 Open Access Article
Open Access ArticleThe role of hollow magnetic nanoparticles in drug delivery†
Ghodsi Mohammadi Ziarani *a,
Masoumeh Malmira,
Negar Lashgari
*a,
Masoumeh Malmira,
Negar Lashgari a and
Alireza Badieib
a and
Alireza Badieib
aDepartment of Chemistry, Alzahra University, Vanak Square, P. O. Box 1993893973, Tehran, Iran. E-mail: gmziarani@hotmail.com; gmohammadi@alzahra.ac.ir
bSchool of Chemistry, College of Science, University of Tehran, 14155-6455, Tehran, Iran
First published on 13th August 2019
Abstract
The increasing number of scientific publications focusing on nanomaterials in the biomedical field indicates growing interest from the broader scientific community. Nanomedicine is a modern science, and research continues into the application of nanoscale materials for the therapy and diagnosis of damaged tissues. In this regard, substantial progress has been made in the synthesis of magnetic materials with desired sizes, morphologies, chemical compositions, and surface chemistry. Among these, magnetic iron oxide nanoparticles have demonstrated great promise as unique carriers in the delivery of chemical drugs due to their combinations of hollow structures. Importantly, due to the combination of the ability to respond to an external magnetic field and the rich possibilities of their coatings, magnetic materials are universal tools for the magnetic separation of small molecules, biomolecules, and cells. This review provides an overview of the synthesis and biological applications of hollow magnetic nanoparticles in drug delivery systems.
1 Introduction
In the past decade, magnetic nanoparticles (MNPs) based on metals such as iron, cobalt and nickel or metal oxides/mixed-metal oxides have aided the efficient development of modern technology.1,2 Nowadays, they are applied in many fields such as bioimaging and sensing; on a smaller scale, they are used as catalysts and in medicine.3–13 Hence, the enormous interest in the efficiency of these materials can be easily understood. Specifically, Frey and co-workers carefully reviewed the synthesis and applications of MNPs in drug delivery.14 The applications of MNPs in drug delivery were also reviewed by Sun's group.15 On the other hand, much attention has been focused on the size and functionalization of iron oxide nanoparticles with various morphologies, such as nanoflowers, nanorods, nanowires and nanocubes.16–20 Recently, hollow nanostructures with high surface areas, low material densities, and controlled pore volumes and shell thicknesses have arisen as an important class of nanomaterials.21–24 Several strategies for the synthesis of hollow structures, such as Ostwald ripening,25,26 the Kirkendall effect,27 reverse micelle transport,28 and layer-by-layer assembly,29 have been developed. These fabrication approaches are conventionally based on the use of well-established templates, including hard templates, soft templates, and sacrificial templates.30–32 On the other hand, magnetic hollow nanostructures can find various biomedical applications, including simultaneous diagnosis and therapy, because the large pore volumes inside the hollow nanostructures can be used to incorporate various drugs and bio-molecules and release them in a controlled manner.33 Additionally, the surfaces of the magnetic hollow nanostructures can be readily functionalized with targeting agents.34 Subsequent to our previous publications,35–38 herein, we wish to review the roles of various pure and modified hollow magnetic nanoparticles in drug delivery processes. Generally, we have classified the uses of HMNPs into three main concepts: preparation, functionalization and the role of HMNPs in drug delivery systems.2 Preparation and surface analysis of HMNPs
2.1. Preparation of HMNPs
Magnetic nanoparticles with unique properties can be used as catalyst supports in organic transformations. Importantly, many attempts have been made to control the size and morphology of magnetic materials via changing reaction parameters such as temperature, time and concentration of reactants to manipulate their magnetic and surface properties. Magnetic nanoparticles can be divided into four categories:39• Metals (Fe, Co, Ni)
• Metal oxides (FeO, Fe2O3, Fe3O4)
• Alloys (FePt, FePd)
• Ferrites (CoFe2O4, CuFe2O4)
Among these categories, metal oxides with hollow structures have attracted much attention because of their simple preparation approach, strong magnetic properties and the sizes, shapes and low densities of the materials. Most of these compounds have been used as catalysts in organic transformations and photocatalysis.40
Recently, Si and co-workers carefully reviewed the synthesis and applications of hollow micro and nanostructures.41 Several synthesis approaches for the fabrication of hollow magnetic nanoparticles, including template-mediated and reaction approaches, have been established. Meanwhile, template-free approaches were established to prepare hollow nano/microspheres, including Ostwald ripening,25 the Kirkendall effect (shell-breaking)27 and surface-protecting etching.30 Currently, various nanomagnetic hollow structures are produced via the Ostwald ripening approach. Additionally, pure nanomagnetic Fe2O3 hollow spheres can be prepared, according to experimental results by Elhampour and coworkers.5 Based on Fig. 1A and B, the nanomagnetic Fe2O3 particles are spherical, with an average diameter of 400 nm; also, the hollow morphology of the nanomagnetic Fe2O3 particles is clearly revealed (Fig. 1).5
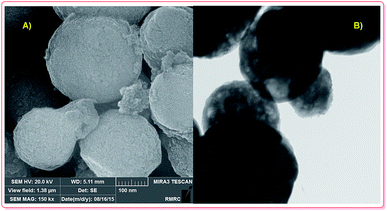 | ||
| Fig. 1 FE-SEM (A) and TEM (B) analysis of Fe2O3 hollow spheres.5 | ||
2.2. Functionalization of HMNPs
A wide range of stabilizing or coating materials, including organic (polymers and surfactants)42 and inorganic (silica and carbon materials),43 have been used as some of the most powerful tools to strengthen the chemical stability of hollow magnetic nanoparticles. For example, various amphiphilic polymers, such as polystyrene-polyacrylic acid block copolymer (PS-PAA), tetradecylphosphonate and polyethylene glycol-2-tetradecyl ether, have been successfully used to transfer hydrophobic magnetic nanoparticles from organic solvents to aqueous solution.44,45 Meanwhile, various commercially available amphiphilic polymers provide different functional groups, including carboxylic acid, thiol, amine, carbonyl, and biotin, for immobilization of various biological moieties, such as peptides, proteins, and oligonucleotides. Moreover, the chemical properties of the coating material can be effective for the surface functionalization of magnetic nanoparticles.3 The roles of pure and modified HMNPs in the adsorption and release of drugs
3.1. Natural drugs (herbal medicines)
Recently, Chen and co-workers demonstrated the preparation of γ-Fe2O3 silica nanotubes (γ-Fe2O3@SiO2 tubes), which were used as a carrier in a controlled RhB delivery system.
The total RhB capacity in the as-prepared tubes was 9.25 mg per g of carrier because of the large open ends from the pore diameter distribution in the magnetic nanotubes. In the UV-Vis spectrum of the tubes in aqueous media, the broad absorption band around 553 nm is characteristic of RhB; thus, its intensity enables estimation of the RhB concentration in the solution. Thus, in the first 5 h, nearly 50% of the RhB was released from the carrier; then, about 80% of the loaded RhB was released within 9 h (Scheme 1).48
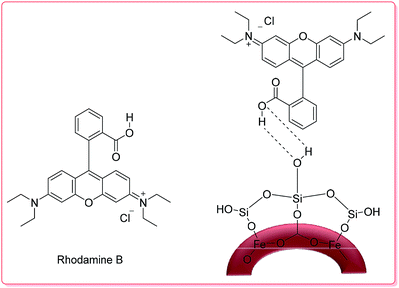 | ||
| Scheme 1 The structures of RhB and RhB-γ-Fe2O3@SiO2.48 | ||
The R6G loading capacity in Fe3O4/PAA was 325.7 mg per mL of carrier. The best drug release rate of 93.0% was achieved in pH 7.4 PBS solution after 14 h. The release efficiency was 86.5% in acidic conditions. Moreover, the solubility parameter can influence the swelling properties of PAA and the binding forces between PAA and R6G.49
3.2. Nonsteroidal anti-inflammatory drugs (NSAIDs)
3.2.1.1. Metal oxides. Lu et al. synthesized hollow-core-double-shell magnetic iron oxide/silica/calcium silicate nanocomposites (MSCN) which were used as-prepared for ibuprofen (IBU) delivery.
