Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Near-infrared fluorescent probe for hydrogen sulfide: high-fidelity ferroptosis evaluation in vivo during stroke†
Tianyu
Liang‡
a,
Taotao
Qiang‡
 *a,
Longfang
Ren
a,
Fei
Cheng
a,
Baoshuai
Wang
a,
Mingli
Li
a,
Wei
Hu
*a,
Longfang
Ren
a,
Fei
Cheng
a,
Baoshuai
Wang
a,
Mingli
Li
a,
Wei
Hu
 *ab and
Tony D.
James
*ab and
Tony D.
James
 *bc
*bc
aCollege of Bioresources and Materials Engineering, Shaanxi Collaborative Innovation Center of Industrial Auxiliary Chemistry & Technology, Shaanxi University of Science & Technology, Xi'an, 710021, China. E-mail: qiangtt515@163.com; huwchem@163.com
bDepartment of Chemistry, University of Bath, Bath, BA27AY, UK. E-mail: t.d.james@bath.ac.uk
cSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453007, China
First published on 21st February 2022
Abstract
Ferroptosis is closely associated with cancer, neurodegenerative diseases and ischemia-reperfusion injury and the detection of its pathological process is very important for early disease diagnosis. Fluorescence based sensing technologies have become excellent tools due to the real-time detection of cellular physiological or pathological processes. However, to date the detection of ferroptosis using reducing substances as markers has not been achieved since the reducing substances are not only present at extremely low concentrations during ferroptosis but also play a key role in the further development of ferroptosis. Significantly, sensors for reducing substances usually consume reducing substances, instigating a redox imbalance, which further aggravates the progression of ferroptosis. In this work, a H2S triggered and H2S releasing near-infrared fluorescent probe (HL-H2S) was developed for the high-fidelity in situ imaging of ferroptosis. In the imaging process, HL-H2S consumes H2S and releases carbonyl sulfide, which is then catalyzed by carbonic anhydrase to produce H2S. Importantly, this strategy does not intensify ferroptosis since it avoids disruption of the redox homeostasis. Furthermore, using erastin as an inducer for ferroptosis, the observed trends for Fe2+, MDA, and GSH, indicate that the introduction of the HL-H2S probe does not exacerbate ferroptosis. In contrast, ferroptosis progression was significantly promoted when the release of H2S from HL-H2S was inhibited using AZ. These results indicate that the H2S triggered and H2S releasing fluorescent probe did not interfere with the progression of ferroptosis, thus enabling high-fidelity in situ imaging of ferroptosis.
Introduction
With strong tissue penetration ability, minor cell and tissue damage, and low interference from autofluorescence, near-infrared (NIR) fluorescence imaging is gradually replacing traditional fluorescence imaging as one of the most popular tools for real-time, in situ, visual tracking of biomolecules.1–5 The recent success of in situ tracking of ferroptosis (an iron-dependent oxidative stress) based on NIR fluorescence sensing has provided a basis for the treatment and drug design for neurodegenerative diseases, acute kidney injuries, and malignant tumors.6,7 However, the current NIR fluorescent probes used to monitor ferroptosis progression detect oxidizing substances such as lipid ROS and free ferrous ions, but the detection of reducing substances is rarely reported.6,8,9 Compared with oxidizing substances, reducing substances such as GSH and GPX4 are considered to be the key biomarkers to directly monitor ferroptosis, because ferroptosis can be regulated by the Xc−/GSH/GPX4 system by controlling the generation of phospholipid hydroperoxides.10 Current methods for the detection of antioxidants GSH or GPX4, for example western blotting make it impossible to achieve high sensitivity, in situ imaging and real-time tracking.11,12 As such the development of probes able to understand cell redox are required in order to understand cell ferroptosis, since the molecular regulation mechanisms of ferroptosis are complicated, and remain to be fully elucidated. In biology, the level of ferroptosis is determined by the simultaneous characterization of multiple targets.13–15As a gaseous signaling molecule, H2S plays an important role in human physiological and pathological processes.16,17 H2S has been defined as an important endogenous neuroprotective agent that exerts protective effects through antioxidant, anti-inflammatory, and anti-apoptotic mechanisms.18,19 A significant amount of evidence suggests that endogenous H2S is produced from cysteine desulfurization catalyzed by cystathionine γ lyase (CSE) or cystathionine β synthase (CBS).20,21 Since ferroptosis inhibits the cystine/glutamate transporter (system xc−), cysteine uptake is decreased, which leads to H2S depletion.22 Thus, H2S can be considered as a representative reducing substance and as such enables monitoring of the ferroptosis process. However, it is difficult for current NIR fluorescent probes to facilitate in situ H2S tracking because extremely low concentrations of H2S will enhance the progression of ferroptosis.21,23 Significantly, traditional sensors for reducing substances require the consumption of the reducing substance, leading to a redox imbalance, which further aggravates the progression of ferroptosis.24–26
Aiming to address these challenges, we have developed a benzyl thiocarbamate with an azide as the recognition site for H2S. Carbonyl sulfide (COS) is released through 1,6-elimination induced by H2S attack, which in turn releases H2S on catalysis by carbonic anhydrase (CA).27–30 This H2S triggered and H2S releasing fluorescent probe has the potential to break through the current bottleneck and thus increase the accuracy in detecting ferroptosis. From our experimental results, the progression of erastin induced ferroptosis in cells with and without HL-H2S were not significantly different. In contrast, the progression of ferroptosis was significantly promoted when the H2S release from HL-H2S was inhibited using AZ. Our results indicate that HL-H2S with both H2S triggered and H2S releasing mechanisms does not induced ferroptosis. Therefore, our probe is capable of in situ high-fidelity ferroptosis analysis, making it a reliable tool for the comprehensive and accurate understanding of ferroptosis progression.
