Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
On the fate of deep eutectic solvents after their use as reaction media: the CO2 production during downstream and ultimate disposal
Pablo
Domínguez de María
 *a and
Selin
Kara
*a and
Selin
Kara
 *bc
*bc
aSustainable Momentum SL, Av. Ansite 3, 4-6, 35011, Las Palmas de Gran Canaria, Canary Is, Spain. E-mail: dominguez@sustainable-momentum.net
bDepartment of Biological and Chemical Engineering, Aarhus University, Gustav Wieds Vej 10, 8000 Aarhus, Denmark
cInstitute of Technical Chemistry, Leibniz University Hannover, Callinstr. 5, 30167, Hannover, Germany. E-mail: selin.kara@iftc.uni-hannover.de
First published on 8th February 2024
Abstract
Deep Eutectic Solvents (DES) have emerged as an alternative for many applications in different chemical sectors (to be used during the upstream and downstream processing, or as performance additives). While traditionally coined as green solvents, the petrochemical and energy-demanding origin of some DES components, together with some reported toxicological data, have been often overlooked. This perspective discusses the possible fate of DES once they have been used as synthetic reaction media, particularly related to the downstream unit to recover the product from the reaction mixture, and to the final DES disposal. The Total Carbon Dioxide Release (TCR) (measured as kg CO2 per kg product) is used to compare different options. After a downstream processing to recover the product often involving an organic solvent which will be incinerated (producing CO2), the used DES media can either be incinerated or diluted to some degree to be divested to a Wastewater Treatment Plant (WWTP). A mild wastewater treatment – involving state-of-the-art microbial processing steps, appears more promising than the incineration option, both in terms of CO2 production, as well as to avoid the potential formation of halide compounds (e.g. from chloride) during the incineration. However, to reach the WWTP, a key factor is the dilution degree of the DES, and the biodegradability that DES (components) may display by wastewater microorganisms. At a range of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 dilution, a production of ∼16 kg CO2 per kg product may be expected in the WWTP, for a synthesis in DES containing 100 g substrate loading per L. Research is urgently needed to assess whether the treatment of DES as diluted wastewater may be a sustainable (and economic) solution for the DES fate.
20 dilution, a production of ∼16 kg CO2 per kg product may be expected in the WWTP, for a synthesis in DES containing 100 g substrate loading per L. Research is urgently needed to assess whether the treatment of DES as diluted wastewater may be a sustainable (and economic) solution for the DES fate.
Sustainability spotlightDeep Eutectic Solvents (DES) have attracted tremendous interest in many chemical sectors over the last two decades. Their broad tunability, together with their straightforward synthesis and potential to involve many bio-based components, have stimulated the research. DES have been used as reaction media, as well as for downstream applications and as performance additives in different synthetic procedures. Despite their broad potential of uses, however, studies concerning their toxicology and, in particular, biodegradability, are rather scarce. A question to be addressed here is What can one do with the DES, after their use in a chemical plant? Being water-miscible solvents, one can envisage either their incineration as organic fraction, or their dilution to be divested to Wastewater Treatment Plants (WWTP). In this perspective some estimations on the possible CO2 production of the different DES-post-treatment alternatives are provided, measured as kg CO2 per kg product. The discussion may provide options to be experimentally assessed by different research groups, to define what the best fate for DES may be, once they have been used in industrial premises. |
1. Introduction. Deep Eutectic Solvents and its holistic cradle-to-grave life-cycle
Deep Eutectic Solvents (DES) have emerged over the last two decades as promising solvent systems that have found (academic) applications in many chemical segments, with use as reaction media, extractive agents, performance additives, etc.1–10 As remarkable features, DES often exert low vapour pressure, can be composed of biogenic materials, and display large tuneability, as their physical–chemical properties depend primarily on the precise combination of their components. In the quest for greener solvents that can replace other more hazardous options, DES have served as inspiration, and the field has broadly flourished.Along with the possible (bio)technological applications of DES, several research groups have also assessed their actual greenness and have challenged the traditional qualitative statements that are often reported in DES publications.11 In that respect, the first holistic life cycle cradle-to-grave assessments (LCA) of DES have begun to appear in the open literature.12 Apart from that, other studies have focused on the sustainability of DES,13,14 its biocompatibility against several (micro)organisms,15–23 and a few studies also on their biodegradability following standard protocols.17,21,24–27 As a result of these investigations, a more realistic picture of the properties of DES and their (allegedly) greenness has emerged. However, considerably more research is needed to understand the options and relationships of DESs with the environment and their ultimate fate after their use.
