Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recreational drug-use as an urban source of nitrous oxide
Kanokrat
Charoenpornpukdee
 a,
Kieran
Stanley
a,
Kieran
Stanley
 a,
Joe
Pitt
a,
Joe
Pitt
 a,
Angelina
Wenger
a,
Alistair
Manning
b,
Dickon
Young
a,
Daniel
Say
a and
Simon
O'Doherty
*a
a,
Angelina
Wenger
a,
Alistair
Manning
b,
Dickon
Young
a,
Daniel
Say
a and
Simon
O'Doherty
*a
aSchool of Chemistry, University of Bristol, Bristol BS81TS, UK. E-mail: s.odoherty@bristol.ac.uk
bMet Office, Exeter, Devon EX1 3PB, UK
First published on 22nd April 2023
Abstract
N2O is a potent greenhouse gas which also contributes to depletion of stratospheric ozone. Its primary sources are from natural and agricultural soils and from the ocean. However, other minor sources exist, including industrial processes, combustion processes in the power generation sector and road transport. Very few atmospheric measurements of these minor sources of N2O exist in the urban environment, particularly in the UK. Nonetheless, they are essential in understanding anthropogenic emissions of N2O. A custom-built automated sampling system was used to make high-frequency measurements of N2O, along with SF6, CO and H2 at an urban site in the city of Bristol, United Kingdom (UK), from 3rd November 2019 to 26th November 2021. These time-series data provide an insight into urban N2O mole fractions, diurnal cycles, and possible sources of N2O during a period of time affected by the COVID-19 pandemic. The data show a pattern of elevated N2O mole fractions during the late evening and early morning hours on weekends, with no significant correlations with other measured species, indicating the apparent use of N2O as a recreational drug. The National Atmospheric Emissions Inventory (NAEI) reports UK emissions for recreational N2O drug use of 12–14 tonnes between 2012 and 2020. We derived a much larger estimate of 89–954 tonnes for the year 2020 using revised estimates of the size of canisters used, the mass of N2O in each canister and the number of times the drug is used at each sitting.
Environmental significanceN2O is a strong and long-lived greenhouse gas, with a Global Warming Potential (GWP) of 265 and an atmospheric lifetime of 114 years. It also plays an important role in strastopheric ozone depletion. Due to its inclusion in the Kyoto Protocol and the Paris Agreement, the UK collates a national emission inventory which is submitted to the UNFCCC. The agricultural sector dominates emissions of N2O within the emissions inventory, with emissions from agricultural soils accounting for 56% of total UK emissions in 2020, and other sources (i.e., road transport, other fuel combustion sources and waste processes) add another 13%. However, there are very few atmospheric measurements of these minor sources of N2O within the urban environment, particularly in the UK. Nonetheless, they are essential in understanding anthropogenic emissions of N2O. Our urban N2O measurement in Bristol, UK, found a clear pattern of significant N2O enhancements indicating a strong source from recreational use and prompting a reassessment of the NAEI emissions estimate (related to recreational use). These findings show that the UK emission inventory underestimates the N2O recreational drug use by between 60% to 680% based on different emission scenarios. Although the new estimates are still a minor source of N2O to the atmosphere; this source would represent over 1.3% of total UK N2O emissions. Given the increasing use of N2O as a recreational drug, it is therefore important to improve the representation of this source in future versions of the inventory. |
1 Introduction
Nitrous oxide (N2O) is a long-lived and potent greenhouse gas with an atmospheric lifetime of 114 years. According to the 5th assessment report of the Intergovernmental Panel on Climate Change (IPCC), N2O has a global warming potential over a 100 year period of 265 (this is the value used for inventory reporting under the Paris Agreement from 2024). N2O accumulates in the atmosphere and is a long-lived, indirect stratospheric ozone-depleting substance.1 The levels of N2O in the atmosphere have increased more rapidly over the past five decades, primarily as a result of increasing agricultural emissions.2 Since 1990, anthropogenic N2O emissions have been reported annually by Annex I parties to the United Nations Framework Convention on Climate Change (UNFCCC). More recently, over 190 national signatories to the Paris Agreement have been required to report their national greenhouse-gas inventory annually with sufficient detail and transparency to track progress towards their nationally determined goals. In the UK National Inventory Report3 (Fig. 1), in 2020 the largest source of N2O was the agricultural sector (48.71 kt y−1). Other sources were land use change and forestry (LULUCF; 5.97 kt y−1), waste management (5.69 kt y−1), transport (3.31 kt y−1), business (2.91 kt y−1), energy supply (2.45 kt y−1), exports (emissions associated with international aviation and shipping; 0.73 kt y−1), industrial process (0.64 kt y−1), and residential (0.51 kt y−1). According to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories, category 2G3b (N2O product uses) describes emissions from using N2O containing products where the N2O is used as a propellant for pressure or aerosol. This includes the usage of N2O cartridges to make whipped cream as well as recreational use of N2O. In 2020 the UK National Atmospheric Emissions Inventory (NAEI) stated the emission estimate for recreational N2O at 14 tonnes.3 Note that the IPCC category 2G3b is associated with industrial processes and product use, but for the purpose of the National Communication Sectors used in Fig. 1, recreational use of N2O is considered to be part of the residential sector.