Open Access Article
Open Access ArticleResolving the controls over the production and emission of ice-nucleating particles in sea spray†
Thomas C. J.
Hill
 *a,
Francesca
Malfatti
*a,
Francesca
Malfatti
 *bc,
Christina S.
McCluskey
*bc,
Christina S.
McCluskey
 d,
Gregory P.
Schill
d,
Gregory P.
Schill
 e,
Mitchell V.
Santander
f,
Kathryn A.
Moore
e,
Mitchell V.
Santander
f,
Kathryn A.
Moore
 a,
Anne Marie
Rauker
a,
Russell J.
Perkins
a,
Anne Marie
Rauker
a,
Russell J.
Perkins
 a,
Mauro
Celussi
a,
Mauro
Celussi
 c,
Ezra J. T.
Levin
g,
Kaitlyn J.
Suski
h,
Gavin C.
Cornwell
i,
Christopher
Lee
c,
Ezra J. T.
Levin
g,
Kaitlyn J.
Suski
h,
Gavin C.
Cornwell
i,
Christopher
Lee
 j,
Paola
Del Negro
c,
Sonia M.
Kreidenweis
j,
Paola
Del Negro
c,
Sonia M.
Kreidenweis
 a,
Kimberly A.
Prather
a,
Kimberly A.
Prather
 fj and
Paul J.
DeMott
fj and
Paul J.
DeMott
 a
a
aDepartment of Atmospheric Science, Colorado State University, Fort Collins, CO, USA. E-mail: Thomas.Hill@colostate.edu
bUniversity of Trieste, Trieste, Italy. E-mail: f.malfatti@gmail.com
cIstituto Nazionale di Oceanografia e di Geofisica Sperimentale, Trieste, Italy
dNational Center for Atmospheric Research, Boulder, CO, USA
eChemical Science Division, Earth System Research Laboratory, National Oceanic and Atmospheric Administration, Boulder, CO, USA
fDepartment of Chemistry and Biochemistry, University of California San Diego, La Jolla, CA, USA
gHandix Scientific, Fort Collins, CO, USA
hJUUL Labs, San Francisco, USA
iPacific Northwest National Laboratory, Richland, WA, USA
jScripps Institution of Oceanography, University of California San Diego, La Jolla, CA, USA
First published on 9th May 2023
Abstract
The role of marine ice-nucleating particles (INPs) in modifying clouds and radiation balance over oceans is uncertain. While recent studies have advanced our understanding of the abundance of marine INPs, characterizing their sources and composition remains a challenge. INP concentrations above oceans are typically low, sometimes extraordinarily so, but there is evidence of elevated levels associated with phytoplankton blooms. Mesocosm experiments have shown that ice-nucleating entities (INEs, which include discrete particles as well as ice-nucleating monolayers) are produced, and INP emissions raised, in the decay phase following bloom collapse. To test if INE production depends upon phytoplankton type, we added dead particulate biomass of a green alga (Nannochloris atomus), a diatom (Skeletonema marinoi) and a cyanobacterium (Prochlorococcus marinus) to a miniature Marine Aerosol Reference Tank filled with seawater. As decomposition progressed, heterotrophic bacteria initially increased and plateaued, then declined, coinciding with an increase in heterotrophic nanoflagellates (HNF) and viruses. Enzyme activities typically increased over several days before plateauing or decreasing, while humic-like substances (HULIS) steadily accumulated. INEs in the seawater peaked 3–5 days after each detritus addition, increasing ∼10- to ∼20-fold. INE concentration was closely correlated with HNF counts, viruses and the concentration of HULIS, but not with bacteria or enzyme activities. Newly-fabricated INEs were organic, primarily heat stable (95 °C), and varied in size. INP concentrations in sea spray aerosol (SSA) tended to peak shortly before the peak of INEs in the seawater, at 4-, 35- and 15-fold higher than at the start in the N. atomus, S. marinoi, and P. marinus incubations, respectively. Using data from the P. marinus incubation, we were able to provide the first estimate of INP enrichment in SSA (over its concentration in the water): it was initially ∼200× for the fresh seawater and increased further after the addition of the P. marinus inoculum. We also tested if a simple nutrient mix (bovine serum albumin (BSA) and three monosaccharides) could stimulate INP production: INEs in the seawater changed little, but INP emissions fell abruptly immediately upon BSA addition due to it forming a monolayer which displaced the sea surface microlayer (SML). These experiments revealed that INE production in the decay phase of a phytoplankton bloom requires the addition of a natural, complex substrate to initiate a realistic succession of decomposers, and that INP emissions are further controlled by their concentration in the SML and, indirectly, by the impact of SML composition upon jet and film drop production.
Environmental significanceOceans emit cloud-active aerosols. One influential class, the ice-nucleating particles (INPs), trigger the freezing of supercooled cloud droplets, reducing cloud reflectivity and longevity whilst facilitating precipitation. Understanding the role of INPs in sea spray aerosols (SSA) is critical for understanding glaciation of marine clouds, especially in remote regions. When phytoplankton blooms collapse, INPs are produced and emitted in the SSA. However, characterizing their sources and dependence upon phytoplankton class remains uncertain. We added dead biomass of three diverse phytoplankton species to seawater in mesocosms. INP production was stimulated by all species, peaked after 3–5 days, and coincided with increases in nanoflagellates and viruses. Emissions were modified by the sea surface microlayer and its control over jet drop production by bubbles. |
1 Introduction
The oceans are a 360 million square kilometre source of cloud-active aerosols. One rare but exceptionally influential class of aerosol is the ice-nucleating particles (INPs). These trigger the freezing of supercooled cloud droplets, which reduces cloud reflectivity and cloud longevity whilst facilitating precipitation. The Southern Ocean (SO) is remote, making long-range transport of INPs from continents of likely minor significance in the marine boundary layer.1 Consequently, the role of locally-emitted marine INPs is critical for understanding glaciation processes of clouds coupled to the marine boundary layer in such remote regions.The emissions and impacts of marine INPs are, however, not well understood,2–4 and discrepancies in simulating mid- and high-latitude southern hemisphere climate5,6 may be a consequence of this deficiency. Improved knowledge of the energy budget of the SO requires an overall greater understanding of ice formation in SO clouds.6–10 Earth system models predict “too low cloud optical thickness and/or too low cloud fraction”5 due in part to them possessing insufficient amounts of supercooled liquid in clouds;6,9,11 the SO's albedo is particularly sensitive to cloud phase and cover due to the low reflectivity of oceans. The result is a positive bias in modelled absorbed solar radiation at the ocean surface.5,12 Vergara-Temprado et al. (2018)10 suggest that the cause of the bias is due to modelled SO clouds behaving as if they have higher INP concentrations than actually occur. Indeed, recent measures show that SO cloud tops can be frequently and deeply supercooled,13 and the boundary layer contain uniquely low INP concentrations4,14–16 that lie at the low end of the range used for parameterization by Vergara-Temprado et al. (2018).10 While biases in simulated SO shortwave radiative fluxes have improved in recent CMIP6 models,17 there is a significant diversity in the representation of cloud phase and freezing processes, and their impact on climate.5–7,9,11,12,18,19 Some studies have demonstrated little influence of ice nucleation on simulated cloud phase and shortwave cloud forcing,19,20 whereas others have demonstrated a strong relationship between available marine INPs and cloud properties.21
Oceans are a generally poor source of INPs. Bulk seawater typically contains ∼1–1000 mL−1 ice-nucleating entities (INEs include both discrete particles and ice-nucleating monolayers) active at −20 °C,22–30 a tiny fraction of the number found, for example, in soils (e.g., 108 g−1 at −20 °C (ref. 31)). Sea spray aerosol (SSA) is also a low efficiency ice nucleator compared with mineral dust.15,32,34 Accordingly, INP concentrations over oceans are typically two or more orders of magnitude lower than over land.3,15,33,34 However, significant and sometimes “phenomenally active”28 excursions from typical background values in seawater and/or boundary layer air have been recorded, associated with a range of factors, such as higher concentrations of biological material, both dissolved23,24,30,34,35 and particulate (e.g., gel-like particles),28,34,35 enhanced productivity in upwelling regions,36–38 and a reduction in salinity caused by melting sea ice.23,24 Phytoplankton blooms appear to be large producers of INEs, especially during the microbial succession of bacteria, viruses and protists that ensues when they collapse and their organic matter is re-worked and decomposed.3,25,29,35,39–41 Emissions of INPs in SSA are also modulated by their enrichment in the sea surface microlayer (SML)22,24,25,30,42 and by the ratio of jet to film drop production, with the former being a more effective INE ejector.40,43 Other factors that may enhance INEs in surface waters include deposited dust35,44 and its stimulation of biological activity via iron fertilization.35
To further resolve the prerequisites, sources, and controls over emissions of marine INPs, we conducted a series of experiments using a miniature Marine Aerosol Reference Tank (miniMART).45 We focused on the decaying phase of a phytoplankton bloom, where bacteria colonize and comminute detrital particles produced from freshly-killed phytoplankton. To test if INE production is dependent upon phytoplankton class we added dead biomass of three phytoplankton species, a green alga, a diatom and a cyanobacterium, sequentially to a miniMART, each time filled with fresh seawater containing natural microbial communities. Secondly, to test if INEs can be generated simply by supplying basic nutrients, we added a mix of one protein and three common marine monosaccharides to a miniMART with the same natural communities. Throughout the ensuing decomposer successions, measurements of the bulk water and SSA included: INE/INP concentrations (including after treatments to measure the abundance of organic ice nucleators and of those deactivated by heat); cell counts of bacteria, viruses and grazers; hydrolytic enzyme activities (e.g. bacterial degradative activities); 16S rRNA profiling of bacteria; emission-excitation matrices; and aerosol size distributions. Collectively, these experiments revealed the importance of the resource quality of the substrate for INE production, and the composition of the SML and its control over jet drop production, for INP emissions.
2 Materials and methods
We studied the production of ice-nucleating entities (INEs) and its link to the emission of ice-nucleating particles (INPs) induced by the decomposition of organic inputs using the miniature Marine Aerosol Reference Tank (miniMART).45 The miniMART uses a plunging water jet, which replicates the bubble plumes generated by small waves, and so simulates the meteorological conditions consistent with the occurrence of small whitecaps generated by wind speeds >∼3 m s−1. We tested three scenarios: (A) microbial degradation of particulate organic carbon (POC) originating from algal detritus from three species representing major marine algal classes; (B) microbial degradation of dissolved organic carbon (DOC) supplied as a simple protein and monosaccharide nutrient enrichment; (C) Microbial degradation of extant DOC (negative control).2.1 Experimental design
Seawater was collected at the Ellen Browning Scripps Memorial Pier (La Jolla, CA, 32.86693°N, 117.2573°E) during high tides by filling a 10 L polypropylene cubitainer (Hedwin Division of Zacros America, Inc., pre-cleaned with 1 M HCl and then triple rinsed with deionized water). During filling, water was filtered through a 100 μm Nitex mesh (Sefar). The cubitainer was shipped overnight to Colorado State University (CSU), and the following morning 7.5 L was used to fill the miniMART (total tank volume of 19 L).45 The fill water was pre-filtered through autoclaved 0.6 μm pore-size polycarbonate filters (Whatman Nuclepore, Cytiva) to remove phytoplankton, that may have produced fresh organic matter via photosynthesis, and larger protists (bacterial grazers) and other larger particles present in the seawater.46 Bacteria, viruses and DOC passed through the filter, as well as some of the small grazers. The miniMART was sealed, the headspace supplied with 6.5 L min−1 HEPA-filtered air, and the rotating water wheel started, producing eight 70 mL water jets min−1 to generate SSA. The miniMART was kept at room temperature (∼23 °C) under low light to prevent growth of any small phytoplankton (e.g., cyanobacteria and picoeukaryotes) that had passed through the 0.6 μm filter. These conditions were maintained continuously for the duration of each experiment.The tank was left to acclimate for 24 h (designated Day -1), and the following day (Day 0) algal detritus or the nutrient mix was added to simulate either the sudden demise of a phytoplankton bloom or an input of labile organic matter, respectively. Experiments were run for 6–18 days, or once air INP number concentrations peaked. Several measures were taken daily of the tank water and SSA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) INE/INP concentrations, bacterial, viral and protist counts, and bacterial degradative activities. Others were taken intermittently at key points: aerosol size distribution, real-time SSA INP concentrations using CSU's Continuous Flow Diffusion Chamber, and water and SSA samples for excitation-emission matrix spectroscopy and DNA extractions.
INE/INP concentrations, bacterial, viral and protist counts, and bacterial degradative activities. Others were taken intermittently at key points: aerosol size distribution, real-time SSA INP concentrations using CSU's Continuous Flow Diffusion Chamber, and water and SSA samples for excitation-emission matrix spectroscopy and DNA extractions.