The maximum loading of IBU–MSCN was 75 mg drug per g. Moreover, the IBU release of IBU–MSCN in phosphate buffer saline (PBS) was rapid in the first 5 h at 37 °C; the drug release was complete at a release time of 60 h (Scheme 2).50
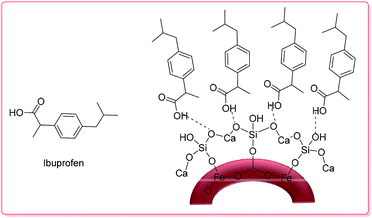 | ||
| Scheme 2 The structure of IBU–MSCN.50 | ||
In another study, to investigate HMNPs as carriers for IBU delivery, α-Fe2O3 and Fe3O4 hollow spheres were prepared by Sasidharan and co-workers. The drug storage capacities of the two nanoparticles were 0.26 to 0.29 g IBU per g of carrier; also, about 96% of the IBU was released into PBS solution at pH 7.3 overnight.51
Cao et al. synthesized PEG-coated Fe3O4 and PEG-coated γ-Fe2O3 hollow spheres (Fig. S1a and b†) from ferrous alkoxide by two different methods. Then, they used the PEG-coated γ-Fe2O3 hollow spheres as a carrier to deliver IBU at pH 7.4 under shaking at a constant rate in simulated body fluid (SBF) at 37 °C. Additionally, IBU was maintained in these hierarchically nanostructured hollow spheres, with uptake amounts of 237 and 297 mg g−1 for PEG-coated γ-Fe2O3 and PEG-coated Fe3O4, respectively; the drug molecules were released in 136 h.52
On the other hand, the PEG-coated Fe3O4 hollow spheres were used in an IBU delivery system. Hence, they designed and synthesized PEG-modified Fe3O4 hollow core/shell hierarchical nanostructures by a solvothermal process (preparation of the precursor) combined with subsequent thermal treatment. Similar to previous work, the samples were used for IBU delivery. After 6 h, 43% of the loaded IBU drug was released, and 78% was released after 24 h; then, the drug release rate decreased and reached a value of 87% after 48 h. However, the drug release rate of the IBU-uncoated Fe3O4 system was higher than that of the IBU–PEG-coated Fe3O4 system. In the first 6 h, about 53% of the loaded IBU was released, and 77% was released in 12 h; a value of 86% was reached after 24 h, which is due to the formation of new hydrogen bonds between the hydroxyl groups of PEG and the carboxyl groups of the IBU molecules in the IBU–PEG-coated Fe3O4 system (Fig. S1a and b†).53
Xia and coworkers synthesized Fe3O4 hollow magnetic core/mesoporous shell (HMMS) structures using carboxylic polystyrene (PS) latex as a hard template that was enclosed within a silica shell via a sol–gel process (Fig. S2†).54 Then, HMMS was applied as a carrier for IBU delivery under an external magnetic field. The amount of IBU drug adsorbed by HMMS was about 20 mg mL−1. This sample was named HMMS-3-IBU-20, where 3 is the sample number and 20 is the concentration of IBU solution. The IBU release from the HMMS-3-IBU-20 system over a 50 h period in phosphate buffered saline solution (pH 7.4) was studied. Generally, 48% of the IBU was released from HMMS-3 after 10 h, and over 70% of IBU was released at the end of the 50 h period (Fig. S3†).54
In a different method, Zhu et al. designed the in situ growth of Cu3(BTC)2 nanomagnetic particles based on the polymerization of a methyl methacrylate (PMMA@Fe3O4/Cu3(BTC)2) hybrid hollow sphere metal framework (MOF) induced by one-pot Pickering emulsion; these nanoparticles were used as a carrier in IBU delivery (Fig. S4†). Additionally, the average size of the IBU molecule (0.5 × 1.0 × 0.8 nm) is exceptionally close to the edge lengths of the square channels in Cu3(BTC)2 (0.95 nm). Moreover, the drug was slowly released from n-hexane solution by magnetic separation within 15 h at 37 °C. The IBU release was complete after a period of 7 h at a higher temperature (45 °C).55
Another rattle-type HMMS with Fe3O4 nanoparticles encapsulated in the cores of mesoporous silica microspheres was successfully synthesized by Zhao et al.56 Importantly, this structure has the merits of both enhanced drug-loading capacity and significant magnetization strength. The as-prepared HMMSs recognize a relatively high storage capacity of up to 302 mg per g of carrier when IBU is used as a model drug, and the IBU–HMMS system has sustained-release properties which follow Fick's law.56 Fig. 2 shows the IBU release behaviour from the system over a 57 h period in neutral (pH 7.1) and acidic (pH 2.4) solutions. Importantly, burst release occurs within 20 h from the HMMSs system at different pH values; then, sustained release follows. Furthermore, the IBU release rate in the neutral solution (pH 7.1) was faster than that in the acidic solution (pH 2.4) (Fig. 2).
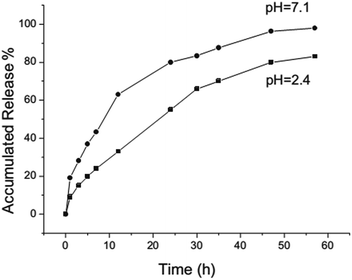 | ||
| Fig. 2 IBU release processes from the HMMS system in a neutral solution (pH 7.1) and an acidic solution (pH 2.4).56 | ||
As a hollow hybrid carrier, super paramagnetic polyelectrolyte hybrid hollow microspheres ((CS/Fe3O4-CA)3–CS–NHCH2–PEG) were synthesized and reported by Zhao and co-workers (Fig. 3).57 This system was used for IBU delivery, and the profile release behaviour in SBF was considered.
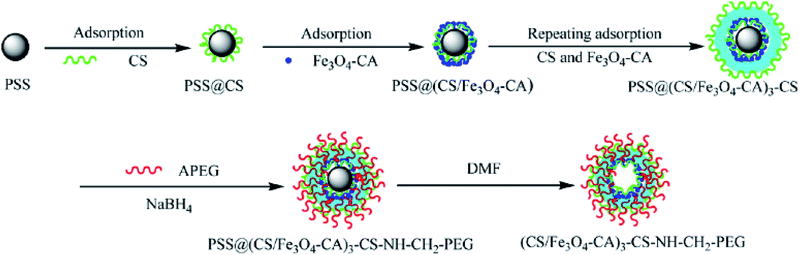 | ||
| Fig. 3 Schematic of the preparation of superparamagnetic polyelectrolyte hybrid hollow microspheres.57 | ||
The drug loading capacities of (CS/Fe3O4-CA)3 and (CS/Fe3O4-CA)3–CS–NHCH2–PEG were found to be about 157 and 185 mg of drug per g of carrier, while the IBU capacity of (CS/Fe3O4-CA)3–CS–NHCH2–PEG was slightly higher than that of (CS/Fe3O4-CA)3 due to hydrogen bonds between the hydroxyl groups of PEG and the carboxyl groups of the IBU drug. Therefore, the cumulative release rates of (CS/Fe3O4-CA)3–CS–NHCH2–PEG and the (CS/Fe3O4-CA)3 hollow carrier in PBS solution at pH 7.4 at 37 °C for 60 h were calculated to be about 91.78% and 81.52%, respectively (Fig. 4).57
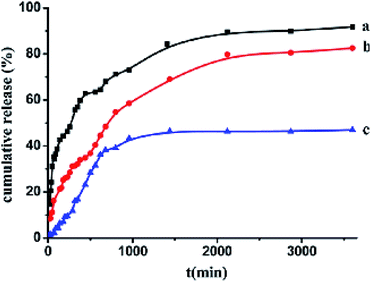 | ||
| Fig. 4 Cumulative ibuprofen release rates from the drug-adsorbed super paramagnetic polyelectrolyte hybrid hollow microspheres (CS/Fe3O4-CA)3–CS in pH 7.4 phosphate buffer at 37 °C (a) and from the biocompatible (CS/Fe3O4-CA)3–CS–NH–CH2–PEG hollow microspheres in phosphate buffer at pH 7.4 (b) or 1.8 (c) at 37 °C, respectively.57 | ||
Zhou and co-workers synthesized porous magnetic hollow silica nanospheres (MHSNs) as a hollow carrier for IBU with drug loading capability. Thus, the uptake capacities of IBU were 14.21% for the hollow carriers with a pore size of 3.7 nm and 8.7% for those with a pore size of 1.5 nm, which was determined by UV-Vis analysis.
The release test was carried out in 50 mL of PBS (pH 7.4). About 15% of the IBU was released from the hollow carrier with a 3.7 pore size in the first 0.5 h; then, about 42% was released overnight. However, in the other case with a pore size of 1.5 nm, more than 80% of the IBU was released in the first half-hour (Fig. S5†).58
In another publication, Lu et al. successfully prepared magnetic Fe3O4/calcium silicate mesoporous nanocomposites (MMCNs) using a two-liquid-phase system by ultrasonic irradiation. According to the UV-Vis results, about 1.03 g of drug per g of carrier could be loaded into the magnetic hollow spheres; also, the drug was slowly released from the MMCNs.59
Recently, Yang and co-workers studied the amino-functionalized hollow Fe3O4/SiO2 core–shell structure and its modification with folic acid (FA) as a carrier for IBU delivery (Fig. S6†).60 The loading capacities of IBU for Fe3O4–SiO2–NH2 and Fe3O4–SiO2–NHFA were 23.3% and 27.7%, respectively. However, due to the higher specific surface area of Fe3O4–SiO2–NHFA than of Fe3O4–SiO2–NH2, the IBU storage capacity was suitable. Further, according to its release behaviours in PBS solution (pH 7.4), the release rate of the Fe3O4–SiO2–NHFA carrier (17.89%) was lower than that of Fe3O4–SiO2–NH2 (37.23%) because of the existence of interactions between the carboxyl groups of IBU and the –CONH groups and hydroxyl groups on Fe3O4–SiO2–NHFA (Fig. S7†).60
In an interesting study, raspberry-like nanomagnetic hollow silica nanospheres (PS@Fe3O4@SiO2) were used as an IBU carrier by Wang et al.61 According to their results, the suitable IBU molecule was introduced into all the pore volumes of the carrier (1.79, 1.33 and 1.18 cm3 g−1); then, about 55% to 70% of loaded IBU was released slowly in all cases after 20 h (Table 1).
| Sample | NH4OH (mL) | TEOS (mL) | BET (m2 g−1) | Total pore volume (cm3 g−1) | Pore size distribution |
|---|---|---|---|---|---|
a PS@Fe3O4@SiO2: ratio of TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4 Fe3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PS PS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH4OH = 1 NH4OH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.b PS@Fe3O4@SiO2: ratio of TEOS 2.b PS@Fe3O4@SiO2: ratio of TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4 Fe3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PS PS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH4OH = 1 NH4OH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.c PS@Fe3O4@SiO2: ratio of TEOS 4.c PS@Fe3O4@SiO2: ratio of TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3O4 Fe3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PS PS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH4OH = 0.5 NH4OH = 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. 4. |
|||||
| 1a | 2 | 1 | 471 | 1.79 | 3.5 to 5.5 |
| 2b | 4 | 1 | 307 | 1.33 | 3.5 to 5.5 |
| 3c | 4 | 1.5 | 265 | 1.18 | 3.5 to 5.5 |
3.2.1.2. Ferrites. Yang et al. synthesized magnetic ZnFe2O4 hollow microsphere silica shells (MZHM-MSS–NH2), functionalized them with folic acid (MZHM-MSS–NHFA) and then used them as an IBU carrier for controlled release (Fig. S8†).62 The IBU storage capacities of MZHM-MSS–NH2 and MZHM-MSS–NHFA were reported to be 16.9% and 22.2%, respectively. The drug release process into a PBS solution at 37 °C was studied. After 48 h, the release amounts of IBU from the MZHM-MSS–NH2 and MZHM-MSS–NHFA systems were 16.90% and 12.11%, respectively, which is due to ionic interactions of the IBU carboxyl groups with the amine groups of MZHM-MSS–NH2 (Fig. S9†).62
Zhang and co-workers studied multiple shell hollow CoFe2O4 as a nanocarrier for IBU delivery. The drug loading capacity of the as-prepared CoFe2O4 was 12.5%, which was attributed to the large specific surface area, mesopores and interconnected macropores of the carrier. The release behaviours of IBU from the drug-loaded magnetic mesoporous calcium nanocomposites (DL-MSHCSs) was considered in PBS over 48 h; it was faster than the release from drug-loaded solid carbon particles (DL-SCPs, Fig. 5).63
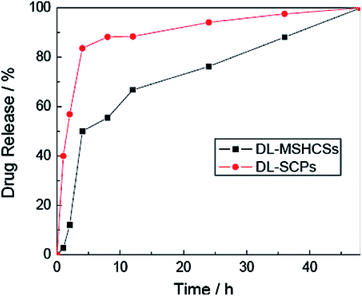 | ||
| Fig. 5 Drug release behaviour of DL-MSHCSs and DL-SCPs.63 | ||
In particular, sodium meclofenamate (pKa = 4.39; soluble in water, ethanol, DMSO and DMF) is a very useful drug for the symptomatic treatment of moderate pain, several forms of arthritis, dysmenorrhea and menorrhagia.65
Lately, Alan and co-workers effectively synthesized hollow magnetic nanocapsules with a high specific surface area as a carrier for SMF loading and significant drug release in vivo.66 SMF was successfully loaded onto the surface of the magnetic nanocapsules; about 18% was loaded after 18 h. Then, the drug release rate of the SMF loaded onto a sample under physiological conditions in PBS buffer at pH 7.2 was studied; about 45% of the loaded SMF was successfully released after 6 hours in aqueous suspension.66
3.3. Antibiotics
The total drug loading amount of Cef was 73 wt% (730 mg of Cef per g of carrier). As a result, the Cef release rates of magnetic and fluorescent hollow composites–cefradine (MFHC–Cef) in three simulated physiological release media with pH 2 (simulated gastric fluid), pH 7.4 (simulated blood fluid) and pH 8.94 (simulated intestinal fluid) were studied; the particles exhibit more compact structures in acidic medium than in basic medium, which leads to differences in their drug permeability and drug release rates (Fig. S10 and S11†).68
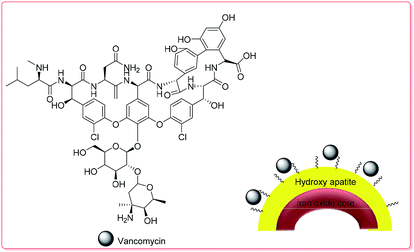 | ||
| Scheme 3 The structure of VAN–HAp.70 | ||
The VAN loading amount of the hollow microspheres reached 28.84 to 35.92 mg g−1, which was higher than that of traditional hollow magnetic hydroxyapatite nanoparticles, due to the formation of stronger affinity hydroxyl groups in HAp and VAN molecules through H-bond interactions. The initial burst release of VAN in the first 9 h at pH 7.4 was around 19 wt% in PBS; VAN was then released completely during the next 2 days (Fig. S12†).70 Table 2 displays the results of the investigation of the capability of the fabricated hollow magnetic HAp microspheres to act as drug carriers using VAN as a model drug. The fabricated hollow magnetic HAp microspheres with higher SBET could successfully provide much more active sites to adsorb higher amounts of the VAN drug.70
| Sample | Fe3O4 amount (wt%) | SBET (m2 g−1) | DLA (mg g−1) | DLE (%) |
|---|---|---|---|---|
| S0 | — | 11.17 | 19.08 ± 2.7 | 47.7 ± 6.75 |
| S1 | — | 34.87 | 35.92 ± 0.12 | 89.8 ± 0.28 |
| S2 | 3.97 | 41.55 | 30.24 ± 0.11 | 75.6 ± 0.28 |
| S3 | 15.38 | 52.32 | 30.16 ± 0.59 | 75.4 ± 1.41 |
| S4 | 40.78 | 67.26 | 28.86 ± 1.95 | 72.2 ± 4.88 |
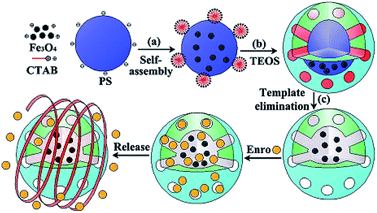 | ||
| Fig. 6 Schematic of the ENR loading and release of MHMS.71 | ||
3.4. Anticancer
In another report, Zhu et al. considered a novel super paramagnetic hollow sphere core–shell structure to study the effects of loading and release of drugs on the surface of nanoparticles. Notably, this delivery method provided a steady aqueous dispersion of hydrophobic drugs with a hydrodynamic size of about 191.9 ± 2.6 nm. DOX was used as a model drug; the endosomal/lysosomal acidic environment enhanced the solubility and drug release of the basic drug. The DOX-loading was measured by UV-Vis spectrometry, and SPIO exhibited a good loading capacity of 10.1 ± 2.5 wt% of drug at pH 7.4. Then, an investigation of the in vitro DOX release from the superparamagnetic iron oxide (SPIO) nanoshells was carried out in PBS for 24 h (pH 7.4); the loading was 26.3 ± 1.8 wt%.76 On the other hand, Park and co-workers established an effective synthesis of monodisperse hollow composite poly(methacrylic acid/ethylene glycol dimethacrylate)/Fe3O4 microcapsules (poly(MAA/EGDMA)/Fe3O4), which were studied as a drug delivery system (Fig. S15†).77 DOX as a model drug was loaded onto the microcapsules, and the successful controlled delivery system enabled 84.6% loading efficiency of the total DOX. The release rate of sample drug from the composite microcapsules was pH dependent in acidic solution because of the weaker electrostatic binding between the anionic carboxyl groups and cationic DOX. Generally, 43.8 wt% of DOX was released in PBS solution at pH 2; a lower drug amount of 28.0 wt% was released at pH 4. In contrast, at pH 7, the release was quite low and remained principally constant (9.5 wt%) (Fig. S16†).77
In addition to the modular approach, Lu and co-workers synthesized multi-functional hollow mesoporous silica nanocapsules with encapsulated iron oxide nanoparticles (IONPs) which were considered for a combination of hyperthermia and chemotherapy applications.78 Hence, DOX was loaded into the pores of the silica shells, with a capacity of 97 mg of drug per g of carrier. The DOX release from synthesized nanocapsules with two different drug loading amounts was investigated at different pH values and under an alternating magnetic field (AMF). However, when DOX release under AMF excitation was applied, the nanocapsule suspension showed a fast magnetic field response at 43 °C for a sample with a concentration of 1.3 mg mL−1 within 7 h (Fig. 6, and 7).78
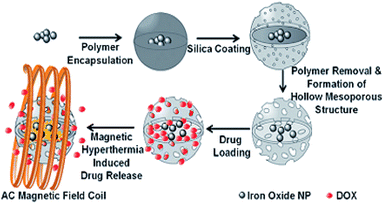 | ||
| Fig. 7 Schematic of DOX loading and magnetic hyperthermia-induced release.78 | ||
Recently, Cheng et al. synthesized Fe3O4@C nanocapsules via a sacrificial-template method by coating SiO2 nanospheres with a Fe3O4@C double-shell structure, followed by etching the SiO2 core under hydrothermal conditions. The nanocapsules exhibited a high loading capacity (1300 mg g−1 for DOX), and the DOX loaded on the surface of the carbon shells showed pH-dependent behavior. DOX release experiments were carried out at three different pH values of 7.4, 6.2 and 5.0. Accordingly, the drug release rate at pH 6.2 was about two times faster than that at pH 7.4 and was even faster at pH 5.0 (Scheme 4).79
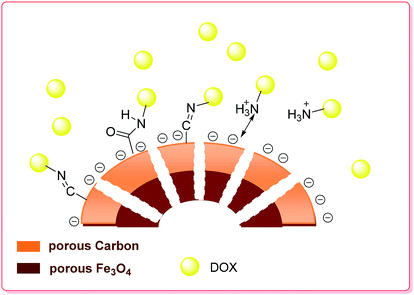 | ||
| Scheme 4 Schematic of the preparation and drug loading of the porous hollow Fe3O4@C nanocapsules.79 | ||
The zeta potentials of the magnetic nanocapsules in solutions with different pH values were also measured; it was found that the surface of the particles was negatively charged at pH values higher than 3, while at pH 5.0 to 7.4, the ionization of the carboxyl groups on the hollow magnetic nanoparticles (HMNPs) formed COO− and the amino groups of DOX combined with the hydrogen ions to form NH3+. The electrostatic interactions of COO− and NH3+ also contributed to the loading of DOX on the HMNPs. As the pH decreased from 7.4 to 5.0, the zeta potential of the HMNPs increased, which indicates that the surface of the HMNPs became less negative. On the other hand, drug release experiments carried out at pH 7.4 can be used to simulate the behavior of DOX–HMNPs when they are injected into blood or enter the intracellular environment or the cytoplasm of normal cells. The release rate at pH 7.4 is quite low (compared to the drug release experiment carried out at pH 6.2).79
Zhou et al. prepared monodispersed yolk-type Au@Fe3O4@C nanospheres with hollow cores 50 nm in diameter by coating Au@SiO2 nanoparticles with Fe3O4@C double layers followed by dissolving the SiO2 (Fig. 8).80 The cytotoxicity of the nanospheres was evaluated by methyl thiazolyltetrazolium assay (MTT assay), which demonstrated their high biocompatibility. As a model drug, DOX was loaded into the yolk-type nanospheres and showed a high DOX loading content of 1237 mg g−1. Moreover, the drug-loaded particles were divided into two groups to examine the release rates; one portion was subjected to magnetic stirring with near infrared irradiation, and the other portion was subjected to magnetic stirring at a constant rate at 37 °C at pH 7.4.
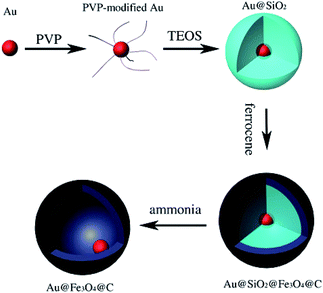 | ||
| Fig. 8 Schematic of the preparation procedure of yolk-type Au@Fe3O4@C.80 | ||
According to the DOX release profiles in Fig. 9, the DOX-Au@Fe3O4@C system obviously demonstrates sustained release; the cumulative release percentage with irradiation of a NIR-laser (red, 60%) was nearly 25% higher than that without laser irradiation (black, 35%) after 100 h.80
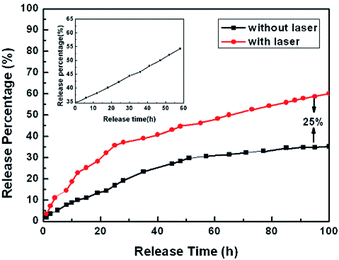 | ||
| Fig. 9 In vitro release profiles of DOX-Au@Fe3O4@C nanoparticles with (red) and without (black) laser irradiation for 100 h. Inset: further DOX release (black) on the balance for another 60 h with laser radiation.80 | ||
Peng and co-workers synthesized hollow iron oxide hydroxide mesoporous silica spheres (FeOOH/HMSS-PEG) and studied their feasibility for in vitro drug delivery (Fig. S17†). The amount of DOX loaded onto the magnetic carrier was 237.1 μg per mg of carrier in PBS solution. The protonated –NH2 groups of the drug became hydrophilic and more water-soluble in an acidic environment; thus, DOX was released completely at pH 6.5.82
Ji and co-workers used DOX as an anticancer agent to study the drug delivery and release efficiency of HPFe3O4@DDACMM-PEG-FA (Fig. 10). The theoretical DOX loading contents were set at 5%, 10% and 50% and the obtained DOX loading efficiencies were 79.40%, 72.30% and 65.86%, respectively. The DOX release process was considered by UV-Vis spectroscopy at λ = 385 nm. Moreover, the in vitro DOX release of the nanocarrier was studied under NIR light exposure at 37 °C. Meanwhile, much more DOX was released from the hollow carriers in the pH 5.0 solution than in the pH 7.0 solution (about 20% increase overnight).81
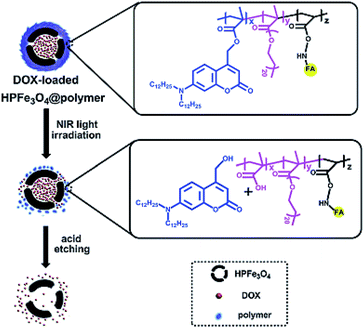 | ||
| Fig. 10 Schematic of the fabrication of HPFe3O4@DDACMM-PEG-FA and its controlled release upon NIR light exposure.81 | ||
To study the folate targeting and magnetic response effects of the drug delivery system, folate modification of HMCNCs was developed by Li et al.83 According to the TGA analysis results, the total weight loss observed for folate-HMCNCs was approximately 10 wt%; similarly, the weight loss behavior of DOX loaded on folate-HMCNCs enabled the calculation of the DOX in the carrier as approximately 24 wt%. Thus, about 69.1%, 76.4% and 79.8% of the DOX in Na3Cit/H3C in buffer solution at pH 5 was released during 48 h from folate-HMCNCs-DOX samples with 0.20, 0.29, and 0.36 cm3 g−1 shell-pore volumes, respectively.83
Recently, Zhu and co-workers synthesized folate-conjugated Fe3O4@SiO2 hollow spheres (Fe3O4@SiO2-FA) to study the loading and subsequent release of DOX as an anticancer drug in Fe3O4@SiO2 and Fe3O4@SiO2-FA hollow spheres (Fig. S18†). The DOX loading efficiencies of Fe3O4@SiO2 and Fe3O4@SiO2-FA were determined to be 76.6% and 54%, respectively. However, the DOX loading capacities in Fe3O4@SiO2 and Fe3O4@SiO2-FA were 27 and 38.3 (μg per mg of carrier), respectively. On the other hand, fast release of DOX from the Fe3O4@SiO2 and Fe3O4@SiO2-FA spheres occurred within 8 h in PBS (pH 7.4) at 37 °C. However, the release rate of the Fe3O4@SiO2-FA spheres was slower than that of the Fe3O4@SiO2 spheres (Fig. S19†).84
Recently, the use of polymers was found to be proficient to efficiently control carried drugs and remarkably limit the side effects and waste of drugs.85 Among these, poly(N-isopropylacrylamide) (PNIPAM) is a thermal-responsive polymer that exhibits a lower critical solution temperature (LCST) and is a suitable candidate for the fabrication of organic–inorganic hollow sphere carriers. Liu and co-workers described the drug delivery properties of the PNIPAM/Fe3O4–ZnS hybrid hollow spheres for DOX as an anticancer agent (Fig. S20†).