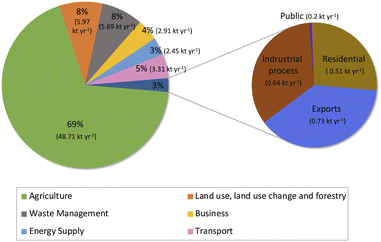 | ||
| Fig. 1 N2O emission budget of 2020 reported by the UK National Atmospheric Emissions Inventory (UK NAEI). | ||
N2O has been used recreationally since the 1960s, often referred to as ‘hippy crack’, ‘whippits’, ‘laughing gas’, or ‘chargers’, and is usually sold in small pressurised silver canisters used for cream whippers. It is typically used by discharging gas from these canisters into another object, such as a balloon, or by inhaling the gas directly into the mouth. Once consumed, it takes a few seconds for the N2O to dissolve into the bloodstream before it reaches the brain, and the entire gas is then released to the atmosphere during exhalation. A common symptom of N2O inhalation is immediate dizziness and a sense of euphoria.4 However, N2O consumption has been linked to a number of short-term risks, including throat muscle spasms and asphyxiation from the oxygen displacement with N2O in the body. The recreational use of N2O has been reported widely in the news and in scientific publications, where N2O canisters intended to make whipped cream are adapted for inhalation.5,6 Kaar et al.7 reported research from the 2014 Global Drug Survey (GDS), showing that lifetime recreational N2O usage was higher in the UK than the other countries in the study (the US, New Zealand, Australia, Switzerland, and Germany). Recreational N2O use was reported to be most often consumed via balloons at festivals and in clubs.7
N2O has been characterised as a new psychoactive substance (NPS), i.e. a drug that mimics the effect of existing drugs. Findings from the Crime Survey for England and Wales (CSEW) showed that, in the last year, 2.4% of adults aged 16 to 59 years and 8.7% of 16 to 24 year-olds had used N2O.8 This made it the second most prevalent drug among young adults aged 16 to 24 years (after cannabis) and the third most prevalent for adults aged 16 to 59 years (after cannabis and cocaine). The sale of N2O is still legal for specific purposes, even though the Psychoactive Substances Act makes possession of N2O with the intent to sell illegal. The sale of N2O for its psychoactive effects was made illegal after the Psychoactive Substances Act in 2016, but it is not currently a crime to be caught in possession of the substance.
Several reports on N2O consumption have found it to be widespread and increasing. In Paris, France, a study investigated the impact of COVID-19 containment on the recreational use of N2O in the Paris area using data reported to Poison Control Centers. This study concluded that the observed number of N2O exposures during the 2019–2022 period showed a pronounced increase compared to earlier years, but the exact impact of the COVID-19 on this increase remains to be determined.9 In Melbourne, Australia, a survey among a group of regular psychostimulant users as part of the Drug Reporting System (EDRS) indicated an increase in the prevalence of N2O use, with recent N2O users typically significantly younger than non-users.10 Nabben et al.11 reported consumption among the Moroccan-Dutch Young Adults in the Netherlands.
Emissions from recreational N2O use have been estimated in the UK NAEI using a bottom-up approach since 2012. However, direct atmospheric measurements of recreational N2O emissions have not yet been widely published. Barker et al.12 reported evidence of recreational N2O emissions based on atmospheric measurements in Manchester, UK. This source was identified based on the temporal pattern of N2O enhancements at an urban measurement site, as well as the lack of correlation between enhancements in N2O and other trace gas species. The authors concluded that further studies in other UK locations are required to better contextualise these observations.
We report in situ, high-frequency observations of N2O at a UK urban measurement station at the School of Chemistry, University of Bristol, between 3rd November 2019 to 26th November 2021. In addition to N2O, we report measurements for carbon monoxide (CO) as a tracer for combustion sources. The focus of this work relates to observations of recreational drug use of N2O in Bristol over this period that spans pre-COVID-19, three UK COVID-19 lockdowns and post-COVID-19 regulations. We then reassess the reported NAEI emissions for this source, based on revised activity data and emission factor estimates.
