A. Ratner and D. F. Shriver, J. Chem. Phys., 1995, 103, 8746–8755 CrossRef CAS.
- P. Johansson, S. P. Gejji, J. Tegenfeldt and J. Lindgren, Solid State Ionics, 1996, 86-88, 297–302 CrossRef CAS.
- P. Johansson, J. Tegenfeldt and J. Lindgren, Polymer, 1999, 40, 4399–4406 CrossRef CAS.
- P. Johansson, Polymer, 2001, 42, 4367–4373 CrossRef CAS.
- N. Shen, R. M. Pope and D. V. Dearden, Int. J. Mass Spectrom., 2000, 195/196, 639–652 CrossRef CAS.
- W. A. Henderson, V. G. Young, Jr, N. R. Brooks and W. H. Smyrl, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2002, 58, m501–m503 Search PubMed.
- W. A. Henderson, N. R. Brooks, W. W. Brennessel and V. G. Young, Jr, J. Phys. Chem. A, 2004, 108, 225–229 CrossRef CAS.
- J. Grondin, D. Talaga, J.-C. Lassègues and W. A. Henderson, Phys. Chem. Chem. Phys., 2004, 6, 938–944 RSC.
- N. R. Dhuaml and S. P. Gejji, Theor. Chem. Acc., 2006, 115, 308–321 CrossRef CAS.
- T. Tamura, K. Yoshida, T. Hachida, M. Tsuchiya, M. Nakamura, Y. Kazue, N. Tachikawa, K. Dokko and M. Watanabe, Chem. Lett., 2010, 39, 753–755 CrossRef CAS.
- K. Yoshida, M. Nakamura, Y. Kazue, N. Tachikawa, S. Tsuzuki, S. Seki, K. Dokko and M. Watanabe, J. Am. Chem. Soc., 2011, 133, 13121–13129 CrossRef CAS PubMed.
- Y. S. Cho, S.-I. Cho, H.-K. Ryua, J. S. Heo, D. H. Lee and S. H. Moon, J. Electrochem. Soc., 2003, 150, F11–F19 CrossRef CAS.
- A. Varnek, G. Wipff, V. P. Solov'ev and A. F. Solotnov, J. Chem. Inf. Model., 2002, 42, 812–829 CrossRef CAS PubMed.
- H. K. Frensdorff, J. Am. Chem. Soc., 1971, 93, 600–606 CrossRef CAS.
- R. M. Izatt, J. S. Bradshaw, S. A. Nielsen, J. D. Lamb, J. J. Christensen and D. Sen, Chem. Rev., 1985, 85, 271–339 CrossRef CAS.
- R. M. Izatt, K. Pawlak, J. S. Bradshaw and R. L. Bruening, Chem. Rev., 1991, 91, 1721–2085 CrossRef CAS.
- G. W. Gokel, D. M. Goli and R. A. Schultz, J. Org. Chem., 1983, 48, 2837–2842 CrossRef CAS.
- H. Zhang and D. V. Dearden, J. Am. Chem. Soc., 1992, 114, 2754–2755 CrossRef CAS.
- W.-Y. Xu and J. Smid, J. Am. Chem. Soc., 1984, 106, 3790–3796 CrossRef CAS.
- C. B. Tsvetanov, E. B. Petrova, D. K. Dimov, I. M. Panayotov and J. Smid, J. Solution Chem., 1990, 19, 425–436 CrossRef.
- W. R. Davidson and P. Kebarle, Can. J. Chem., 1976, 54, 2594–2599 CrossRef CAS.
- A. Plewa-Marczewska, M. Kalita, M. Marczewski and M. Siekierski, Electrochim. Acta, 2010, 55, 1389–1395 CrossRef CAS.
- L. L. Chan and J. Smid, J. Am. Chem. Soc., 1967, 89, 4547–4549 CrossRef CAS.
- L. L. Chan and J. Smid, J. Am. Chem. Soc., 1968, 90, 4654–4661 CrossRef CAS.
- L. L. Chan, K. H. Wong and J. Smid, J. Am. Chem. Soc., 1970, 92, 1955–1963 CrossRef CAS.
- U. Takaki and J. Smid, J. Am. Chem. Soc., 1974, 96, 2588–2593 CrossRef CAS.
- C. Detellier and P. Laszlo, Helv. Chim. Acta, 1976, 59, 1333–1345 CrossRef CAS.
- W. R. Gilkerson and M. D. Jackson, J. Am. Chem. Soc., 1982, 104, 1218–1223 CrossRef CAS.
- A. Plewa, M. Kalita and M. Siekierski, Electrochim. Acta, 2007, 53, 1527–1534 CrossRef CAS.
- J. Smid and A. M. Grotens, J. Phys. Chem., 1973, 77, 2377–2382 CrossRef CAS.
- E. de Boer, A. A. K. Klaassen, J. J. Mooij and J. H. Noordik, Pure Appl. Chem., 1979, 51, 73–83 CrossRef CAS.
- C. P. Rhodes and R. Frech, Macromolecules, 2001, 34, 2660–2666 CrossRef CAS.
- R. Frech, C. P. Rhodes and M. Khan, Macromol. Symp., 2002, 186, 41–49 CrossRef CAS.
- C. P. Rhodes, M. Khan and R. Frech, J. Phys. Chem. B, 2002, 106, 10330–10337 CrossRef CAS.
- W. A. Henderson, N. R. Brooks, W. W. Brennessel and V. G. J. Young, Chem. Mater., 2003, 15, 4679–4684 CrossRef CAS.
- W. A. Henderson, N. R. Brooks and V. G. J. Young, Chem. Mater., 2003, 15, 4685–4690 CrossRef CAS.
- W. A. Henderson, J. Phys. Chem. B, 2006, 110, 13177–13183 CrossRef CAS PubMed.
- W. A. Henderson, Macromolecules, 2007, 40, 4963–4971 CrossRef CAS.