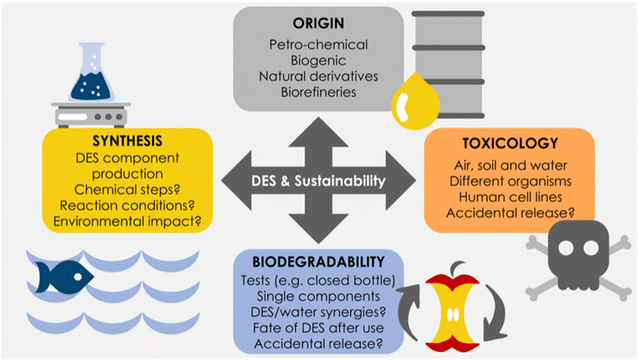
To consider the overall greenness of a solvent in general (and DES in particular), four main aspects must be assessed: (i) its origin, (ii) synthetic production, (iii) toxicological profile, and (iv) biodegradability (Fig. 1). Other aspects, like the environmental impact of solvent transportation, may also play a role in the overall chemical pipeline.28
As the first aspect to be considered, the origin of the raw materials employed for the solvent production needs to be addressed. Currently, raw materials can be derived from petrochemical sources or from biomass-based biorefineries. Not all DES components are bio-based, and some of them have indeed a petrochemical origin. Herein, the paradigmatic case may be choline chloride, which is currently chemically produced at a large scale – and competitive cost – through alkylations in an energy-intensive process.11,14 For this, and for many other examples, the option of using naturally occurring, or biorefinery-derived compounds, would be of interest, provided that the associated costs would be aligned too. Likewise, apart from the origin of the DES, the synthetic steps needed to generate the individual DES components must be discussed, with particular emphasis on their environmental impact.11 The used solvents, reagents, the generated wastes, etc., must be evaluated in detail to determine the environmental “invoice” of the DES production.
Next to the origin and synthesis routes of the DES components, studies related to biocompatibility and biodegradability must be carefully defined. In the concrete case of DES, despite some works have been performed assessing the most prominent DES,15–23 most of the studies covers only the use of aquatic organisms. Hence, more studies are needed to assess other toxicological profiles and environments (e.g. air and soil).11 Importantly, a few recent studies assess the difference in biocompatibility of the DES components separately, and whether toxicological synergies (positive or negative) are created when DES are formed, and charges are more delocalized.14,15 As a general trend reported, some toxicity is observed, which is usually related to the DES properties, like pH, viscosity, water content, etc.11,16 In fact, the antibacterial toxicity of some DES has been put forth to deliver antimicrobial solvents and dental resin composites, leading to unexpected application fields of DES that may be of interest in the future.17,29–32 Last but not least, it must be noted that while classic DES display low-to-moderate toxicities – although more studies are needed including the assessment of wide range of the DES components – there is always the risk of eutrophication if large volumes of DES are accidently disposed in the environment, due to the sudden increase in nutrients, that would trigger large biomass production and oxygen depletion in contaminated areas.13
Finally, a fundamental but surprisingly largely overlooked aspect is that of DES biodegradability (Fig. 1), presumably because the use of “naturally occurring” compounds implies intrinsically that biodegradability can be taken for granted.11 It must be noted that the biodegradability of commonly used DES components has been previously assessed, before the term DES was coined (e.g. polyols, urea, in natural river waters),33,34 an aspect that it is also stated in their individual material safety data sheets (MSDS). With respect to the biodegradability of DES components and their combinations, few papers have been published recently,17,21,24–27 most of which have used standard procedures for biodegradation measurements, such as the closed-bottle or the respirometry test methods recommended by the Organization for Economic Co-operation and Development (OECD). The majority of the reported studies deal with choline chloride DES types, and use classic hydrogen-bond donors like carboxylic acids (e.g. malonic acid), amino acids, urea, ethylene glycol, glycerol, etc. Results show, as a general trend, that all DES display acceptable biodegradability, yet with some striking differences among studies, like the impact of water on the DES biodegradability, where apparently the addition of water leads to less biodegradable media.18 Clearly much more data are needed to generate solid heuristics in the DES fate, and to evaluate their ultimate impact when disposed of after use.