The concentration of DOX was measured by a UV-Vis spectrometer (480 nm); the loading capacity of DOX in the carrier was found to be about 70 μg per mg of carrier, and the DOX-loading efficiency was 35.5%. However, according to the drug release of DOX from the hybrid hollow spheres in PBS at pH 7 at different temperatures (25 °C, 37 °C, and 42 °C), about 20.9% cumulative DOX was released after 51 h at 25 °C; meanwhile, the release increased to 25.6% at 37 °C and 29.1% at 42 °C. Due to the expulsion of hydrophobically bound water from the polymer chains, the DOX release was faster at higher temperatures than at 25 °C (Fig. S21†).86 The effects of a hollow hybrid nanogel system (poly(AA-co-MEA)-g-mPEG/PNIPA) as a carrier were studied on the delivery and release of DOX as a drug agent.87 The results showed a high DOX loading efficiency (88.3%) and DOX loading capacity (9.6 wt%) for the hollow hybrid nanogels. The in vitro release of the loaded DOX from the hybrid nanogels was dependent on both pH and temperature. Furthermore, among various pH values (7.4, 5 and 4), remarkably enhanced drug release (>50%) at pH 5 was obtained at 37 °C over a period of 24 h.
Huang et al. synthesized tubular silica particles with hollow/porous structures based on Fe3O4 MNPs and hyaluronic acid and then used them as a DOX carrier for controlled release.88 The DOX storage capacity of the nanocarrier was reported to be 18.7%. The drug release process into PBS solution at 37 °C was studied. The nanocarrier-DOX revealed sustained drug release behaviour over 36 h, reaching 23% and 55% of the initial DOX loading amount at pH 7.4 and 5.5, respectively.88
Recently, Zhang and co-workers successfully synthesized hollow mesoporous silica nanochains with movable maghemite cores (γ-Fe2O3@mSiO2) as a carrier for doxorubicin hydrochloride (DOX) loading and release (Fig. S22†).89 The as-synthesised hollow mesoporous nanochains exhibited high drug loading and a good controlled release process due to their high specific surface area (197.2 m2 g1). The drug loading capacity of γ-Fe2O3@mSiO2 was 167.5 l g per mg of carrier. Moreover, the drug release rate of DOX loaded onto the sample under natural conditions was very slow in the first 7 h and reached about 51% when extended to 80 h (Fig. S23†).89
The magnetite dual-targeting of methylene bis acryl amide–meta acrylic acid P(MBAAm-co-MAA) with folic acid (FA) linkage as a DOX carrier was studied by Yang et al (Fig. 11). The DOX loading capacity of the dual-targeting hollow P(MBAAm-co-MAA) microspheres (as high as 176 mg per mg of carrier) was measured, and about 61% encapsulation efficiency was reported in the case of an initial DOX concentration of 230 mg mL−1. On the other hand, the release behavior of the dual-targeting hollow microspheres was dependent on the pH values in the environment. It was found that about 28% of loaded DOX was released from the carrier after 8 h in near-neutral conditions (pH 7.4). Finally, about 42%, 48% and 95% of the DXR drugs loaded onto the carrier were released at pH 6.0, 5.0 and 4.0 after 10 h, respectively, which was noticeably faster than the release rate under neutral conditions (Fig. S24†).90
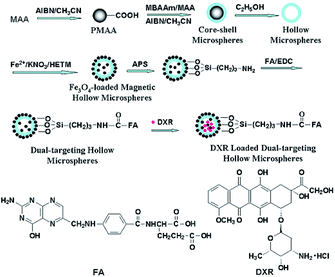 | ||
| Fig. 11 Preparation of magnetite and tumor dual-targeting P(MBAAm-co-MAA) hollow microspheres as anticancer drug-carriers and the chemical structures of FA and DXR molecules.90 | ||
Li and co-workers synthesized and characterized hollow magnetic nanoparticles (HMNPs) and studied their drug delivery as a phase-change material (PCM). In this method, DXR as a model drug was loaded into the HMNPs carrier; about 4% of DXR was released from HMNP@PCM@DXR at physiological temperature, whereas around 80% of DXR was released in 30 min at 42 °C.91
Zhou and co-workers studied multiple shell hollow Fe3O4 NPs assembled with lignin and grafted with folic acid as a nanocarrier for DXR delivery.92 The drug loading capacity of the as-prepared NPs was 67.5 ± 6%. The release behaviour of DXR from the drug-loaded NPs was considered in PBS buffer solutions at pH 5.5 and 7.4 over 8 h; only 19% and 13.4% of DXR leaked into the buffer solutions, respectively. In less than 30 h, DXR was released smoothly from the drug-loaded NPs under different pH conditions; this was attributed to the existence of magnetite NPs and folic acid.92
In another report, Deng and co-workers synthesized hollow Fe3O4/SiO2@PEG–poly(L,D-lactide) (Fe3O4/SiO2@PEG–PLA) nanoparticles and studied their use in drug delivery (Fig. S26†).96 Hence, CDDP as a model drug was loaded into the hollow carrier Fe3O4/SiO2@PEG–PLA. Additionally, the CDDP loading efficiencies in HMS and HMS@PEG–PLA were 50.62% and 37.34%, while the loading amounts of CDDP in HMS and HMS@PEG–PLA were 172 and 127 μg per mg of carrier. Also, in vitro drug release studies were carried out under physiological conditions in PBS at 37 °C. Meanwhile, high release rates were attained due to the presence of the drug near the surface of the particles. Generally, HMS@PEG–PLA presented a slower release than HMS in the medium because of the strong chemical bonds between the drugs and the high polymer material (Fig. S27†).96
Cheng and co-workers synthesized porous hollow nanoparticles (PHNPs) and modified them with herceptin to study the loading and release of CDDP as an anticancer drug by a diffusion-controlled slow process (Fig. S28†).97 The CDDP loading efficiency of PHNPs was determined to be up to 25%. Moreover, fast release of CDDP from PHNPs occurred in physiological buffer, with t1/2 = 4 h. However, the fabricated PHNPs with open pores (∼2 to 4 nm) and stable porous shells in neutral or basic physiological conditions could successfully provide many more active sites to adsorb higher amounts of the CDDP drug. On the other hand, the CDDP-PHNPs could target breast cancer SK-BR-3 cells, with IC50 values reaching 2.9 μM, much lower than that of 6.8 μM for free CDDP.97
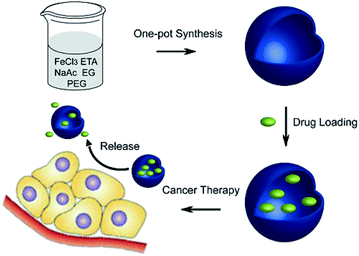 | ||
| Fig. 12 Schematic of the one-pot synthesis of Fe3O4 hollow spheres and their application for chemotherapeutics.99 | ||
Generally, the Fe3O4 hollow spheres were loaded with CPT drug via soaking them in DMSO for 24 h; the capacity was 176 μg. Then, CPT release from the Fe3O4 hollow spheres occurred in PBS at pH 7.4 and in DMSO with different incubation periods. However, once CPT–Fe3O4 was dispersed in DMSO for 0.5 h, most of the CPT was released and could be detected in the supernatant. Additionally, the negligible drug leakage of the sample in PBS has great significance in the minimization of side effects.