- W. A. Henderson and N. R. Brooks, Inorg. Chem., 2003, 42, 4522–4524 CrossRef CAS PubMed.
- J. M. Timko, R. C. Helgeson, M. Newcomb, G. W. Gokel and D. J. Cram, J. Am. Chem. Soc., 1974, 96, 7097–7099 CrossRef CAS.
- R. A. Bartsch and P. N. Juri, Tetrahedron Lett., 1979, 20, 407–410 CrossRef.
- R. A. Bartsch, P. N. Juri and M. A. Mills, Tetrahedron Lett., 1979, 20, 2499–2502 CrossRef.
- J. Otera, T. Shiomi, K. Murakami and Y. Kawasaki, Bull. Chem. Soc. Jpn., 1981, 54, 2964–2967 CrossRef CAS.
- Y. Hirashima, K. Ito and J. Shiokawa, Chem. Lett., 1983, 12, 9–10 CrossRef.
- Y. Hirashima, K. Kanetsuki, I. Yonezu, K. Kamakura and J. Shiokawa, Bull. Chem. Soc. Jpn., 1983, 56, 738–743 CrossRef CAS.
- Y. Inoue and T. Hakushi, J. Chem. Soc., Perkin Trans. 2, 1985, 935–946 RSC.
- M. A. Guerra, T. R. Bierschenk and R. J. Lagow, J. Am. Chem. Soc., 1986, 108, 4103–4105 CrossRef CAS.
- P. R. Markies, O. S. Akkerman, F. Bickelhaupt, W. J. J. Smeets and A. L. Spek, Organometallics, 1994, 13, 2616–2627 CrossRef CAS.
- M. Meot-Ner, L. W. Sieck, J. F. Liebman and S. Scheiner, J. Phys. Chem., 1996, 100, 6445–6450 CrossRef CAS.
- K. M. Fromm, H. Goesmann and G. Bernardinelli, Polyhedron, 2000, 19, 1783–1789 CrossRef CAS.
- S. Mishra, S. Daniele, L. G. Hubert-Pfalzgraf and E. Jeanneau, Eur. J. Inorg. Chem., 2007, 2208–2215 CrossRef CAS.
- M. K. J. Chantooni, D. Britton and I. M. Kolthoff, J. Crystallogr. Spectrosc. Res., 1993, 23, 497–503 CrossRef CAS.
- G. D. Jaycox, R. Sinta and J. Smid, J. Polym. Sci., Polym. Chem. Ed., 1982, 20, 1629–1638 CrossRef CAS.
- M. Saraswathi and J. M. Miller, J. Am. Soc. Mass Spectrom., 1996, 7, 42–49 CrossRef CAS PubMed.
- R. V. Slates and M. Szwarc, J. Am. Chem. Soc., 1967, 89, 6043–6050 CrossRef CAS.
- G. W. Canters, A. A. K. Klassen and E. de Boer, J. Phys. Chem., 1970, 74, 3299–3302 CrossRef CAS.
- W. H. Smyrl, S. R. Kurtz, J. M. Zeigler and D. S. Ginley, J. Chem. Soc., Chem. Commun., 1983, 1155–1156 RSC.
- J. S. Foos, T. S. Stolki and X. Beebe, J. Electrochem. Soc., 1989, 136, 2748–2749 CrossRef CAS.
- U. Sharma and V. K. Bhagwat, Asian J. Chem., 1992, 4, 758–763 Search PubMed.
- R. Pyati and R. W. Murray, J. Am. Chem. Soc., 1996, 118, 1743–1749 CrossRef CAS.
- D. Teeters, R. G. Neuman and B. D. Tate, Solid State Ionics, 1996, 85, 239–245 CrossRef CAS.
- Y. Choquette, G. Brisard, M. Parent, D. Brouillette, G. Perron, J. E. Desnoyers, M. Armand, D. Gravel and N. Slougui, J. Electrochem. Soc., 1998, 145, 3500–3507 CrossRef CAS.
- A. Plewa, F. Chyliński, M. Kalita, M. Bukat, P. Parzuchowski, R. Borkowska, M. Siekierski, G. Z. Żukowska and W. Wieczorek, J. Power Sources, 2006, 159, 431–437 CrossRef CAS.
- Y. Katayama, S. Miyashita and T. Miura, J. Power Sources, 2010, 195, 6162–6166 CrossRef CAS.
- K. Izutsu, T. Nakamura, K. Miyoshi and K. Kurita, Electrochim. Acta, 1996, 41, 2523–2527 CrossRef CAS.
- D. Aurbach and E. Granot, Electrochim. Acta, 1997, 42, 697–718 CrossRef CAS.
- D. Brouillette, G. Perron and J. E. Desnoyers, J. Solution Chem., 1998, 27, 151–182 CrossRef CAS.
- K. Hayamizu, Y. Aihara, S. Arai and C. G. Martinez, J. Phys. Chem. B, 1999, 103, 519–524 CrossRef CAS PubMed.
- K. Hayamizu, E. Akiba, T. Bando and Y. Aihara, J. Chem. Phys., 2002, 117, 5929–5939 CrossRef CAS.
- W. A. Henderson, F. McKenna, M. A. Khan, N. R. Brooks, V. G. J. Young and R. Frech, Chem. Mater., 2005, 17, 2284–2289 CrossRef CAS.
- V. S. Kolosnitsyn, E. V. Karaseva, D. Y. Seung and M. D. Cho, Russ. J. Electrochem., 2003, 39, 1089–1093 CrossRef CAS.
- V. S. Kolosnitsyn, E. V. Karaseva, N. V. Shakirova, D. Y. Seung and M. D. Cho, Russ. J. Electrochem., 2002, 38, 1360–1363 CrossRef CAS.
- S. Tobishima, H. Morimoto, M. Aoki, Y. Saito, T. Inose, T. Fukumoto and T. Kuryu, Electrochim. Acta, 2004, 49, 979–987 CrossRef CAS.
