Open Access Article
Open Access ArticleSustainable artificial coral reef restoration using nanoclays and composite hydrogel microcapsules†
Mohammad
Fahimizadeh
 ab,
Febrianne
Sukiato
c,
Kok Lynn
Chew
cd,
Yang Amri
Affendi
ab,
Febrianne
Sukiato
c,
Kok Lynn
Chew
cd,
Yang Amri
Affendi
 c,
Pooria
Pasbakhsh
c,
Pooria
Pasbakhsh
 *a,
Joash Ban
Lee Tan
*a,
Joash Ban
Lee Tan
 ef,
R. K. Singh
Raman
g and
Peng
Yuan
b
ef,
R. K. Singh
Raman
g and
Peng
Yuan
b
aSchool of Engineering, Monash University Malaysia, Jalan Lagoon Selatan, 47500 Bandar Sunway, Selangor, Malaysia. E-mail: Pooria.pasbakhsh@monash.edu
bSchool of Environmental Science and Engineering, Guangdong University of Technology, Guangzhou, 510006, China
cInstitute of Ocean and Earth Sciences (IOES), Universiti Malaya, 50603 Kuala Lumpur, Wilayah, Kuala Lumpur, Malaysia
dCoralku Solutions, 6000 Kuala Lumpur, 6000 Wilayah Kuala Lumpur, Malaysia
eSchool of Science, Monash University Malaysia, Jalan Lagoon Selatan, 47500 Bandar Sunway, Selangor, Malaysia
fTropical Medicine and Biology Multidisciplinary Platform, Monash University Malaysia, Jalan Lagoon Selatan, 47500 Bandar Sunway, Selangor, Malaysia
gDepartment of Chemical and Biological Engineering, Department of Mechanical and Aerospace Engineering, Monash University, Clayton 3168, Australia
First published on 8th January 2024
Abstract
This report assesses the potential of sustainably-produced artificial coral reef materials metakaolin clay, hydrogel microcapsules, and nanocomposite microcapsule-encapsulated calcifying bacteria as artificial coral reef constituents. The findings show that nanoclays and hydrogels can be considered safe, potential artificial reef constituents, and can improve the sustainability of artificial reefs.
Sustainability spotlightIn July 2023, the global ocean surface temperatures reached unprecedented levels due to anthropogenic global warming. Current projections for the coming decades spell doom for marine organisms not adapted to survive in warmer and more acidic seas. Among the most vulnerable are coral reefs, which form the backbone of numerous marine ecosystems. Coral reefs are under tremendous pressure from global warming, disease outbreaks, and pollution, losing substantial coverage every year. This short communication describes an interdisciplinary effort to assess the potential of novel, environmentally sustainable construction additives for coral reef restoration. The findings can lead to the construction of better performing and more sustainable artificial reefs, coastal and offshore structures, and coral nursery materials, firmly aligning the progress of the field with several United Nations Sustainable Development Goals (SDGs), namely SDG9 (Industry, Innovation and Infrastructure), SDG13 (Climate Action), and SDG 14 (Life Below Water), to address and mitigate the threats posed by global warming. |
Coral reefs are mostly composed of hard corals with skeletons made of calcium carbonate. They are found in shallow waters of tropical and subtropical oceans worldwide, providing habitats for various marine organisms.1,2 The Coral Triangle is an area with the highest marine biodiversity in the world,3 and Malaysia is one of the countries located in this area. It is estimated that Malaysia contains 1595.21 km2 of coral reefs and gains multiple environmental and economic benefits from its coral reefs.4,5 The Department of Marine Park Malaysia evaluated that its marine protected areas with coral reefs generated approximately USD 1.93 billion in total economic value from its ecosystem services from 2011 to 2015.6
In recent decades, global coral reef cover has declined due to anthropogenic factors such as pollution, unsustainable coastal development, and destructive fishing practices.7,8 However, climate change brought about by global warming has been identified as the main cause of the decline of coral reefs worldwide,8,9 and various coral restoration efforts have been taken to assist in the recovery of degraded reefs.10 According to a recent coral restoration review, 21% of coral restoration projects worldwide deploy artificial reef structures to increase habitat for marine life and fisheries production of coral reefs, provide coastal recreational activities (i.e., SCUBA diving), and prevent destructive fishing activities.2 In Malaysia, artificial reef structures have been deployed since 1975 to conserve marine areas by preventing trawling activities in nearshore areas, providing habitat for marine life, and boosting commercial fish populations to improve the catches of artisanal fishermen.11,12 Another notable use of artificial reef structure is to provide an area for coral larvae to settle and thrive.13 Mobile coral larvae depend on physical cues to determine their settlement location and often seek deposits of crustose coralline algae (CCA) with a rugose-textured surface.14 Moreover, an in situ study by Goreau15 found that increased calcium carbonate formation can stimulate the settlement of coral larvae, growth of coral, and other calcifying organisms. The importance of the presence of calcium carbonate for coral attachment has been highlighted in other studies.16,17
Artificial reef structures are commonly fabricated using standard construction materials, such as cement and steel bars. Such materials are widely available and have predictable performance and service life. However, cement and steel production has a high environmental impact.18,19 The durability of steel-reinforced cement-based structures is significantly lowered by crack formation and ingress of corrosive elements such as chloride, which makes the artificial reef material susceptible to strong waves and surges, lowering the service life of the artificial reef material.20 Considering the intensifying pressure on coral reef ecosystems and cement-based structures due to global warming and ocean acidification, artificial coral reefs are candidates for multidisciplinary research. Self-healing of concrete is a novel and sustainable technique that can autonomously fix structural concrete cracks and fill small pores, extending the service life of concrete structures.18 Concrete self-healing systems rely on chemical and biological healing agents, of which the biological pathway is considered the most sustainable. Biological concrete self-healing relies on calcium carbonate precipitation by alkaliphilic spore-forming bacteria that can withstand the harsh cement environment. Calcium carbonate precipitation is triggered only after structural faults introduce water and oxygen into the cement matrix, resulting in spore germination.
When the self-healing system is concentrated on the structure's outer surface, this trigger is no longer needed to initiate biomineralization as the bacterial spores would readily germinate. Bacterial self-healing concrete systems can also increase calcium–silicate–hydrate (CSH) gel formation in concrete.18 Alongside the increased calcium carbonate formation, this aspect can improve concrete's structural resistance and surface complexity.
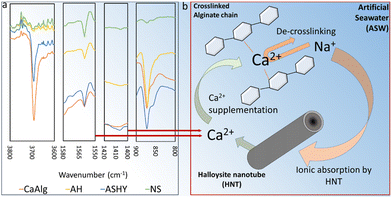
Biological healing agents are susceptible to the harsh alkaline cement environment and therefore require protection, often provided by solid carriers such as expanded clay,21 nanoclay,22 lightweight aggregate,23 and hydrogels such as calcium alginate (CaAlg) microcapsules.24,25 Alginate is harvested from brown algae and readily cross-links into a biopolymer with divalent cations such as calcium ions. As a hydrogel, CaAlg can store water beneficial for biological activity and cement hydration through internal curing. Also, cross-linked CaAlg capsules possess a complex surface that may benefit coral settlement and attachment. However, CaAlg can degrade due to the high alkalinity of the cement environment,24 and can benefit from structural reinforcement by additives such as halloysite clay nanoparticles (HNT).26,27 HNTs can further add to the surface complexity of CaAlg microcapsules and improve coral attachment while strengthening the cement matrix and CaAlg-cement bonding through pozzolanic reactions.28–30 Other pozzolans, like metakaolin, can also benefit coral attachment by providing complex surfaces and pozzolanic reactions that improve the cement matrix.31,32 Another interesting aspect is the potential of CaAlg to be colonized by beneficial reef microorganisms that can help sustain a healthy reef.33
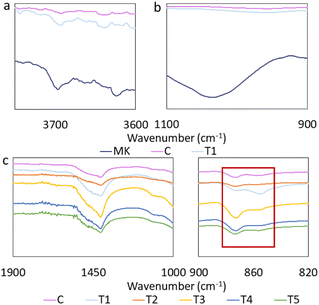
In the current report, the impact of metakaolin (MK) and various microcapsules added to cement paste samples on the health and attachment of coral fragments and the structure of the cement paste samples were investigated. The summary of the samples is presented in Table 1. A full description of the cement paste samples preparation and constituents has been provided in ESI Appendix A, Table SI2.† Adding metakaolin to the cement paste resulted in a loss of structural order in metakaolin. The metakaolin in-plane Si–O–Si stretching band at 1035 cm−1 disappeared after cement paste incorporation (Fig. 1b). The band at 3620 cm−1 is attributed to the stretching vibration of the inner –OH groups, and the bands at 3696 cm−1, 3669 cm−1 and 3656 cm−1, that are attributed to the stretching of inner octahedral surface –OH groups all experienced intensity loss, and peak broadening (Fig. 1a). This observation has been previously considered as a sign of geopolymerization.34
| Cement paste series | Capsule type | MK | Alg | HNT | YE | S |
|---|---|---|---|---|---|---|
| C | − | − | − | − | − | − |
| T1 | − | + | − | − | − | − |
| T2 | CaAlg | + | + | − | − | − |
| T3 | AH | + | + | + | − | − |
| T4 | ASHY | + | + | + | + | + |
| T5 | ASY | + | + | − | + | + |
The intensity of the bands attributed to CaCO3 increased after the addition of metakaolin to the cement paste; however, the relative intensity of 873 cm−1 was lowered while the relative intensity of 855 cm−1 increased in metakaolin-containing cement paste samples, indicating higher vaterite content relative to calcite (Fig. 1c).24,35 Comparison of the relative intensities of the 873 cm−1 and 855 cm−1 peaks suggests a higher calcite proportion in T2, T3, T4, and T5 samples than in cement paste control samples (Fig. 1c).
FTIR spectra of microcapsules separated from cement paste samples after nine weeks of artificial seawater exposure displayed evidence of CaCO3 formation in all microcapsules, with greater intensity of the corresponding peak for the ASHY microcapsules. Furthermore, Ca-alginate de-crosslinking is evidenced by shifts in 1575 cm−1 and 1418 cm−1 peaks towards wavenumbers associated with Na-alginate for all microcapsules (Fig. 2b). The trend in peak shift suggests that AH microcapsules display the highest resistance to de-crosslinking than other microcapsules. The likely reason behind Ca-alginate de-crosslinking is the substitution of Ca2+ with Na+ and Mg2+ (Fig. 2c). The presence of HNTs in the AH microcapsules seems to decrease the rate of dissociation of Ca2+ from the Ca-alginate polymer chains, hypothetically by carrying forward Ca2+ ions from the Ca2+-alginate crosslinking step of the microcapsule preparation process, into the cement-embedded microcapsules (Fig. 2c). The presence of an intense band at 3692 cm−1 in the spectra of CaAlg and ASHY microcapsules can be attributed to non-structural –OH (Fig. 2a),36 which also indicates the presence of Na or Ca hydroxides in the CaAlg and AH microcapsules.
No significant differences were detected in coral color scores between treatments throughout the experiment between treatments or across different weeks (Fig. 3, statistics in ESI Appendix B†). The highest coral health scores observed were from coral fragments attached to C and T4 cement paste samples, reaching the approximate mean health score of 5.3 after six weeks. Coral fragments attached to different cement paste samples recovered to varying degrees from the stress of sampling and transplantation. The assessment-wide drop in the health scores at week nine occurred possibly due to an increase in water and atmospheric temperatures caused by a local heatwave. Such stresses were experienced by all coral fragments, and all fragments had recovered from the initial stress prior to the experiment. The stresses and recovery were judged based on significant colour changes, visible signs of stress post fragmentation, transportation and during treatment.
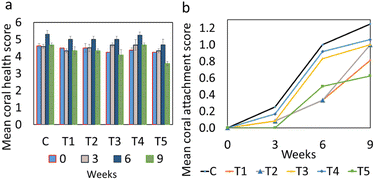 | ||
| Fig. 3 Nine-week measurements of mean coral (a) health score and (b) attachment score. The error bars for health score measurements are based on standard deviation. | ||
The coral health score findings confirm that the elements in the artificial reef samples, i.e., the cement, metakaolin, calcium alginate, halloysite nanotubes, the bacteria, and the nutrients, may have no adverse effects on coral health.
It should be noted that among all components, only the bacteria, the nutrients, and minute quantities of calcium ions or calcium lactate leftover from the capsule formation steps would diffuse into the aqueous phase. No sudden changes in water parameters were observed during the initial days of placing the cement paste samples into the closed aquarium systems despite the expected diffusion of constituents, such as Ca(OH)2 and Ca2+. Therefore, the constituents of the cement paste samples can be considered safe for coral attachment.
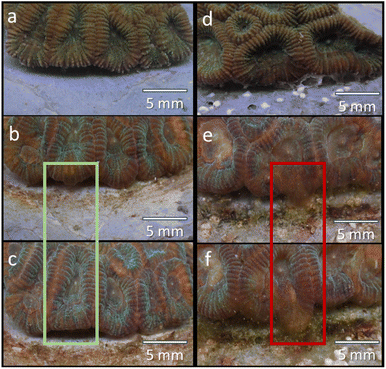
Coral attachment to different cement paste samples was visually evident after three weeks (Fig. 3 and 4). Coral fragments in all treatments gradually initiated the attachment process, as evident from the formation of soft tissue in the fragment-cement paste interface (Fig. 4). The retraction of this new tissue formation was also observed in some fragments (Fig. 4b and c). Overall, no significant differences were detected in attachment scores between treatments on any day of the experiment (Fig. 3 and 4, statistics in ESI Appendix C†). Coral attachment first became noticeable in control samples after 18 days, and all fragments initiated attachment after 36 days. This finding suggests that the microcapsules did not negatively impact coral attachment. The findings infer that the coral attachment was not affected by the increase in the surface complexity brought upon by the inclusion of the microcapsules, as well as the presence of varying textures, surface charges, and active chemical groups on the cement surface.
This study found that 25% cement replacement by hydrogel microcapsules and the nanoclays did not significantly impact either measurement or result in any mortality of the coral. Hence, it can be concluded that cement replacement with metakaolin and hydrogel microcapsules had no negative impact on coral health and attachment and can therefore be considered a valid cement replacement for artificial coral reefs. The findings can have substantial implications in the regions where coral reefs are the most vulnerable, particularly in tropical South East Asia.
Moreover, the potential of geopolymers based on sustainably-sourced nanoclays such as kaolin and halloysite, biomineralized CaCO3, hydrogels, and pozzolanic nanoclays as artificial coral reef materials can be elucidated from the findings of this study. Future research is needed to more clearly ascertain the influence of these constituents on coral health and attachment, and to assess the durability of artificial coral reefs composed of such materials.
Conflicts of interest
There are no conflicts to declare.Notes and references
- N. Knowlton, R. E. Brainard, R. Fisher, M. Moews, L. Plaisance and M. J. Caley, in Life in the World's Oceans: Diversity, Distribution and Abundance, 2010 Search PubMed.
- G. J. Williams, N. A. J. Graham, J. B. Jouffray, A. V. Norström, M. Nyström, J. M. Gove, A. Heenan and L. M. Wedding, Funct. Ecol., 2019, 33(6), 1014–1022 CrossRef.
- L. Burke, K. Reytar, M. Spalding and A. Perry, Reefs at Risk Revisited, 2011 Search PubMed.
- L. Boström-Einarsson, R. C. Babcock, E. Bayraktarov, D. Ceccarelli, N. Cook, S. C. A. Ferse, B. Hancock, P. Harrison, M. Hein, E. Shaver, A. Smith, D. Suggett, P. J. Stewart-Sinclair, T. Vardi and I. M. McLeod, PLoS One, 2020, 15(1), e0226631 CrossRef PubMed.
- P. L. Choo, S. Magupin, M. A. Syed Hussein, Z. Waheed and A. Yang Amri, in The Marine Ecosystems of Sabah, Penerbit Universiti Malaysia Sabah, 2022, pp. 173–187 Search PubMed.
- Jabatan Taman Laut Malaysia, Total Economic Value of Marine Biodiversity, 2015 Search PubMed.
- M. Safuan, H. B. Wee, Y. S. Ibrahim, I. Idris and Z. Bachok, J. Sustain. Sci. Manag., 2016, 108 Search PubMed.
- O. Hoegh-Guldberg, E. S. Poloczanska, W. Skirving and S. Dove, Front. Mar. Sci., 2017, 4(158) DOI:10.3389/fmars.2017.00158.
- T. P. Hughes, J. T. Kerry, A. H. Baird, S. R. Connolly, A. Dietzel, C. M. Eakin, S. F. Heron, A. S. Hoey, M. O. Hoogenboom, G. Liu, M. J. McWilliam, R. J. Pears, M. S. Pratchett, W. J. Skirving, J. S. Stella and G. Torda, Nature, 2018, 556, 492–496 CrossRef CAS PubMed.
- A. J. Edwards, J. Guest, S. Shafir, D. Fisk, E. Gomez, B. Rinkevich, A. Heyward, M. Omori, K. Iwao, R. Dizon, A. Morse, C. Boch, S. Job, L. Bongiorni, G. Levy, L. Shaish and S. Wells, Reef Rehabilitation Manual, 2010 Search PubMed.
- A. N. Umar, R. Anwar, O. H. Hassan and Z. Zakaria, in Proceedings of the International Symposium on Research of Arts, Design and Humanities (ISRADH 2014), 2015 Search PubMed.
- A. R. bin Latun and M. P. bin Abdullah, Pap. Present. Symp. Artif. Reefs Fish Aggregating Devices as Tools Manag. Enhanc. Mar. Fish. Resour., Colombo, Sri Lanka, 1990 Search PubMed.
- E. Higgins, A. Metaxas and R. E. Scheibling, PLoS One, 2022, 17 Search PubMed.
- L. Harrington, K. Fabricius, G. De’Ath and A. Negri, Ecology, 2004, 85, 3428–3437 CrossRef.
- T. J. Goreau, Nat. Resour., 2014, 5, 527–537 Search PubMed.
- M. G. Sabater and H. T. Yap, J. Exp. Mar. Biol. Ecol., 2002, 272, 131–146 CrossRef CAS.
- S. M. Strömberg, T. Lundälv and T. J. Goreau, J. Exp. Mar. Biol. Ecol., 2010, 395, 153–161 CrossRef.
- M. Fahimizadeh, P. Pasbakhsh, L. S. Mae, J. B. L. Tan and R. K. S. Raman, Engineering, 2022, 13, 217–237 CrossRef CAS.
- A. Røyne, Y. J. Phua, S. Balzer Le, I. G. Eikjeland, K. D. Josefsen, S. Markussen, A. Myhr, H. Throne-Holst, P. Sikorski and A. Wentzel, PLoS One, 2019, 14, e0212990 CrossRef PubMed.
- V. Wiktor and H. M. Jonkers, Smart Mater. Struct., 2016, 25, 84006 CrossRef.
- V. Wiktor and H. M. Jonkers, Cem. Concr. Compos., 2011, 33, 763–770 CrossRef CAS.
- M. Fahimizadeh, P. Pasbakhsh, L. S. Mae, J. B. L. Tan and R. K. S. Raman, Appl. Clay Sci., 2023, 240, 106979 CrossRef CAS.
- W. Khaliq and M. B. Ehsan, Constr. Build. Mater., 2016, 102, 349–357 CrossRef CAS.
- M. Fahimizadeh, A. D. Abeyratne, L. S. Mae, R. K. Raman Singh and P. Pasbakhsh, Materials, 2020, 13, 3711 CrossRef CAS PubMed.
- V. W. Wiktor and H. M. Jonkers, Smart Mater. Struct., 2016, 25, 84008 CrossRef.
- L. Liu, Y. Wan, Y. Xie, R. Zhai, B. Zhang and J. Liu, Chem. Eng. J., 2012, 187, 210–216 CrossRef CAS.
- Y. Jin, R. Yendluri, B. Chen, J. Wang and Y. Lvov, J. Colloid Interface Sci., 2016, 466, 254–260 CrossRef CAS PubMed.
- T. Yu, B. Zhang, P. Yuan, H. Guo, D. Liu, J. Chen, H. Liu and L. Setti Belaroui, Constr. Build. Mater., 2023, 389, 131709 CrossRef CAS.
- T. T. Haw, F. Hart, A. Rashidi and P. Pasbakhsh, Appl. Clay Sci., 2020, 188, 105533 CrossRef CAS.
- B. Zhang, H. Guo, P. Yuan, Y. Li, Q. Wang, L. Deng and D. Liu, Appl. Clay Sci., 2020, 185, 105375 CrossRef CAS.
- M. Li, X. Zhu, A. Mukherjee, M. Huang and V. Achal, J. Hazard. Mater., 2017, 329, 178–184 CrossRef CAS PubMed.
- B. Zhang, T. Yu, H. Guo, J. Chen, Y. Liu and P. Yuan, Clays Clay Miner., 2022, 70, 882–902 CrossRef CAS.
- J. Li, Q. Yang, J. Dong, M. Sweet, Y. Zhang, C. Liu, Y. Zhang, X. Tang, W. Zhang and S. Zhang, Engineering, 2022, 28, 105–116 CrossRef.
- S. Hollanders, R. Adriaens, J. Skibsted, Ö. Cizer and J. Elsen, Appl. Clay Sci., 2016, 132, 552–560 CrossRef.
- K. S. Singh and S. G. Sawant, Indian J. Geo-Marine Sci., 2022, 51 Search PubMed.
- T. Zhao, X. Han, L. He, Y. Jia and R. C. Yu, ACS Omega, 2023, 8, 702–708 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00330b |
| This journal is © The Royal Society of Chemistry 2024 |