2 Methods
2.1 Site descriptions
Measurements of N2O, CO, SF6 and H2 were made at an urban sampling site located at the Atmospheric Chemistry Research Group (ACRG), School of Chemistry, University of Bristol, UK (51.456°N, 2.600°W; 62 m above sea level and 26 m above ground level). Air was sampled from the rooftop of the building through 1/2′′ O. D. Synflex 1300 tubing (Hose Tech Ltd, UK). An oil-less linear pump (DBM20-801, GAST Group Ltd, UK) continuously flushed the sample line at a flow rate of 10 L min−1. Air from this inlet line was filtered through a 40 μm inline filter (SS-8TF-40, Swagelok, UK) to trap particles. Note that the measurements of SF6 and H2 are not reported in this study, as these species are not typically co-emitted with N2O, and so they do not act as useful tracers for N2O sources. However, these gases are briefly mentioned in the instrument description below so as to fully describe the analytical process.Clean air baseline measurements of N2O were performed at Mace Head (MHD) (53.327°N, 9.905°W, 18 m above sea level and 10 m above ground level). The MHD atmospheric research station is representative of mid-latitude northern hemispheric background air. MHD is located remotely; the closest city is Galway, which is 55 km easterly. The area immediately surrounding MHD is very sparsely populated, providing very low local anthropogenic emissions.13In situ measurements of dried whole air were collected from 10 m inlet height at a 20 min measurement frequency, with standards traceable to the SIO-16 scale.13–15
2.2 Sampling instrumentation
A custom-built automated sampling system (GC-MD) was deployed in the ACRG laboratory, and the system setup has previously been described by Stanley et al.13 Briefly, the system is equipped with gas chromatography coupled with a micro electron capture detector (GC-μECD, Agilent 6890, Agilent Technologies, California, US), and a reduction gas analyser (RGA; Peak Performer 1, Trace Analytical, Inc., California, US). The GC-μECD measured N2O and SF6, and the GC-RGA measured CO and H2. The measurements on the two detector channels were performed simultaneously. Ambient air for analysis was sub-sampled from the air inlet line described above using a KNF pump (N86 STE, KNF Neuberger UK Ltd, Oxfordshire, UK) at a flow rate of 40 mL min−1. Calibration standards were set at the same inlet pressure as the pump delivery pressure. The sample was flushed through a Nafion drier (Perma Pure, MD-050-72S-1, USA) operated in counter-purge mode to dry the sample and then through a ten-port, two-position valve (VICI Valco AG International, Switzerland) containing two gas sample loops. The flow through the sample loops was controlled with a mass flow controller (GSC-A4TA-BB22, Voeglin Instruments AG, Switzerland) and the pressure inside the loop was measured using a pressure sensor (100PSI-A-DO, All Sensors, BS-Rep GmbH, Germany). After flushing with the sample at 40 mL min−1 for 1 min, the sample loops were isolated at 20 psig and allowed to equilibrate to ambient pressure. The pressures were logged, and the samples were then injected into the ECD and RGA instrument channels for separation and detection during the 10 min analysis time. Further details of the instrument have been described by Stanley et al.13 and Grant et al.16The working standard (a calibrated whole air sample of N2O mole fraction at 322.56 nmol mol−1) was run after every four air measurements to account for short-term instrumental drift. The mole fraction of the working standard was close to the baseline atmospheric mole fraction of the air sample to avoid sample matrix effects and minimise non-linearity issues. Dry air mole fractions have been reported using the following calibration scales: N2O (SIO-16), SF6 (SIO-05), CO (CSIRO-94) and H2 (MPI-2009). The response of the GC-μECD and GC-RGA is not linear, and a non-linearity assessment was performed by measuring calibration cylinders containing a range of mole fractions spanning ambient air mole fractions. A second-order polynomial curve was fitted to the results, and the correction was implemented using a custom software package GCWerks (GCWerks™, https://www.gcwerks.com). The precision of the instrument was calculated from the standard deviation with a 3-sigma filter of bracketing standards; the precision of N2O, SF6, CO and H2 have been reported at 0.40 ± 0.12, 0.03 ± 0.005 and 0.80 ± 0.23, and 2.3 ± 0.5 nmol mol−1, respectively.
Local meteorological data (wind speed and direction) were used in this study. From the beginning of the project until March 2021, the wind data were obtained from meteorological equipment located on the roof of the physics building (approximately 500 m away from the sample inlet in chemistry) and provided wind speed (Pro-D sensor, Logicenergy, UK) and wind direction (WindDirection, Logicenergy, UK) in 10 minute averaging intervals. Unfortunately, this equipment broke down in March 2021. A second meteorological data set located on the roof of the Bio-Medical School, approximately 20 m away from the sampling inlet, was available from August 2021 to the end of the study. The wind data from the Bio-Medical School was measured by a sonic anemometer (Gill Wind Master, Gill Instrument, UK) with a sample rate of 20 Hz, averaged into 10 minute intervals.