Importantly, almost no death occurred in cells in the Fe3O4 group, indicating its negligible toxicity in vitro.99 In another report, Sahu and co-workers synthesized a hollow magnetic mesoporous silica-based multimodal theranostic nanoagent as an efficient carrier for high loading and controlled release of CPT (Fig. S29†).100 According to their results, these multifunctional nanoparticles are not only extremely stable in aqueous buffer but also possess appreciably good cytotoxicity through the induction of apoptosis. The drug-loading capacity of the as-synthesised nanomagnetic carrier was 17.5%. Generally, this high CPT loading is a result of the high surface area of hollow mesoporous silica, which provides more interior spaces and conjugation sites. The drug release is higher at neutral pH and less acidic pH compared to other systems in which the drug is covalently attached to the carrier through ester linkages. At pH 5.3, an immediate release of 25% was observed after 10 h, which gradually increased to 83% after 80 h. The nanomagnetic product can be used as a carrier for CPT without premature release of the drug in blood vessels, and it also shows a sustained release pattern over a prolonged period of time inside the lysosomal compartment (Fig. S30†).100
Hollow magnetic core mesoporous double-shell nanostructures (HMMNSs) were studied as a nanocarrier for DOC and CPT delivery by Wu and co-workers. Based on UV-Vis data, about 150 mg of DOC or 140 mg of CPT as drug agents were loaded into 1 g of the HMMNSs. Meanwhile, only 1.8% (DOC) and 2.0% (CPT) of the loaded drugs were released into PBS solution at pH 7.4 for up to 72 h.101
In another study, to investigate superparamagnetic hollow spheres as carriers for CPT delivery, Fe3O4 hollow spheres functionalized with (3-aminopropyl)triethoxysilane (APTES) with an average size of about 200 nm were prepared by Patil and co-workers. The drug storage capacity of the nanoparticles was 20 μg of CPT per mL of carrier; also, about 30% of the CPT was released into PBS solution at pH 7.4 during 4 h.102
4 Conclusions
In this review, we outline the recent advances in multifunctional HMNPs for adsorption and delivery of various natural and chemical pharmaceutical applications. HMNPs have the desired properties for safe use as pharmaceutical excipients. Hollow morphology, low density, large pore size, magnetic separation and high surface area are some advantages of these materials for drug delivery. These systems have great utility in controlled release and targeting of almost all classes of bioactive molecules, as discussed in this review. Despite the numerous challenges of these materials, HMNPs are indeed promising candidates for pharmaceutical and biological applications.At the end, SWOT analysis of hollow magnetic nanomaterials provides understanding of their synthesis methods and how these materials can be used to create smart drug delivery systems.
Generally, these materials can target specific locations in the body. The dosage of drug can be more readily adjusted compared with traditional magnetic target drug carriers. Moreover, they are among the most beneficial compounds due to their low density and non-toxicity, which can provide more opportunities for cancer therapy and provide a pathway toward the treatment of challenging diseases.
However, one of the major weakness of hollow magnetic nanoparticles for drug delivery applications is the synthesis of specific magnetic hollow particles by various approaches and the simultaneous controlling and tuning of the shapes and sizes of the final particles. In addition, the drug molecules cannot remain in circulating systems in the body.
Conflicts of interest
There are no conflicts to declare.List of abbreviations
| AMF | Alternating magnetic field |
| APTES | (3-Aminopropyl)triethoxysilane |
| CPT | Camptothecin |
| CDDP | Cisplatin |
| CM | Casein micelle |
| DL | Drug loading |
| DOX | Doxorubicin |
| DOX | Doxorubicin hydrochloride |
| ENR | Enrofloxacin |
| FA | Folic acid |
| FR | Folate receptor |
| 5-FU | 5-Fluorouracile |
| HAp | Hydroxy apatite |
| HMMNSs | Hollow magnetic mesoporous Nanostructures |
| HMMS | Hollow magnetic mesoporous shell |
| HMSPs | Hollow structure superparamagnetic particles |
| HMNPs | Hollow magnetic nanoparticles |
| HPNSs | Hollow porous nanocrystal shells |
| IBU | Ibuprofen |
| IONPs | Iron oxide nano particles |
| LCST | Lower critical solution temperature |
| MAA | Meta acrylic acid |
| MBAAm | Methylene bis acryl amide |
| MHMS | Magnetic hollow mesoporous silica |
| MHSNs | Magnetic hollow silica nanosphere |
| MMCNs | Magnetic mesoporous calcium nano composites |
| MNPs | Magnetic nano particles |
| MOF | Metal organic framework |
| MTT | Methyl thiazolyl tetrazolium |
| MZHM-MSs | Magnetic ZnFe2O4 hollow microsphere silica shells |
| PBS | Phosphate buffer saline |
| PCM | Phase change material |
| PEG | Poly ethylene glycol |
| PHNPs | Porous hollow nanoparticles |
| PLA | Poly(L,D-lactide) |
| PTXL | Paclitaxel |
| SBF | Simulated body fluid |
| SCPs | Solid carbon particles |
| SPIO | Super paramagnetic iron oxide |
| TSCHMSs | Silanized hollow Fe3O4/carbon/poly(N-isopropyl acrylamide) magnetic spheres |
| TXL | Docetaxel |
| UV-Vis | Ultraviolet-visible |
| VAN | Vancomycin |
Acknowledgements
The authors gratefully acknowledge financial support from the Research Council of Alzahra University.Notes and references
- S. Kralj and D. Makovec, ACS Nano, 2015, 9, 9700–9707 CrossRef CAS PubMed.
- M. Tadic, S. Kralj, M. Jagodic, D. Hanzel and D. Makovec, Appl. Surf. Sci., 2014, 322, 255–264 CrossRef CAS.
- J. W. Bulte, Methods Mol. Med., 2006, 124, 419–439 Search PubMed.
- A. Amoozadeh, M. Malmir, N. Koukabi and S. Otokesh, J. Chem. Res., 2015, 39, 694–697 CrossRef CAS.
- A. Elhampour, M. Malmir, E. Kowsari, F. Boorboor ajdari and F. Nemati, RSC Adv., 2016, 6, 96623–96634 RSC.
- V. F. Vavsari, G. M. Ziarani, S. Balalaie, M. Karimi, A. Latifi and A. Badiei, Synfacts, 2016, 12, 1215 CrossRef.
- G. M. Ziarani, Z. Kazemi, P. Gholamzadeh, A. Badiei and M. Afshar, Appl. Organomet. Chem., 2017, 31, e3830–e3836 CrossRef.
- G. M. Ziarani, P. Gholamzadeh, A. Badiei and V. F. Vavsari, Res. Chem. Intermed., 2018, 44, 277–288 CrossRef.
- V. F. Vavsari, G. M. Ziarani, S. Balalaie, A. Badiei, F. Golmahammadi, S. Ramezanpour and F. Rominger, ChemistrySelect, 2017, 2, 3496–3499 CrossRef.
- G. M. Ziarani, L. Seiedakbari, P. Gholamzadeh and A. Badiei, Iran. J. Catal., 2017, 7, 137–145 CAS.
- S. Sadjadi, M. Malmir and M. M. Heravi, RSC Adv., 2017, 7, 36807–36818 RSC.
- S. Sadjadi, M. M. Heravi and M. Malmir, J. Taiwan Inst. Chem. Eng., 2018, 86, 240–251 CrossRef CAS.
- K. Ulbrich, K. Holá, V. Šubr, A. Bakandritsos, J. Tuček and R. Zbořil, Chem. Rev., 2016, 116, 5338–5431 CrossRef CAS PubMed.
- N. F. Frey, S. Peng, K. Cheng and S. Sun, Chem. Soc. Rev., 2009, 38, 2532–2542 RSC.
- Z. Sun and S. Sun, in Biomedical Nanotechnology: Methods and Protocols, Methods in Molecular Biology, ed. S. H. Petrosko and E. S. Day, Springer, 2017, vol. 1570, pp. 73–90 Search PubMed.
- L. P. Zhu, H. M. Xiao and S. Y. Fu, Cryst. Growth Des., 2007, 7, 177–182 CrossRef CAS.
- X. G. Wen, S. H. Wang, Y. Ding, Z. L. Wang and S. H. Yang, J. Phys. Chem. B, 2005, 109, 215–220 CrossRef CAS PubMed.
- B. Y. Geng, F. M. Zhan, H. Jiang, Y. J. Guo and Z. J. Xing, Chem. Commun., 2008, 5773–5775 RSC.
- W. Xie, Z. Guo, F. Gao, Q. Gao, D. Wang, B. Liaw, Q. Cai, X. Sun, X. Wang and L. Zhao, Theranostics, 2018, 8, 3284–3307 CrossRef CAS PubMed.
- K. E. Albinali, M. M. Zagho, Y. Deng and A. Elzatahry, Int. J. Nanomed., 2019, 14, 1707–1723 CrossRef PubMed.
- X. W. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987–4019 CrossRef CAS.
- H. J. Fan, U. Gösele and M. Zacharias, Small, 2007, 3, 1660–1671 CrossRef CAS PubMed.
- J. Y. Zhong, C. B. Cao, Y. Y. Liu, Y. N. Li and W. S. Khan, Chem. Commun., 2010, 46, 3869–3871 RSC.
- Y. Wang, Q. S. Zhu and L. Tao, CrystEngComm, 2011, 13, 4652–4657 RSC.
- W. Ostwald, Z. Phys. Chem., 1897, 22, 289–330 CAS.
- W. Cheng, K. B. Tang, Y. X. Qi, J. Sheng and Z. P. Liu, J. Mater. Chem., 2010, 20, 1799–1805 RSC.
- A. D. Smigelskas and E. O. Kirkendall, Trans. AIME, 1947, 171, 130–142 Search PubMed.
- B. Jia and L. Gao, J. Phys. Chem. C, 2008, 112, 666–671 CrossRef CAS.
- P. Hu, L. Yu, A. Zuo, C. Guo and F. Yuan, J. Phys. Chem. C, 2009, 113, 900–906 CrossRef CAS.
- S. W. Kim, M. Kim, W. Y. Lee and T. Hyeon, J. Am. Chem. Soc., 2002, 124, 7642–7643 CrossRef CAS PubMed.
- B. Tan and S. E. Rankin, Langmuir, 2005, 21, 8180–8187 CrossRef CAS PubMed.
- Y. Ding, Y. Hu, X. Jiang, L. Zhang and C. Yang, Angew. Chem., Int. Ed., 2004, 43, 6369–6372 CrossRef CAS PubMed.
- Q. He, Z. Wu and C. Huang, J. Nanosci. Nanotechnol., 2012, 12, 2943–2954 CrossRef CAS PubMed.
- S. E. Skarabalak, J. Chen, Y. Sun, X. Lu, L. Au and C. M. Cobley, Acc. Chem. Res., 2008, 41, 1587–1595 CrossRef PubMed.
- V. F. Vavsari, G. M. Ziarani and A. Badiei, RSC Adv., 2015, 5, 91686–91707 RSC.
- Z. Bahrami, A. Badiei and G. M. Ziarani, Int. J. Bio-Inorg. Hybrid Nanomater., 2015, 4, 121–128 Search PubMed.
- Z. Bahrami, A. Badiei and G. M. Ziarani, J. Nanopart. Res., 2015, 125, 1–12 Search PubMed.
- A. Badiei, I. Haririan, A. Jahangir and G. M. Ziarani, Dyn. Biochem. Process Biotechnol. Mol. Biol., 2009, 3, 48–50 Search PubMed.
- B. Karimi, F. Mansouri and H. M. Mirzaei, ChemCatChem, 2015, 7, 1736–1789 CrossRef CAS.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064–2210 CrossRef CAS PubMed.
- Y. Si, M. Chen and L. Wu, Chem. Soc. Rev., 2016, 45, 690–714 RSC.
- L. H. Reddy, J. L. Arias, J. Nicolas and P. Couvreur, Chem. Rev., 2012, 112, 5818–5878 CrossRef CAS PubMed.
- A. K. Guptaa and M. Gupta, Biomaterials, 2005, 26, 3995–4021 CrossRef PubMed.
- D. B. Robinson, H. H. J. Persson, H. Zeng, G. Li, N. Pourmand, S. Sun and S. X. Wang, Langmuir, 2005, 21, 3096–3103 CrossRef CAS PubMed.
- S. W. Kim, S. Kim, J. B. Tracy, A. Jasanoff and M. G. Bawendi, J. Am. Chem. Soc., 2005, 127, 4556–4557 CrossRef CAS PubMed.
- F. M. Zehentbauer, C. Moretto, R. Stephen, T. Thevar, J. R. Gilchrist, D. Pokrajac, K. L. Richard and J. Kiefer, Spectrochim. Acta, Part A, 2014, 121, 147–151 CrossRef CAS PubMed.
- E. J. K. Al -Yasari, Med. J. Babylon, 2014, 11, 768–775 Search PubMed.
- X. Chen, R. Klingeler, M. Kath, A. A. El Gendy, K. Cendrowski, R. J. Kalenczuk and E. Borowiak-Palen, ACS Appl. Mater. Interfaces, 2012, 4, 2303–2309 CrossRef CAS PubMed.
- Q. He, J. Liu, J. Liang, X. Liu, D. Tuo and W. Li, Materials, 2018, 11, 247–263 CrossRef PubMed.
- B. Q. Lu, Y. J. Zhu, G. F. Cheng and Y. J. Ruan, Mater. Lett., 2013, 104, 53–56 CrossRef CAS.
- M. Sasidharan, H. N. Luitel, N. Gunawardhana, M. Inoue, S.-i. Yusa, T. Watari and K. Nakashima, Mater. Lett., 2012, 73, 4–7 CrossRef CAS.
- S. W. Cao, Y. J. Zhu, M. Y. Ma, L. Li and L. Zhang, J. Phys. Chem. C, 2008, 112, 1851–1856 CrossRef CAS.
- S. W. Cao and Y. J. Zhu, J. Phys. Chem. C, 2008, 112, 12149–12156 CrossRef CAS.
- L. Y. Xia, M. Q. Zhang, C. Yuan and M. Z. Rong, J. Mater. Chem., 2011, 21, 9020–9026 RSC.
- X. Zhu, S. Zhang, L. Zhang, H. Liu and J. Hu, RSC Adv., 2016, 6, 58511–58515 RSC.
- W. Zhao, H. Chen, Y. Li, L. Li, M. Lang and J. Shi, Adv. Funct. Mater., 2008, 18, 2780–2788 CrossRef CAS.
- X. Zhao, P. Du and P. Liu, Mol. Pharmaceutics, 2012, 9, 3330–3339 CrossRef CAS PubMed.
- J. Zhou, W. Wu, D. Caruntu, M. H. Yu, A. Martin, J. F. Chen, C. J. O'Connor and W. L. Zhou, J. Phys. Chem. C, 2007, 111, 17473–17477 CrossRef CAS.
- B.-Q. Lu, Y.-J. Zhu, H.-Y. Ao, C. Qi and F. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 6969–6974 CrossRef CAS PubMed.
- Y. Yang, X. Guo, K. Wei, L. Wang, D. Yang, L. Lai, M. Cheng and Q. Liu, J. Nanopart. Res., 2014, 16, 2210–2214 CrossRef.
- C. Wang, J. Yan, Z. Li, H. Wang and X. Cui, J. Nanopart. Res., 2013, 15, 1937–1942 CrossRef.
- D. Yang, K. Wei, Q. Liu, Y. Yang, X. Guo, H. Rong, M. L. Cheng and G. Wang, Mater. Sci. Eng., C, 2013, 33, 2879–2884 CrossRef CAS PubMed.
- L. Zhang, Y. Sun, W. Jia, S. Ma, B. Song, Y. Li, H. Jiu and J. Liu, Ceram. Int., 2014, 40, 8997–9002 CrossRef CAS.
- E. V. Hersh, S. Cooper, N. Betts, D. Wedell and K. MacAfee, Oral Surg., Oral Med., Oral Pathol., 1993, 76, 680–687 CrossRef CAS.
- J. M. Vargyas, J. D. Campeau and D. R. J. Mishell, Am. J. Obstet. Gynecol., 1987, 157, 944–950 CrossRef CAS PubMed.
- V. G. B. Alan, C. P. L. Isabel, M. M. Eugenia, G. R. Roberto, J. L. Coffer and M.-R. Miguel A, J. Nanomed. Res., 2016, 3, 1–5 Search PubMed.
- J. E. Dolfini, H. E. Applegate, G. Bach, H. Basch, J. Bernstein, J. Schwartz and F. L. Weisenborn, J. Med. Chem., 1971, 14, 117–119 CrossRef CAS PubMed.
- L. Li, H. Li, D. Chen, H. Liu, F. Tang, Y. Zhang, J. Ren and Y. Li, J. Nanosci. Nanotechnol., 2009, 9, 2540–2545 CrossRef CAS PubMed.
- L. Cui, A. Iwamoto, J. Q. Lian, H. Neoh, T. Maruyama, Y. Horikawa and K. Hiramatsu, Antimicrob. Agents Chemother., 2006, 50, 428–438 CrossRef CAS PubMed.
- K. Lin, L. Chen, P. Liu, Z. Zou, M. Zhang, Y. Shen, Y. Qiao, X. Liu and J. Chang, CrystEngComm, 2013, 15, 2999–3008 RSC.
- F. Liu, J. Wang, Q. Cao, H. Deng, G. Shao, D. Y. B. Deng and W. Zhou, Chem. Commun., 2015, 51, 2357–2360 RSC.
- R. T. Chlebowski, West. J. Med., 1979, 131, 364–368 CAS.
- P. G. Upton, K. T. Yamaguchi, S. Myers, T. P. Kidwell and R. J. Anderson, Cancer Treat Rep., 1986, 70, 503–507 CAS.
- A. Kumar, B. Gautam, C. Dubey and P. K. Tripath, Int. J. Pharm. Sci. Rev. Res., 2014, 3, 4117–4128 Search PubMed.
- S. Xu, B. Yin, J. Guo and C. Wang, J. Mater. Chem. B, 2013, 1, 4079–4087 RSC.
- X.-M. Zhu, J. Yuan, K. C.-F. Leung, S.-F. Lee, K. W. Y. Sham, C. H. K. Cheng, D. W. T. Au, G.-J. Teng, A. T. Ahuja and Y.-X. J. Wang, Nanoscale, 2012, 4, 5744–5754 RSC.
- S.-J. Park, H.-S. Lim, Y. M. Lee and K. D. Suh, RSC Adv., 2015, 5, 10081–10088 RSC.
- F. Lu, A. Popa, S. Zhou, J.-J. Zhu and A. C. S. Samia, Chem. Commun., 2013, 49, 11436–11438 RSC.
- K. Cheng, Z. Sun, Y. Zhou, H. Zhong, X. Kong, P. Xia, Z. Guo and Q. Chen, Biomater. Sci., 2013, 1, 965–974 RSC.
- Y.-M. Zhou, H.-B. Wang, M. Gong, Z.-Y. Sun, K.-C. Cheng, X.-k. Kong, Z. Guo and Q. W. Chen, Dalton Trans., 2013, 42, 9906–9913 RSC.
- W. Ji, N. Li, D. Chen, Y. Jiao, Q. Xu and J. Lu, RSC Adv., 2014, 4, 51055–51061 RSC.
- Y.-K. Peng, Y.-J. Tseng, C.-L. Liu, S.-W. Chou, Y.-W. Chen, S. C. Edman Tsang and P.-T. Chou, Nanoscale, 2015, 7, 2676–2687 RSC.
- D. Li, J. Tang, J. Guo, S. Wang, D. Chaudhary and C. Wang, Chem.–Eur. J., 2012, 18, 16517–16524 CrossRef CAS PubMed.
- Y. Zhu, Y. Fang and S. Kaskel, J. Phys. Chem. C, 2010, 114, 16382–16388 CrossRef CAS.
- M. Karg and T. Hellweg, Curr. Opin. Colloid Interface Sci., 2009, 14, 438–450 CrossRef CAS.
- G. Liu, D. Hu, M. Chen, C. Wang and L. Wu, J. Colloid Interface Sci., 2013, 397, 73–79 CrossRef CAS PubMed.
- W.-H. Chiang, V. T. Ho, H.-H. Chen, W.-C. Huang, Y.-F. Huang, S.-C. Lin, C.-S. Chern and H.-C. Chiu, Langmuir, 2013, 29, 6434–6443 CrossRef CAS PubMed.
- L. Huang, L. Ao, W. Wang, D. Hu, Z. Sheng and W. Su, Chem. Commun., 2015, 51, 3923–3926 RSC.
- W. Zhang, X. Si, B. Liu, G. Bian, Y. Qi, X. Yang and C. Li, J. Colloid Interface Sci., 2015, 456, 145–154 CrossRef CAS PubMed.
- X. Yang, L. Chen, B. Han, X. Yang and H. Duan, Polymer, 2010, 51, 2533–2539 CrossRef CAS.
- J. Li, Y. Hu, Y. Hou, X. Shen, G. Xu, L. Dai, J. Zhou, Y. Liu and K. Cai, Nanoscale, 2015, 7, 9004–9012 RSC.
- Y. Zhou, Y. Han, G. Li, S. H. Yang, F. Xiong and F. Chu, Nanomaterials, 2019, 9, 188–201 CrossRef CAS PubMed.
- M. Rozencweig, D. D. von Hoff, M. Slavik and F. M. Muggia, Ann. Intern. Med., 1977, 86, 803–812 CrossRef CAS PubMed.
- L. H. Einhorn and S. D. Williams, N. Engl. J. Med., 1979, 300, 289–291 CrossRef CAS PubMed.
- M. Lei, T. Chao and Z. Lei, J. Nanopart. Res., 2014, 16, 2410–2416 CrossRef.
- H. Deng and Z. Lei, Composites, Part B, 2013, 54, 194–199 CrossRef CAS.
- K. Cheng, S. Peng, C. Xu and S. Sun, J. Am. Chem. Soc., 2009, 131, 10637–10644 CrossRef CAS PubMed.
- M. E. Wall, M. C. Wani, C. E. Cook, K. H. Palmer, A. T. McPhail and G. A. Sim, J. Am. Chem. Soc., 1966, 88, 3888–3890 CrossRef CAS.
- Y. Zhu, J. Lei and Y. Tian, Dalton Trans., 2014, 43, 7275–7281 RSC.
- S. Sahu, N. Sinha, S. K. Bhutia, M. Majhi and S. Mohapatra, J. Mater. Chem. B, 2014, 2, 3799–3808 RSC.
- H. Wu, S. Zhang, J. Zhang, G. Liu, J. Shi, L. Zhang, X. Cui, M. Ruan, Q. He and W. Bu, Adv. Funct. Mater., 2011, 21, 1850–1862 CrossRef CAS.
- P. B. Patil, V. C. Karade, P. P. Waifalkar, S. C. Sahoo, P. Kollu, M. S. Nimbalkar, A. D. Chougale and P. S. Pati, IEEE Trans. Magn., 2017, 53, 5200604–5200607 Search PubMed.
- T. M. Mekhail and M. Markman, Expert Opin. Pharmacother., 2002, 3, 755–766 CrossRef CAS PubMed.
- B. Luo, S. Xu, W.-F. Ma, W.-R. Wang, S.-L. Wang, J. Guo, W.-L. Yang, J.-H. Hu and C.-C. Wang, J. Mater. Chem., 2010, 20, 7107–7113 RSC.
- J. L. Grem, Invest. New Drugs, 2000, 18, 299–313 CrossRef CAS PubMed.
- T. Maria, A. Panagiotis and P. Ioannis, J. Cancer Ther., 2015, 6, 345–355 CrossRef.
- L. Chen, H. Zhang, L. Li, Y. Yang, X. Liu and B. Xu, Appl. Polym. Sci., 2015, 132, 42617–42627 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra01589b |
| This journal is © The Royal Society of Chemistry 2019 |