Results and discussion
Design strategy and synthesis of fluorescent probe HL-H2S
The response mechanism of the H2S triggered and H2S releasing probe HL-H2S are shown in Scheme 1. With NIR emission, HL-H2S is endowed with good tissue penetration ability, which results in minor cell and tissue damage, and low interference from autofluorescence.31 An azido benzene was used as the H2S recognition site32,33 which was linked to the fluorophore using a thiocarbamate (H2S precursor). When HL-H2S responds to H2S, COS is released through 1,6-elimination, which is then catalyzed by CA to release H2S. Thus, a novel H2S triggered and H2S releasing system results. Most notably, quinolinemalonitrile is linked to the thiophene in HL-NH2 by σ-bonds, so molecular rotation of its structure will be regulated by viscosity. This enabled us to accurately detect the ferroptosis process, because cell viscosity increases during ferroptosis.6,8,34HL-H2S could be used to detect the ferroptosis of cells of high viscosity, but there was no significant change in normal cells.6,35 In a high-viscosity environment, the rotation of HL-NH2 is hindered, resulting in an increase of quantum yield from 0.01 to 0.67, as shown in Table S1.† As such the sensitivity of HL-NH2, is enhanced in a viscous environment facilitating accurate detection of ferroptosis. The detailed synthetic procedures and characterization data for probe HL-H2S are described in Scheme S1.† The response mechanism of the probe was confirmed by monitoring the 1H NMR and verifying the molecular weight before and after reaction using HR-MS (Fig. S1 and S2†). When HL-H2S reacts with NaHS (H2S donor), the signal at δ 11.4 (Ha) disappears, and a molecular ion peak m/z 429.11169 using HR-MS confirms the formation of HL-NH2 (m/z 429.11444, [M + Na]+).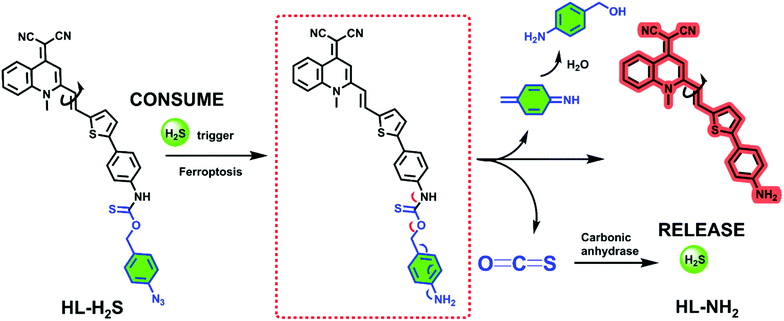 | ||
| Scheme 1 H2S triggered and H2S releasing probe for high-fidelity ferroptosis evaluation and mechanistic details for COS/H2S release by HL-H2S. | ||
Photophysical properties of HL-NH2
With probe HL-H2S in hand, the photophysical properties of the fluorophore HL-NH2 in different solvents were systematically investigated to optimize the detection system. Fig. S3a and b† show the absorption and fluorescence spectra of HL-NH2, respectively, and the photophysical parameters are given in Table S1.† In glycerol, the fluorescence intensity of HL-NH2 was the highest at 646 nm (quantum yield = 0.67), the molar absorptivity reached 0.64 × 104 M−1 cm−1 (λabs = 430 nm), and the Stokes shift was 216 nm. Therefore, light scattering due to excitation was avoided, and the resolution and accuracy of the probe improved. According to Fig. S3d,† the fluorescence intensity of HL-NH2 was highest in glycerol, indicating that the fluorescence intensity of HL-NH2 is affected by viscosity. The sensitivity of HL-NH2 to polarity was also analyzed. As shown in Fig. S3c,† the fluorescence intensity of HL-NH2 does not change as the dielectric constant of the solvent increases, indicating that fluorescence emission of HL-NH2 is not affected by the polarity of the solvent. According to the literature,36,37 quinolinemalonitrile derivates exhibit aggregation-induced emission (AIE). The AIE performance of HL-NH2 was investigated in a mixture of tetrahydrofuran (THF) and water, where THF was a good solvent for HL-NH2 and water was a poor solvent. As shown in Fig. S4a,† the absorption intensity of HL-NH2 remains low without significant changes as the water content (fw) decreased from 100% to 70%. As fw drops below 70%, the absorption intensity of HL-NH2 increases abruptly at 450 nm, which may be due to the scattering effect of the aggregates generated in situ.38 Unlike absorption intensity, the fluorescence intensity of HL-NH2 was negligible at 0% and 100% fw, and reaches a maximum at 40% (Fig. S4b–d†), which is different from conventional AIE luminescence.39 We believe that the reason for the difference observed for our system and traditional AIE fluorophores based on quinolinemalonitrile are due to the primary amino group of HL-NH2 exhibiting enhanced solubility is PBS through hydrogen bonding with water, which results in reduced AIE.37 The response of HL-NH2 to viscosity was also evaluated. As shown in Fig. S5a–c,† the fluorescence intensity of HL-NH2 