2.2 Algal detritus preparation and labile organic carbon amendment
Samples of detritus from three species representing diverse groups of marine phytoplankton were prepared for addition to the miniMART (see Table 1). Nannochloris atomus (phylum Chlorophyta), Skeletonema marinoi (phylum Bacillariophyta) and Prochlorococcus marinus MED 04 (phylum Cyanobacteria, Prochlorococcus marinus subsp. Pastoris, str. CMP1986) cultures were grown under 12/12 h light/dark cycles at 23 °C. Subsequently, the cells were harvested, washed with 0.2 μm-filtered and autoclaved seawater and subjected to seven freeze–thaw cycles47 to produce algal detritus. The detritus was then washed to remove the dissolved cellular fraction. Prior to harvesting, the chlorophyll a (Chl a) of each culture was measured48 to calculate the dose to be added in chlorophyll equivalent units (Table 1). The labile organic carbon amendment was composed of a mixture of one protein (bovine serum albumin, BSA) and three sugars (glucose, galactose and mannose).| Amendment | Period | Supplier, strain, medium | Concentration | Notes |
|---|---|---|---|---|
| Nannochloris atomus detritus | 10–16 Feb | Bigelow, CCMP509, f/2 (ref. 54) | 14 μg L−1 Chl a equiv. | Green microalga, phylum Chlorophyta. Has an organic layered cell wall composed of cellulose and other polysaccharides |
| Skeletonema marinoi detritus | 17–26 Feb | OGS, —, f/2 (ref. 54) | 2.5 μg L−1 Chl a equiv. | Diatom, phylum Bacillariophyta. Cells are usually connected in long chains. Cosmopolitan in coastal (not polar) seas, and forms extensive spring blooms. Skeletonema costatum was the most abundant phytoplankton species in IMPACTS blooms39,52 |
| Prochlorococcus marinus MED4 detritus | 30 Aug–9 Sep | Bigelow, CCMP2389, Pro99 (ref. 55) | 2.5 μg L−1 Chl a equiv. | Phylum Cyanobacteria. From eastern Mediterranean. High light adapted. Prochlorococcus dominate temperate and tropical oceans |
| None (control) | 23–29 Aug | — | — | Native DOC control |
| BSA | 11–29 Nov | Sigma, A6003 | 1 mg L−1 | Typical DOC in seawater is ∼0.5–1.0 mg L−1 (ref. 53) |
| Glucose | Sigma, G7021 | 0.33 mg L−1 | ||
| Galactose | Sigma, G5288 | 0.33 mg L−1 | ||
| Mannose | Sigma, M6020 | 0.33 mg L−1 |
INEs were measured in the axenic N. atomus and P. marinus cultures, but not in the S. marinoi detritus, which had been prepared in Italy. After subtraction of INPs in the media, INP concentration was calculated on the basis of chlorophyll equivalent units (Fig. S1†). While N. atomus showed very low activity >−24 °C, P. marinus appeared to possess some activity extending to around −15 °C. However, since the cultures were not prepared specifically to test for IN activity (i.e. after subculturing in INE-free media), the apparent activity may have been due to contaminating INEs in the stock inoculum. Ladino et al. (2016)49 tested exudates (exopolymer secretions, but also possibly cellular debris) of N. atomus at −40 °C and found they nucleated ice at low RHice values, but the activated fractions were low. Skeletonema marinoi was shown by Alpert et al. (2011)50 to have IN activity at −29 °C, while Ickes et al. (2020)42 measured a T50 of ice nucleation in culture samples at approximately −26 to −21 °C; the INEs were also sensitive to a heat treatment. Interestingly, Xi et al. (2021)51 recently measured significant IN activity beginning at ∼−15 °C in a sea ice sample enriched with Nitzschia stellata, a diatom from the same class as S. marinoi. From its heat sensitivity they suggested the source was a protein-containing colloidal nanogel.
2.3 Bacterial and viral abundance
Seawater was sampled every day and fixed with EM-grade glutaraldehyde at 0.5% final concentration, flash frozen, and stored at −80 °C until flow cytometric analysis was performed (within one month). Heterotrophic bacterial and viral abundances were determined by flow cytometry using a BD FACSCanto™ II (Becton Dickinson). For heterotrophic bacteria and heterotrophic nanoflagellates (HNF), 1 mL of thawed sample was stained with SYBRGreen I at room temperature for 10 min (10−4 dilution of the commercial stock) in the dark.56,57 For viral abundance, 2.5 μL of thawed sample was diluted in freshly prepared 1× TE buffer (pH 8) and stained with SYBRGreen I at 80 °C for 10 min in the dark (5 × 10−5 dilution of the commercial stock).58 Then, the samples were run at low flow to avoid overlapping events (65 μL for 1 min) through the flow cytometer, and green fluorescence (FL1) and 90° side light scatter (SSC) intensity recorded (the trigger was set on FL1). The samples were weighed before and after the run to measure the analysed volume. Heterotrophic bacteria and viral populations were discriminated based on their signature in the FL1 vs. SSC specific cytograms. FL1 threshold was used for bacteria, HNF and viruses.SSA sampling was done every day by impinging freshly produced SSA into 0.2 μm filtered autoclaved seawater (FASW) at 1 L min−1 for 30 min. Five millilitres of the resulting solution were fixed with EM-grade glutaraldehyde at 5% final concentration, flash frozen, and stored at −80 °C until microscopic analysis (within one month). Thawed samples were filtered onto 0.02 μm alumina oxide membranes (Anodisc, GE Healthcare) and stained with SYBRGreen I following the protocol of Noble and Fuhrman (1998)59 for viral and bacterial estimates. The membranes were then mounted and immediately imaged using an inverted epifluorescence microscope Olympus BX51 with a UPlanFI 100× oil immersion objective equipped with a 490/528 nm excitation/emission filter cube (U-MSWB2). Bacteria and viruses were counted in 20–30 fields.
2.4 Bacterial degradative activities
The bacterial degradative activities of the bulk seawater and SSA samples were measured using fluorogenic substrate analogs at saturating concentrations (20 μM).60–62 The enzymes alkaline phosphatase (APase, AP), protease (leucine, L), lipase (oleate, O and butyrate, B) and beta-glucosidase (G) were measured with, respectively, substrates 4-methyl-umbelliferyl-phosphate (Sigma, M8883), L-leucine-7-amido-4-methyl-coumarin (Sigma, L2145), 4-methyl-umbelliferyl oleate (Sigma, 75164), 4-methyl-umbelliferyl butyrate (Sigma, 19362) and 4-methylumbelliferyl β-D-glucopyranoside (Sigma, M3633). Fluorescence resulting from enzymatic release of the free fluorophores (4-methylumbelliferone (MUF) and 7-amido-4-methylcoumarin (AMC)) was measured by a multimode reader (Synergy™ H1, BioTek) on 96-well microtitre plates incubated in the dark at in situ temperature for 1 h. Fluorescence was measured immediately after adding substrates and again at the end of the incubation, at 355 nm excitation and 460 nm emission.Enzyme activities were expressed as the concentration of substrate hydrolysed per unit time of incubation and normalized by volume of air impinged for SSA samples, or by volume of bulk seawater for water samples. Six replicates for each sample-substrate combination were analysed on the microtitre plate. Standard curves of the free fluorophores AMC and MUF were used to relate fluorescence to free fluorophore concentration. Enzyme activity values for aerosol samples are reported as the difference between the sample and a blank generated by impinging particle-free air in FASW prepared at the beginning of every experiment cycle.
2.5 Measurement of ice-nucleating entities (INEs) and particles (INPs)
To re-suspend collected aerosol, one filter was placed in a 50 mL Falcon tube, 6 mL of 0.1 μm-filtered deionized water added, and the tube tumbled on a Roto-Torque (Cole-Palmer) for 20 min. INEs/INPs were measured in each water sample and aerosol suspension, and in 10- to 20-fold and 225- to 400-fold dilutions of each to estimate concentrations of INEs/INPs active at colder temperatures (to −27 °C). To estimate INPs in each sample and dilution, 32 × 50 μL aliquots were dispensed into two 96-well PCR trays (Optimum Ultra, Life Science Products) in a laminar flow cabinet, and the trays inserted into blocks of the Colorado State University Ice Spectrometer. Immersion freezing temperature spectra per millilitre of water and per litre of miniMART headspace air were obtained as described in Hiranuma et al. (2015),63 except that the spectrometer headspace was purged with 0.25–1 L min−1 of HEPA-filtered and cooled nitrogen, with flow decreasing as temperature was lowered. Ninety-five percent confidence intervals were derived from the frozen fraction of wells at each temperature using formula 2 from Agresti and Coull (1998).64 For undiluted water samples, freezing temperatures were adjusted by +2 °C to offset freezing point depression caused by salinity. A negative control of 0.1 μm-filtered deionized water was included in each measurement to adjust for INEs in the water used for re-suspensions and dilutions. To account for background INPs on filters, three filter blanks (loaded into inline filter units, which were attached to the miniMART but without flow) were also processed, and a regression derived from the mean INPs per filter at each temperature (background INPs per filter = 5.1 × 10−6 × e−0.633×°C, R2 = 0.98). The significance of differences between samples (p < 0.05) was assessed using Fisher's Exact Test using counts of wells frozen and unfrozen in each sample to derive exact p values for differences in proportions of wells frozen between samples.
To determine the contribution of heat-labile biological INEs/INPs (e.g., IN proteins), 2 mL aliquots of filter suspensions and water samples were heated to 95 °C for 20 min and then tested in the Ice Spectrometer to estimate the reduction in ice nucleation activity caused by heating. On select water samples, the predominance of organic INEs was assessed by using hydrogen peroxide digestions, followed by analysis in the Ice Spectrometer. Two millilitres of seawater were combined with 1 mL of 30% H2O2 (Sigma Aldrich) and the mixture immersed in water heated to 95 °C for 20 min while being illuminated with two, 26 W UVB fluorescent bulbs (Exo Terra) to generate hydroxyl radicals. To remove residual H2O2 and prevent otherwise significant freezing point depression, catalase (Cat. No. 100429, MP Biomedicals) was added to the cooled solution until effervescence completely ceased. Finally, to assess INE size, some samples were filtered with a 0.2 μm syringe filter (DISMIC-13cp, Advantec; pre-rinsed with deionized water and 0.5 mL of sample) and the filtrate used to measure INEs <0.2 μm (i.e., viruses, vesicles and dissolved organic carbon such as cellular debris and cell-free molecules).65
2.6 Aerosol distribution measurements
Aerosol size distributions were monitored with a scanning mobility particle sizer (SMPS, TSI Model 3080, 0.014 μm < D < 0.75 μm) and an aerodynamic particle sizer (APS, TSI Model 3321, 0.54 μm < D < 20 μm). Aerosol diffusion driers were used to dry aerosol upstream of the SMPS and APS to <20% relative humidity. The SMPS was operated with an aerosol flow rate of 1.0 Lpm and sheath flow rate of 3 Lpm. The APS inlet has a total flow rate of 5 Lpm, consisting of a 1 Lpm aerosol flow pulled through an inner nozzle surrounded by sheath flow (4 Lpm). Due to flow restrictions of the miniMART system, the APS configuration used a sampling inlet that pulled 1 Lpm from the miniMART and 4 Lpm filtered sheath flow from the room. Several corrections were applied to the APS data: a size calibration using polystyrene latex spheres of known sizes was applied, the first three size bins of the APS were removed due to poor counting statistics, and the particle density of SSA was assumed to be 1.8 g cm−3 (ref. 68) to convert aerodynamic diameter to physical SSA diameter (shape factor assumed to be 1). The last 11 size bins were removed from the SMPS distributions due to poor counting statistics and the impactor 50% cut-off (D50 = 700 nm). These corrected SMPS and APS data were then merged to form the aerosol size distribution. Aerosol surface area was calculated assuming a spherical shape for all particles.2.7 Fluorescence excitation-emission matrix (EEM) spectroscopy
EEM spectroscopy was used to characterize and obtain relative concentrations of fluorescent organic compounds in tank water and SSA on key days in each succession. EEMs were obtained for all samples using a spectrofluorometer (Horiba Scientific, Aqualog). Excitation wavelengths ranged from 235–450 nm, and emission from 250–800 nm. A background spectrum acquired with ultrapure water was subtracted from all EEMs. EEMs were corrected for inner-filter effects, and Rayleigh scatter (1st and 2nd order) was removed. EEMs were also normalized to the area of the Raman Scattering peak of water at 350 nm excitation to convert fluorescence intensities to Raman Units (R.U.). For aerosol samples, Raman Units were adjusted to a constant volume of filtered air (1 m3). Fluorescent regions included those indicative of humic-like substances (excitation/emission ranges: 360/445–460 nm, 260/425–475 nm, and 320/400–420 nm), tryptophan-like (excitation/emission: 235/329 nm), tyrosine-like (excitation/emission: 235/302 nm) and an unusual protein-like peak with an excitation peak at 240 nm and an emission range of 300–350 nm.2.8 DNA sequencing and diversity analyses
Next-generation sequencing of the bacterial 16S rRNA gene was performed on DNA extracted from tank water and SSA in the N. atomus detritus addition experiment. DNA was extracted from frozen tank water from days 2 and 3, and from SSA filtered overnight on days 1 → 2 and 2 → 3, corresponding to one day prior to and the date of peak INP concentrations. For water samples, 6 mL was centrifuged at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×g for 5 min, the supernatant removed, and the remaining 50 μL used for DNA extraction. For SSA, half a filter was cut into strips and these were added directly to the bead beating tube. DNA extraction was performed using the DNeasy PowerLyzer Microbial Kit (QIAGEN), with homogenization by bead beating using a FastPrep-24 instrument (MP Biomedicals). DNA was extracted using the high recovery modification of the standard method as detailed in Hill et al. (2014).69 All procedures were carried out using sterile utensils in a laminar flow cabinet. The centrifuge, bead beater and pipets were cleaned with DNA AWAY (MBP).