2.3 Statistical analyses
Statistical analysis of data was performed using the OpenAir package17 in R.182.4 National Atmospheric Emissions Inventory (NAEI)
The NAEI reports annual N2O emissions from 1990 to the most current publication year as part of the UK's commitments under the Kyoto Protocol, and as part of the EU's GHG monitoring mechanism until 2022, and under the Paris Agreement from 2024. Emissions of N2O are reported by sector, and the sector detailing N2O emissions related to recreational drug use is contained in the industrial processes sector under section 2G3b – other product uses of N2O.3This emission estimate is based on the estimated total UK usage:
| S = ∑NiFiTi | (1) |
Annual N2O emissions (Q) are then calculated using:
| Q = Sml | (2) |
This calculation uses the mass of N2O in a canister (m) and the number of doses (canisters used) per usage (l). The UK National Inventory Report 2022 estimates total UK emissions for the year 2020 of 14 tonnes based on the following assumptions: canisters are assumed to be 10 mL in volume and to contain N2O at 60 bar. The ideal gas law (P1 × V1 = P2 × V2) is solved for V2, and this volume is converted to a mass by multiplying by the density of N2O to produce a mass of 1.17 g N2O per 10 mL canister. All N2O within the canister is assumed to be emitted during usage. Each usage is assumed to consist of a single dose.
3 Results and discussions
3.1 N2O urban observations in Bristol
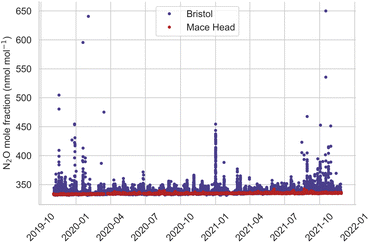
Atmospheric measurements of N2O in Bristol cover just over two years, from 3rd November 2019 to 26th November 2021 and are shown in Fig. 2. Bristol N2O mole fractions are elevated compared to measurements at MHD, a rural baseline research station located on the west coast of Ireland. MHD predominantly samples from a clean ocean sector in the North Atlantic and is representative of the Northern Hemispheric average mole fraction for N2O, but it also receives air that has travelled over Europe and the UK. The MHD and Bristol baseline data increase from 332.7 nmol mol−1 in Nov 2019 to 335.5 nmol mol−1 in Nov 2021: a growth rate of 1.4 nmol mol−1 year−1. Although the Bristol urban data shows similar ‘baseline’ mole fractions to MHD, the frequency and magnitude of above baseline events (pollution events) are significantly greater, indicating many more local sources. At MHD, the highest pollution event reaches 342.5 nmol mol−1, whereas the pollution events in Bristol reach levels as high as 650.0 nmol mol−1. A clear pattern of pollution events are exhibited in Fig. 2, a section of high mole fractions occur between 17th November 2019 to 14th March 2020, the mole fractions drop until a single high event on 1st January 2021 then reduce until mole fractions increase again from 15th August 21 onwards.Fig. 3(a) shows hourly averaged N2O observations separated by hour of the day, and day of the week. These data show that the hourly averaged N2O observations in Bristol display a diurnal cycle, with maxima in the early hours (01:00) of the morning and minima at around midday (12:00), which most likely reflect local emissions of N2O into a shallow nocturnal boundary layer under low wind speed conditions at 01:00 and a well-mixed boundary layer at 12:00; the highest hourly averaged mole fractions occur at the weekend. The hourly averaged CO observations do not follow the same enhancements in mole fraction as N2O (Fig. 3(b)). CO follows a more traditional double cycle in enhancements that reflect increased emissions of CO from morning and evening traffic volume. Likewise, the CO emission showed no significant correlation with N2O enhancements over the weekends in Bristol. Barker et al.12 studied the in situ measurement of N2O in Fallowfield, Manchester; a similar pattern of N2O emission was found, appearing with larger enhancement on weekends than weekdays and an N2O enhancement