gradually increases as the viscosity of the solution is increased from 1.1 cP (0 vol% glycerol) to 1410 cP (99 vol% glycerol) by increasing the proportion of glycerol. According to Fig. S5b and c,† two different linear relationships emerge for volume fraction ranges from 0–40% and 50–99%. For each viscosity range the linear correlation coefficient between the fluorescence intensity of the probe and the viscosity are 0.996, indicating that the probe HL-H2S can quantify the viscosity of a target solution. Finally, the aqueous solubility of the probe HL-H2S was investigated. The probe was soluble in a water solution at a concentration of 20 μM and exhibited a good linear relationship between solubility and concentration (R2 = 0.974). Therefore, the solubility of HL-H2S is sufficient for in vivo and in vitro experiments (Fig. S6†).Spectroscopic response of HL-H2S to H2S
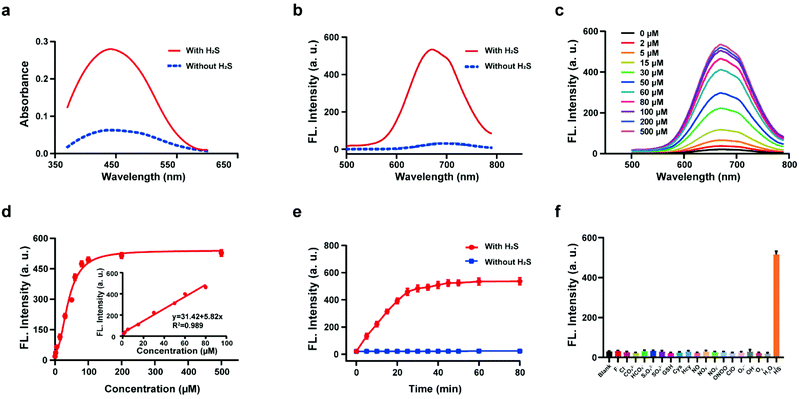
The response of the probe HL-H2S to H2S (from NaHS) was investigated in phosphate buffer solution (PBS, pH = 7.4, containing 80% glycerol and 2% DMSO). The addition of 100 μM H2S resulted in a significant increase in the spectral intensity of both the UV and fluorescence (Fig. 1a and b), indicating excellent response of HL-H2S towards H2S. The fluorescence titration experiments (Fig. 1c) indicated that as the H2S concentration increased from 0 to 500 μM, the fluorescence intensity of the solution (λem = 670 nm) increased by around 25-fold before reaching a plateau at a H2S concentration of 100 μM (Fig. 1d). While the fluorescence intensity exhibited a good linear relationship with concentrations of H2S over a range from 1–80 μM (Fig. 1d inset). The detection limit was calculated to be 1.3 nM. The above experiments indicated that HL-H2S exhibits a dual response capability for viscosity and H2S. The response of HL-H2S was then analyzed in terms of kinetics, pH stability, and selectivity to further investigate the performance towards H2S. Kinetic analysis indicated that the reaction between HL-H2S and H2S (100 μM) was completed within 40 min, indicating that HL-H2S is capable of quickly identifying H2S (Fig. 1e). The effect of different pH environments on HL-H2S and its response to H2S were then evaluated. As shown in Fig. S7,† over a pH range from 5.0 to 8.5, the fluorescence intensity of HL-H2S does not change significantly regardless of the presence of H2S, indicating that the response of HL-H2S to H2S is not affected by changes in environmental pH. Finally, the influence of reactive oxygen (ClO−, O2˙−, ˙OH, 1O2, and H2O2), reactive nitrogen (NO2−, NO3−, NO, and ONOO−), reactive sulfur (GSH, Cys, Hcy, S2O32−, SO42−and HS−) and other anions (F−, Cl−, CO32−, HCO3−) on the response of HL-H2S were evaluated. As shown in Fig. 1f, these interfering species hardly affect the fluorescence intensity of the system, while the introduction of H2S significantly enhanced the fluorescence intensity of the system, indicating that HL-H2S exhibits a high selectivity for H2S.In vitro H2S release of HL-H2S
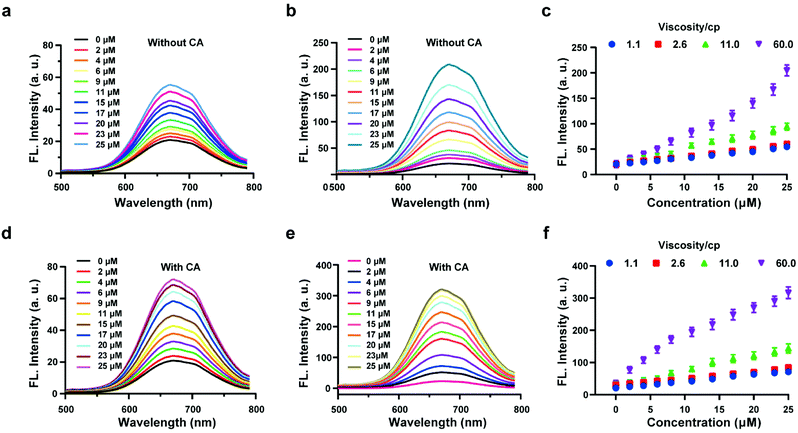
The above experiments confirmed that the probe HL-H2S is capable of H2S-specific recognition in a high-viscosity environment. Next, ferroptosis was simulated in vitro and the detection and release capacity of the probe for H2S was evaluated to facilitate the accurate detection of ferroptosis using the probe. CA has been reported to catalyze the conversion of COS, to produce H2S.27,28 As shown by Fig. 2a, in the absence of CA and glycerol, a ca. ∼3-fold enhancement of the fluorescence with increasing H2S concentration was achieved. However, the fluorescence increases 10-fold as the glycerol content increases to 80% (viscosity: 60 cP), and the effect of environmental viscosity on the response of HL-H2S to H2S is significant (Fig. 2b and c). Compared to the absence of CA, the sensitivity of HL-H2S to H2S is slightly enhanced in the presence of CA (Fig. 2d). Importantly, Fig. 2e and f show that the fluorescence intensity increases 15-fold with addition of H2S, which was attributed to the release of H2S from HL-H2S following reaction with H2S. To verify this conjecture, acetazolamide (AZ), an inhibitor of CA,40 was used to regulate the release of H2S. Fig. S8 a and b† shows that the fluorescence intensity of the solution with AZ and CA is almost identical to that of the solution without AZ or CA, and both are lower than that of the solution with CA, indicating that under these conditions HL-H2S can be catalyzed by CA to produce H2S. To verify the conversion of decomposed HL-H2S to H2S in the presence of CA, the well-known methylene blue (MB) method29 was used to measure the generation of H2S under the above conditions. The results indicated that the changes of H2S monitored using MB were consistent with the fluorescence results obtained for the probe, indicating that under these conditions HL-H2S can be catalyzed by CA to produce H2S (Fig. S8c†), which confirms that HL-H2S can release H2S in the presence of CA. Fig. S8d† is the calibration curve between concentrations of H2S (0–20 μM) and absorption intensity at 670 nm.Imaging of H2S and cytoplasmic viscosity in living cells with HL-H2S
After confirming the H2S response and H2S release capacity of HL-H2S for in vitro experiments, we anticipated that HL-H2S can keep the level of cell ferroptosis form being aggravated during ferroptosis monitoring, which excludes the possibility of HL-H2S-induced ferroptosis, thus enabling high-fidelity imaging and analysis of ferroptosis. Before performing cellular confocal imaging, the biocompatibility of HL-H2S was evaluated. As shown in Fig. S9,† the survival rate of PC12 cells is over 90% after 24 h coincubation with HL-H2S at different concentrations (0–30 μM) according to MTT assay, indicating that HL-H2S has good biocompatibility and is suitable for in situ imaging analysis. The dual response of HL-H2S to H2S and viscosity was then evaluated at the cellular level. Intracellular H2S content and viscosity levels were regulated using NaHS (10 μM) to release H2S, viscosity inducer monensin41 (10 μM), and different cell incubation temperatures (25 °C and 4 °C) since lowering the temperature increases the intracellular viscosity.42,43 As shown in Fig. S10a and b,† the red channel fluorescence intensity of the groups with NaHS or monensin were slightly higher than those of the control group. However, the red channel fluorescence intensity of the group with NaHS and monensin was significantly higher than that of the first three groups. In addition, as the incubation temperature decreased, the red channel fluorescence intensity in the group with NaHS increased significantly, while that of the group without NaHS remained unchanged (Fig. S10c and d†). The above experiments indicate that HL-H2S has dual response to viscosity and H2S at the cellular level. The ability of HL-H2S to detect exogenous and endogenous H2S was then assessed. As shown in Fig. S11,† PC12 cells were incubated with HL-H2S (10 μM) for 10 min to ensure complete entry into the cells. Then the cells were incubated for 40 min with monensin (10 μM), sulfhydryl scavenger N-ethylmaleimide44 (NEM, 0.5 mM), and different concentrations of NaHS (H2S donor) (0, 5, 10, or 20 μM). The experimental results indicated that the red channel fluorescence intensity gradually increased with an increase of H2S concentration (Fig. S11a and b†), indicating that HL-H2S could detect exogenous H2S. It should be noted that the fluorescence intensity of the groups with NEM dropped significantly compared to the control group, which was due to the removal of endogenous H2S by NEM. Therefore, HL-H2S could detect endogenous H2S. CA activity was modulated using AZ to illustrate the H2S releasing capacity of the probe. According to the results shown in Fig. S12,† the red channel fluorescence intensity of the group with AZ decreases significantly compared to the groups without AZ. Meanwhile, since exogenous H2S is present in cells, regardless of whether the cells were co-incubated with AZ, the result is consistent with Fig. S8.† However, the fluorescence intensity difference is negligible between the groups with and without NEM. The above experiments show that HL-H2S is capable of H2S response and release at the cellular level.Imaging of ferroptosis during OGD/R
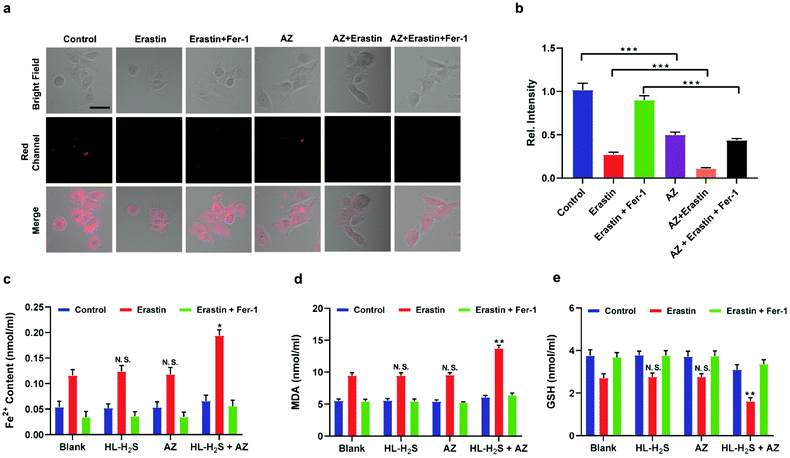
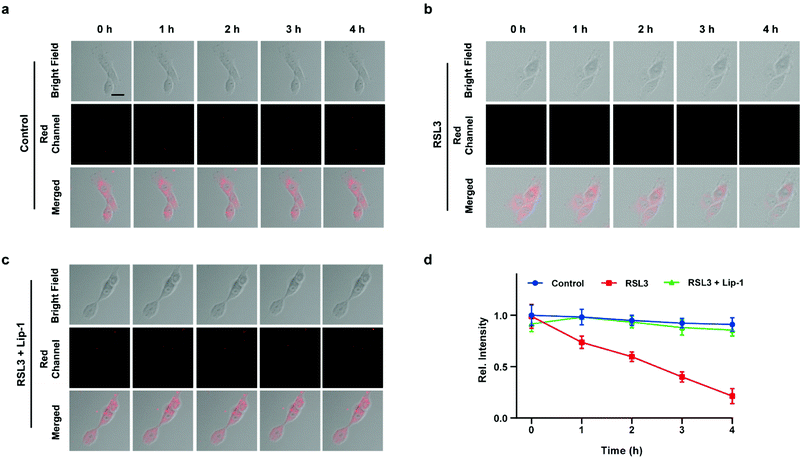
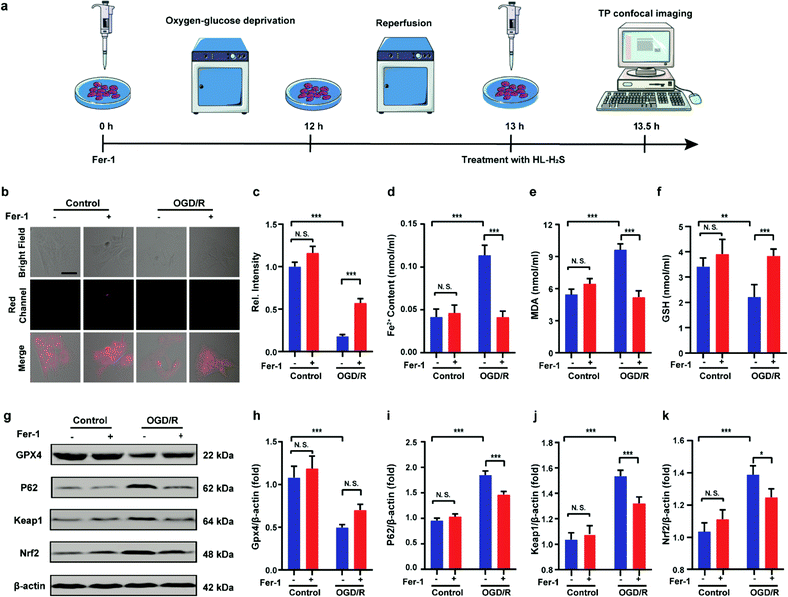
Inspired by the above experiments, the high-fidelity response of the H2S triggered and H2S releasing probe HL-H2S to ferroptosis was evaluated. As shown in Fig. 3a and b, a ferroptosis model was built by incubating PC12 cells with the ferroptosis inducer erastin,45 in which the red channel fluorescence intensity decreases significant compared to the control group, indicating that endogenous H2S is consumed in the ferroptosis process. Meanwhile, the addition of ferroptosis inhibitor ferrostatin-1 (Fer-1)45 resulted in a significant increase of red channel fluorescence intensity, indicating that HL-H2S is capable of the in situ imaging of ferroptosis. Given that Fe2+, MDA, and GSH levels are common indicators of ferroptosis progression, the simultaneous increase in Fe2+ and MDA and decrease in GSH indicate that a ferroptosis model was successfully constructed46,47 (Fig. 3c–e, blank group). Importantly, the Fe2+, MDA, and GSH levels were not significantly different between cells with and without HL-H2S (As shown in Fig. 3c–e, blank group vs.HL-H2S group). In addition, regardless of whether the cells undergo ferroptosis, the red channel fluorescence intensity was significantly decreased in cells where H2S release was inhibited from HL-H2S by AZ (Fig. 3a and b). While Fe2+ and MDA levels increased and the GSH level decreased significantly compared to the groups without HL-H2S (as shown in Fig. 3c–e, blank group vs.HL-H2S + AZ group). Moreover, there is no change of Fe, MDA, or GSH content for the AZ only control experiment compared to the control or probe alone, further indicating that AZ has no effect on the degree of cell ferroptosis, and only inhibits the ability of the probe to release H2S by inhibiting the activity of CA (as shown in Fig. 3c–e, blank group vs. AZ group). These results indicated that the H2S triggered and H2S releasing fluorescent probe HL-H2S maintained the progression of ferroptosis, thus enabling high-fidelity in situ imaging of ferroptosis. To further evaluate the capacity of HL-H2S for real time monitoring of ferroptosis, confocal imaging of PC12 cells stained with HL-H2S were recorded at different times (0, 1, 2, 3, 4 h) under different cell culture conditions including control, RSL3, and RSL3 + liproxstatin-1 (Lip-1). As shown in Fig. 4, the fluorescence of cells with RSL3 (ferroptosis inducer) decreased gradually with time. However, the fluorescence for control cells (only HL-H2S) and those pre-treated with Lip-1 (ferroptosis inhibitor48) remained constant. Thus, fluorescence intensity change of HL-H2S could be used to monitor RSL3-induced ferroptosis in live cells.Finally, PC12 cells were used to construct an oxygen glucose deprivation/re-oxygenation (OGD/R) model and simulate cells undergoing ischemia-reperfusion,49 with which the relationship between cellular OGD/R and ferroptosis were explored (Fig. 5a). According to Fig. 5b and c, the red channel fluorescence intensity in the OGD/R group decreases significantly compared to the control group (p < 0.001). However, the fluorescence intensity of the group with Fer-1 is again significantly higher compared to the group without Fer-1 (p < 0.001). Therefore, the cellular ischemia-reperfusion process was accompanied by ferroptosis. It should be noted that the red channel fluorescence intensity of the control group with the addition of Fer-1 is slightly increased compared to the group without Fer-1, indicating the presence of ferroptosis among normal cells. The trends of Fe2+, MDA, and GSH levels are comparable with those observed for the fluorescence intensity (Fig. 5d–f), indicating the capacity of the probe to monitor ferroptosis progression induced by OGD/R. The p62–Keap1–Nrf2 signaling pathway was examined to further explore the pathological mechanisms by which the cellular ischemia-reperfusion process induces ferroptosis.47,50 Western blotting assay shown in Fig. 5g–k indicated that the p62–Keap1–Nrf2 signaling pathway was activated in the OGD/R cells, while GPX4 expression was significantly downregulated. After incubation with Fer-1, the p62–Keap1–Nrf2 signaling pathway was suppressed and GPX4 was upregulated. The p62–Keap1–Nrf2 signaling pathway and the GPX4 expression in the control group indicated no significant changes with or without the addition of Fer-1. Therefore, the ischemia-reperfusion process inhibited GPX4 and in turn affected the p62–Keap1–Nrf2 signaling pathway to induce ferroptosis.
In vivo imaging of cerebral apoplexy in mice
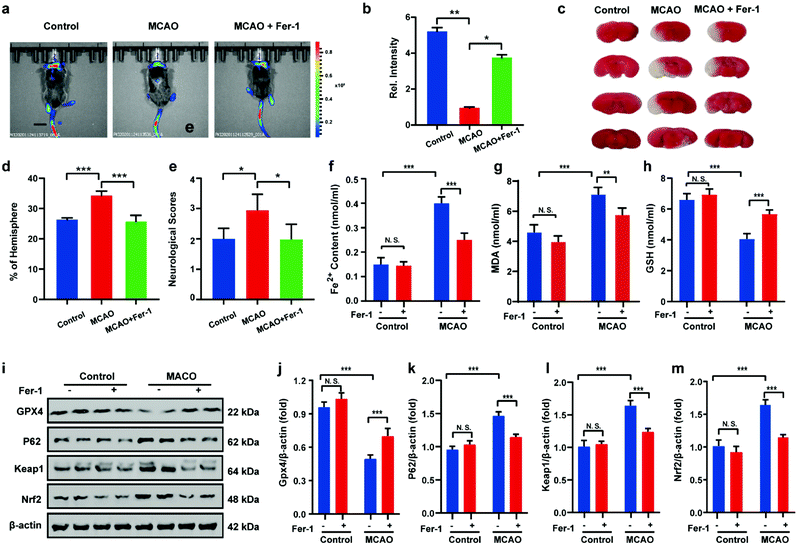
After uncovering the relationship between ischemia-reperfusion and ferroptosis, the cerebral apoplexy in mice was simulated using a middle cerebral artery occlusion (MCAO) model. Prior to imaging, the biocompatibility of HL-H2S in each tissue was assessed. After intravenous injection of HL-H2S, the different organs, including brain, heart, liver, spleen, lung, and kidney were stained by hematoxylin and eosin (H&E). As shown in Fig. S13,†HL-H2S exerted no obvious organ or tissue damage, indicating its good tissue biocompatibility for in vivo imaging analysis in mice. The findings acquired from the mouse MCAO model were like those obtained from the cellular model. The fluorescence intensity in the brains of mice in the MCAO model group decreased significantly compared to that of normal mice (p < 0.01). However, adding ferroptosis inhibitor Fer-1 caused a significant rebound of fluorescence intensity in the brains of mice (Fig. 6a and b, p < 0.05), indicating that MCAO in mice could lead to ferroptosis in their brains. 2,3,5-Triphenyltetrazolium chloride (TTC, measures tissue viability used to evaluate infarct size) staining and behavioral experiment results are in high agreement with in vivo imaging, indicating the viability of the mouse MCAO model (Fig. 6c–e). The changes in Fe2+, MDA, and GSH levels again demonstrate that ferroptosis occurred in the brain of mice during stroke (Fig. 6f–h). Similarly, the relationship between ferroptosis and GPX4 activity and the p62–Keap1–Nrf2 signaling pathway was explored in mice, and the results were consistent with those at the cellular level. Therefore, the MCAO process inhibits GPX4 activity in mice, which in turn affects the p62–Keap1–Nrf2 signaling pathway and ultimately leads to ferroptosis (Fig. 6i–m).Conclusions
In summary, a H2S triggered and H2S releasing NIR fluorescent probe HL-H2S was developed, which exhibits dual response to viscosity and H2S. After reacting with H2S, the probe releases COS through 1,6-elimination, which in turn releases H2S catalyzed by CA. In vitro experiments confirm a low detection limit of 1.3 nM, significant fluorescence enhancement and good selectivity amongst various ROS/RNS species. In addition, the probe exhibited excellent characteristics in terms of large Stokes shift (216 nm), favourable water solubility, excellent pH stability, and low cytotoxicity. While the cell experiments indicated that the progression of erastin induced ferroptosis in cells with and without HL-H2S were not significantly different. In contrast, the progression of ferroptosis was significantly promoted when the H2S release from HL-H2S was inhibited using AZ. Significantly, by using H2S triggered and H2S releasing mechanisms during the imaging process, the probe could avoid the induction of ferroptosis. Therefore, the probe is capable of in situ high-fidelity ferroptosis analysis, making it a reliable tool for the comprehensive and accurate understanding of ferroptosis progression. A cellular OGD/R model was constructed, and a mouse MCAO model was built to simulate MCAO in mice, and ferroptosis inducer erastin and ferroptosis inhibitor Fer-1 were used to regulate the progression of ferroptosis during MCAO. Confocal imaging revealed that the MCAO process could induce ferroptosis.Data availability
All data supporting this study are provided as ESI† accompanying this paper.Author contributions
Tianyu Liang: methodology, investigation, data curation, validation, formal analysis, writing – original draft. Taotao Qiang: project administration, writing – review & editing. Longfang Ren: data curation, investigation. Fei Cheng: validation. Baoshuai Wang: data curation, formal analysis. Mingli Li: software, data curation. Wei Hu: investigation, data curation, formal analysis, writing – original draft. Tony D. James: conceptualization, writing – review & editing, supervision.Conflicts of interest
TDJ acts as an academic consultant for TQ as part of a guest professorship at SUST.Acknowledgements
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of South-Central University of Nationalities and experiments were approved by the Animal Ethics Committee of College of Biology (South-Central University of Nationalities). This work was funded by the Open Project of Shaanxi Collaborative Innovation Center of Industrial Auxiliary Chemistry & Technology (No. XTKF-2020-05); the Key Scientific Research Group of Shaanxi Province (2020TD-009), Key Scientific Research Program of Shaanxi Provincial Education Department (Collaborative Innovation Center project) (20JY003), Science and Technology Plan Project of Xi'an Weiyang District (No. 201907) and the Youth Innovation Team of Shaanxi Universities. TDJ wishes to thank the Royal Society for a Wolfson Research Merit Award and the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University for support (2020ZD01).References
- L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622–661 RSC.
- X. Zhen, J. Zhang, J. Huang, C. Xie, Q. Miao and K. Pu, Angew. Chem., Int. Ed., 2018, 57, 7804–7808 CrossRef CAS PubMed.
- Y. Yang, Y. Hu, W. Shi and H. Ma, Chem. Sci., 2020, 11, 12802–12806 RSC.
- M. Weber, H. Han, B. Li, M. L. Odyniec, C. E. F. Jarman, Y. Zang, S. D. Bull, A. B. Mackenzie, A. C. Sedgwick, J. Li, X. He and T. D. James, Chem. Sci., 2020, 11, 8567–8571 RSC.
- X. Tian, L. C. Murfin, L. Wu, S. E. Lewis and T. D. James, Chem. Sci., 2021, 12, 3406–3426 RSC.
- H. Li, W. Shi, X. Li, Y. Hu, Y. Fang and H. Ma, J. Am. Chem. Soc., 2019, 141, 18301–18307 CrossRef CAS PubMed.
- K.-N. Wang, L.-Y. Liu, D. Mao, S. Xu, C.-P. Tan, Q. Cao, Z.-W. Mao and B. Liu, Angew. Chem., Int. Ed., 2021, 60, 2–8 CrossRef.
- B. Dong, W. Song, Y. Lu, Y. Sun and W. Lin, ACS Sens., 2021, 6, 22–26 CrossRef CAS PubMed.
- L. Shi, Q. Guan, X. Gao, X. Jin, L. Xu, J. Shen, C. Wu, X. Zhu and C. Zhang, Anal. Chem., 2018, 90, 9218–9225 CrossRef CAS PubMed.
- T. M. Seibt, B. Proneth and M. Conrad, Free Radical Biol. Med., 2019, 133, 144–152 CrossRef CAS PubMed.
- X. Song, X. Wang, Z. Liu and Z. Yu, Front. Oncol., 2020, 10, 597434 CrossRef PubMed.
- Y. Sun, Y. Zheng, C. Wang and Y. Liu, Cell Death Discovery, 2018, 9, 753 CrossRef PubMed.
- J. Li, F. Cao, H.-l. Yin, Z.-j. Huang, Z.-t. Lin, N. Mao, B. Sun and G. Wang, Cell Death Discovery, 2020, 11, 88 CrossRef PubMed.
- B. Zhou, J. Liu, R. Kang, D. J. Klionsky, G. Kroemer and D. Tang, Semin. Cancer Biol., 2020, 66, 89–100 CrossRef CAS PubMed.
- M. Conlon, C. D. Poltorack, G. C. Forcina, D. A. Armenta, M. Mallais, M. A. Perez, A. Wells, A. Kahanu, L. Magtanong, J. L. Watts, D. A. Pratt and S. J. Dixon, Nat. Chem. Biol., 2021, 17, 665–674 CrossRef CAS PubMed.
- M. R. Filipovic, J. Zivanovic, B. Alvarez and R. Banerjee, Chem. Rev., 2018, 118, 1253–1337 CrossRef CAS PubMed.
- B. Wang, C. Huang, L. Chen, D. Xu, G. Zheng, Y. Zhou, X. Wang and X. Zhang, ACS Biomater. Sci. Eng., 2020, 6, 798–812 CrossRef CAS PubMed.
- M. D. Hartle and M. D. Pluth, Chem. Soc. Rev., 2016, 45, 6108–6117 RSC.
- X. Zhang and J.-S. Bian, ACS Chem. Neurosci., 2014, 5, 876–883 CrossRef CAS PubMed.
- C. M. Levinn, M. M. Cerda and M. D. Pluth, Acc. Chem. Res., 2019, 52, 2723–2731 CrossRef CAS PubMed.
- C. D. McCune, S. J. Chan, M. L. Beio, W. Shen, W. J. Chung, L. M. Szczesniak, C. Chai, S. Q. Koh, P. T. H. Wong and D. B. Berkowitz, ACS Cent. Sci., 2016, 2, 242–252 CrossRef CAS PubMed.
- J. Zhang, H. Shan, L. Tao and M. Zhang, J. Mol. Neurosci., 2020, 70, 2020–2030 CrossRef CAS PubMed.
- M. D. Hammers, M. J. Taormina, M. M. Cerda, L. A. Montoya, D. T. Seidenkranz, R. Parthasarathy and M. D. Pluth, J. Am. Chem. Soc., 2015, 137, 10216–10223 CrossRef CAS PubMed.
- S. Gong, Z. Zheng, X. Guan, S. Feng and G. Feng, Anal. Chem., 2021, 93, 5700–5708 CrossRef CAS PubMed.
- H. Zhu, C. Liu, C. Liang, B. Tian, H. Zhang, X. Zhang, W. Sheng, Y. Yu, S. Huang and B. Zhu, Chem. Commun., 2020, 56, 4086–4089 RSC.
- T. Chiku, D. Padovani, W. D. Zhu, S. Singh, V. Vitvitsky and R. Banerjee, J. Biol. Chem., 2009, 284, 11601–11612 CrossRef CAS PubMed.
- Y. Zhao and M. D. Pluth, Angew. Chem., Int. Ed., 2016, 55, 14638–14642 CrossRef CAS PubMed.
- A. K. Steiger, S. Pardue, C. G. Kevil and M. D. Pluth, J. Am. Chem. Soc., 2016, 138, 7256–7259 CrossRef CAS PubMed.
- T. Liang, D. Zhang, W. Hu, C. Tian, L. Zeng, T. Wu, D. Lei, T. Qiang, X. Yang and X. Sun, Talanta, 2021, 235, 122719 CrossRef CAS PubMed.
- Y. Hu, X. Li, Y. Fang, W. Shi, X. Li, W. Chen, M. Xian and H. Ma, Chem. Sci., 2019, 10, 7690–7694 RSC.
- W. Hu, L. Zeng, S. Zhai, C. Li, W. Feng, Y. Feng and Z. Liu, Biomaterials, 2020, 241, 119910 CrossRef CAS PubMed.
- S. K. Bae, C. H. Heo, D. J. Choi, D. Sen, E.-H. Joe, B. R. Cho and H. M. Kim, J. Am. Chem. Soc., 2013, 135, 9915–9923 CrossRef CAS PubMed.
- S. Gong, E. Zhou, J. Hong and G. Feng, Anal. Chem., 2019, 91, 13136–13142 CrossRef CAS PubMed.
- H. Wei, Y. Yu, G. Wu, Y. Wang, S. Duan, J. Han, W. Cheng, C. Li, X. Tian and X. Zhang, Sens. Actuators, B, 2022, 350, 130862 CrossRef CAS.
- Z. Yang, J. Cao, Y. He, J. H. Yang, T. Kim, X. Peng and J. S. Kim, Chem. Soc. Rev., 2014, 43, 4563–4601 RSC.
- A. Shao, Y. Xie, S. Zhu, Z. Guo, S. Zhu, J. Guo, P. Shi, T. D. James, H. Tian and W.-H. Zhu, Angew. Chem., Int. Ed., 2015, 54, 7275–7280 CrossRef CAS PubMed.
- W. Fu, C. Yan, Z. Guo, J. Zhang, H. Zhang, H. Tian and W.-H. Zhu, J. Am. Chem. Soc., 2019, 141, 3171–3177 CrossRef CAS PubMed.
- D. Wang, M. M. S. Lee, G. Shan, R. T. K. Kwok, J. W. Y. Lam, H. Su, Y. Cai and B. Z. Tang, Adv. Mater., 2018, 30, 1802105 CrossRef PubMed.
- R. Hu, N. L. C. Leung and B. Z. Tang, Chem. Soc. Rev., 2014, 43, 4494–4562 RSC.
- A. K. Steiger, M. Marcatti, C. Szabo, B. Szczesny and M. D. Pluth, ACS Chem. Biol., 2017, 12, 2117–2123 CrossRef CAS PubMed.
- J. Yin, M. Peng and W. Lin, Anal. Chem., 2019, 91, 8415–8421 CrossRef CAS PubMed.
- X. J. Peng, Z. G. Yang, J. Y. Wang, J. L. Fan, Y. X. He, F. L. Song, B. S. Wang, S. G. Sun, J. L. Qu, J. Qi and M. Yan, J. Am. Chem. Soc., 2011, 133, 6626–6635 CrossRef CAS PubMed.
- L. Hou, P. Ning, Y. Feng, Y. Ding, L. Bai, L. Li, H. Yu and X. Meng, Anal. Chem., 2018, 90, 7122–7126 CrossRef CAS PubMed.
- C. Wu, D. Fan, C. Zhou, Y. Liu and E. Wang, Anal. Chem., 2016, 88, 2899–2903 CrossRef CAS PubMed.
- H. Yuan, Z. Han, Y. Chen, F. Qi, H. Fang, Z. Guo, S. Zhang and W. He, Angew. Chem., Int. Ed., 2021, 60, 8174–8181 CrossRef CAS PubMed.
- C. Ou, W. Na, W. Ge, H. Huang, F. Gao, L. Zhong, Y. Zhao and X. Dong, Angew. Chem., Int. Ed., 2021, 60, 8157–8163 CrossRef CAS PubMed.
- J. Li, K. Lu, F. Sun, S. Tan, X. Zhang, W. Sheng, W. Hao, M. Liu, W. Lv and W. Han, J. Transl. Med., 2021, 19, 96 CrossRef CAS PubMed.
- B. Zhang, X. Chen, F. Ru, Y. Gan, B. Li, W. Xia, G. Dai, Y. He and Z. Chen, Cell Death Discovery, 2021, 12, 843 CrossRef CAS PubMed.
- Y. Li, X. Wang, J. Yang, X. Xie, M. Li, J. Niu, L. Tong and B. Tang, Anal. Chem., 2016, 88, 11154–11159 CrossRef CAS PubMed.
- J. Cheng, T. Xu, C. Xun, H. Guo, R. Cao, S. Gao and W. Sheng, Life Sci., 2021, 266, 118905 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and characterization probe HL-H2S supplementary spectral and cells experiments results. See DOI: 10.1039/d1sc05930k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |