Synthesis, crystal structures, and in vitro anticancer properties of new N-heterocyclic carbene (NHC) silver(i)- and gold(i)/(iii)-complexes: a rare example of silver(i)–NHC complex involved in redox transmetallation†
Abstract
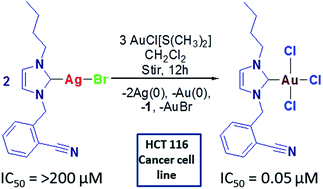
A series of cationic, linearly coordinated silver(I)– (4–6) and gold(I)/(III)–NHC (NHC = N-heterocyclic carbene) (7–9) complexes of (benz)imidazol-2-ylidene ligands was prepared and successfully characterized. Complexes 4–6 were prepared by in situ deprotonation of azolium salts and 7–9 by NHC transfer from their respective silver(I) complexes. All new compounds were characterized by elemental analysis and 1H, 13C NMR, and FTIR spectroscopy techniques, and four of the complexes were unambiguously characterized by single-crystal X-ray diffraction method. Results of structural studies on single crystals of 5a, 6, and 8 revealed a linear coordination geometry about the metal center, whereas gold(III) in complex 7 had a consistent square-planar geometry. The reaction of complex 4 with gold(I) source formed a gold(III)–NHC complex following redox transmetallation. In all these cases, metal⋯H and π⋯π stacking interactions were observed, which affected the Ccarbene–M–Ccarbene bond angle. Preliminary anticancer activities of all new compounds were also studied in vitro against four human derived cancer cell lines by MTT assay method. Cationic silver(I) complexes displayed better anticancer potentials than their gold(I) counterparts. In particular, gold(III) complex displayed superior activity, with IC50 values at low-micro and nanomolar levels; thus, this complex showed superior selectivity toward all four tested cancer cell lines.

 Please wait while we load your content...
Please wait while we load your content...