independent of CO and a range of other traffic related species, such as CH4, CO2, NOx, PM2.5, and PM10. Another significant source of urban N2O emissions is from wastewater treatment plants. For this source, the temporal emission pattern is dependent on the incoming nitrogen load and the competing nitrification and denitrification processes occurring within a plant (which are dependent on the plant type; Gruber et al.19). However, the weekly pattern observed at our Bristol measurement site is not consistent with wastewater treatment plants being the main source. We propose that the weekend enhancements in Bristol are related to the use of N2O as a recreational drug, a similar conclusion suggested for measurements carried out in Manchester.12
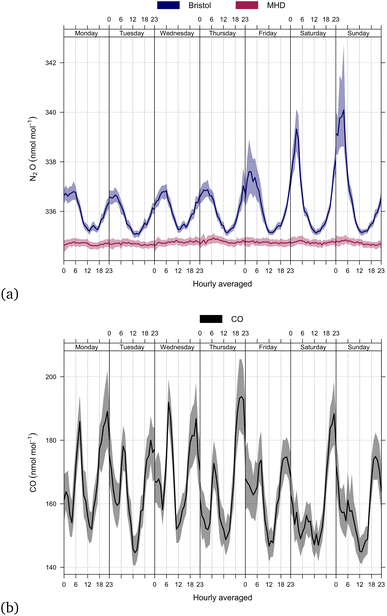 | ||
| Fig. 3 (a) Hourly averaged observations of N2O in Bristol and MHD (b) the hourly average of CO in Bristol. The shades represent the bootstrap 95% confidence interval in the mean. | ||
Occasionally, we observe very large pollution events (over 400 nmol mol−1), such as during 31st December 2020 to 1st January 2021 – the night of New Year's Eve gatherings and fireworks across Bristol. The MHD data reveal no significant N2O diurnal cycle due to a lack of local anthropogenic sources; the median mole fraction was 334.7 nmol mol−1.
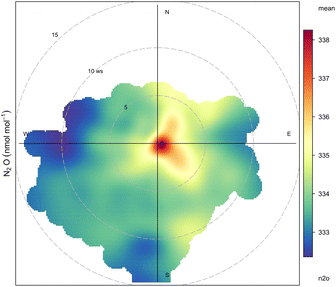
A bivariate polar plot of Bristol N2O observations and wind data is shown in Fig. 4. This plot uses a function for plotting mole fraction in polar coordinates and shows the N2O mole fraction by wind speed and direction. The prevailing wind direction during this study is south-westerly. It is clear that an N2O hotspot is centred towards the middle of the plot indicating the potential source of N2O emissions occurs at low wind speeds (less than 2 ms−1) and without a dominant wind direction. This is consistent with nightlife activity located in an area close (approx. 0.5 km) to the sampling site.
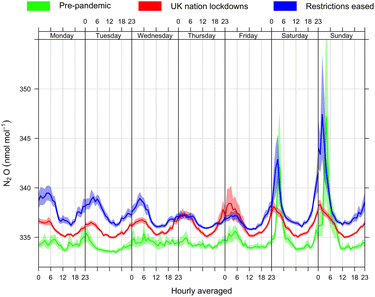
The influence of numerous UK national lockdowns, in response to the COVID pandemic, are explored in Fig. 5. The data are split into hourly averaged N2O observations for, (a) pre-pandemic from the 3rd November 2019–22nd March 2020 (green line), (b) restrictions imposed during the various UK national lockdown periods from the 23rd March 2020–19th July 2021 (red line) and (c) after restrictions were lifted on the entertainment and nightlife industry from the 20th July 2021 onwards (blue line). Significant N2O enhancements that occurred over the weekend in the pre-pandemic phase disappeared entirely during the UK national lockdown restrictions (red line). At the time the UK government announced the first national lockdown on 23rd March 2020, all non-essential high street businesses were closed, and people were ordered to stay home, with permission to leave for essential purposes only. On 5th November 2020, the second national restrictions were reintroduced in England. The unusual enhancements visible in the Friday section of the lockdown data (red line) are attributed to a single event, New Year's Eve celebrations from Friday (31st December 2020 to 1st January 2021), where despite COVID restrictions people gathered in small groups. During these celebrations there were spikes in both N2O and CO, possibly implying a combustion source of these gases related to firework usage in addition to recreational N2O usage. The maximum mole fractions of N2O and CO were 650.0 and 965.5 nmol mol−1 respectively. On the 19th July, England removed all legal limits on social contact, and the most restricted sectors of the economy reopened (e.g. the entertainment sector, including pubs and nightclubs). Since then, the pre-pandemic N2O enhancements over the weekend have reappeared suggesting a return to nightlife activities that involved the use of N2O (dark blue line).
3.2 Reassessment of the UK national emission inventory for recreational use of N2O
The large N2O enhancements observed at our Bristol measurement site, along with the similar observations in Manchester presented by Barker et al.,12 present clear qualitative evidence for significant local N2O emissions associated with recreational use. However, it is not possible to derive a robust top-down estimate of recreational N2O emissions based on these data without further constraining information. Prior knowledge of the spatial and temporal distributions of these emissions is very limited, such that a single measurement site cannot be used to yield a reliable posterior emission rate. In addition, challenges in modelling the complex dispersion patterns associated with the night-time urban environment further limit the application of a top-down approach to emission estimation.Nonetheless, the large urban N2O enhancements observed in this study prompted us to revisit the methodology used by the NAEI to calculate bottom-up emission estimates for recreational use (as described in Section 2.3). On closer inspection of the assumptions used to determine emissions in the NAEI, it was clear that there were a number of inconsistencies. Firstly, there was an error in the calculation of the mass of N2O contained in a canister; secondly, the assumption that a single size of the canisters was used (10 mL) was inconsistent with the range of canister sizes available for purchase; and finally, the amount of N2O consumed at each sitting by individual users was based on outdated and conservative assumptions.
When determining the mass contained in a 10 mL canister it was assumed that N2O followed the ideal gas law. However, this is not applicable in this case as N2O condenses into a liquid at pressures above 51 bar, but the N2O canisters are pressurised to around 60 bar. The pressure in the canister will remain constant until all of the liquid N2O has been vaporised into gas form. Therefore, the volume of gas released from a canister is far in excess of that derived using the ideal gas law.
It was also assumed that all canisters used were 10 mL, whereas in reality a wide range of canister sizes are used. In industrial production, the maximum mass of N2O that is filled into a canister is determined by the filling density, which is the ratio of the mass of N2O to the water capacity of the canister (in kg per liter). This value is 0.75 kg L−1 and is stipulated in respective regulation.20,21 Hence, canisters with a water capacity of 10 mL and 21.3 mL can be filled up to 7.5 g and 16 g of N2O, respectively, much higher than the mass (1.17 g) derived in Section 2.3. Even larger canisters can now be purchased on the internet, and some nightclubs sell N2O balloons filled from commercial 50 L cylinders.
The assumption that a single canister is used per sitting is also inconsistent with published values. A number of publications in Europe have reported values ranging from one to hundreds of balloons per session.9,22 A survey of university students in New Zealand reported the average number of N2O bulbs used in one session ranged from two to six.10 In the UK, Kaar et al.7 reported the number of hits per session, where a hit was estimated to be a balloon which is usually equivalent to the amount of N2O in a whipped cream canister. This study reported that the consumption of N2O ranges from 3 to 10 canisters, and the median of doses per usage is 5 canisters. Meanwhile, Ehirim et al.5 reported that the majority (53.8%) of users surveyed had more than 3 intakes in a single sitting, with some users reporting over 20 intakes per sitting. Here, we calculate emission estimates assuming both 1 and 5 canisters per sitting.
Using these updated assumptions, we have reassessed recreational emissions of N2O in the UK for 2020. The methodology used to determine the frequency of use categories and number of users in each of these categories was not changed. It was assumed that 2.4% of adults aged 16–59 years old were N2O users in the UK; this approximated to 894![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 704 people.8 This number was used to generate the annual total usage (eqn (1) from Section 2.3). The frequency and number of users are shown in Table 1; it is clear that in the UK a majority of users (93%) fall into the less frequent use categories.
704 people.8 This number was used to generate the annual total usage (eqn (1) from Section 2.3). The frequency and number of users are shown in Table 1; it is clear that in the UK a majority of users (93%) fall into the less frequent use categories.
| Frequency of use | Frequency of use (times/year) | Number of users (%) | Number of usage events |
|---|---|---|---|
| High frequency | |||
| Everyday | 365 | 0.94 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 065 065![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 810 810 |
| 3–5 days/week | 208 | 0.51 | 958![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 004 004 |
| 1–2 days/week | 78 | 4.66 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 882 882 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Low frequency | |||
| 2–3 times/month | 30 | 6.64 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 782 782![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570 570 |
| 1/month | 12 | 10.07 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 081 081![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 524 524 |
| 1/2 months | 6 | 18.51 | 993![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 624 624 |
| 1–2/years | 1.5 | 58.66 | 787![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 306 306 |
| Estimated total usage | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 919 919![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 720 720 |
||
The N2O emission from recreational use was then estimated using eqn (2) detailed in Section 2.3. Table 2 illustrates a range of emission scenarios for recreational use of N2O in 2020. The UK inventory estimated the present emission by applying the mass of N2O at 1.17 g; the annual emission was then reported at 14 tonnes. Meanwhile, our suggested calculations show that UK emissions reach up to 89 and 191 tonnes per year for 10 mL (7.5 g) and 21.3 mL (16 g) canisters, respectively, as these canister volumes are more commonly used. In addition, the consumption of N2O per sitting was adjusted from 1 canister per sitting to 5 canisters. As a result, the new estimate with a realistic number of canisters per session, with the 10 mL and 21.3 mL canister volumes, yields yearly emissions of 447 and 954 tonnes. These results indicate that the estimates reported in the NAEI are underestimating the emission of recreational N2O drug use by 60–680%. These findings raise the important concern about the impact of N2O recreational use and actions needed to mitigate N2O emission from unnecessary sources.
| Mass of N2O in a canister | Hits per sitting | N2O emissions (tonnes) |
|---|---|---|
| 1.17 g | 1 canister | 14 (UK inventory) |
| 7.5 g | 1 canister | 89 |
| 16 g | 1 canister | 191 |
| 7.5 g | 5 canisters | 447 |
| 16 g | 5 canisters | 954 |
4 Conclusions
In situ measurements of N2O were performed at an urban site in Bristol over two years, providing a calibrated time series of mole fractions with a time resolution of ten minutes. The observational data shows a baseline consistent with the N2O observations from the MHD background site on the west coast of Ireland. The Bristol observations showed positive deviations (pollution events) from the baseline that were far larger than those at MHD, indicating local sources specific to Bristol. A clear pattern of weekend N2O enhancements was shown to be dominated by usage of N2O as a recreational drug. This was further confirmed by cessation of emissions during the COVID lockdown months and a return to emissions once restrictions to the entertainment sector were removed.Estimates of N2O emissions related to recreational drug use reported in the NAEI were found to be incorrect due to a combination of calculation errors and incorrect/outdated assumptions. This work has proposed a range of new estimates by considering the number of canisters per sitting, the correct mass of N2O in a single canister and use of a range of canister sizes. These findings show that the UK emission inventory underestimates the N2O recreational drug use by between 60% to 680% based on the emission scenario used. Although the new estimates are still a minor source of N2O to the atmosphere, at the upper end of our estimated range (954 tonnes per year) this source would represent over 1.3% of total UK N2O emissions. Given the increasing use of N2O as a recreational drug, it is therefore important to improve the representation of this source in future versions of the NAEI.
Author contributions
Kanokrat Charoenpornpukdee: writing – original draft, methodology, data curation, conceptualization. Kieran Stanley: conceptualization, investigation, writing – review editing. Joe Pitt: validation, writing – review editing. Angelina Wenger; investigation, writing – review editing. Alistair Manning: investigation. Dickon Young: investigation. Daniel Say: investigation. Simon O'Doherty: supervision, conceptualization, resources, writing – review editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the provision of Met data for both stations, Shane P Windsor, Department of Aerospace Engineering and James Mathew, Atmospheric Chemistry Research Group, School of Chemistry and Sam Gunner, Faculty of Engineering. We also thank Mr Gerard Spain, the MHD station operator. The Department for Business, Energy Industrial Strategy (BEIS) in the UK supported the University of Bristol for operations at Mace Head, Ireland (contract 5488/11/2021). Kanokrat Charoenpornpukdee is supported by the Royal Thai Government Scholarship provided by the Office of the Civil Service Commission (OCSC), Royal Government of Thailand.Notes and references
- Climate Change 2014: Synthesis Report, ed. R. K. Pachauri and L. Mayer, Intergovernmental Panel on Climate Change, Geneva, Switzerland, 2015 Search PubMed.
- H. Tian, R. Xu, J. G. Canadell, R. L. Thompson, W. Winiwarter, P. Suntharalingam, E. A. Davidson, P. Ciais, R. B. Jackson, G. Janssens-Maenhout, M. J. Prather, P. Regnier, N. Pan, S. Pan, G. P. Peters, H. Shi, F. N. Tubiello, S. Zaehle, F. Zhou, A. Arneth, G. Battaglia, S. Berthet, L. Bopp, A. F. Bouwman, E. T. Buitenhuis, J. Chang, M. P. Chipperfield, S. R. S. Dangal, E. Dlugokencky, J. W. Elkins, B. D. Eyre, B. Fu, B. Hall, A. Ito, F. Joos, P. B. Krummel, A. Landolfi, G. G. Laruelle, R. Lauerwald, W. Li, S. Lienert, T. Maavara, M. MacLeod, D. B. Millet, S. Olin, P. K. Patra, R. G. Prinn, P. A. Raymond, D. J. Ruiz, G. R. van der Werf, N. Vuichard, J. Wang, R. F. Weiss, K. C. Wells, C. Wilson, J. Yang and Y. Yao, Nature, 2020, 586, 248–256 CrossRef CAS PubMed.
- P. Brown, L. Cardenas, S. Choudrie, S. Del Vento, E. Karagianni, J. MacCarthy, P. Mullen, N. Passant, B. Richmond, G. Thistlethwaite, A. Thomson and D. Wakeling, UK Greenhouse Gas Inventory, 1990 to 2020: Annual Report for Submission under the Framework Convention on Climate Change, 2022 Search PubMed.
- Drug Science, Nitrous Oxide (Laughing Gas), 2019, https://www.drugscience.org.uk/drug-information/nitrous-oxide/ Search PubMed.
- E. M. Ehirim, D. P. Naughton and A. Petróczi, Front. Psychiatr., 2018, 8, 312 CrossRef PubMed.
- G. Randhawa and A. Bodenham, Br. J. Anaesth., 2016, 116, 321–324 CrossRef CAS PubMed.
- S. J. Kaar, J. Ferris, J. Waldron, M. Devaney, J. Ramsey and A. R. Winstock, J. Psychopharmacol., 2016, 30, 395–401 CrossRef CAS PubMed.
- Office for National Statistics, Drug Misuse in England and Wales, 2020 Search PubMed.
- L. Dufayet, W. Caré, H. Laborde-Casterot, L. Chouachi, J. Langrand and D. Vodovar, La Revue de Médecine Interne, 2022, 43, 402–405 CrossRef CAS PubMed.
- C. Papanastasiou and P. Dietze, Just a laughing matter? Nitrous oxide use among a group of regular psychostimulant users in Melbourne, Victoria, 2013, https://ndarc.med.unsw.edu.au/sites/default/files/ndarc/resources/Poster_VICEDRS2013.pdf Search PubMed.
- T. Nabben, J. Weijs and J. van Amsterdam, Int. J. Environ. Res. Public Health, 2021, 18, 5574 CrossRef CAS PubMed.
- P. A. Barker, G. Allen, M. Flynn, S. Riddick and J. R. Pitt, Urban Clim., 2022, 46, 101282 CrossRef.
- K. M. Stanley, A. Grant, S. O'Doherty, D. Young, A. J. Manning, A. R. Stavert, T. G. Spain, P. K. Salameh, C. M. Harth, P. G. Simmonds, W. T. Sturges, D. E. Oram and R. G. Derwent, Atmos. Meas. Tech., 2018, 11, 1437–1458 CrossRef CAS.
- R. G. Prinn, R. F. Weiss, J. Arduini, T. Arnold, H. L. DeWitt, P. J. Fraser, A. L. Ganesan, J. Gasore, C. M. Harth, O. Hermansen, J. Kim, P. B. Krummel, S. Li, Z. M. Loh, C. R. Lunder, M. Maione, A. J. Manning, B. R. Miller, B. Mitrevski, J. Mühle, S. O'Doherty, S. Park, S. Reimann, M. Rigby, T. Saito, P. K. Salameh, R. Schmidt, P. G. Simmonds, L. P. Steele, M. K. Vollmer, R. H. Wang, B. Yao, Y. Yokouchi, D. Young and L. Zhou, Earth Syst. Sci. Data, 2018, 10, 985–1018 CrossRef.
- R. G. Prinn, R. F. Weiss, P. J. Fraser, P. G. Simmonds, D. M. Cunnold, F. N. Alyea, S. O'Doherty, P. Salameh, B. R. Miller, J. Huang, R. H. J. Wang, D. E. Hartley, C. Harth, L. P. Steele, G. Sturrock, P. M. Midgley and A. McCulloch, J. Geophys. Res.: Atmos., 2000, 105, 17751–17792 CrossRef CAS.
- A. Grant, K. F. Stanley, S. J. Henshaw, D. E. Shallcross and S. O'Doherty, Atmos. Chem. Phys., 2010, 10, 4715–4724 CrossRef CAS.
- D. C. Carslaw and K. Ropkins, Environ. Model. Software, 2012, 27–28, 52–61 CrossRef.
- R Core Team, R: A Language and Environment for Statistical Computing, 2022, https://www.R-project.org/ Search PubMed.
- W. Gruber, L. von Känel, L. Vogt, M. Luck, L. Biolley, K. Feller, A. Moosmann, N. Krähenbühl, M. Kipf, R. Loosli, M. Vogel, E. Morgenroth, D. Braun and A. Joss, Water Res.: X, 2021, 13, 100122 CAS.
- S. Das, S. Chattopadhyay and P. Bose, Indian J. Anaesth., 2013, 57, 489 CrossRef PubMed.
- S. Love-Jones and P. Magee, Anaesth. Intensive Care Med., 2007, 8, 2–6 CrossRef.
- J. van Amsterdam, T. Nabben and W. van den Brink, Regul. Toxicol. Pharmacol., 2015, 73, 790–796 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |