Enantioselective synthesis of 1,2,5,6-tetrahydropyridines (THPs) via proline-catalyzed direct Mannich-cyclization/domino oxidation–reduction sequence: application for medicinally important N-heterocycles†
Abstract
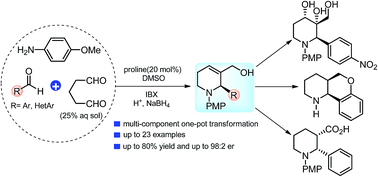
An enantioselective multi-component synthesis of 1,2,5,6-tetrahydropyridines (THPs) has been developed through a one-pot domino-process. This transformation proceeds through proline-catalyzed direct Mannich reaction-cyclization of glutaraldehyde with in situ generated imines, followed by site-selective oxidation–reduction sequence under mild conditions. Chiral 1,2,5,6-THPs are obtained in good to high yields (up to 80%) and with the excellent enantioselectivity (up to 98 : 2 er). The usefulness of this operationally simple method is also shown to synthesize other medicinally important nitrogen-heterocycles.

 Please wait while we load your content...
Please wait while we load your content...