000×g for 5 min, the supernatant removed, and the remaining 50 μL used for DNA extraction. For SSA, half a filter was cut into strips and these were added directly to the bead beating tube. DNA extraction was performed using the DNeasy PowerLyzer Microbial Kit (QIAGEN), with homogenization by bead beating using a FastPrep-24 instrument (MP Biomedicals). DNA was extracted using the high recovery modification of the standard method as detailed in Hill et al. (2014).69 All procedures were carried out using sterile utensils in a laminar flow cabinet. The centrifuge, bead beater and pipets were cleaned with DNA AWAY (MBP).
Sequencing was performed by the Joint Genome Institute using Illumina MiSeq paired-end sequencing of the V4–V5 region of the 16S rRNA gene using primers 515F-y and 926R (Parada et al. 2016).70 Sequencing generated 1.2–1.5 million reads per sample. Percent of reads with an average base calling Phred quality Q score >30 was >80%. iTagger v2.1, which uses Usearch and Qiime to analyse amplicon libraries, was used for processing. Contaminants were essentially all chloroplast or mitochondrial rRNA. Operational taxonomic units (OTUs) were clustered using a 97% identity threshold. Classification primarily used the taxonomy report integrated within the National Center for Biotechnology Information's Basic Local Alignment Search Tool (BLAST), and the Seqmatch tool of the Ribosomal Database Project, Release 11.71
Basic diversity analyses were performed on each dataset. To estimate total OTUs we used Chao 1, a non-parametric estimator that uses the number of singletons and doubletons to predict total OTU abundance. In practice it provides a lower limit of total diversity. Two general measures of diversity were chosen:72 the Shannon index (H′), a measure of the difficulty in predicting the identity of the next individual sampled, which is positively correlated with both species richness and evenness, giving more weight to rare than common species; and the log series index (α), which requires only total number of species/OTUs (S) and total counts (N) to calculate and is a fitted constant in the equation describing the log series model of species abundance. α responds approximately exponentially to changes in S/N ratio and was recommended by Magurran (1988)73 as a possible universal diversity statistic. We also calculated Simpson's index (D), which gives the probability that two sequences chosen at random will be from the same OTU. It gives a strong weighting to the dominants, with a higher value indicating lower diversity. Principal components analysis was performed using a correlation matrix of standardized scores with missing values excluded pairwise.
3 Results and discussion
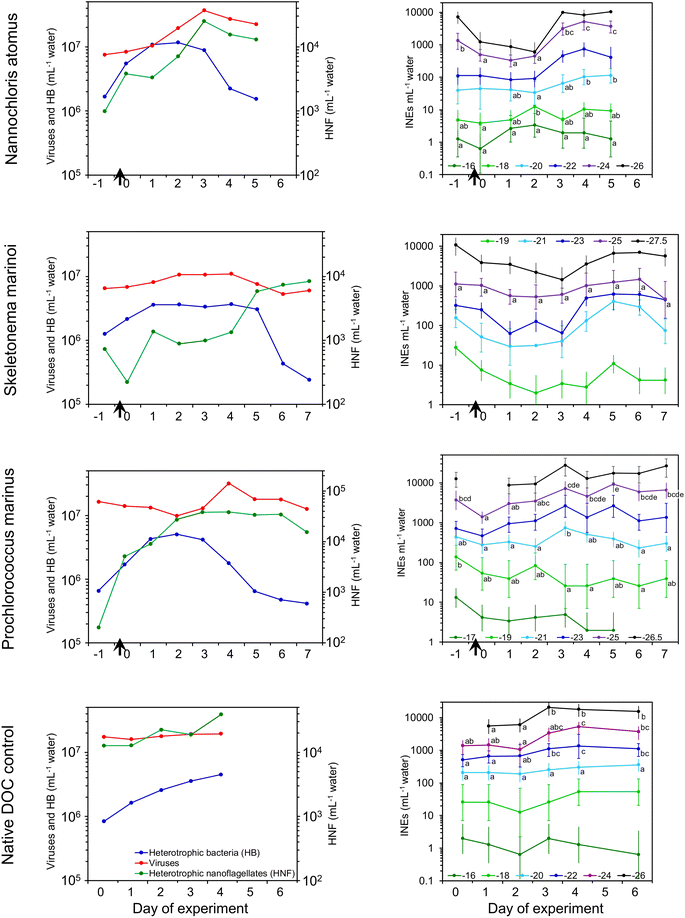
Each experiment was initiated with seawater that had been 0.6 μm-filtered to remove particulate organic carbon (POC).74,75 Ammerman et al. (1984)46 reported that about 70% of bacteria in Scripps Pier seawater were ≤0.6 μm in diameter, and all passed through a 1 μm filter (i.e., would pass through the not uncommon coincident multiple holes in the 0.6 μm membranes). Hence, filtering will have left the standing bacterial community largely intact. The starter filtrate thus contained bacteria, viruses, vesicles76 and “a broad, polydisperse mix of biopolymers, including polysaccharides, proteins, nucleic acids, and lipids”.75 Dead algal biomass, or a simple nutrient mix comprising one protein and three monosaccharides, was then added to simulate the pulse of decomposable organic matter that follows the collapse of a phytoplankton bloom, initiating a microbial succession. We present the results in decreasing POC → DOC ratio. That is, three experiments involving the addition of algal POC followed by one experiment in which a protein + monosaccharide DOC nutrient mix was added.3.1 Changes in the seawater during incubations
INEs in the water initially decreased or remained level (Fig. 1). Note that the added detritus itself contained few INEs (Fig. S1†). After 1–4 days, INE concentrations rebounded, increasing by up to 18-, 14- and 7-fold in the N. atomus, S. marinoi, and P. marinus incubations, respectively. Increases tended to be confined to INEs active below −20 °C. Interestingly, there was also a 5-fold increase in INEs active below −22 °C in the control incubation, driven by the native DOC in the seawater.
Heating the seawater to 95 °C to denature biological INEs produced small changes (Fig. S3†). In fresh, 0.6 μm filtered seawater, INEs active above ∼−22 °C were heat sensitive in two of the incubations, as also found in McCluskey et al. (2018),25 but at peak days the populations were generally unaffected by it, indicating new INEs weren't proteins.
Testing of filtrates after 0.2 μm filtration produced varied effects (Fig. S3†). At Day 0, three of the incubations (S. marinoi, P. marinus and the control experiment) had significantly lower INE concentrations in the filtrate, indicating most were >0.2 μm and therefore potentially bacteria, grazers or INEs within microgels. At peak INE days different patterns had emerged: the S. marinoi INE population was uniformly <0.2 μm (i.e., viruses, vesicles, cellular debris and/or cell-free molecules), the P. marinus and control experiments had INEs both smaller and larger than 0.2 μm, with cross-overs occurring at ∼−22 °C, while the N. atomus INEs were all >0.2 μm. This variation suggests that different INE sources developed in different incubations. McCluskey et al. (2018)25 found most INEs were >0.2 μm in a waveflume bloom, persisting throughout that entire 5 weeks experiment.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, which was also evident in the control experiment since, as mentioned, the filtered water contained enough DOC to initiate a modest succession.
1 ratio, which was also evident in the control experiment since, as mentioned, the filtered water contained enough DOC to initiate a modest succession.
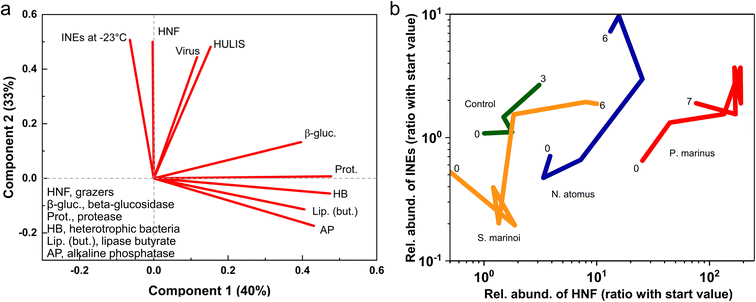
Heterotrophic nanoflagellates are protist grazers, 2–20 μm in diameter and possessing flagella. They are the main predators of bacteria, which they consume via a process called phagocytosis. If the HNFs are the primary source of the INEs then this could arise directly from IN-active molecules associated with HNFs, or indirectly via their production of IN-active bacterial decomposition products or the fragmentation of pre-existing INEs in the consumed bacteria. If viruses drive INE production it could occur, similarly, via growth of an IN phage population or via bacterial lysis and disintegration producing an apparent multiplication of INEs. The proliferating HNFs and viruses produce new organic matter pools, with one fraction being ready utilizable79,80 and the other more recalcitrant, possibly funnelling into the HULIS pool. Fragmentation and dispersal of INE-containing entities was suggested as the principal mechanism of apparent proliferation by Wilbourn et al. (2020).81 Indeed, during the P. marinus incubation the disappearance of remnant intact (countable) P. marinus cells coincided with INEs increasing in the water.
3.2 Changes in SSA emissions during incubations
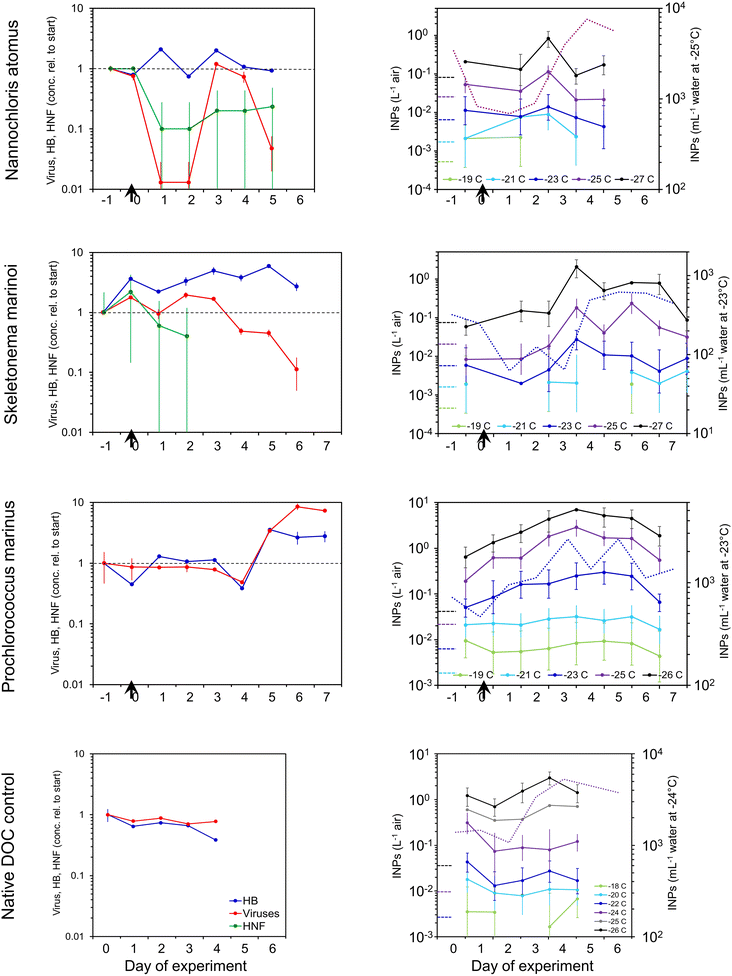
Changes in concentrations of INPs in the SSA generally paralleled those in the water (Fig. 3). INP concentrations in the SSA peaked three to four days after the addition of detritus, being 4-, 35- and 15-fold higher than emissions at the start in the N. atomus, S. marinoi, and P. marinus incubations, respectively. These increases were seen in INPs active below around −24 °C. Paralleling the INPs, HULIS and protein-like substances peaked in the middle of the N. atomus and S. marinoi incubations, before declining (Fig. S4†).
In previous waveflume and mesocosm studies, INP emissions in SSA were seen to increase, similarly, by 13- and 30-fold three to four days after the collapse of a phytoplankton bloom with the resulting injection of decomposable biomass.39
There were differences in INE and INP dynamics in the SSA and water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SSA INP emissions tended to peak a day or so earlier than in the water and, in at least two cases, decline faster. These differences could be caused by several mechanisms, all mediated by the interaction between the INEs and the SML. Firstly, the characteristics (e.g., hydrophobicity) of the INEs may have changed over time, with the initial INE population being more enriched in the SML, and hence emitted, than those predominating later. Secondly, enmeshment of INEs within aggregating gels may have curtailed INP emissions after a couple of days due to the increasing size of the gels reducing their likelihood of being ejected. Enmeshment of INEs within growing gels would manifest itself as an apparent increase in INE size and, therefore, a reduction in INEs passing through a 0.2 μm filter. This was evident in the N. atomus incubation (Fig. S3†), but not in the S. marinoi incubation. However, both showed peak SSA INP emissions a day earlier than in the water. Thirdly, changes in SML composition and thickness may have altered INE emissions. The effect of this may have been directly linked to increased INE enrichment within the SML, and/or indirect and caused by changed bubble longevity affecting the time for enrichment of INEs in films or a shift in bubble size altering the relative abundances of film vs. jet drops.
SSA INP emissions tended to peak a day or so earlier than in the water and, in at least two cases, decline faster. These differences could be caused by several mechanisms, all mediated by the interaction between the INEs and the SML. Firstly, the characteristics (e.g., hydrophobicity) of the INEs may have changed over time, with the initial INE population being more enriched in the SML, and hence emitted, than those predominating later. Secondly, enmeshment of INEs within aggregating gels may have curtailed INP emissions after a couple of days due to the increasing size of the gels reducing their likelihood of being ejected. Enmeshment of INEs within growing gels would manifest itself as an apparent increase in INE size and, therefore, a reduction in INEs passing through a 0.2 μm filter. This was evident in the N. atomus incubation (Fig. S3†), but not in the S. marinoi incubation. However, both showed peak SSA INP emissions a day earlier than in the water. Thirdly, changes in SML composition and thickness may have altered INE emissions. The effect of this may have been directly linked to increased INE enrichment within the SML, and/or indirect and caused by changed bubble longevity affecting the time for enrichment of INEs in films or a shift in bubble size altering the relative abundances of film vs. jet drops.
Heat tests (95 °C) on aerosol INP samples produced minimal changes (Fig. S5†), similar to the results in the water (a lack of available suspension precluded other tests). The only consistent impact occurred in the control experiment where, just as in the water, a modest reduction was apparent at Day 2–3, suggesting the INPs were predominantly biological. By contrast, previous waveflume SSA emissions showed a more general INP heat sensitivity, especially for those active above −20 °C.25
Among all samples, a total of 317 OTUs were detected. The Chao I estimator, which provides a lower limit of total diversity,82 predicted ∼210–230 OTUs in each of the four samples (Table S1†). The somewhat greater diversity detected in the SSA samples was reflected in their higher log series (α) and Shannon index (H′) values; H′ is positively correlated with both species richness and evenness. The presence of some very dominant OTUs in all samples generated relatively high Simpson's dominance index (D) values.
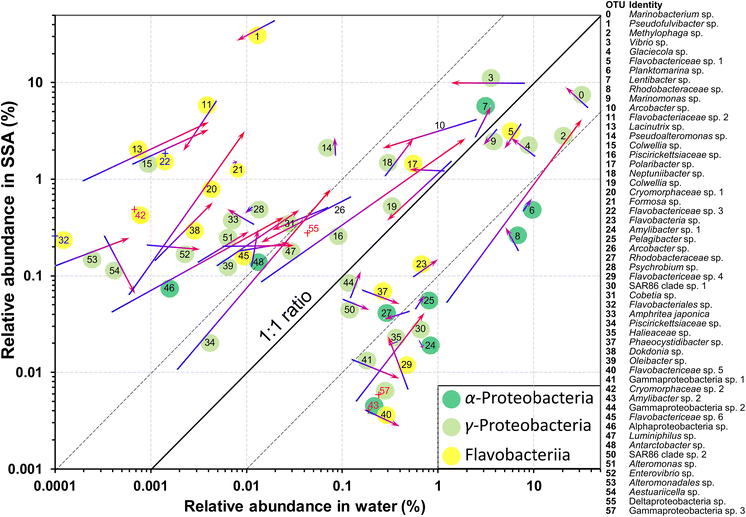
The identities and relative abundances of the more common OTUs (average relative abundances >0.1%, or >0.2% in a single sample) in the water and SSA are given in Fig. 4 and Table S2.† Three phylogenetic groups, which accounted for 51/54 of the most abundant OTUs in the water (Fig. 4), are found to consistently dominate bloom-associated bacterial communities.83–87 They are the Rhodobacteraceae within the Alphaproteobacteria, the Gammaproteobacteria, such as members of the Alteromonadaceae, and the Flavobacteriia class within the Bacteroidetes. Several of the most abundant genera (e.g., Pseudofulvibacter, Methylophaga, Vibrio, Glaciecola) have also been recorded as predominating in natural successions in hydrocarbon/biomass-enriched or bloom-associated environments88 and mesocosm experiments.52,85,89,90
Several OTUs were particularly notable. A Marinobacterium sp. (OTU 0) was very abundant in the water, while OTU 2, a Methylophaga sp., increased greatly in relative abundance in the water between days 2 and 3 while maintaining a relatively low emission efficiency (Fig. 4). In contrast to the Marinobacterium and Methylophaga spp., a species of Pseudofulvibacter (OTU 1) was extraordinarily enriched in the aerosol while comprising only 0.01% of seawater sequences (Fig. 4). Explanations for the dominance of these species and why the Flavobacteriia predominated among OTUs enriched in the SSA (Fig. 4) are given with Table S2.†
Several OTUs increased greatly in their relative abundances in both the water and SSA over the 24 h period (Fig. 4). Most marked were the Methylophaga sp. (OTU 2), two Piscirickettsiaceae spp. (OTUs 16 and 34), an Alphaproteobacteria sp. (OTU 46) and a Cryomorphaceae sp. (OTU 20). In general, however, the ratios of the relative abundances in the SSA and in the water remained fairly constant.
None of the genera or families that showed marked increases have been associated with ice nucleation in previous studies. Of the known ice nucleation active bacteria (the genera Pseudomonas, Xanthomonas, Pantoea and Lysinibacillus) only one potential species, a Pseudomonad (OTU 88), was recorded. However, it was barely detectable, with only 3 reads among a total of >2.5 million in the two water samples.
3.3 Pure nutrient addition experiment
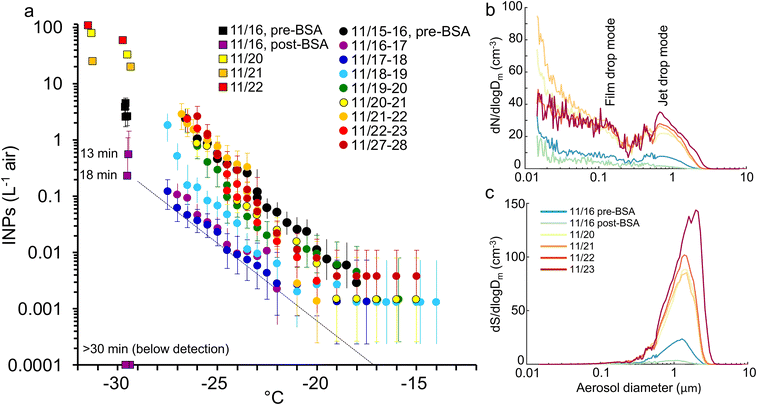
We also tested whether adding a simple DOC mix could initiate the production of INPs, mimicking the surge of DOC expected during the death phase of a phytoplankton bloom. This experiment is treated separately from the other incubations because it used an unrealistically simple and easily metabolized cocktail and higher DOC concentration (∼2× than is typical in seawater), and so will have stimulated the growth of an atypical and opportunistic subset of heterotrophic bacteria. The labile DOC amendment was composed of one protein, bovine serum albumin (BSA, 1 mg L−1), plus three sugars (glucose, galactose and mannose each at 0.33 mg L−1) that are common in both marine colloids and bulk seawater.75 The response of the decomposer community was similar to the previous incubations (Fig. S6†) except that a collapse in heterotrophic bacteria, typically coincident with a surge in nanoflagellates, was not seen. INE numbers remained unchanged apart from a modest increase at lower temperatures. The INEs were predominantly organic, heat resistant and <0.2 μm.In contrast to their stability in the water, INP emissions in the SSA decreased dramatically immediately upon nutrient addition (Fig. 5). The suppression was caused by the formation of a monolayer, ∼4 nm deep, of protein adsorbed at the air–water interface.94,95 Two hours after addition, a single layer of bubbles had accumulated around the edge of the tank covering ∼10–15% of the surface. This decreased to ∼5% a day later and disappeared after three days, presumably due to the consumption of the BSA by the heterotrophic community (Fig. S6†), by which time the aerosol and INP emissions were rebounding (Fig. 5).
The BSA monolayer will most likely have inhibited INP emissions in two ways. Firstly, it will have displaced the pre-existing SML and the INEs enriched within it. Secondly, it changed bubble bursting behaviour in a way that dampened emissions in general. Bubble bursting produces both film drops, from the disintegration of the thin bubble film, and jet drops, from the breakup of the water jet formed by the collapse of the bubble cavity. Larger bubbles with a radius ≥1 mm generate mainly film drops when the cap disintegrates. Film drops produce SSA with a broad range of sizes and a dry diameter mode at approximately 0.1 μm,43 as indicated in Fig. 5b. By contrast, bubbles <0.5 mm radius produce only jet drops,96,97 which are larger than film drops with a dry SSA mode at ∼0.7 μm (Fig. 5b). Since the diameter of a dry SSA particle is ∼¼ of the diameter of its parent droplet,98 this equates to a fresh jet drop mode diameter of ∼3 μm. Further, the radius of the original bubble is ∼20× the diameter of the dry SSA particle produced by the top jet drop,43,96,97,99 which translates to a bubble radius of ∼14 μm. The mode is comparable with the maximal production of bubbles of 20–40 μm radius generated in the miniMART with fresh seawater.45
Addition of BSA reduced film drop emissions, but especially diminished jet drop emissions (Fig. 5b and c). Modini et al. (2013)100 observed a similar quenching (79–98%) of aerosol production following the addition of a surfactant, Triton X-100, and ascribed several mechanisms to the reduction: the surfactant increased bubble longevity allowing films to drain and thin out before breaking, while the reduced surface tension caused a less explosive contraction of the film upon rupture. Both actions reduced the production of SSA upon film disintegration. Due to the reduction in surface tension, surfactants also reduce the jet height, speed and width, and, hence, the number of jet drops released.101 The formation of stable islands of bubbles will also have reduced jet drop production.
Jet drops accounted for the bulk of the surface area and volume of emissions before BSA addition. They may also be enriched in INEs due to organic particles adsorbing to the outside of bubbles combined with boundary layer flow concentrating these organics at the bubble bases through which the jet drops erupt.43,102,103 Hence, a strong quenching of jet drop production by BSA likely caused the collapse in INP emissions.43 Finally, it is possible that the BSA bound directly to the INEs, forming flocs too large to be ejected.
3.4 Controls over INP emissions
The previous experiments have shown that changes in INP emissions over the incubations may be distilled into the following equation:| ΔINPSSA = ΔINEwater × ΔINESML × ΔSSA |
We can use the P. marinus addition experiment to attempt to apportion these controls over production and release of INPs in nascent sea spray. Upon addition of dead P. marinus detritus, aerosol emissions initially decreased modestly (Fig. 6a), but then over the following days rebounded, reaching a peak 4 days later. The initial suppression followed by the progressive increase in across-the-board particle emissions suggests that an SML with surfactant properties initially formed from the release of material in the added detritus, inhibiting film and especially jet drop emissions (Fig. 6b) just as occurred with BSA. Presumably, this SML was then consumed by the heterotrophic bacteria or progressively removed by continuous SSA production. Interestingly, Christiansen et al. (2019)105 also observed a reduction in SSA number concentration with increasing additions of previously frozen S. marinoi cells to a tank using plunging jets to generate SSA.
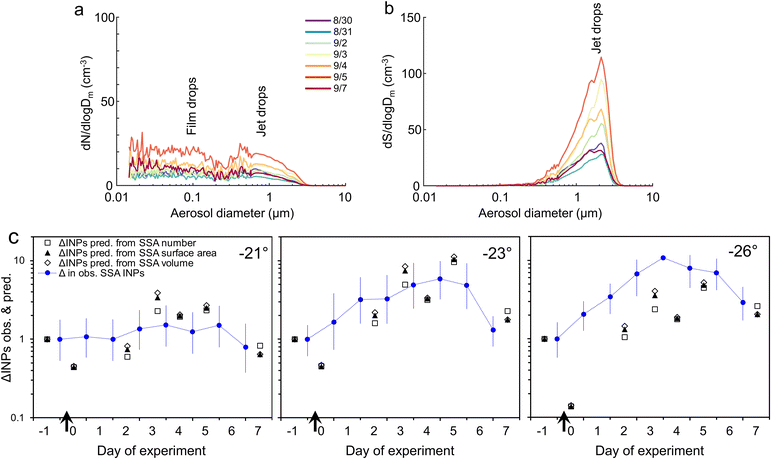
If we assume (for the sake of this exercise) that level of INE enrichment in the SML was constant, we can use the relative changes (value on Day x/value at the start) in the other two terms to predict INPs in the aerosol (Fig. 6c). For SSA INPs active at −21 and −23 °C there is good correspondence between observed and predicted values. However, at −26 °C the observed SSA INP concentration is generally, although improving later in the incubation, several times higher than predicted, presumably due to higher enrichment of INEs active at this temperature in the SML. (No consistent improvement is seen when SSA volume is used in place of surface area to predict INPs, as suggested by Mitts et al. (2021).40) Bacteria are typically enriched >10-fold in the SML,91,92 partly due their association with gels.91 Amino acids and protein-like substances also accumulate in the SML.78,106 Up to ∼10-fold enrichment of INEs in the SML is commonly observed in mesocosm experiments,25,107 as well as in field samples.22,24,30,35,108,109
Data from the P. marinus addition experiment can also be used to estimate the enrichment of INPs in the SSA relative to the bulk water. INPs per volume of freshly-emitted SSA were derived from the integrated volume of dry SSA. Dry SSA diameters were scaled up to obtain total droplet volume by assuming 4× the dry SSA particle diameter represented the diameter of the fresh drop.98 INPs in the SSA were thus converted to INPs per mL of fresh sea spray, and this was divided by the INEs per mL of bulk water to obtain enrichment factors (EFBulk→SSA) (Fig. 7). At the start, before inoculum addition, the EFBulk→SSA values at all INP temperatures were 160–280. After inoculum addition, the EFBulk→SSA increased several-fold and tended to be higher for colder INPs.
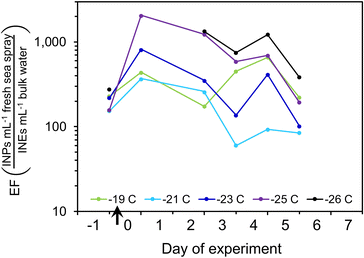 | ||
| Fig. 7 Enrichment factors for INPs active at different temperatures over the course of the P. marinus addition experiment. | ||
These estimated enrichment factors correspond to values found in previous studies of SSA organics, which often range from ∼100- to >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold,91,104,110–112 although they can be higher for lipids, proteins and carbohydrates.93,113,114 Organic matter in submicron particles is almost entirely water insoluble and was found to consist of colloids and aggregates exuded by phytoplankton,115 whereas Bio (i.e., protein and phosphate-containing) particles were dominant at sizes >1 μm.68,116
000-fold,91,104,110–112 although they can be higher for lipids, proteins and carbohydrates.93,113,114 Organic matter in submicron particles is almost entirely water insoluble and was found to consist of colloids and aggregates exuded by phytoplankton,115 whereas Bio (i.e., protein and phosphate-containing) particles were dominant at sizes >1 μm.68,116
4 Conclusions
When phytoplankton blooms collapse, the ensuing decomposer community of bacteria, and the viruses and grazers that prey upon them, comminute, consume and transform the biomass. The constituents and/or by-products of this community consistently possess ice nucleation activity.3,25,39,40 To test if INE production depends upon phytoplankton type we added dead POC biomass of a green alga (N. atomus), a diatom (S. marinoi) and a cyanobacterium (P. marinus) to a miniMART filled with fresh seawater. Over the next two days, heterotrophic bacteria concentrations increased then plateaued, and after several more days decreased, coincident with an increase in HNF and, in two cases, viruses. Enzyme activities typically increased over the first few days, while HULIS steadily accumulated.INEs in the water initially decreased or remained stable, but after 1–4 days they rebounded, increasing by up to 18-, 14- and 7-fold in the N. atomus, S. marinoi, and P. marinus incubations, respectively. Newly fabricated INEs were organic, mostly not heat labile, and varied in size (all <0.2, a mix of sizes, or all >0.2 μm). INE concentration active at −23 °C was closely correlated with HNF, and also with viruses and the concentration of HULIS, but not with heterotrophic bacteria concentrations. Sources of INEs may thus include:
• Components of the new populations of HNF and/or viruses, and/or debris of bacteria consumed by HNF and viruses.
• Newly-formed HULIS.
• Progressive fragmentation of INEs or release of IN-monolayers composed of fatty alcohols/acids and possible crystallites of these117,118 from the consumed bacteria or phytoplankton.
Changes in emissions of INPs in the SSA generally paralleled those in the water, but tended to peak a day or so earlier. The most pronounced increases occurred with the diatom S. marinoi (up to 35-fold higher) and cyanobacterium P. marinus (up to 15-fold higher) incubations. Several mechanisms could explain the early peak in INP emissions: (1) initially-formed INEs had characteristics that led to their greater enrichment in the SML than those produced later, (2) progressive enmeshment of INEs within larger gels reduced their emissions after a few days, (3) changes in the SML may have altered INE emissions by altering enrichment and/or changing surface tension and bubble longevity. We should note that in the ocean algae are often nutrient depleted and the bacterial degradation of algal detritus may create a different molecular fingerprint, affecting INE emissions differently than observed here. Hence, it would be premature to extrapolate from this and similar studies to estimate INE production in situ.
Sequencing of bacteria in the N. atomus addition experiment showed three groups, known to dominate bloom-associated bacterial communities, comprising ∼95% of the abundant OTUs at the peak of INP production. Several OTUs greatly increased their relative abundances in both the water and SSA over the peak period of INPs emissions, although none are currently associated with known groups of ice nucleation-active bacteria.
To test if INE production is simply the result of an injection of nutrients, we added a simple DOC cocktail (BSA + three monosaccharides) to a fourth incubation. INE concentrations in the water remained essentially unchanged. Unexpectedly, INP emissions in the SSA abruptly fell due to the formation of a BSA monolayer, which will have displaced the pre-existing SML and INEs enriched within it, and altered bubble bursting behaviour, in particular, by reducing jet drop emissions. Since jet drops accounted for the bulk of the surface area and volume of the SSA, their quenching effectively shut down INP emissions. This serendipitous result revealed the strong control of the SML over INP emissions, directly via INP enrichment and indirectly via bubble bursting.
Overall, changes in INP emissions are the complex product of (1) changes of INPs in the water, (2) INE enrichment in the SML, and (3) SSA emissions, due to altered SML composition modifying jet drop production. From the P. marinus incubation we estimate that INP enrichment in the SSA was around 160–280 for fresh seawater, and likely increases during the decay phase of the phytoplankton bloom. We believe this is the first estimate of EFBulk→SSA for marine INPs.
Collectively, these experiments revealed that the production of INEs following the collapse of phytoplankton blooms requires a heterogeneous substrate to initiate a complex natural succession of decomposers. INP emissions are enhanced by INE enrichment in the SML and enhanced or reduced by the SML's influence over jet drop production.
If phytoplankton blooms consistently generate and emit INPs, their atmospheric contribution would be most pronounced in remote regions of the SO.2 Chl a concentrations range widely (annual mean >0.1 to >2 mg m−3) across the SO,119 with a timing, scale, and biomass varying latitudinally and regionally. Latitudinally, blooms are driven by sunlight: in temperate “bioregions”, phytoplankton blooms occur in October, whereas near Antarctica they peak in January/February.119 In regions where currents interact with continental shelves, islands and hydrothermal vents, large blooms develop every spring and summer, fertilized by upwelling of nutrients, especially iron and silicate.120,121 Some marine and phytoplankton bloom-induced INEs/INPs can possess activity at the exceptionally warm temperatures required for secondary ice multiplication,22,36,39,108 a process that can generate ice particle concentrations orders of magnitude higher than the numbers of INPs present.
Author contributions
FM, TH, CM, GS, PD and KP designed the study. GC, CL and MS collected and shipped the seawater samples. TH and AR performed the INP measurements. RP automated the Ice Spectrometer. CM, GS, EL and KS operated the CFDC, while GS and KM processed the CFDC results. TH prepared filters and took the INP samples, and also performed the DNA extractions. FM prepared the phytoplankton biomass, and performed the collection and analysis of microbial counts and enzyme activities. MS provided the EEM analyses. PDN provided the Skeletonema culture. KM, GS and CM analysed the aerosol sizing data and merged the distributions. TH, FM, KM, CM, MS, PD, RP and MC contributed significantly to the analyses and presentation of figures. All authors contributed to the writing and editing of the article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by NSF through the NSF Center for Aerosol Impacts on Chemistry of the Environment (NSF-CAICE), CHE-1801971. Sequencing was performed by the Joint Genome Institute (U.S. Department of Energy) under JGI minigrant proposal 2792 managed/coordinated by Susannah Tringe and Christa Pennacchio. We thank Dr Alfred Beran for providing inoculum of the algal culture Skeletonema marinoi (CoSMi: http://cosmi.inogs.it/), and Wil Biddle and Dr J. Nick Fisk for the use of their fluorometer.References
- J. Uetake, T. C. J. Hill, K. A. Moore, P. J. DeMott, A. Protat and S. M. Kreidenweis, Airborne bacteria confirm the pristine nature of the Southern Ocean boundary layer, Proc. Natl. Acad. Sci. U.S.A., 2020, 117, 13275–13282, DOI:10.1073/pnas.2000134117.
- S. M. Burrows, C. Hoose, U. Pöschl and M. G. Lawrence, Ice nuclei in marine air: Biogenic particles or dust?, Atmos. Chem. Phys., 2013, 13, 245–267, DOI:10.5194/acp-13-245-2013.
- P. J. DeMott, T. C. J. Hill, C. S. McCluskey, K. A. Prather, D. B. Collins, R. C. Sullivan, M. J. Ruppel, R. H. Mason, V. E. Irish, T. Lee, C. Y. Hwang, T. S. Rhee, J. R. Snider, G. R. McMeeking, S. Dhaniyala, E. R. Lewis, J. J. B. Wentzell, J. Abbatt, C. Lee, C. M. Sultana, A. P. Ault, J. L. Axson, M. Diaz Martinez, I. Venero, G. Santos-Figueroa, M. D. Stokes, G. B. Deane, O. L. Mayol-Bracero, V. H. Grassian, T. H. Bertram, A. K. Bertram, B. F. Moffett and G. D. Franc, Sea spray aerosol as a unique source of ice nucleating particles, Proc. Natl. Acad. Sci. U.S.A., 2016, 113, 5797–5803, DOI:10.1073/pnas.1514034112.
- G. M. McFarquhar, C. S. Bretherton, R. Marchand, A. Protat, P. J. DeMott, S. P. Alexander, G. C. Roberts, C. H. Twohy, D. Toohey, S. Siems, Y. Huang, R. Wood, R. M. Rauber, S. Lasher-Trapp, J. Jensen, J. L. Stith, J. Mace, J. Um, E. Järvinen, M. Schnaiter, A. Gettelman, K. J. Sanchez, C. S. McCluskey, L. M. Russell, I. L. McCoy, R. L. Atlas, C. G. Bardeen, K. A. Moore, T. C. J. Hill, R. S. Humphries, M. D. Keywood, Z. Ristovski, L. Cravigan, R. Schofield, C. Fairall, M. D. Mallet, S. M. Kreidenweis, B. Rainwater, J. D'Alessandro, Y. Wang, W. Wu, G. Saliba, E. J. T. Levin, S. Ding, F. Lang, S. C. H. Truong, C. Wolff, J. Haggerty, M. J. Harvey, A. R. Klekociuk and A. McDonald, Observations of clouds, aerosols, precipitation, and surface radiation over the Southern Ocean: An overview of Capricorn, Marcus, Micre, and Socrates, Bull. Am. Meteorol. Soc., 2021, 102, E894–E928, DOI:10.1175/BAMS-D-20-0132.1.
- Y.-T. Hwang and D. M. W. Frierson, A link between the double-Intertropical Convergence Zone problem and cloud biases over the Southern Ocean, Proc. Natl. Acad. Sci. U.S.A., 2013, 110, 4935–4940, DOI:10.1073/pnas.1213302110.
- J. E. Kay, C. Wall, V. Yettella, B. Medeiros, C. Hannay, P. Caldwell and C. Bitz, Global climate impacts of fixing the Southern Ocean shortwave radiation bias in the Community Earth System Model (CESM), J. Clim., 2016, 29, 4617–4636, DOI:10.1175/JCLI-D-15-0358.1.
- D. T. McCoy, D. L. Hartmann, M. D. Zelinka, P. Ceppi and D. P. Grosvenor, Mixed-phase cloud physics and Southern Ocean cloud feedback in climate models, J. Geophys. Res.: Atmos., 2015, 120, 9539–9554, DOI:10.1002/2015JD023603.
- I. Tan, T. Storelvmo and M. D. Zelinka, Observational constraints on mixed-phase clouds imply higher climate sensitivity, Science, 2016, 352(6282), 224–227, DOI:10.1126/science.aad5300.
- A. Bodas-Salcedo, P. G. Hill, K. Furtado, K. D. Williams, P. R. Field, J. C. Manners, P. Hyder and S. Kato, Large contribution of supercooled liquid clouds to the solar radiation budget of the Southern Ocean, J. Clim., 2016, 29, 4213–4228, DOI:10.1175/JCLI-D-15-0564.1.
- J. Vergara-Temprado, A. K. Miltenberger, K. Furtado, D. P. Grosvenor, B. J. Shipway, A. A. Hill, J. M. Wilkinson, P. R. Field, B. J. Murray and K. S. Carslaw, Strong control of Southern Ocean cloud reflectivity by ice-nucleating particles, Proc. Natl. Acad. Sci. U.S.A., 2018, 115, 2687–2692, DOI:10.1073/pnas.1721627115.
- É. Vignon, S. P. Alexander, P. J. DeMott, G. Sotiropoulou, F. Gerber, T. C. J. Hill, R. Marchand, A. Nenes and A. Berne, Challenging and improving the simulation of mid-level mixed-phase clouds over the high-latitude Southern Ocean, J. Geophys. Res.: Atmos., 2021, 126, e2020JD033490, DOI:10.1029/2020JD033490.
- K. E. Trenberth and J. T. Fasullo, Simulation of present-day and twenty-first-century energy budgets of the southern oceans, J. Climatol., 2010, 23, 440–454, DOI:10.1175/2009JCLI3152.1.
- T. J. Zaremba, R. M. Rauber, G. M. McFarquhar, M. Hayman, J. A. Finlon and D. M. Stechman, Phase characterization of cold sector Southern Ocean cloud tops: results from Socrates, J. Geophys. Res.: Atmos., 2020, 125, e2020JD033673, DOI:10.1029/2020JD033673.
- S. Kremser, M. Harvey, P. Kuma, S. Hartery, A. Saint-Macary, J. McGregor, A. Schuddeboom, M. von Hobe, S. T. Lennartz, A. Geddes, R. Querel, A. McDonald, M. Peltola, K. Sellegri, I. Silber, C. S. Law, C. J. Flynn, A. Marriner, T. C. J. Hill, P. J. DeMott, C. C. Hume, G. Plank, G. Graham and S. Parsons, Southern Ocean Cloud and Aerosol data: a compilation of measurements from the 2018 Southern Ocean Ross Sea Marine Ecosystems and Environment voyage, Earth Syst. Sci. Data, 2021, 13, 3115–3153, DOI:10.5194/essd-13-3115-2021.
- C. S. McCluskey, J. Ovadnevaite, M. Rinaldi, J. Atkinson, F. Belosi, D. Ceburnis, M. Salvatore, T. C. J. Hill, U. Lohmann, Z. A. Kanji, C. O'Dowd, S. M. Kreidenweis and P. J. DeMott, Marine and terrestrial organic ice-nucleating particles in pristine marine to continentally influenced Northeast Atlantic air masses, J. Geophys. Res.: Atmos., 2018, 123, 6196–6212, DOI:10.1029/2017JD028033.
- A. Welti, E. K. Bigg, P. J. DeMott, X. Gong, M. Hartmann, M. Harvey, S. Henning, P. Herenz, T. C. J. Hill, B. Hornblow, C. Leck, M. Löffler, C. S. McCluskey, A. M. Rauker, J. Schmale, C. Tatzelt, M. van Pinxteren and F. Stratmann, Ship-based measurements of ice nuclei concentrations over the Arctic, Atlantic, Pacific and Southern oceans, Atmos. Chem. Phys., 2020, 20, 15191–15206, DOI:10.5194/acp-20-15191-2020.
- M. D. Zelinka, T. A. Myers, D. T. McCoy, S. Po-Chedley, P. M. Caldwell, P. Ceppi, S. A. Klein and K. E. Taylor, Causes of higher climate sensitivity in CMIP6 models, Geophys. Res. Lett., 2020, 47, e2019GL085782, DOI:10.1029/2019GL085782.
- A. Gettelman, C. G. Bardeen, C. S. McCluskey, E. Järvinen, J. Stith, C. Bretherton, G. McFarquhar, C. Twohy, J. D'Alessandro and W. Wu, Simulating observations of Southern Ocean clouds and implications for climate, J. Geophys. Res.: Atmos., 2020, 125, e2020JD032619, DOI:10.1029/2020JD032619.
- W. T. K. Huang, L. Ickes, I. Tegen, M. Rinaldi, D. Ceburnis and U. Lohmann, Global relevance of marine organic aerosol as ice nucleating particles, Atmos. Chem. Phys., 2018, 18, 11423–11445, DOI:10.5194/acp-18-11423-2018.
- X. Zhao, X. Liu, S. M. Burrows and Y. Shi, Effects of marine organic aerosols as sources of immersion-mode ice-nucleating particles on high-latitude mixed-phase clouds, Atmos. Chem. Phys., 2021, 21, 2305–2327, DOI:10.5194/acp-21-2305-2021.
- T. Raatikainen, M. Prank, J. Ahola, H. Kokkola, J. Tonttila and S. Romakkaniemi, The effect of marine ice-nucleating particles on mixed-phase clouds, Atmos. Chem. Phys., 2022, 22, 3763–3778, DOI:10.5194/acp-22-3763-2022.
- M. Hartmann, X. Gong, S. Kecorius, M. van Pinxteren, T. Vogl, A. Welti, H. Wex, S. Zeppenfeld, H. Herrmann, A. Wiedensohler and F. Stratmann, Terrestrial or marine–indications towards the origin of ice-nucleating particles during melt season in the European Arctic up to 83.7°N, Atmos. Chem. Phys., 2021, 21, 1613–11636, DOI:10.5194/acp-21-11613-2021.
- V. E. Irish, P. Elizondo, J. Chen, C. Chou, J. Charette, M. Lizotte, L. A. Ladino, T. W. Wilson, M. Gosselin, B. J. Murray and E. Polishchuk, Ice-nucleating particles in Canadian Arctic sea-surface microlayer and bulk seawater, Atmos. Chem. Phys., 2017, 17, 10583–10595, DOI:10.5194/acp-17-10583-2017.
- V. E. Irish, S. J. Hanna, X. Yu, M. Boyer, E. Polishchuk, M. Ahmed, J. Chen, J. P. Abbatt, M. Gosselin, R. Chang, L. A. Miller and A. K. Bertram, Revisiting properties and concentrations of ice-nucleating particles in the sea surface microlayer and bulk seawater in the Canadian Arctic during summer, Atmos. Chem. Phys., 2019, 19, 7775–7787, DOI:10.5194/acp-19-7775-2019.
- C. S. McCluskey, T. C. J. Hill, C. M. Sultana, O. Laskina, J. Trueblood, M. V. Santander, C. M. Beall, J. M. Michaud, S. M. Kreidenweis, K. A. Prather, V. H. Grassian and P. J. DeMott, A mesocosm double feature: Insights into the chemical make-up of marine ice nucleating particles, J. Atmos. Sci., 2018, 75, 2405–2423, DOI:10.1175/JAS-D-17-0155.1.
- B. F. Moffett, Fresh water ice nuclei, Fundam. Appl. Limnol., 2016, 188, 19–23, DOI:10.1127/fal/2016/0851.
- J. Rosinski, P. L. Haagenson, C. T. Nagamoto, B. Quintana, F. Parungo and S. D. Hoyt, Ice-forming nuclei in air masses over the Gulf of Mexico, J. Aerosol Sci., 1988, 19, 539–551, DOI:10.1016/0021-8502(88)90206-6.
- R. Schnell, Ice nuclei in seawater, fog water and marine air off the Coast of Nova Scotia: summer 1975, J. Atmos. Sci., 1977, 34, 1299–1305, DOI:10.1175/1520-0469(1977)034%3C1299:INISFW%3E2.0.CO;2.
- R. C. Schnell and G. Vali, Freezing nuclei in marine waters, Tellus, 1975, 27, 321–323, DOI:10.1111/j.2153-3490.1975.tb01682.x.
- T. W. Wilson, L. A. Ladino, P. A. Alpert, M. N. Breckels, I. M. Brooks, J. Browse, S. M. Burrows, K. S. Carslaw, J. A. Huffman, C. Judd, W. P. Kilthau, R. H. Mason, G. McFiggans, L. A. Miller, J. J. Nájera, E. Polishchuk, S. Rae, C. L. Schiller, M. Si, J. V. Temprado, T. F. Whale, J. P. S. Wong, O. Wurl, J. D. Yakobi-Hancock, J. P. D. Abbatt, J. Y. Aller, A. K. Bertram, D. A. Knopf and B. J. Murray, A marine biogenic source of atmospheric ice-nucleating particles, Nature, 2015, 525, 234–238, DOI:10.1038/nature14986.
- T. C. J. Hill, P. J. DeMott, Y. Tobo, J. Fröhlich-Nowoisky, B. F. Moffett, G. D. Franc and S. M. Kreidenweis, Sources of organic ice nucleating particles in soils, Atmos. Chem. Phys., 2016, 16, 7195–7721, DOI:10.5194/acp-16-7195-2016.
- Z. A. Kanji, L. A. Ladino, H. Wex, Y. Boose, M. Burkert-Kohn, D. J. Cziczo and M. Krämer, Overview of ice nucleating particles, Meteorol. Monogr., 2017, 58, 1.1–1.33, DOI:10.1175/AMSMONOGRAPHS-D-16-0006.1.
- E. K. Bigg, Ice nucleus measurements in remote areas, J. Atmos. Sci., 1973, 30, 1153–1157 CrossRef.
- C. S. McCluskey, T. C. J. Hill, R. S. Humphries, A. M. Rauker, S. Moreau, P. G. Strutton, S. D. Chambers, A. G. Williams, I. McRobert, J. Ward, M. D. Keywood, J. Harnwell, W. Ponsonby, Z. M. Loh, P. B. Krummel, A. Protat, S. M. Kreidenweis and P. J. DeMott, Observations of ice nucleating particles over Southern Ocean waters, Geophys. Res. Lett., 2018, 45, 11989–11997, DOI:10.1029/2018GL079981.
- J. V. Trueblood, A. Nicosia, A. Engel, B. Zäncker, M. Rinaldi, E. Freney, M. Thyssen, I. Obernosterer, J. Dinasquet, F. Belosi and A. Tovar-Sánchez, A two-component parameterization of marine ice-nucleating particles based on seawater biology and sea spray aerosol measurements in the Mediterranean Sea, Atmos. Chem. Phys., 2021, 21, 4659–4676, DOI:10.5194/acp-21-4659-2021.
- R. C. Schnell and G. Vali, Biogenic ice nuclei: part I. Terrestrial and marine sources, J. Atmos. Sci., 1976, 33, 1554–1564, DOI:10.1175/1520-0469(1976)033%3C1554:BINPIT%3E2.0.CO;2.
- J. Rosinski, P. L. Haagenson, C. T. Nagamoto and F. Parungo, Ice-forming nuclei of maritime origin, J. Aerosol Sci., 1986, 17, 23–46, DOI:10.1016/0021-8502(86)90004-2.
- J. M. Creamean, J. N. Cross, R. Pickart, L. McRaven, P. Lin, A. Pacini, R. Hanlon, D. G. Schmale, J. Ceniceros, T. Aydell, N. Colombi, E. Bolger and P. J. DeMott, Ice nucleating particles carried from below a phytoplankton bloom to the Arctic atmosphere, Geophys. Res. Lett., 2019, 46, 8572–8581, DOI:10.1029/2019GL083039.
- C. S. McCluskey, T. C. J. Hill, F. Malfatti, C. M. Sultana, C. Lee, M. V. Santander, C. M. Beall, K. A. Moore, G. C. Cornwell, D. B. Collins, K. A. Prather, T. Jayarathne, E. A. Stone, F. Azam, S. M. Kreidenweis and P. J. DeMott, A dynamic link between ice nucleating particles released in nascent sea spray aerosol and oceanic biological activity during two mesocosm experiments, J. Atmos. Sci., 2017, 74, 151–166, DOI:10.1175/JAS-D-16-0087.1.
- B. A. Mitts, X. Wang, D. D. Lucero, C. M. Beall, G. B. Deane, P. J. DeMott and K. A. Prather, Importance of supermicron ice nucleating particles in nascent sea spray, Geophys. Res. Lett., 2021, 48, e2020GL089633, DOI:10.1029/2020GL089633.
- X. Wang, C. M. Sultana, J. Trueblood, T. C. J. Hill, F. Malfatti, C. Lee, O. Laskina, K. A. Moore, C. M. Beall, C. S. McCluskey, G. C. Cornwell, Y. Zhou, J. L. Cox, M. A. Pendergraft, M. V. Santander, T. H. Bertram, C. D. Cappa, F. Azam, P. J. DeMott, V. H. Grassian and K. A. Prather, Microbial control of sea spray aerosol composition: A tale of two blooms, ACS Cent. Sci., 2015, 1, 124–131, DOI:10.1021/acscentsci.5b00148.
- L. Ickes, G. C. Porter, R. Wagner, M. P. Adams, S. Bierbauer, A. K. Bertram, M. Bilde, S. Christiansen, A. M. Ekman, E. Gorokhova, K. Höhler, A. A. Kiselev, C. Leck, O. Möhler, B. J. Murray, T. Schiebel, R. Ullrich and M. E. Salter, The ice-nucleating activity of Arctic sea surface microlayer samples and marine algal cultures, Atmos. Chem. Phys., 2020, 20, 11089–11117, DOI:10.5194/acp-20-11089-2020.
- X. Wang, G. B. Deane, K. A. Moore, O. S. Ryder, M. D. Stokes, C. M. Beall, D. B. Collins, M. V. Santander, S. M. Burrows, C. M. Sultana and K. A. Prather, The role of jet and film drops in controlling the mixing state of submicron sea spray aerosol particles, Proc. Natl. Acad. Sci. U.S.A., 2017, 114, 6978–6983, DOI:10.1073/pnas.1702420114.
- G. C. Cornwell, C. M. Sultana, M. Prank, R. E. Cochran, T. C. Hill, G. P. Schill, P. J. DeMott, N. Mahowald and K. A. Prather, Ejection of dust from the ocean as a potential source of marine ice nucleating particles, J. Geophys. Res.: Atmos., 2020, e2020JD033073, DOI:10.1029/2020JD033073.
- M. D. Stokes, G. Deane, D. B. Collins, C. Cappa, T. Bertram, A. Dommer, S. Schill, S. Forestieri and M. Survilo, A miniature Marine Aerosol Reference Tank (miniMART) as a compact breaking wave analogue, Atmos. Meas. Tech., 2016, 9, 4257–4267, DOI:10.5194/amt-9-4257-2016.
- J. W. Ammerman, J. A. Fuhrman, A. Hagström and F. Azam, Bacterioplankton growth in seawater: I. Growth kinetics and cellular characteristics in seawater cultures, Mar. Ecol. Prog. Ser., 1984, 18, 31–39, DOI:10.3354/meps018031.
- K. D. Bidle and F. Azam, Accelerated dissolution of diatom silica by marine bacterial assemblages, Nature, 1999, 397(6719), 508–512, DOI:10.1038/17351.
- O. Holm-Hansen and B. Riemann, Chlorophyll a determination: improvements in methodology, Oikos, 1978, 30, 438–447, DOI:10.2307/3543338.
- L. A. Ladino, J. D. Yakobi-Hancock, W. P. Kilthau, R. H. Mason, M. Si, J. Li, L. A. Miller, C. L. Schiller, J. A. Huffman, J. Y. Aller, D. A. Knopf, A. K. Bertram and J. P. D. Abbatt, Addressing the ice nucleating abilities of marine aerosol: A combination of deposition mode laboratory and field measurements, Atmos. Environ., 2016, 132, 1–10, DOI:10.1016/j.atmosenv.2016.02.028.
- P. A. Alpert, J. Y. Aller and D. A. Knopf, Initiation of the ice phase by marine biogenic surfaces in supersaturated gas and supercooled aqueous phases, Phys. Chem. Chem. Phys., 2011, 13, 19882–19894, 10.1039/C1CP21844A.
- Y. Xi, A. Mercier, C. Kuang, J. Yun, A. Christy, L. Melo, M. T. Maldonado, J. A. Raymond and A. K. Bertram, Concentrations and properties of ice nucleating substances in exudates from Antarctic sea-ice diatoms, Environ. Sci.: Processes Impacts, 2021, 23, 323–334, 10.1039/d0em00398k.
- J. M. Michaud, L. R. Thompson, D. Kaul, J. L. Espinoza, R. A. Richter, Z. Z. Xu, C. Lee, K. M. Pham, C. M. Beall, F. Malfatti, F. Azam, R. Knight, M. D. Burkart, C. L. Dupont and K. A. Prather, Taxon-specific aerosolization of bacteria and viruses in an experimental ocean-atmosphere mesocosm, Nat. Commun., 2018, 9(1), 1–10, DOI:10.1038/s41467-018-04409-z.
- R. Benner, J. D. Pakulski, M. McCarthy, J. I. Hedges and P. G. Hatcher, Bulk chemical characteristics of dissolved organic matter in the ocean, Science, 1992, 255, 1561–1564, DOI:10.1126/science.255.5051.1561.
- R. R. L. Guillard, Culture of phytoplankton for feeding marine invertebrates, in Culture of Marine Invertebrate Animals, ed. W. L., Smith and M. H., Chanley, Springer, Boston, MA, 1975, pp. 29–60, DOI:10.1007/978-1-4615-8714-9.
- L. R. Moore, A. F. Post, G. Rocap and S. W. Chisholm, Utilization of different nitrogen sources by the marine cyanobacteria Prochlorococcus and Synechococcus, Limnol. Oceanogr., 2002, 47, 989–996, DOI:10.4319/lo.2002.47.4.0989.
- U. Christaki, C. Courties, R. Massana, P. Catala, P. Lebaron, J. M. Gasol and M. V. Zubkov, Optimized routine flow cytometric enumeration of heterotrophic flagellates using SYBR Green I, Limnol. Oceanogr. Methods, 2011, 9, 329–339, DOI:10.4319/lom.2011.9.329.
- J. M. Gasol and P. A. Del Giorgio, Using flow cytometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities, Sci. Mar., 2000, 64, 197–224, DOI:10.3989/scimar.2000.64n2197.
- D. Marie, F. Partensky, S. Jacquet and D. Vaulot, Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I, Appl. Environ. Microbiol., 1997, 63, 186–193, DOI:10.1128/aem.63.1.186-193.1997.
- R. T. Noble and J. A. Fuhrman, Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria, Aquat. Microb. Ecol., 1998, 14, 113–118, DOI:10.3354/ame014113.
- H. G. Hoppe, Significance of exoenzymatic activities in the ecology of brackish water: measurements by means of methylumbelliferyl-substrates, Mar. Ecol. Prog. Ser., 1983, 11, 299–311, DOI:10.3354/meps011299.
- F. Malfatti, C. Lee, T. Tinta, M. A. Pendergraft, M. Celussi, Y. Zhou, C. M. Sultana, A. Rotter, J. L. Axson, D. B. Collins, M. V. Santander, A. L. Anides Morales, L. I. Aluwihare, N. Riemer, V. H. Grassian, F. Azam and K. A. Prather, Detection of active microbial enzymes in nascent sea spray aerosol: implications for atmospheric chemistry and climate, Environ. Sci. Technol. Lett., 2019, 6, 171–177, DOI:10.1021/acs.estlett.8b00699.
- J. Martinez, D. C. Smith, D. F. Steward and F. Azam, Variability in ectohydrolytic enzyme activities of pelagic marine bacteria and its significance for substrate processing in the sea, Aquat. Microb. Ecol., 1996, 10, 223–230, DOI:10.3354/ame010223.
- N. Hiranuma, S. Augustin-Bauditz, H. Bingemer, C. Budke, J. Curtius, A. Danielczok, K. Diehl, K. Dreischmeier, M. Ebert, F. Frank, N. Hoffman, K. Kandler, A. Kiselev, T. Koop, T. Leisner, O. Möhler, B. Nillius, A. Peckhaus, D. Rose, S. Weinbruch, H. Wex, Y. Boose, P. J. DeMott, J. D. Hader, T. C. J. Hill, Z. A. Kanji, G. Kulkarni, E. J. T. Levin, C. S. McCluskey, M. Murakami, B. J. Murray, D. Niedermeier, M. D. Petters, D. O'Sullivan, A. Saito, G. P. Schill, T. Tajiri, M. A. Tolbert, A. Welti, T. F. Whale, T. P. Wright and K. Yamashita, A comprehensive laboratory study on the immersion freezing behavior of illite NX particles: a comparison of 17 ice nucleation measurement techniques, Atmos. Chem. Phys., 2015, 15, 1–30, DOI:10.5194/acp-15-2489-2015.
- A. Agresti and B. A. Coull, Approximate is better than “exact” for interval estimation of binomial proportions, Am. Stat., 1998, 52, 119–126, DOI:10.1080/00031305.1998.10480550.
- F. Azam and F. Malfatti, Microbial structuring of marine ecosystems, Nat. Rev. Microbiol., 2007, 5, 782–791, DOI:10.1038/nrmicro1747.
- D. C. Rogers, P. J. DeMott, S. M. Kreidenweis and Y. Chen, A continuous-flow diffusion chamber for airborne measurements of ice nuclei, J. Atmos. Oceanic Technol., 2001, 18, 725–741, DOI:10.1175/1520-0426(2001)018%3C0725:ACFDCF%3E2.0.CO;2.
- P. J. DeMott, A. J. Prenni, G. R. McMeeking, R. C. Sullivan, M. D. Petters, Y. Tobo, M. Niemand, O. Möhler, J. R. Snider, Z. Wang and S. M. Kreidenweis, Integrating laboratory and field data to quantify the immersion freezing ice nucleation activity of mineral dust particles, Atmos. Chem. Phys., 2015, 15, 393–409, DOI:10.5194/acp-15-393-201.
- K. A. Prather, T. H. Bertram, V. H. Grassian, G. B. Deane, M. D. Stokes, P. J. Demott, L. I. Aluwihare, B. P. Palenik, F. Azam, J. H. Seinfeld, R. C. Moffet, M. J. Molina, C. D. Cappa, F. M. Geiger, G. C. Roberts, L. M. Russell, A. P. Ault, J. Baltrusaitis, D. B. Collins, C. E. Corrigan, L. A. Cuadra-Rodriguez, C. J. Ebben, S. D. Forestieri, T. L. Guasco, S. P. Hersey, M. J. Kim, W. F. Lambert, R. L. Modini, W. Mui, B. E. Pedler, M. J. Ruppel, O. S. Ryder, N. G. Schoepp, R. C. Sullivan and D. Zhao, Bringing the ocean into the laboratory to probe the chemical complexity of sea spray aerosol, Proc. Natl. Acad. Sci. U.S.A., 2013, 110, 7550–7555, DOI:10.1073/pnas.1300262110.
- T. C. J. Hill, D. G. Georgakopoulos, P. J. DeMott, W. L. Stump and G. D. Franc, Measurement of ice nucleation-active bacteria on plants and in precipitation by quantitative PCR, Appl. Environ. Microbiol., 2014, 80, 1256–1267, DOI:10.1128/AEM.02967-13.
- A. E. Parada, D. M. Needham and J. A. Fuhrman, Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples, Environ. Microbiol., 2016, 18, 1403–1414, DOI:10.1111/1462-2920.13023.
- J. R. Cole, Q. Wang, J. A. Fish, B. Chai, D. M. McGarrell, Y. Sun, C. T. Brown, A. Porras-Alfaro, C. R. Kuske and J. M. Tiedje, Ribosomal Database Project: data and tools for high throughput rRNA analysis, Nucleic Acids Res., 2014, 42, D633–D642, DOI:10.1093/nar/gkt1244.
- T. C. J. Hill, K. A. Walsh, J. A. Harris and B. F. Moffett, Using ecological diversity measures with bacterial communities, FEMS Microbiol. Ecol., 2003, 43, 1–11, DOI:10.1111/j.1574-6941.2003.tb01040.x.
- A. E. Magurran, Ecological Diversity and its Measurement, Chapman and Hall, London, 1988, p. 179, DOI:10.1007/978-94-015-7358-0.
- E. Fuentes, H. Coe, D. Green and G. McFiggans, On the impacts of phytoplankton derived organic matter on the properties of the primary marine aerosol-part 2: composition, hygroscopicity and cloud condensation activity, Atmos. Chem. Phys., 2011, 11, 2585–2602, DOI:10.5194/acp-11-2585-2011.
- P. Verdugo, Marine microgels, Annu. Rev. Mar. Science, 2012, 4, 375–400, DOI:10.1146/annurev-marine-120709-142759.
- S. J. Biller, F. Schubotz, S. E. Roggensack, A. W. Thompson, R. E. Summons and S. W. Chisholm, Bacterial vesicles in marine ecosystems, Science, 2014, 343, 183–186, DOI:10.1126/science.1243457.
- M. V. Santander, Insights into Ocean-To-Atmosphere Transfer of Humic-like Substances and Bacteria, PhD thesis, University of California San Diego, U.S.A., p. 192, 2022.
- C. Lee, C. M. Sultana, D. B. Collins, M. V. Santander, J. L. Axson, F. Malfatti, G. C. Cornwell, J. R. Grandquist, G. B. Deane, M. D. Stokes, F. Azam, V. H. Grassian and K. A. Prather, Advancing model systems for fundamental laboratory studies of sea spray aerosol using the microbial loop, J. Phys. Chem. A, 2015, 119, 8860–8870, DOI:10.1021/acs.jpca.5b03488.
- J. A. Fuhrman and R. T. Noble, Viruses and protists cause similar bacterial mortality in coastal seawater, Limnol. Oceanogr., 1995, 40, 1236–1242, DOI:10.4319/lo.1995.40.7.1236.
- H. Ogawa, Y. Amagai, I. Koike, K. Kaiser and R. Benner, Production of refractory dissolved organic matter by bacteria, Science, 2001, 292, 917–920, DOI:10.1126/science.1057627.
- E. K. Wilbourn, D. C. O. Thornton, C. Ott, J. Graff, P. K. Quinn, T. S. Bates, R. Betha, L. M. Russell, M. J. Behrenfeld and S. D. Brooks, Ice nucleation by marine aerosols over the North Atlantic Ocean in late spring, J. Geophys. Res.: Atmos., 2020, 125, e2019JD030913, DOI:10.1029/2019JD030913.
- N. J. Gotelli and R. K. Colwell, Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness, Ecol. Lett., 2001, 4, 379–391, DOI:10.1046/j.1461-0248.2001.00230.x.
- A. Buchan, G. R. LeCleir, C. A. Gulvik and J. M. González, Master recyclers: features and functions of bacteria associated with phytoplankton blooms, Nat. Rev. Microbiol., 2014, 12, 686–698, DOI:10.1038/nrmicro3326.
- H. P. Grossart, F. Levold, M. Allgaier, M. Simon and T. Brinkhoff, Marine diatom species harbour distinct bacterial communities, Environ. Microbiol., 2005, 7, 860–873, DOI:10.1111/j.1462-2920.2005.00759.x.
- J. Pinhassi, M. M. Sala, H. Havskum, F. Peters, O. Guadayol, A. Malits and C. Marrasé, Changes in bacterioplankton composition under different phytoplankton regimens, Appl. Environ. Microbiol., 2004, 70, 6753–6766, DOI:10.1128/AEM.70.11.6753-6766.2004.
- H. Teeling, B. M. Fuchs, D. Becher, C. Klockow, A. Gardebrecht, C. M. Bennke, M. Kassabgy, S. Huang, A. J. Mann, J. Waldmann, M. Weber, A. Klindworth, A. Otto, J. Lange, J. Bernhardt, C. Reinsch, M. Hecker, J. Peplies, F. D. Bockelmann, U. Callies, G. Gerdts, A. Wichels, K. H. Wiltshire, F. O. Glöckner, T. Schweder and R. Amann, Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom, Science, 2012, 336, 608–611, DOI:10.1126/science.1218344.
- F. Thomas, J.-H. Hehemann, E. Rebuffet, M. Czjzek and G. Michel, Environmental and gut Bacteroidetes: the food connection, Front. Microbiol., 2011, 2, 16, DOI:10.3389/fmicb.2011.00093.
- J.-H. Wang, J. Lu, Y.-X. Zhang, J. Wu, C. Zhang, X. Yu, Z. Zhang, H. Liu and W.-H. Wang, High-throughput sequencing analysis of the microbial community in coastal intensive mariculture systems, Aquacult. Eng., 2018, 83, 93–102, DOI:10.1016/j.aquaeng.2018.10.001.
- M. Cunliffe, A. S. Whiteley, H. Schäfer, L. Newbold, A. Oliver and J. C. Murrell, Comparison of bacterioneuston and bacterioplankton dynamics during a phytoplankton bloom in a fjord mesocosm, Appl. Environ. Microbiol., 2009, 75, 7173–7181, DOI:10.1128/AEM.01374-09.
- A. Krolicka, C. Boccadoro, M. M. Nilsen and T. Baussant, Capturing early changes in the marine bacterial community as a result of crude oil pollution in a mesocosm experiment, Microbes Environ., 2017, 32, 358–366, DOI:10.1264/jsme2.ME17082.
- M. Cunliffe, A. Engel, S. Frka, B. Gašparovic, C. Guitart, J. C. Murrell, M. Salter, C. Stolle, R. Upstill-Goddard and O. Wurl, Sea surface microlayers: a unified physicochemical and biological perspective of the air–ocean interface, Prog. Oceanogr., 2013, 109, 104–116, DOI:10.1016/j.pocean.2012.08.004.
- J. Y. Aller, M. R. Kuznetsova, C. J. Jahns and P. F. Kemp, The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols, J. Aerosol Sci., 2005, 36, 801–812, DOI:10.1016/j.jaerosci.2004.10.012.
- E. Rastelli, C. Corinaldesi, A. Dell'Anno, M. Lo Martire, S. Greco, M. C. Facchini, M. Rinaldi, C. O'Dowd, D. Ceburnis and R. Danovaro, Transfer of labile organic matter and microbes from the ocean surface to the marine aerosol: an experimental approach, Sci. Rep., 2017, 7, 1–10, DOI:10.1038/s41598-017-10563-z.
- K. Engelhardt, A. Rumpel, J. Walter, J. Dombrowski, U. Kulozik, B. Braunschweig and W. Peukert, Protein adsorption at the electrified air–water interface: implications on foam stability, Langmuir, 2012, 28, 7780–7787, DOI:10.1021/la301368v.
- J. R. Lu, T. J. Su and J. Penfold, Adsorption of serum albumins at the air/water interface, Langmuir, 1999, 15, 6975–6983, DOI:10.1021/la990131h.
- J. S. Lee, B. M. Weon, S. J. Park, J. H. Je, K. Fezzaa and W.-K. Lee, Size limits the formation of liquid jets during bubble bursting, Nat. Commun., 2011, 2, 367, DOI:10.1038/ncomms1369.
- E. R. Lewis and S. E. Schwartz, Sea Salt Aerosol Production: Mechanisms, Methods, Measurements and Models – a Critical Review, American Geophysical Union, Washington, DC, 2004, DOI:10.1029/GM152.
- F. Veron, Ocean Spray, Annu. Rev. Fluid. Mech., 2015, 47, 507–538, DOI:10.1146/annurev-fluid-010814-014651.
- C. F. Brasz, C. T. Bartlett, P. L. Walls, E. G. Flynn, Y. E. Yu and J. C. Bird, Minimum size for the top jet drop from a bursting bubble, Phys. Rev. Fluids, 2018, 3, 074001, DOI:10.1103/PhysRevFluids.3.074001.
- R. L. Modini, L. M. Russell, G. B. Deane and M. D. Stokes, Effect of soluble surfactant on bubble persistence and bubble-produced aerosol particles, J. Geophys. Res.: Atmos., 2013, 10, 1388–1400, DOI:10.1002/jgrd.50186.
- D. Dey, J. M. Boulton-Stone, A. N. Emery and J. R. Blake, Experimental comparisons with a numerical model of surfactant effects on the burst of a single bubble, Chem. Eng. Sci., 1997, 52, 2769–2783, DOI:10.1016/S0009-2509(97)00083-3.
- P. K. Quinn, D. B. Collins, V. H. Grassian, K. A. Prather and T. S. Bates, Chemistry and related properties of freshly emitted sea spray aerosol, Chem. Rev., 2015, 115, 4383–4399, DOI:10.1021/cr500713g.
- R. L. Stefan and A. J. Szeri, Surfactant scavenging and surface deposition by rising bubbles, J. Colloid Interface Sci., 1999, 212, 1–13, DOI:10.1006/jcis.1998.6037.
- M. van Pinxteren, T.-B. Robinson, S. Zeppenfeld, X. Gong, E. Bahlmann, K. Wadinga Fomba, N. Triesch, F. Stratmann, O. Wurl, A. Engel, H. Wex and H. Herrmann, High number concentrations of transparent exopolymer particles in ambient aerosol particles and cloud water – a case study at the tropical Atlantic Ocean, Atmos. Chem. Phys., 2022, 22, 5725–5742, DOI:10.5194/acp-22-5725-2022.
- S. Christiansen, M. E. Salter, E. Gorokhova, Q. T. Nguyen and M. Bilde, Sea spray aerosol formation: laboratory results on the role of air entrainment, water temperature, and phytoplankton biomass, Environ. Sci. Technol., 2019, 53, 3107–13116, DOI:10.1021/acs.est.9b04078.
- M. Kuznetsova, C. Lee, J. Aller and N. M. Frew, Enrichment of amino acids in the sea-surface microlayers at coastal and open ocean sites in the North Atlantic Ocean, Limnol. Oceanogr., 2004, 49, 1605–1619, DOI:10.4319/lo.2004.49.5.1605.
- P. Roy, L. E. Mael, T. C. J. Hill, L. Mehndiratta, G. Peiker, M. L. House, P. J. DeMott, V. H. Grassian and C. S. Dutcher, Ice nucleating activity and residual particle morphology of bulk seawater and sea surface microlayer, ACS Earth Space Chem., 2021, 5, 1916–1928, DOI:10.1021/acsearthspacechem.1c00175.
- X. Gong, H. Wex, M. V. Pinxteren, N. Triesch, K. W. Fomba, J. Lubitz, C. Stolle, T. B. Robinson, T. Müller, H. Herrmann and F. Stratmann, Characterization of aerosol particles at Cabo Verde close to sea level and at the cloud level-part 2: ice-nucleating particles in air, cloud and seawater, Atmos. Chem. Phys., 2020, 20, 1451–1468, DOI:10.5194/acp-20-1451-2020.
- M. J. Wolf, M. Goodell, E. Dong, L. A. Dove, C. Zhang, L. J. Franco, C. Shen, E. G. Rutkowski, D. N. Narducci, S. Mullen, A. R. Babbin and D. J. Cziczo, A link between the ice nucleation activity and the biogeochemistry of seawater, Atmos. Chem. Phys., 2020, 20, 15341–15356, DOI:10.5194/acp-20-15341-2020.
- P. K. Quinn, T. S. Bates, K. S. Schulz, D. J. Coffman, A. A. Frossard, L. M. Russell, W. C. Keene and D. J. Kieber, Contribution of sea surface carbon pool to organic matter enrichment in sea spray aerosol, Nat. Geosci., 2014, 7, 228–232, DOI:10.1038/ngeo2092.
- L. M. Russell, L. N. Hawkins, A. A. Frossard, P. K. Quinn and T. S. Bates, Carbohydrate-like composition of submicron atmospheric particles and their production from ocean bubble bursting, Proc. Natl. Acad. Sci. U.S.A., 2010, 107, 6652–6657, DOI:10.1073/pnas.0908905107.
- M. van Pinxteren, S. Barthel, K. W. Fomba, K. Müller, W. Von Tümpling and H. Herrmann, The influence of environmental drivers on the enrichment of organic carbon in the sea surface microlayer and in submicron aerosol particles – measurements from the Atlantic Ocean, Elem. Sci. Anth., 2017, 5, 35, DOI:10.1525/elementa.
- E. S. Hasenecz, T. Jayarathne, M. A. Pendergraft, M. V. Santander, K. J. Mayer, J. Sauer, C. Lee, W. S. Gibson, S. M. Kruse, F. Malfatti, K. A. Prather and E. A. Stone, Marine bacteria affect saccharide enrichment in sea spray aerosol during a phytoplankton bloom, ACS Earth Space Chem., 2020, 4, 638–1649, DOI:10.1021/acsearthspacechem.0c00167.
- S. Zeppenfeld, M. van Pinxteren, D. van Pinxteren, H. Wex, E. Berdalet, D. Vaqué, M. Dall'Osto and H. Herrmann, Aerosol marine primary carbohydrates and atmospheric transformation in the Western Antarctic Peninsula, ACS Earth Space Chem., 2021, 5, 1032–1047, DOI:10.1021/acsearthspacechem.0c00351.
- M. C. Facchini, M. Rinaldi, S. Decesari, C. Carbone, E. Finessi, M. Mircea, S. Fuzzi, D. Ceburnis, R. Flanagan, E. D. Nilsson, G. de Leeuw, M. Martino, J. Woeltjen and C. D. O'Dowd, Primary submicron marine aerosol dominated by insoluble organic colloids and aggregates, Geophys. Res. Lett., 2008, 35, L17814, DOI:10.1029/2008GL034210.
- M. V. Santander, B. A. Mitts, M. A. Pendergraft, J. Dinasquet, C. Lee, A. N. Moore, L. B. Cancelada, K. L. A. Kimble, F. Malfatti and K. A. Prather, Tandem fluorescence measurements of organic matter and bacteria released in sea spray aerosols, Environ. Sci. Technol., 2021, 55, 5171–5179, DOI:10.1021/acs.est.0c05493.
- P. J. DeMott, R. H. Mason, C. S. McCluskey, T. C. J. Hill, R. J. Perkins, Y. Desyaterik, A. K. Bertram, J. V. Trueblood, V. H. Grassian, Y. Qiu, V. Molinero, Y. Tobo, C. M. Sultana, C. Lee and K. A. Prather, Ice nucleation by particles containing long-chain fatty acids of relevance to freezing by sea spray aerosols, Environ. Sci. Process Impacts, 2018, 20, 1559–1569, 10.1039/C8EM00386F.
- R. J. Perkins, M. G. Vazquez de Vasquez, E. E. Beasley, T. C. J. Hill, E. A. Stone, H. C. Allen and P. J. DeMott, Relating structure and ice nucleation of mixed surfactant systems relevant to sea spray aerosol, J. Phys. Chem. A, 2020, 124, 8806–8821, DOI:10.1021/acs.jpca.0c05849.
- M. Ardyna, H. Claustre, J.-B. Sallée, F. D'Ovidio, B. Gentili, G. van Dijken, F. D'Ortenzio and K. R. Arrigo, Delineating environmental control of phytoplankton biomass and phenology in the Southern Ocean, Geophys. Res. Lett., 2017, 44, 5016–5024, DOI:10.1002/2016GL072428.
- J. Robinson, E. E. Popova, M. A. Srokosz and A. Yool, A tale of three islands: downstream natural iron fertilization in the Southern Ocean, J. Geophys. Res.: Oceans, 2016, 121, 3350–3371, DOI:10.1002/2015JC011319.
- C. M. S. Schine, A.-C. Alderkamp, G. van Dijken, L. J. A. Gerringa, S. Sergi, P. Laan, H. van Haren, W. H. van de Poll and K. R. Arrigo, Massive Southern Ocean phytoplankton bloom fed by iron of possible hydrothermal origin, Nat. Commun., 2021, 12, 1211, DOI:10.1038/s41467-021-21339-5.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ea00154c |
| This journal is © The Royal Society of Chemistry 2023 |