2. Green Chemistry metrics to assess the environmental impact
Modern synthetic processes need to be aligned not only with the expected efficiency and robust economics, but also with environmental standards that may secure the sustainability of the process. Since the establishment of the Green Chemistry principles, some decades ago, several quantitative metrics covering both the mass and the energy environmental impact of a process have been developed.35–40 Among them, the E-factor measures the kilograms of waste generated by a kilogram of product and represents a rapid tool to determine the environmental impact of a given reaction (or a part of it).37–39 Thus, higher E-factors would imply more significant waste generation, being therefore less environment-friendly processes. Moreover, the E-factor can be assessed for different parts of the reaction, thus enabling the identification of hot-spots in which the environmental impact may be higher, and where mitigation actions should be conducted.41 One relevant conclusion derived from the E-factor is the need to establish intensified processes in which the use of resources may be properly optimized, namely reactions with high substrate loadings to minimize the use of solvents and water, and recycling systems that may reduce the waste.39,41–44As a downside, however, the E-factor does not allow the direct comparison among types of waste and allocates the same environmental burden to one kilogram of waste, regardless of its nature and hazardousness. To overcome this, and to complement environmental metrics, recently the concept “Total Carbon Dioxide Release” (TCR) has been introduced.45,46 The TCR results from converting all wastes into CO2, which enables fair comparisons by determining the kg CO2 per kg product that are produced in each reaction, synthetic step, functional unit, etc. The TCR allows the assessment of different reaction media for a given reaction and can calculate the impact of solvents or processes, based on the final CO2 production values.41,43,45,46 For the calculation of TCR, it is assumed that all wastes are incinerated (worst-case scenario). Herein, the organic fractions would produce ∼2.3 kg CO2 per kg solvent, assuming as model the incineration of a mixture of tetrahydrofuran, methanol, and heptane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Likewise, a mild wastewater treatment plant (WWTP) following the classical digestion steps would produce ∼0.073 kg CO2 per kg wastewater, while the treatment of recalcitrant wastewater effluent would be incineration too, rendering ∼0.63 kg CO2 per kg wastewater.45,46 With those estimations in hand, the calculation of TCR for organic reactions is straightforward. Albeit being approximate, the tool becomes useful to assess processes that are at early development phases. Based on the TCR analysis, meaningful recommendations to reach more sustainable systems can be judiciously provided.
1). Likewise, a mild wastewater treatment plant (WWTP) following the classical digestion steps would produce ∼0.073 kg CO2 per kg wastewater, while the treatment of recalcitrant wastewater effluent would be incineration too, rendering ∼0.63 kg CO2 per kg wastewater.45,46 With those estimations in hand, the calculation of TCR for organic reactions is straightforward. Albeit being approximate, the tool becomes useful to assess processes that are at early development phases. Based on the TCR analysis, meaningful recommendations to reach more sustainable systems can be judiciously provided.
As it can be rapidly deduced from the TCR metrics, the divestment of wastes to WWTP would be preferred, since this would lead to less CO2 production. However, waste effluents need to fulfil with certain standards to be accepted in WWTP.46 In that respect, chemicals are classified according to their hazardousness, under the “Water Hazard Class” concept (WGK, Wassergefährdungsklasse). Thus, chemicals can be “non-hazardous to water”, “slightly hazardous to water” (WGK1), “obviously hazardous to water” (WGK2), or “highly hazardous to water” (WGK3). If compounds of higher WGK classes are present in the wastewater, the effluent might demand a special pre-treatment before disposing it to conventional WWTP, to assure that biodegradability standards are fulfilled.46 Likewise, the same would apply to compounds with lower WGK, but present in high concentration in the water effluent (e.g. a pre-extraction to remove a significant fraction of a cosolvent). Moreover, a careful analysis of all by-products formed during the reaction and waste treatment need to be performed as well, because non-degradable or hazardous by-products can be formed during those processing steps.45,46 Importantly, the WGK of many compounds are publicly available in databases on the internet47 and can be accessed rapidly to assess the waste composition on a case-by-case basis. Thus, when new wastewater effluents are produced, some biodegradability tests need to be conducted for the specific mixture, to assure that they are on-spec for the WWTP. As result, if some (by)products are hampering the adequate biodegradation of the effluent (before divesting to WWTP), some extra pretreatments steps may be necessary (e.g. pre-extraction to remove the problematic chemicals, and send that fraction to incineration).43,45,46
3. Assessing DES from a sustainability perspective
Once considerations about the greenness and sustainability of DES (Section 1), and measurements related to CO2 production, TCR (Section 2) are made, an assessment on the fate of DES after being used as reaction media in a synthetic reaction can be conducted. It must be noted that, to our knowledge, there are no publications discussing what to do with the DES, once they have been used as reaction media. The research field is rather new, and possibly the industrial vision on how to handle these used solvents is necessary for future implemented reactions using DES. In any case, some preliminary assumptions can be made here to stimulate the research and the debate.The starting point would be to consider a (bio)catalytic process using DES as reaction media. Given the outstanding capacity of DES to dissolve substrates with even impaired solubility,1–10 it is assumed that a relatively high loading of 100 g substrate per LDES can be established, and that full conversion is achieved in the reaction. As functional unit for the assessment, the production of one kilogram of product can be considered. Therefore, 10 L DES as reaction media would be needed for such one-kilogram production if no solvent recycling loops are established (an approximate E-factor of 10, considering the solvent use). Clearly, introducing several solvent (DES) re-use steps in the process would obviously improve the figures significantly, and this should be always matter of research when assessing new synthetic reactions (although industrial recycling may not always be as straightforward as one would expect).43
3.1. The downstream processing after the reaction in DES
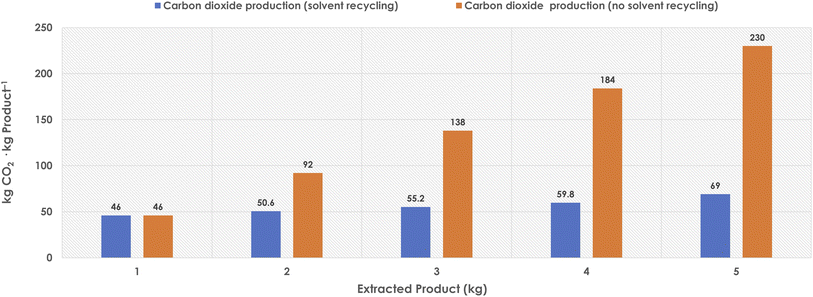
Once the reaction is concluded, the product needs to be recovered from the DES media and purified to on-spec levels to reach marketable forms. An ideal process would be such that while substrates are well dissolved in DES, the product would turn out insoluble, precipitating from the reaction media when the synthesis proceeds. Although feasible in some cases,41 such a situation cannot be considered as a frequent case, and thus other options for the downstream processing need to be introduced. Other potentially interesting options would be the use of supercritical fluids to extract products, or to trigger the selective precipitation of it. Research in these directions would be welcome to set up sustainable downstream options. A commonly reported strategy is the addition of a surplus of water to trigger the precipitation of the product, followed by water removal at high temperature to recover the DES components. The energy consumption should be considered in that case, to evaluate the environmental burden that the approach may render at large scale, together with water and solvent (DES) recycling options.In any case, the most often reported downstream processing approach for DES in literature is the product extraction by means of an organic solvent – insoluble with the DES phase – followed by solvent distillation, product recovery and DES recycling. In many of the reported cases, ethyl acetate is the solvent choice, as a DES-immiscible, readily available, inexpensive, low toxic, and potentially biogenic option. Typically, the extraction uses two-fold the reaction volume (two extraction rounds). For this downstream processing step, the CO2 production (TCR) would be related to the solvent use during extraction, when it finally goes to incineration. Importantly, if the solvent can be reused several cycles (assuming 10% solvent loss per cycle), the environmental burden can be lowered considerably, as resources are more properly used (Fig. 2).
As observed (Fig. 2), solvent recycling is crucial to reach decent TCR values in that downstream processing part.41,43 In the case of reusing the solvent five times (even with a 10% loss per cycle), the solvent TCR contribution would be reduced to ca. 14 kg CO2 per kg product. Furthermore, the combination of biogenic solvents with renewable fuels for the incineration unit would generate a proportion of neutral CO2, since the carbon present in those chemicals and fuels would be from life-based cycles. In any case, although oftentimes overlooked, the solvents used in the downstream processing step need always to be assessed for their environmental burden, and they account often for a significant amount of the waste generated in chemical processes, with or without DES.28,41,43
3.2. The fate of the DES after the reaction and downstream processing
Once the synthetic reaction has been conducted using DES as reaction media, and the product downstream is finalized, the DES “waste” remains to be disposed – ideally after its reuse for a few operations that can contribute to lowering the environmental impact and improving process economics. After some (re)uses, DES components may be partly decomposed, by-products may be formed, and in many cases colour formation appears, leading to darker solutions that must be replaced by fresh solvent. As stated above (Section 2), waste media from chemical industries can be separated in an organic fraction, and in an aqueous effluent, both collecting all respective wastes generated during the reaction (covering both upstream and downstream of the reaction, water and solvents used). The typical fate for the organic fractions is incineration, while the aqueous effluent can be sent to different procedures – incineration or WWTP – depending on the nature and recalcitrance of the fraction.45,46 Thus, if the wastewater is highly recalcitrant, incineration would be its fate as well. However, if the wastewater can be mildly treated through conventional Wastewater Treatment Plants (WWTP), this involves less severe steps and generates water that can be recycled back into the environment (see Section 2). Regardless of the option (or combinations thereof), the Total Carbon Dioxide Release (TCR) can be rapidly estimated to compare the possibilities.45,46When DES are used as reaction media, an effluent containing DES and possibly some (miscible) water parts (e.g. up to 10% (v/v) as cosolvent) will be generated. Interestingly, DES may be, in principle, treated either as an organic fraction – non-aqueous waste, or diluted in water to a concentration that grants its consideration as wastewater (reaching a certain threshold needed for an adequate mild treatment, see below). Depending on the approach, a different production of CO2 (TCR) may be expected (Fig. 3).