- T. Inose, D. Watanabe, H. Morimoto and S. Tobishima, J. Power Sources, 2006, 162, 1297–1303 CrossRef CAS.
- T. V. Kaulgud, N. R. Dhumal and S. P. Gejji, J. Phys. Chem. A, 2006, 110, 9231–9239 CrossRef CAS PubMed.
- K. Yoshida, M. Tsuchiya, N. Tachikawa, K. Dokko and M. Watanabe, J. Phys. Chem. C, 2011, 115, 18384–18394 CAS.
- A. Orita, K. Kamijima, M. Yoshida, K. Dokko and M. Watanabe, J. Power Sources, 2011, 196, 3874–3880 CrossRef CAS.
- T. Tamura, T. Hachida, K. Yoshida, N. Tachikawa, K. Dokko and M. Watanabe, J. Power Sources, 2010, 195, 6095–6100 CrossRef CAS.
- S. Seki, K. Takei, H. Miyashiro and M. Watanabe, J. Electrochem. Soc., 2011, 158, A769–A774 CrossRef CAS.
- S. Seki, N. Serizawa, K. Takei, K. Dokko and M. Watanabe, J. Power Sources, 2013, 243, 323–327 CrossRef CAS.
- B. L. Ellis, K. T. Lee and L. F. Nazar, Chem. Mater., 2010, 22, 691–714 CrossRef CAS.
- N. Tachikawa, K. Yamauchi, E. Takashima, J.-W. Park, K. Dokko and M. Watanabe, Chem. Commun., 2011, 47, 8157–8159 RSC.
- C. Barchasz, J.-C. Leprêtre, S. Patoux and F. Alloin, Electrochim. Acta, 2013, 89, 737–743 CrossRef CAS.
- D. Aurbach, Y. Gofer, Z. Lu, A. Schechter, O. Chusid, H. Gizbar, Y. Cohen, V. Ashkenazi, M. Moshkovich, R. Turgeman and E. Levi, J. Power Sources, 2001, 97–98, 28–32 CrossRef CAS.
- D. Aurbach, H. Gizbar, A. Schechter, O. Chusid, H. E. Gottlieb, Y. Gofer and I. Goldberg, J. Electrochem. Soc., 2002, 149, A115–A121 CrossRef CAS.
- D. Aurbach, I. Weissman, Y. Gofer and E. Levi, Chem. Rec., 2003, 3, 61–73 CrossRef CAS PubMed.
- B. M. L. Rao and L. P. Klemann, J. Electrochem. Soc., 1980, 127, 761–762 CrossRef CAS.
- C. Zhang, Y. G. Andreev and P. G. Bruce, Angew. Chem., Int. Ed., 2007, 46, 2848–2850 CrossRef CAS PubMed.
- C. Zhang, E. Staunton, Y. G. Andreev and P. G. Bruce, J. Mater. Chem., 2007, 17, 3222–3228 RSC.
- C. Zhang, D. Ainsworth, Y. G. Andreev and P. G. Bruce, J. Am. Chem. Soc., 2007, 129, 8700–8701 CrossRef CAS PubMed.
- C. Zhang, S. J. Lilley, D. Ainsworth, E. Staunton, Y. G. Andreev, A. M. Z. Slawin and P. G. Bruce, Chem. Mater., 2008, 20, 4039–4044 CrossRef CAS.
- D. W. Xia and J. Smid, J. Polym. Sci., Polym. Lett. Ed., 1984, 22, 617–621 CrossRef CAS.
- L. Holzer, F. P. Wenzl, S. Tasch, G. Leising, B. Winkler, L. Dai and A. W. H. Mau, Appl. Phys. Lett., 1999, 75, 2014–2016 CrossRef CAS.
- J. S. J. McConaghy and J. J. Bloomfield, J. Org. Chem., 1968, 33, 3425–3428 CrossRef CAS.
- R. D. Rieke and P. M. Hudnall, J. Am. Chem. Soc., 1969, 91, 3678–3679 CrossRef CAS.
- G. Moshuk, G. Petrowski and S. Winstein, J. Am. Chem. Soc., 1968, 90, 2179–2181 CrossRef CAS.
- R. Rieke, M. Ogliaruso, R. McClung and S. Winstein, J. Am. Chem. Soc., 1966, 88, 4729–4730 CrossRef CAS.
- L. L. Miller and L. J. Jacoby, J. Am. Chem. Soc., 1969, 91, 1130–1134 CrossRef CAS.
- H. M. Walborsky, M. S. Aronoff and M. F. Schulman, J. Org. Chem., 1971, 36, 1036–1040 CrossRef CAS.
- L. Wei, A. Bell, K. H. Ahn, M. M. Holl, S. Warner, I. D. Williams and S. J. Lippard, Inorg. Chem., 1990, 29, 825–837 CrossRef CAS.
- L. Wei, A. Bell, S. Warner, I. D. Williams and S. J. Lippard, J. Am. Chem. Soc., 1986, 108, 8302–8303 CrossRef CAS.
- H. Riesner and E. Winterfeldt, J. Chem. Soc., Chem. Commun., 1972, 786–787 RSC.
- A. R. Dahl, C. A. Heil and A. D. Norman, Inorg. Chem., 1975, 14, 1095–1098 CrossRef CAS.
- J. E. Saavedra, J. Org. Chem., 1979, 44, 860–861 CrossRef CAS.
- T. Ohsawa, T. Takagaki, F. Ikehara, Y. Takahashi and T. Oishi, Chem. Pharm. Bull., 1982, 30, 3178–3186 CrossRef CAS.
- A. V. Kavaliunas, A. Taylor and R. D. Rieke, Organometallics, 1983, 2, 377–383 CrossRef CAS.
- G. L. Rochfort and R. D. Rieke, Inorg. Chem., 1986, 25, 348–355 CrossRef CAS.
- S. Inaba, H. Matsumoto and R. D. Rieke, J. Org. Chem., 1984, 49, 2093–2098 CrossRef CAS.
- S. Inaba and R. D. Rieke, J. Org. Chem., 1985, 50, 1373–1381 CrossRef CAS.
- G. T. King and N. E. Miller, Inorg. Chem., 1986, 25, 4309–4311 CrossRef CAS.
- P. A. Bianconi, R. N. Vrtis, C. P. Rao, I. D. Williams, M. P. Engeler and S. J. Lippard, Organometallics, 1987, 6, 1968–1977 CrossRef CAS.
- P. E. Fanwick, D. R. Root and R. A. Walton, Inorg. Chem., 1989, 28, 395–397 CrossRef CAS.
- P. E. Fanwick, D. R. Root and R. A. Walton, Inorg. Chem., 1989, 28, 3203–3209 CrossRef CAS.
- C. Yang and C. U. J. Pittman, Tetrahedron Lett., 1997, 38, 6561–6564 CrossRef CAS.
- C. Yang and C. U. J. Pittman, Synth. Commun., 1998, 28, 517–525 CrossRef CAS.
- C. U. J. Pittman and C. Yang, J. Hazard. Mater., 2001, 82, 299–311 CrossRef CAS PubMed.
- J. V. B. Kanth and H. C. Brown, Inorg. Chem., 2000, 39, 1795–1802 CrossRef CAS PubMed.
- R. J. Ouellette and C. Levin, J. Am. Chem. Soc., 1971, 93, 471–476 CrossRef CAS.
- M. Ochiai and E. Fujita, J. Chem. Soc., Chem. Commun., 1975, 967–968 RSC.
- E. Fujita and M. Ochiai, J. Chem. Soc., Perkin Trans. 1, 1977, 1948–1953 RSC.
- A. McKillop, B. P. Swann, M. E. Ford and E. C. Taylor, J. Am. Chem. Soc., 1973, 95, 3641–3645 CrossRef CAS.
- A. McKillop, O. H. Oldenziel, B. P. Swann, E. C. Taylor and R. L. Robey, J. Am. Chem. Soc., 1973, 95, 1296–1301 CrossRef CAS.
- G. Stork and P. F. Hudrlik, J. Am. Chem. Soc., 1968, 90, 4464–4465 CrossRef CAS.
- G. Stork and P. F. Hudrlik, J. Am. Chem. Soc., 1968, 90, 4462–4464 CrossRef CAS.
- R. V. Stevens and L. E. DuPree, J. Chem. Soc. D, 1970, 1585–1586 RSC.
- R. V. Stevens and M. P. Wentland, J. Am. Chem. Soc., 1968, 90, 5580–5583 CrossRef CAS PubMed.
- J. L. Kice and G. J. Kasperek, J. Am. Chem. Soc., 1970, 92, 3393–3397 CrossRef CAS.
- J. L. Kice, C. A. Walters and S. B. Burton, J. Org. Chem., 1974, 39, 346–351 CrossRef CAS.
- W. T. Reichle, J. Org. Chem., 1972, 37, 4254–4257 CrossRef CAS.
- S. D. Pastor and E. T. Hessell, J. Org. Chem., 1985, 50, 4812–4815 CrossRef CAS.
- K. S. Chen, J. Kleinberg and J. A. Landgrebe, Inorg. Chem., 1973, 12, 2826–2828 CrossRef CAS.
- J. White and G. McGillivray, J. Org. Chem., 1974, 39, 1973–1974 CrossRef CAS.
- N. C. Brown, J. Gambino and G. E. Wright, J. Med. Chem., 1977, 20, 1186–1189 CrossRef CAS PubMed.
- T. Izumi and S. I. Miller, J. Org. Chem., 1978, 43, 871–875 CrossRef CAS.
- E. H. Banitt and G. J. Conard, J. Labelled Compd. Radiopharm., 1981, 18, 713–720 CrossRef CAS.
- A. Okamoto, T. Hayashi and I. Mita, Polym. J., 1983, 15, 423–427 CrossRef CAS.
- C. L. Stephens, H. L. Nyquist and K. I. Hardcastle, J. Org. Chem., 2002, 67, 3051–3056 CrossRef CAS PubMed.
- Q. Zhu, J. Wu, R. Fathi and Z. Yang, Org. Lett., 2002, 4, 3333–3336 CrossRef CAS PubMed.
- S. Kim, B. K. Yoo, K. Chun, W. Kang, J. Choo, M.-S. Gong and S.-W. Joo, J. Mol. Catal. A: Chem., 2005, 226, 231–234 CrossRef CAS.
- S. W. Smith and G. C. Fu, J. Am. Chem. Soc., 2008, 130, 12645–12647 CrossRef CAS PubMed.
- S. W. Smith and G. C. Fu, Angew. Chem., Int. Ed., 2008, 47, 9334–9336 CrossRef CAS PubMed.
- J. J. Fitt and H. W. Gschwend, J. Org. Chem., 1984, 49, 209–210 CrossRef CAS.
- J. M. Schomaker and T. J. Delia, J. Org. Chem., 2001, 66, 7125–7128 CrossRef CAS PubMed.
- T. J. Delia, J. M. Schomaker and A. S. Kalinda, J. Heterocycl. Chem., 2006, 43, 127–131 CrossRef CAS.
- R. A. Geanangel and S. G. Shore, J. Am. Chem. Soc., 1967, 89, 6771–6772 CrossRef CAS.
- N. S. Hosmane, J. R. Wermer, Z. Hong, T. D. Getman and S. G. Shore, Inorg. Chem., 1987, 26, 3638–3639 CrossRef CAS.
- S. H. Lawrence, J. R. Wermer, S. K. Boocock, M. A. Banks, P. C. Keller and S. G. Shore, Inorg. Chem., 1986, 25, 367–372 CrossRef CAS.
- A. Pelter, T. Levitt and K. Smith, J. Chem. Soc. D, 1969, 435–436 RSC.
- L. A. Peacock and R. A. Geanangel, Inorg. Chem., 1976, 15, 244–246 CrossRef CAS.
- R. N. Leyden, B. P. Sullivan, R. T. Baker and M. F. Hawthorne, J. Am. Chem. Soc., 1978, 100, 3758–3765 CrossRef CAS.
- J. R. Wermer and S. G. Shore, Inorg. Chem., 1987, 26, 1644–1645 CrossRef CAS.
- T. D. Getman, J. A. Krause, P. M. Niedenzu and S. G. Shore, Inorg. Chem., 1989, 28, 1507–1510 CrossRef CAS.
- C.-H. Kang, S.-J. Kim, J.-J. Ko, K.-B. Lee and S. O. Kang, Bull. Korean Chem. Soc., 1993, 14, 537–539 CAS.
- J. Holub, D. L. Ormsby, J. D. Kennedy, R. Greatrex and B. Štíbr, Inorg. Chem. Commun., 2000, 3, 178–181 CrossRef CAS.
- S. Rosen and D. Sworm, Anal. Chem., 1966, 38, 1392–1397 CrossRef CAS.
- P. G. Gassman, J. T. Lumb and F. V. Zalar, J. Am. Chem. Soc., 1967, 89, 946–952 CrossRef CAS.
- B. W. Finucane and J. B. Thomson, J. Chem. Soc. D, 1969, 1220 RSC.
- A. B. Lateef, J. A. Reeder and L. Rand, J. Org. Chem., 1971, 36, 2295–2298 CrossRef.
- T. P. Karpetsky and E. H. White, J. Org. Chem., 1972, 37, 339–341 Search PubMed.
- C. U. J. Pittman, P. L. Grube, O. E. Ayers, S. P. McManus, M. D. Rausch and G. A. Moser, J. Polym. Sci., Part A-1, 1972, 10, 379–386 CrossRef CAS.
- G. Stork and T. L. MacDonald, J. Am. Chem. Soc., 1975, 97, 1264–1265 CrossRef CAS.
- J. W. Wilt and R. R. Rasmussen, J. Org. Chem., 1975, 40, 1031–1036 CrossRef CAS.
- P. J. Stang and M. G. Mangum, J. Am. Chem. Soc., 1975, 97, 3854–3856 CrossRef CAS.
- P. J. Stang and M. G. Mangum, J. Am. Chem. Soc., 1977, 99, 2597–2601 CrossRef CAS.
- J. L. Vidal, R. A. Fiato, L. A. Cosby and R. L. Pruett, Inorg. Chem., 1978, 17, 2574–2582 CrossRef CAS.
- J. L. Vidal, Inorg. Chem., 1981, 20, 243–249 CrossRef CAS.
- C. J. Collins, H. P. Hombach, B. Maxwell, M. C. Woody and B. M. Benjamin, J. Am. Chem. Soc., 1980, 102, 851–853 CrossRef CAS.
- M. Ladika and P. J. Stang, J. Chem. Soc., Chem. Commun., 1981, 459–460 RSC.
- T. E. Paxson, C. A. Reilly and D. R. Holecek, J. Chem. Soc., Chem. Commun., 1981, 618–619 RSC.
- E. E. van Tamelen and D. A. Seeley, J. Am. Chem. Soc., 1969, 91, 5194 CrossRef CAS.
- G. P. Pez, P. Apgar and R. K. Crissey, J. Am. Chem. Soc., 1982, 104, 482–490 CrossRef CAS.
- K.-H. Budt, J.-M. Vatele and Y. Kishi, J. Am. Chem. Soc., 1986, 108, 6080–6082 CrossRef CAS PubMed.
- C.-M. T. Hayward and J. R. Shapley, Organometallics, 1988, 7, 448–452 CrossRef CAS.
- O. S. Tee and J. A. Enos, Can. J. Chem., 1988, 66, 3027–3030 CrossRef CAS.
- J. R. Briggs, J. Chem. Soc., Chem. Commun., 1989, 674–675 RSC.
- W. Maringgele, U. Seebold, A. Heine, D. Stalke, M. Noltemeyer, G. M. Sheldrick and A. Meller, Organometallics, 1991, 10, 2097–2098 CrossRef CAS.
- M.-H. Hung, W. B. Farnham, A. E. Feiring and S. Rozen, J. Am. Chem. Soc., 1993, 115, 8954–8959 CrossRef CAS.
- M. L. Hays and T. P. Hanusa, Tetrahedron Lett., 1995, 36, 2435–2436 CrossRef CAS.
- N. V. Kirij, Y. L. Yagupolskii, N. Maggiarosa, W. Tyrra and D. Naumann, J. Fluorine Chem., 2001, 112, 213–218 CrossRef CAS.
- L. M. Yagupolskii, S. V. Shelyazhenko, I. I. Maletina, V. N. Petrik, E. B. Rusanov and A. N. Chernega, Eur. J. Org. Chem., 2001, 1225–1233 CrossRef CAS.
- O. Israelsohn, K. P. C. Vollhardt and J. Blum, J. Mol. Catal. A: Chem., 2002, 184, 1–10 CrossRef CAS.
- W. Tyrra, D. Naumann, B. Hoge and Y. L. Yagupolskii, J. Fluorine Chem., 2003, 119, 101–107 CrossRef CAS.
- W. Tyrra, N. V. Kirij, D. Naumann and Y. L. Yagupolskii, J. Fluorine Chem., 2004, 125, 1437–1440 CrossRef CAS.
- H. Zook and J. Miller, J. Org. Chem., 1971, 36, 1112–1116 CrossRef.
- M. Shinohara, J. Smid and M. Szwarc, J. Am. Chem. Soc., 1968, 90, 2175–2177 CrossRef CAS.
- M. A. Guerra and R. J. Lagow, J. Chem. Soc., Chem. Commun., 1990, 65–66 RSC.
- L. Liang and S. Ying, Makromol. Chem., 1993, 194, 581–600 CrossRef CAS.
- J. S. Wang, R. Jerome, R. Warin, H. Zhang and P. Teyssie, Macromolecules, 1994, 27, 3376–3382 CrossRef CAS.
- L. J. Krause and J. A. Morrison, J. Chem. Soc., Chem. Commun., 1980, 671–672 RSC.
- L. J. Krause and J. A. Morrison, J. Am. Chem. Soc., 1981, 103, 2995–3001 CrossRef CAS.
- L. J. Krause and J. A. Morrison, J. Chem. Soc., Chem. Commun., 1981, 1282–1283 RSC.
- C. D. Ontiveros and J. A. Morrison, Organometallics, 1986, 5, 1446–1448 CrossRef CAS.
- J. K. Galiotos and J. A. Morrison, Organometallics, 2000, 19, 2603–2607 CrossRef CAS.
- H. H. Murray, J. P. J. Fackler, L. C. Porter, D. A. Briggs, M. A. Guerra and R. J. Lagow, Inorg. Chem., 1987, 26, 357–363 CrossRef CAS.
- H. K. Nair and J. A. Morrison, Inorg. Chem., 1989, 28, 2816–2820 CrossRef CAS.
- D. C. Loizou, J. Castillo, A. R. Oki, N. S. Hosmane and J. A. Morrison, Organometallics, 1992, 11, 4189–4193 CrossRef CAS.
- K. Ludovici, D. Naumann, G. Siegemund, W. Tyrra, H.-G. Varbelow and H. Wrubel, J. Fluorine Chem., 1995, 73, 273–274 CrossRef CAS.
- S. Daniele, L. G. Hubert-Pfalzgraf and J. Vaissermann, Polyhedron, 2003, 22, 127–132 CrossRef CAS.
- K. Sukata, Bull. Chem. Soc. Jpn., 1984, 57, 613–614 CrossRef CAS.
- D. G. Lee and V. S. Chang, J. Org. Chem., 1978, 43, 1532–1536 CrossRef CAS.
- C. Paradisi, U. Quintily and G. Scorrano, J. Org. Chem., 1983, 48, 3022–3026 CrossRef CAS.
- D. E. Bergbreiter and J. R. Blanton, J. Org. Chem., 1987, 52, 472–473 CrossRef CAS.
- K. Sukata and T. Akagawa, J. Org. Chem., 1989, 54, 1476–1479 CrossRef CAS.
- D. Landini, A. Maia, L. Corda, A. Maccioni and G. Podda, Tetrahedron Lett., 1989, 30, 5781–5784 CrossRef CAS.
- D. Landini, A. Maia, L. Corda, A. Maccioni and G. Podda, Tetrahedron, 1991, 47, 7477–7488 CrossRef CAS.
- A. A. Varnek, A. Maia, D. Landini, A. Gamba, G. Morosi and G. Podda, J. Phys. Org. Chem., 1993, 6, 113–121 CAS.
- H. B. Lazrek, M. Taourirte, J.-L. Barascut and J.-L. Imbach, Nucleosides Nucleotides, 1991, 10, 1285–1293 CAS.
- I. Y. Kudryavtsev and L. S. Zakharov, Russ. Chem. Bull., 2001, 50, 1457–1460 CrossRef CAS.
- T. Y. Kochergina and V. F. Anufriev, Russ. J. Org. Chem., 2002, 38, 534–537 CrossRef CAS.
- H. J. Koh, K. L. Han and I. Lee, J. Org. Chem., 1999, 64, 4783–4789 CrossRef CAS PubMed.
- J. C. Hogan and R. D. Gandour, J. Am. Chem. Soc., 1980, 102, 2865–2866 CrossRef CAS.
- J. C. Hogan and R. D. Gandour, J. Org. Chem., 1991, 56, 2821–2826 CrossRef CAS.
- J. C. Hogan and R. D. Gandour, J. Org. Chem., 1992, 57, 55–61 CrossRef CAS.
- N. Basilio, L. García-Río, J. R. Leis, J. C. Mejuto and M. Pérez-Lorenzo, Chem. Commun., 2005, 3817–3819 RSC.
- N. Basilio, L. García-Río, J. C. Mejuto and M. Pérez-Lorenzo, J. Org. Chem., 2006, 71, 4280–4285 CrossRef CAS PubMed.
- L. García-Río, J. C. Mejuto and M. Pérez-Lorenzo, J. Phys. Chem. B, 2007, 111, 11149–11156 CrossRef PubMed.
- X. Dai, N. A. Strotman and G. C. Fu, J. Am. Chem. Soc., 2008, 130, 3302–3303 CrossRef CAS PubMed.
- R. A. Bartsch and I. W. Yang, Tetrahedron Lett., 1979, 20, 2503–2504 CrossRef.
- Y. Kimura and S. L. Regen, J. Org. Chem., 1982, 47, 2493–2494 CrossRef CAS.
- M. S. Newman and W. C. Liang, J. Org. Chem., 1973, 38, 2438–2441 CrossRef CAS.
- H. Ishii, Y. Hou and T. Fuchigami, Tetrahedron, 2000, 56, 8877–8881 CrossRef CAS.
- S. A. Grabovskiy, Q. K. Timerghazin and N. N. Kabal'nova, Russ. Chem. Bull., 2005, 54, 2384–2393 CrossRef CAS.
- C. W. Yoon, P. J. Carroll and L. G. Sneddon, J. Am. Chem. Soc., 2009, 131, 855–864 CrossRef CAS PubMed.
- I. Yoshpe-Besancon, D. Auriol, F. Paul, P. Monsan, J. C. Gripon and B. Ribadeau-Dumas, Biotechnol. Appl. Biochem., 1993, 18, 93–102 CAS.
- C. M. Rosell, M. Terreni, R. Fernandez-Lafuente and J. M. Guisan, Enzyme Microb. Technol., 1998, 23, 64–69 CrossRef CAS.
- D. B. Berkowitz, R. E. Hartung and S. Choi, Tetrahedron: Asymmetry, 1999, 10, 4513–4520 CrossRef CAS.
- C. G. P. H. Schroën, V. A. Nierstrasz, P. J. Kroon, R. Bosma, A. E. M. Janssen, H. H. Beeftink and J. Tramper, Enzyme Microb. Technol., 1999, 24, 498–506 CrossRef.
- A. Illanes and A. Fajardo, J. Mol. Catal. B: Enzym., 2001, 11, 587–595 CrossRef CAS.
- H. Ohno and N. Yamaguchi, Bioconjugate Chem., 1994, 5, 379–381 CrossRef CAS PubMed.
- N. Y. Kawahara and H. Ohno, Bioconjugate Chem., 1997, 8, 643–648 CrossRef CAS PubMed.
- F. Kurusu and H. Ohno, Electrochim. Acta, 2000, 45, 2911–2915 CrossRef CAS.
- J. Chen, S. K. Spear, J. G. Huddleston and R. D. Rogers, Green Chem., 2005, 7, 64–82 RSC.
- L. Cao, F. van Rantwijk and R. A. Sheldon, Org. Lett., 2000, 2, 1361–1364 CrossRef CAS PubMed.
- B. R. Taylor and S. M. Kauzlarich, Chem. Mater., 1998, 10, 22–24 CrossRef CAS.
- B. R. Taylor and S. M. Kauzlarich, Chem. Mater., 1999, 11, 2493–2500 CrossRef CAS.
- B. R. Taylor, G. A. Fox, L. J. Hope-Weeks, R. S. Maxwell, S. M. Kauzlarich and H. W. H. Lee, Mater. Sci. Eng., B, 2002, 96, 90–93 CrossRef.
- C.-S. Yang, R. A. Bley, S. M. Kauzlarich, H. W. H. Lee and G. R. Delgado, J. Am. Chem. Soc., 1999, 121, 5191–5195 CrossRef CAS.
- C.-S. Yang, Q. Liu and S. M. Kauzlarich, Chem. Mater., 2000, 12, 983–988 CrossRef CAS.
- E. E. Johnston, J. D. Bryers and B. D. Ratner, Langmuir, 2005, 21, 870–881 CrossRef CAS PubMed.
- F. Brétagnol, L. Ceriotti, M. Lejeune, A. Papadopoulou-Bouraoui, M. Hasiwa, D. Gilliland, G. Ceccone, P. Colpo and F. Rossi, Plasma Processes Polym., 2006, 3, 30–38 CrossRef.
- F. Brétagnol, M. Lejeune, A. Papadopoulou-Bouraoui, M. Hasiwa, H. Rauscher, G. Ceccone, P. Colpo and F. Rossi, Acta Biomater., 2006, 2, 165–172 CrossRef PubMed.
- F. Brétagnol, L. Sirghi, S. Mornet, T. Sasaki, D. Gilliland, P. Colpo and F. Rossi, Nanotechnology, 2008, 19, 125306 CrossRef PubMed.
- C.-S. Yang, D. D. Awschalom and G. D. Stucky, Chem. Mater., 2001, 13, 594–598 CrossRef CAS.
- C.-S. Yang, D. D. Awschalom and G. D. Stucky, Chem. Mater., 2002, 14, 1277–1284 CrossRef CAS.
- H. W. Chiu and S. M. Kauzlarich, Chem. Mater., 2006, 18, 1023–1028 CrossRef CAS.
- A. L. Pickering, C. Mitterbauer, N. D. Browning, S. M. Kauzlarich and P. P. Power, Chem. Commun., 2007, 580–582 RSC.
- J. Cho, Electrochim. Acta, 2008, 54, 461–466 CrossRef CAS.
- N. Shirahata and Y. Sakka, J. Ceram. Soc. Jpn., 2010, 118, 932–939 CrossRef CAS.
- S. Mishra, G. Ledoux, E. Jeanneau, S. Daniele and M.-F. Joubert, Dalton Trans., 2012, 41, 1490–1502 RSC.
- M. Shirai, H. Ishida and M. Tanaka, J. Polym. Sci., Part B: Polym. Phys., 1988, 26, 2075–2091 CrossRef CAS.
- J. Morgado, F. Cacialli, R. H. Friend, B. S. Chuah, H. Rost, S. C. Moratti and A. B. Holmes, Synth. Met., 2001, 119, 595–596 CrossRef CAS.
- J. A. Moore, J.-H. Kim and P. R. Seidel, Chem. Mater., 1991, 3, 742–745 CrossRef CAS.
- P. J. Fazen, E. E. Remsen, J. S. Beck, P. J. Carroll, A. R. McGhie and L. G. Sneddon, Chem. Mater., 1995, 7, 1942–1956 CrossRef CAS.
- P. J. Fazen, J. S. Beck, A. T. Lynch, E. E. Remsen and L. G. Sneddon, Chem. Mater., 1990, 2, 96–97 CrossRef CAS.
- K. Moraes, J. Vosburg, D. Wark and L. V. Interrante, Chem. Mater., 2004, 16, 125–132 CrossRef CAS.
- G. P. López and B. D. Ratner, Plasmas Polym., 1996, 1, 127–151 CrossRef.
- B. Sandner, N. Kotzian, J. Tubke, S. Wartewig and O. Lange, Macromol. Chem. Phys., 1997, 198, 2715–2727 CrossRef CAS.
- R. T. Baker, P. J. Krusic, T. H. Tulip, J. C. Calabrese and S. S. Wreford, J. Am. Chem. Soc., 1983, 105, 6763–6765 CrossRef CAS.
- I. Baxter, S. R. Drake, M. B. Hursthouse, K. M. A. Malik, J. McAleese, D. J. Otway and J. C. Plakatouras, Inorg. Chem., 1995, 34, 1384–1394 CrossRef CAS.
- S. R. Drake, S. A. S. Miller and D. J. Williams, Inorg. Chem., 1993, 32, 3227–3235 CrossRef CAS.
- S. R. Drake, A. Lyons, D. J. Otway, A. M. Z. Slawin and D. J. Williams, J. Chem. Soc., Dalton Trans., 1993, 2379–2386 RSC.
- V.-C. Arunasalam, I. Baxter, S. R. Drake, M. B. Hursthouse, K. M. A. Malik, S. A. S. Miller, D. M. P. Mingos and D. J. Otway, J. Chem. Soc., Dalton Trans., 1997, 1331–1336 RSC.
- F. J. Arnáiz, R. Aguado, M. R. Pedrosa, J. Mahía and M. A. Maestro, Polyhedron, 2001, 20, 2781–2785 CrossRef.
- A. Crochet and K. M. Fromm, Z. Anorg. Allg. Chem., 2010, 636, 1484–1496 CrossRef CAS.
- G. Malandrino, R. Licata, F. Castelli, I. L. Fragalà and C. Benelli, Inorg. Chem., 1995, 34, 6233–6234 CrossRef CAS.
- G. Malandrino, C. Benelli, F. Castelli and I. L. Fragalà, Chem. Mater., 1998, 10, 3434–3444 CrossRef CAS.
- K. D. Pollard, J. J. Vittal, G. P. A. Yap and R. J. Puddephatt, J. Chem. Soc., Dalton Trans., 1998, 1265–1268 RSC.
- G. Malandrino, R. Lo Nigro, I. L. Fragalà and C. Benelli, Eur. J. Inorg. Chem., 2004, 500–509 CrossRef CAS.
- S.-J. Kang, Y. S. Jung and I.-H. Suh, Bull. Korean Chem. Soc., 1999, 20, 95–98 CAS.
- K. D. Pollard, H. A. Jenkins and R. J. Puddephatt, Chem. Mater., 2000, 12, 701–710 CrossRef CAS.
- G. Malandrino, R. Lo Nigro, C. Benelli, F. Castelli and I. L. Fragalà, Chem. Vap. Deposition, 2000, 6, 233–238 CrossRef CAS.
- G. Malandrino, M. Bettinelli, A. Speghini and I. L. Fragalà, Eur. J. Inorg. Chem., 2001, 1039–1044 CrossRef CAS.
- G. G. Condorelli, S. Gennaro and I. L. Fragalà, Chem. Vap. Deposition, 2000, 6, 185–192 CrossRef CAS.
- R. Lo Nigro, R. G. Toro, M. E. Fragalà, P. Rossi, P. Dapporto and G. Malandrino, Inorg. Chim. Acta, 2009, 362, 4623–4629 CrossRef CAS.
- A. J. Blake, J. A. Darr, S. M. Howdle, M. Poliakoff, W.-S. Li and P. B. Webb, J. Chem. Crystallogr., 1999, 29, 547–554 CrossRef CAS.
- D. Pfeiffer, M. J. Heeg and C. H. Winter, Inorg. Chem., 2000, 39, 2377–2384 CrossRef CAS PubMed.
- E. Buncel, J. M. Dust, A. Jonczyk, R. A. Manderville and I. Onyido, J. Am. Chem. Soc., 1992, 114, 5610–5619 CrossRef CAS.
- E. Buncel and R. A. Manderville, J. Phys. Org. Chem., 1993, 6, 71–82 CrossRef CAS.
- R. A. Manderville and E. Buncel, J. Am. Chem. Soc., 1993, 115, 8985–8989 CrossRef CAS.
- R. A. Manderville and E. Buncel, J. Chem. Soc., Perkin Trans. 2, 1993, 1887–1894 RSC.
- C. A. Rouse, A. C. Finlinson, B. J. Tarbet, J. C. Pixton, N. M. Djordjevic, K. E. Markides, J. S. Bradshaw and M. L. Lee, Anal. Chem., 1988, 60, 901–905 CrossRef CAS.
- A. J. Schuetz, M. G. Weller and R. Niessner, Fresenius' J. Anal. Chem., 1999, 363, 777–782 CrossRef CAS.
- S. F. Sciamanna and S. Lynn, Ind. Eng. Chem. Res., 1988, 27, 492–499 CrossRef CAS.
- A. Henni, P. Tontiwachwuthikul and A. Chakma, Can. J. Chem. Eng., 2005, 83, 358–361 CrossRef CAS.
- H. E. Mishmash and C. E. Meloan, Anal. Chem., 1972, 44, 835–836 CrossRef CAS PubMed.
- J. Dasilva-Carbalhal, L. García-Río, D. Gómez-Díaz, J. C. Mejuto and M. Pérez-Lorenzo, J. Colloid Interface Sci., 2005, 292, 591–594 CrossRef CAS PubMed.
- Y. Gu and F. Jérôme, Green Chem., 2010, 12, 1127–1138 RSC.
- A. Wolfson, C. Dlugy and Y. Shotland, Environ. Chem. Lett., 2007, 5, 67–71 CrossRef CAS.
- A. Behr, J. Eilting, K. Irawadi, J. Leschinski and F. Lindner, Green Chem., 2008, 10, 13–30 RSC.
- S. Tang, G. A. Baker and H. Zhao, Chem. Soc. Rev., 2012, 41, 4030–4066 RSC.
- J. I. García, H. García-Marín, J. A. Mayoral and P. Pérez, Green Chem., 2010, 12, 426–434 RSC.
| This journal is © The Royal Society of Chemistry 2014 |