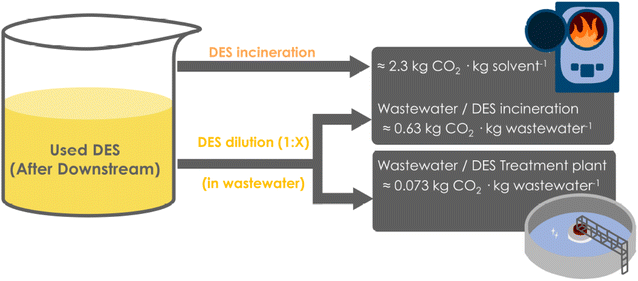 | ||
| Fig. 3 Options to dispose of the DES after their use as reaction media, either the direct incineration as “organic” waste (top), or the aqueous dilution and incineration of the wastewater (middle), or the further aqueous dilution and final mild wastewater treatment in WWTP (below). Data for TCR calculations were retrieved from the literature.45,46 | ||
As observed, the incineration of DES by treating it as “organic media” would produce a higher TCR than when aqueous dilutions are applied (Fig. 3).43,45,46 However, the dilution step will lead to larger volumes of wastewater to be treated, and thus the TCR values will also increase accordingly. Assuming different water-dilution degrees, Fig. 4 depicts the obtained TCRs when treating 10 L of DES media (assumed as necessary to produce 1 kg product at 100 g substrate loading per LDES), and considering the different options, namely the direct organic incineration, a water-dilution and incineration, or a mild wastewater treatment (WWTP) if possible.
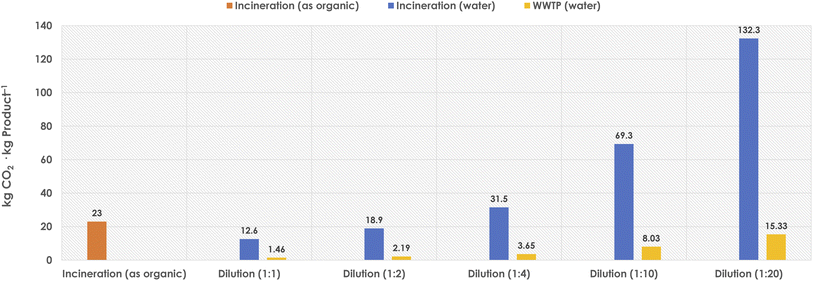 | ||
| Fig. 4 Obtained TCRs (kg CO2 per kg product) when treating 10 L DES as expended reaction media (needed to produce 1 kg product at 100 g substrate per LDES), and following different options such as organic incineration, or different aqueous dilution ratios and establishing water incineration or mild wastewater treatment (WWTP). Calculations based on TCR methods retrieved from literature.43,45,46 | ||
Concerning the “organic incineration” option, the direct treatment of the DES as a “non-aqueous solvent” would produce around 25 kg CO2 per kg product, provided that a behaviour and energy demand analogous to other conventional solvents would be applicable for a DES (at this point, this is an estimation, and empirical data are needed).45,46 It must be noted, though, that the ionic nature of the DES may create further needs during the incineration step, and it may become possible that a higher CO2 production would be generated during the DES treatment as “organic fraction”. Moreover, the presence of halides in many DES (e.g. chloride from choline chloride) should not be overlooked either. During incineration, it becomes obvious that chloride must go somewhere, and it may be expected that some forms of volatile chlorinated species will be generated during the incineration and disposed of to the environment.48 This aspect needs clearly to be considered before adopting organic incineration as the disposal path for exhausted DES coming from synthetic reactions.
As stated above, DES may be treated as a non-aqueous solvent (to be incinerated), or may be diluted to generate aqueous water–DES mixtures, with different properties depending on the dilution degree, until disaggregation of DES into its components takes place.49 Obviously, at higher dilution levels, larger volumes of wastewater will need to be treated, either as incineration, or as WWTP mildly treatable effluent. Following the above-discussed rationale (Fig. 3), the incineration of the aqueous effluent might improve the CO2 production compared to the organic incineration (Fig. 4, dilutions 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), but would not solve the presence of halide wastes and their fate to the environment. Therefore, it appears that proceeding to mild WWTP would be the most promising option for DES. Herein, large dilutions may lead to treatable wastewater effluents, which would produce more decent CO2 levels when treated. For instance, in the case of a 20-times dilution of the DES would render approximately 16 kg CO2 per kg product for its final fate after use (Fig. 4, up to dilution 1
4), but would not solve the presence of halide wastes and their fate to the environment. Therefore, it appears that proceeding to mild WWTP would be the most promising option for DES. Herein, large dilutions may lead to treatable wastewater effluents, which would produce more decent CO2 levels when treated. For instance, in the case of a 20-times dilution of the DES would render approximately 16 kg CO2 per kg product for its final fate after use (Fig. 4, up to dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20).
20).
Based on the proposed simulation, there is one important question remaining: to what extent should one dilute the DES media to reach adequate values for sending the effluent to mild WWTP? The higher the dilution, the higher production of CO2, because a larger volume must be treated (Fig. 4). Thus, the dilution degree should be kept to a minimum, as long as the biodegradability tests show that it can be accepted to the WWTP. As stated above (Section 2), the data provided until now for the biodegradability of DES (and components) are, in general, promising, as most of the assessments classify them as “readily biodegradable”, and furthermore the usual DES components are regarded as WGK1, “slightly hazardous to water”.43 Admittedly, all these studies cover only a rather narrow range of the DES possibilities (choline chloride, glycerol, urea, etc.), and under relatively low concentrated conditions (as biodegradability tests so establish). The influence of the concentration, namely the critical DES concentration that wastewater microorganisms can manage, is crucial to determining the dilution degree.14,17,24,50 In that respect, some works have proposed to assess the influence of the composition of the wastewater microorganisms in the degradation of DES.14,17 Herein, a particular aspect to be addressed is the presence of chloride – or other halides, and its toxicity for the aquatic environment,14,51–53 which may force to lower the thresholds significantly during the dilution step (at the cost of treating larger aqueous volumes and producing more CO2). To overcome these issues, the use of more resistant extremophiles has been proposed for wastewater treatment as well.16 Other options might include the use of DES components as carbon source for microorganisms in bacterial fuel cells, generating energy while reducing wastes. In a different line, the reported formation of by-products, due to the inherent reactivity of DES, should be assessed in-depth, since some of these unexpectedly formed chemicals can end up in the wastewater and jeopardize the overall biodegradability of the effluent.11 In particular for DES, the interaction of the components with other chemicals present in the effluent – which may act as cosolvents, needs to be assessed to, as unexpected interactions and new properties may appear.54 In general, the mild wastewater treatment seems to be promising for the DES fate, but clearly more research needs to be devoted to validating the extent of the dilution to reach a WWTP. A compromise may possibly be needed, combining an acceptable dilution degree with a decent, low CO2 production.
4. Concluding remarks
To validate the greenness and sustainability of DES (and solvents in general), aspects related to the chemical origin, the synthetic steps, and the biocompatibility and biodegradability of DES need to be considered. Besides that, this paper has addressed a largely unexplored aspect related to DES, namely their fate after being used as synthetic reaction media. What can be done with used DES once they must be discarded? After a process is conducted in DES, the downstream unit is implemented to deliver the product in a marketable form. The traditional ways of doing this with DES are either the addition of a large surplus of water, which triggers product precipitation and further recovery of DES upon water evaporation, or the use of an extractive organic solvent, which will be ultimately incinerated, after some cycles. Later, the used DES media can have, in principle, several alternatives: either incineration as an organic fraction, or dilution in water to be divested to WWTP. Due to the lower CO2 formed during WWTP and the downside of the potential halide compounds formation during incineration, the WWTP route seems in principle more environmentally feasible. Research is needed to determine the “dilution degree” that will make DES–water effluents acceptable in WWTP, enabling a trade-off between the lowest dilution degree possible with acceptable biodegradation patterns for the WWTP. We hope that this article will stimulate research in these areas, to provide sustainable options for the DES fate, in particular, and for other solvents in general.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
PDdM acknowledges financial support from the European Union's Horizon 2020 research and innovation programme RADICALZ (grant number: 101000560) is gratefully acknowledged. SK acknowledges Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, grant number: 391127961) for the financial support. This project has received funding from the European Union's Horizon Europe research and innovation program under the Marie Skłodowska-Curie grant agreement no. 101072731. The authors thank Dr Johanna Meyer (Institute of Technical Chemistry, Leibniz University Hannover) for the graphical representations.References
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhik, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 CrossRef CAS PubMed.
- A. A. Quintana, A. M. Sztapka, V. Santos Ebinuma and C. Agatemor, Angew. Chem., Int. Ed., 2022, 134, e202205609 CrossRef.
- A. Prabhune and R. Dey, J. Mol. Liq., 2023, 379, 121676 CrossRef CAS.
- D. Procopio, X. Marset, G. Guillena, M. L. Di Gioia and D. J. Ramon, Adv. Synth. Catal., 2023 DOI:10.1002/adsc.202300725.
- X. Marset and G. Guillena, Molecules, 2022, 27, 8445 CrossRef CAS PubMed.
- D. A. Alonso, S. J. Burlingham, R. Chinchilla, G. Guillena, D. J. Ramón and M. Tiecco, Eur. J. Org Chem., 2021, 4065–4071 CrossRef CAS.
- D. Amodo, E. Maffeis, F. Marra, S. Nejrotti and C. Prandi, Molecules, 2023, 28, 516 CrossRef PubMed.
- N. Guajardo, C. R. Müller, R. Schrebler, C. Carlesi and P. Domínguez de María, ChemCatChem, 2016, 8, 1020–1027 CrossRef CAS.
- M. Pätzold, S. Siebenhaller, S. Kara, A. Liese, C. Syldatk and D. Holtmann, Trends Biotechnol., 2019, 37, 943–959 CrossRef PubMed.
- D. O. Abranches and J. A. P. Coutinho, Annu. Rev. Chem. Biomol. Eng., 2023, 14, 141–163 CrossRef CAS PubMed.
- Q. Zaib, M. J. Eckelman, Y. Yang and D. Kyung, Green Chem., 2022, 24, 7924–7930 RSC.
- M. Vieira Sanches, R. Freitas, M. Oliva, A. Mero, L. De Marchi, A. Cuccaro, G. Fumagalli, A. Mezzetta, G. Colombo Dugoni, M. Ferro, A. Mele, L. Guazzelli and C. Pretti, Environ. Sci. Pollut. Res., 2023, 30, 17268–17279 CrossRef CAS PubMed.
- S. Nejrotti, A. Antenucci, C. Pontremoli, L. Gontrani, N. Barbero, M. Carbone and M. Bonomo, ACS Omega, 2022, 7, 47449–47461 CrossRef CAS PubMed.
- L. Lomba, M. P. Ribate, E. Sangüesa, J. Concha, M. P. Garralaga, D. Errazquin, C. B. García and B. Giner, Appl. Sci., 2021, 11, 10061 CrossRef CAS.
- G. Martínez Martínez, G. Guillena Townley and R. M. Martínez-Espinosa, Heliyon, 2022, e12567 CrossRef PubMed.
- Z. Yang, in Deep Eutectic Solvents: Synthesis, Properties and Applications, ed. D. J. Ramón and G. Guillena, Wiley-VCH, Weinheim, Germany, 2020 Search PubMed.
- D. Lapeña, D. Errazquin, L. Lomba, C. Lafuente and B. Giner, Environ. Sci. Pollut. Res., 2021, 28, 8812–8821 CrossRef PubMed.
- K. Radosevic, M. Cvjetko Bubalo, V. G. Srcek, D. Grgas, T. L. Dragivecic and I. R. Redovnikovic, Ecotoxicol. Environ. Saf., 2015, 112, 46–53 CrossRef CAS PubMed.
- I. Juneidi, M. Hayyan and M. A. Hashim, RSC Adv., 2015, 5, 83636–83647 RSC.
- X. D. Hou, Q. P. Lui, T. J. Smith, N. Li and M. H. Zong, PLoS One, 2013, 8, e59145 CrossRef CAS PubMed.
- M. Marchel, H. Cieslinski and G. Boczkaj, J. Hazard. Mater., 2022, 425, 127963 CrossRef CAS PubMed.
- J. Torregrosa-Crespo, X. Marset, G. Guillena, D. J. Ramón and R. M. Martínez-Espinosa, Sci. Total Environ., 2020, 704, 135382 CrossRef CAS PubMed.
- A. Azzouz and M. Hayyan, Process Saf. Environ. Prot., 2023, 176, 1021–1025 CrossRef CAS.
- Y. Huang, F. Feng, J. Jiang, Y. Qiao, T. Wu, J. Voglmeir and Z. G. Chen, Food Chem., 2017, 221, 1400–1405 CrossRef CAS PubMed.
- B. Y. Zhao, P. Zu, F. X. Yang, H. Wu, M. H. Zong and W. Y. Lou, ACS Sustainable Chem. Eng., 2015, 3, 2746–2755 CrossRef CAS.
- D. Coleman and N. Gathergood, Chem. Soc. Rev., 2010, 39, 600–637 RSC.
- P. Domínguez de María, EFB Bioeconomy J., 2023, 3, 100056 CrossRef.
- K. O. Wikene, H. V. Rukke, E. Bruzell and H. H. Tonnesen, J. Photochem. Photobiol., B, 2017, 171, 27–33 CrossRef CAS PubMed.
- K. O. Wikene and E. Bruzell, J. Photochem. Photobiol., B, 2015, 148, 188–196 CrossRef CAS PubMed.
- K. O. Wikene, H. V. Rukke, E. Bruzell and H. H. Tonnesen, Eur. J. Pharm. Biopharm., 2016, 105, 75–84 CrossRef CAS PubMed.
- J. Wang, X. Dong, Q. Yua, S. N. Baker, H. Li, N. E. Larm and G. A. Baker, Dent. Mater., 2017, 33, 1445–1455 CrossRef CAS PubMed.
- W. H. Evans and E. J. David, Water Res., 1974, 8, 97–100 CrossRef CAS.
- W. H. Evans, E. J. David and S. J. Patterson, Water Res., 1973, 7, 975–985 CrossRef CAS.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- B. M. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS PubMed.
- R. A. Sheldon, ACS Sustainable Chem. Eng., 2018, 6, 32–48 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2023, 25, 1704–1728 RSC.
- P. Domínguez de María, Curr. Opin. Green Sustainable Chem., 2021, 31, 100514 CrossRef.
- E. Tieves, F. Tonin, E. Fernández-Fueyo, J. M. Robbins, B. Bommarius, A. S. Bommarius, M. Alcalde and F. Hollmann, Tetrahedron, 2019, 75, 1311–1314 CrossRef.
- P. Domínguez de María, Green Chem., 2022, 24, 9620–9628 RSC.
- L. E. Meyer, M. Hobisch and S. Kara, Curr. Opin. Biotechnol., 2022, 78, 102835 CrossRef CAS PubMed.
- P. Domínguez de María, S. Kara and F. Gallou, Molecules, 2023, 28, 6452 CrossRef PubMed.
- B. Burek, A. Dawood, F. Hollmann, A. Liese and D. Holtmann, Front. Catal., 2022, 2, 858706 CrossRef.
- U. Onken, A. Koettgen, H. Scheidat, P. Schuepp and F. Gallou, Chimia, 2019, 73, 730–736 CrossRef CAS PubMed.
- C. Krell, R. Schreiber, L. Hueber, L. Sciascera, X. Zheng, Z. Clarke, R. Haenggi, M. Parmentier, H. Baguia, S. Rodde and F. Gallou, Org. Process Res. Dev., 2021, 25, 900–915 CrossRef CAS.
- https://webrigoletto.uba.de/rigoletto/public/welcome.do, accessed January 2024.
- W. Ma, W. Terrence, F. J. Frandsen, Y. Beibei and C. Guany, Prog. Energy Combust. Sci., 2020, 76, 100789 CrossRef.
- O. S. Hammond, D. T. Bowron and K. J. Edler, Angew. Chem., Int. Ed., 2017, 33, 9782–9785 CrossRef PubMed.
- Q. Wen, J. X. Chen, Y. L. Tang, J. Wang and Z. Yang, Chemosphere, 2015, 132, 63–69 CrossRef CAS PubMed.
- G. De Boeck, A. Vlaeminck, A. van der Linden and R. Blust, Physiol. Biochem. Zool., 2000, 73, 102–111 CrossRef CAS PubMed.
- J. R. F. Elphick, K. D. Bergh and H. D. Bailey, Environ. Toxicol. Chem., 2011, 30, 239–246 CrossRef CAS PubMed.
- D. J. Soucek, T. K. Linton, C. D. Tarr, A. Dickinson, N. Wickramanayake, G. G. Delos and L. A. Cruz, Environ. Toxicol. Chem., 2011, 30, 930–938 CrossRef CAS PubMed.
- M. Busato, A. Del Giudice, V. Di Lisio, P. Tomai, V. Migliorati, A. Gentili, A. Martinelli and P. D'Angelo, ACS Sustainable Chem. Eng., 2021, 9, 12252–12261 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |