A comparative assessment of PM2.5 exposures in light-rail, subway, freeway, and surface street environments in Los Angeles and estimated lung cancer risk†
Winnie
Kam
a,
Ralph J.
Delfino
b,
James J.
Schauer
c and
C.
Sioutas
*a
aDepartment of Environmental Engineering, University of Southern California, Los Angeles, CA 90089, USA. E-mail: sioutas@usc.edu; Fax: +1 (213) 744-1425; Tel: +1 (213) 740-6134
bDepartment of Epidemiology, University of California, Irvine, CA, USA
cEnvironmental Chemistry and Technology Program, University of Wisconsin-Madison, Madison, WI, USA
First published on 4th December 2012
Abstract
According to the U.S. Census Bureau, 570![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000+ commuters in Los Angeles travel for over 60 minutes to work. Studies have shown that a substantial portion of particulate matter (PM) exposure can occur during this commute. This study represents the integration of the results from five commute environments in Los Angeles. Personal PM exposures are discussed for the: (1) METRO gold line, a ground-level light-rail route, (2) METRO red line, a subway line, (3) the 110, a high volume freeway with low heavy-duty vehicle (HDV) fraction, (4) the 710, a major corridor for HDVs from the Port of Los Angeles, and (5) Wilshire/Sunset Boulevards, major surface streets. Chemical analysis including total and water-soluble metals and trace elements, elemental and organic carbon (EC/OC), and polycyclic aromatic hydrocarbons (PAHs) was performed. The focus of this study is to compare the composition and estimated lung cancer risk of PM2.5 (dp < 2.5 μm) for the five differential commute environments. Metals associated with stainless steel, notably Fe, Cr, and Mn, were elevated for the red line (subway), most likely from abrasion processes between the rail and brakes; elements associated with tire and brake wear and oil additives (Ca, Ti, Sn, Sb, and Pb) were elevated on roadways. Elemental concentrations on the gold line (light-rail) were the lowest. For water-solubility, metals observed on the red line (subway) were the least soluble. PAHs are primarily derived from vehicular emissions. Overall, the 710 exhibited high levels of PAHs (3.0 ng m−3), most likely due to its high volume of HDVs, while the red and gold lines exhibited low PAH concentrations (0.6 and 0.8 ng m−3 for red and gold lines, respectively). Lastly, lung cancer risk due to inhalation of PAHs was calculated based on a commuter lifetime (45 years for 2 hours per workday). Results showed that lung cancer risk for the 710 is 3.8 and 4.5 times higher than the light-rail (gold line) and subway (red line), respectively. With low levels of both metal and PAH pollutants, our results indicate that commuting on the light-rail (gold line) may have potential health benefits when compared to driving on freeways and busy roadways.
000+ commuters in Los Angeles travel for over 60 minutes to work. Studies have shown that a substantial portion of particulate matter (PM) exposure can occur during this commute. This study represents the integration of the results from five commute environments in Los Angeles. Personal PM exposures are discussed for the: (1) METRO gold line, a ground-level light-rail route, (2) METRO red line, a subway line, (3) the 110, a high volume freeway with low heavy-duty vehicle (HDV) fraction, (4) the 710, a major corridor for HDVs from the Port of Los Angeles, and (5) Wilshire/Sunset Boulevards, major surface streets. Chemical analysis including total and water-soluble metals and trace elements, elemental and organic carbon (EC/OC), and polycyclic aromatic hydrocarbons (PAHs) was performed. The focus of this study is to compare the composition and estimated lung cancer risk of PM2.5 (dp < 2.5 μm) for the five differential commute environments. Metals associated with stainless steel, notably Fe, Cr, and Mn, were elevated for the red line (subway), most likely from abrasion processes between the rail and brakes; elements associated with tire and brake wear and oil additives (Ca, Ti, Sn, Sb, and Pb) were elevated on roadways. Elemental concentrations on the gold line (light-rail) were the lowest. For water-solubility, metals observed on the red line (subway) were the least soluble. PAHs are primarily derived from vehicular emissions. Overall, the 710 exhibited high levels of PAHs (3.0 ng m−3), most likely due to its high volume of HDVs, while the red and gold lines exhibited low PAH concentrations (0.6 and 0.8 ng m−3 for red and gold lines, respectively). Lastly, lung cancer risk due to inhalation of PAHs was calculated based on a commuter lifetime (45 years for 2 hours per workday). Results showed that lung cancer risk for the 710 is 3.8 and 4.5 times higher than the light-rail (gold line) and subway (red line), respectively. With low levels of both metal and PAH pollutants, our results indicate that commuting on the light-rail (gold line) may have potential health benefits when compared to driving on freeways and busy roadways.
Environmental impactOver half a million commuters in Los Angeles travel for over two hours on a daily basis. Studies have shown that a substantial portion of particulate matter (PM) exposure can occur during commutes. The link between elevated levels of PM exposure and adverse human health effects has been well established. The components of PM are highly complex, some of which may have toxic, and potentially carcinogenic, effects on humans. This study assesses the PM exposure for various commute environments in Los Angeles and estimates lung cancer risk based on PM inhalation. Results from this study are of interest not only for commuters, but also for residents and pedestrians who are in the vicinity of major roadways that are sources of these pollutants. |
1 Introduction
As a major metropolitan area characterized by urban sprawl, over 570![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 commuters in Los Angeles travel for over 60 minutes to work, yielding a daily commute of over two hours (American Community Survey, U.S. Census Bureau). Previous studies have shown that a substantial portion of particulate matter (PM) exposure can occur during commute depending on the type and route of travel.1,2 The link between elevated levels of PM, particularly PM2.5 (aerodynamic diameter, dp, less than 2.5 μm), and adverse human health effects has been well established.3 Numerous health studies have also indicated that long-term ambient PM exposure can be related to risk of lung cancer,4,5 cardiovascular disease,6 and neurodegenerative disorders.7,8 Earlier studies have also shown that 33–45% of total ultrafine particle (dp < 180 nm) exposure for Los Angeles residents occurs during time spent in their vehicles.9
000 commuters in Los Angeles travel for over 60 minutes to work, yielding a daily commute of over two hours (American Community Survey, U.S. Census Bureau). Previous studies have shown that a substantial portion of particulate matter (PM) exposure can occur during commute depending on the type and route of travel.1,2 The link between elevated levels of PM, particularly PM2.5 (aerodynamic diameter, dp, less than 2.5 μm), and adverse human health effects has been well established.3 Numerous health studies have also indicated that long-term ambient PM exposure can be related to risk of lung cancer,4,5 cardiovascular disease,6 and neurodegenerative disorders.7,8 Earlier studies have also shown that 33–45% of total ultrafine particle (dp < 180 nm) exposure for Los Angeles residents occurs during time spent in their vehicles.9
The composition of PM is highly complex, and is composed of multiple components including elemental and organic carbon (EC and OC), metals and trace elements, and organic species, some of which may have toxic, and potentially carcinogenic, effects on humans. Transition metals (i.e. Fe, Cr, Co, Mn) have been shown to be significantly correlated with oxidative stress in cell systems,10,11 and ultimately lead to DNA damage.12 Water-soluble metals are also of particular interest in terms of public exposure as solubility contributes to its bioavailability to human cells.13 A number of polycyclic aromatic hydrocarbons (PAHs) have been identified as being reasonably anticipated to be human carcinogens according to the U.S. Department of Health and Human Services and probable or possible carcinogens according to the California Office of Environmental Health Hazard Assessment (OEHHA). Earlier studies have also estimated lung cancer risk based on inhalation of particle-bound PAHs.14,15
The focus of this study is to assess and compare PM2.5 exposures between five different transport environments in the Los Angeles area (subway, light-rail, surface street, and two major freeways with a low and high truck fraction). Key species for comparison are EC, OC, total and water-soluble metals and trace elements, and PAHs. They are presented as mass per m3 of air. Sources of PM components and species will be discussed in detail as well as its contribution from the commute environment. Lastly, estimates of lung cancer risk based on concentrations of PAHs are calculated to determine the risk associated with each commute environment. The novelty of the current study lies in its focus on the exposure risk assessment for commuters of various microenvironments of Los Angeles. The PM species presented and discussed in this study have either been identified as carcinogens or as hazardous to human health. In addition, results from this study are of primary interest not only for commuters of these specific commute environments, but also for residents and pedestrians who are in the vicinity of major roadways that are sources of these pollutants.
2 Experimental methodology
This study represents the integration of two major campaigns that were conducted independently but with common measurement methods. The two campaigns will be referred to as the METRO study and the on-road study. Table 1 shows a summary of the sampling dates and times, routes, and meteorological parameters including average temperature, relative humidity (RH), prevailing wind direction, and wind speed. Meteorological data are based on a South Coast Air Quality Management District (SCAQMD) monitoring site. Average temperatures varied from 17–24 °C for the two campaigns due to different sampling periods. Note that the differences in the variation of relative humidity were due to the longer sampling time period for the on-road campaign (6 AM–5 PM) as compared to the METRO campaign (9:30 AM–1:00 PM). Overall, the wind direction and wind speed are comparable in the two campaigns. The University of Southern California (USC) site, which is centrally located near downtown Los Angeles, was sampled concurrently during both campaigns as a reference site. Previous studies have also been conducted at this site.16,17Fig. 1 shows a map of the five commute environments, the USC reference site, and the SCAQMD monitoring site.| Route | Dates of sampling | Sampling times | Temperature (°C) | RH (%) | Prevailing wind direction | Wind speed (m s−1) |
|---|---|---|---|---|---|---|
| METRO study | ||||||
| METRO lines | 5/3–8/13/10 | 9:30 AM–1:00 PM | 24.0 ± 3.5 | 55 ± 9.7 | SW | 3.2 ± 0.9 |
| On-road study | ||||||
| 110 | 3/1–3/8/11, 4/11–4/18/11 | 6:00 AM–5:00 PM | 18.3 ± 4.5 | 57.2 ± 21.5 | W | 3.2 ± 1.2 |
| 710 | 3/17–3/25/11, 4/19–4/25/11 | 6:00 AM–5:00 PM | 17.3 ± 3.6 | 59.9 ± 18.2 | W | 3.5 ± 1.3 |
| Wilshire/Sunset | 3/9–3/16/11, 4/26–5/2/11 | 6:00 AM–5:00 PM | 23.8 ± 5.7 | 42.0 ± 20.9 | SW | 3.3 ± 1.6 |
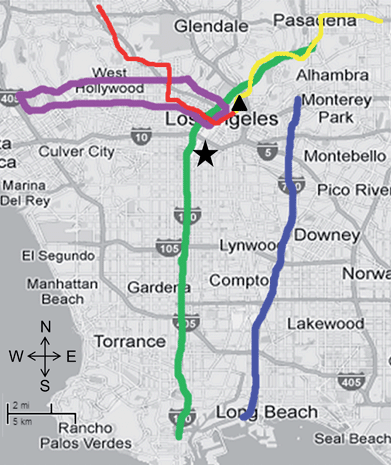 | ||
| Fig. 1 Map of five commute environments: 110 (green), 710 (blue), Wilshire/Sunset (purple), METRO red line (red), and METRO gold line (yellow). The USC reference site is denoted by the star, and the South Coast Air Quality Management District (SCAQMD) monitoring site is denoted by the triangle. | ||
2.1 Sampling methodology
Both campaigns used the same instruments to collect PM for the purpose of comparing results. PM2.5 was collected using the compact Personal Cascade Impactor Sampler (PCIS) (SKC Inc., Eighty-Four, PA),18,19 which was operated with portable Leland Legacy pumps (SKC Inc., Eighty-Four, PA). The PCIS was prepared using an impaction stage with a cutpoint of 2.5 μm and an after filter stage, which collected PM2.5. For the purpose of comprehensive chemical analysis, two types of filters were used—PTFE (Teflon) filters (Pall Life Sciences, Ann Arbor, MI) and quartz microfiber filters (Whatman International Ltd, Maidstone, England). Two sets of samples (N = 2) were collected for both campaigns.For the METRO campaign, subjects used suitcases to carry the instruments and spent 25% of their time waiting at stations and 75% of their time inside the train to simulate a typical commuter. The sampling duration for the METRO campaign was on weekdays from May to August 2010 from 9:30 AM to 1:00 PM. For the on-road campaign, the sampling vehicle was a Honda Insight Hybrid 2011 equipped with a curved inlet for roadway air entry to the sampling instruments. Since anisokinetic effects, which may result in the overestimation or underestimation of relatively larger particles (i.e. PM10–2.5),20 need to be considered for on-road PM measurements, it was determined that PM2.5, the PM fraction of interest in this study, is largely unaffected by anisokinetic sampling.21 The sampling duration for the on-road campaign was on weekdays from March to April 2011 from 6:00 AM to 5:00 PM.
2.2 Route description
A detailed description of the methodology of the METRO study has been described by Kam et al. (2011),22 so only a brief description follows. In the METRO study, two lines (the red line and the gold line) were sampled concurrently using identical instruments. The red line is a subway line powered by an electric third-rail that connects Downtown Los Angeles to North Hollywood and has the highest ridership of the METRO system; the gold line is a ground-level light rail line powered by overhead electric lines that connects Downtown Los Angeles to Pasadena. Trains pass every 8–12 minutes depending on the hour.The on-road study has been described in detail by Kam et al. (2012),21 so a summary of the routes and methodology follows. On-road sampling was conducted for three roadways during different time periods (Table 1). The three roadways each represent differential private commute environments. The 110 is a high-traffic freeway that runs from the Port of Los Angeles through Downtown Los Angeles to Pasadena, and is composed mostly of light-duty vehicles (LDVs); the 710 is less trafficked but serves as the main corridor for heavy-duty vehicles (HDVs) traveling to and from the Port of Los Angeles; Wilshire/Sunset Boulevards are major surface street routes that are composed primarily of LDVs and negligible HDVs and characterized by “stop-and-go” (frequent acceleration and deceleration) driving conditions. In the Los Angeles Basin, the 110 and 710 represent the lowest HDV (3.9%) and highest HDV (11.3%) traffic composition of the freeways in the area, respectively. Traffic data from the CalTrans PeMS 2011 database showed that total traffic flows for the 110 and 710 in one direction are 6378 and 4247 vehicles per hour, respectively, and truck flows are 243 and 470 trucks per hour. Wilshire/Sunset has a total flow of 1839 vehicles per hour and negligible truck flows.
2.3 Sample analysis
Both campaigns were analyzed using the same analytical methodology. The Teflon filters were gravimetrically analyzed to determine mass concentration using a MT5 Microbalance (Mettler-Toledo Inc., Columbus, OH). For chemical analysis, Teflon filters were extracted by acid and subsequently analyzed by magnetic-sector Inductively Coupled Plasma Mass Spectroscopy (SF-ICPMS) to determine metals and trace elements.23 The quartz filters were analyzed using the Thermal Evolution/Optical Transmittance analysis to measure elemental and organic carbon (EC/OC).24 Water extracts of the samples determined WSOC and water-soluble metals and trace elements. Quartz filters were also analyzed for polycyclic aromatic hydrocarbons (PAHs) concentrations by gas chromatography mass spectroscopy (GC/MS) using an established solvent extraction and molecular quantification analysis protocol as initially developed by Mazurek et al. (1987)25 and subsequently advanced by other studies.26,273 Results and discussion
3.1 Comparability of the two campaigns
The interpretation of the results of this study largely depends on the comparability of PM components and chemical speciation data across the two sampling campaigns. Chemical speciation results from PM collected at the USC site (N = 2) were compared for the two campaigns since sampling there occurred concurrently as a reference site while using the same sampling instrumentation and analyzed with the same methodology. To distinguish the two datasets, ‘USC on-road’ refers to reference results from the on-road campaign and ‘USC METRO’ refers to the reference results from the METRO campaign. Fig. 2 shows the average concentrations (μg m−3) of major PM components and the range, unless otherwise noted. Organic carbon (OC) is a major component of PM2.5 in the Los Angeles Basin, and can constitute approximately 30–40% of PM2.5 in the Basin.28 According to mass balance analysis from the previously published manuscripts at the USC site, organic matter (OM), which is OC multiplied by a correction factor of 1.6 ± 0.2 for an urban aerosol,29 constitutes the second largest component of PM2.5 after inorganic ions.21,22 The current study presents OC with no correction factor. The OC levels at USC are 3.5 and 3.1 μg m−3 for the METRO and on-road campaigns, respectively, varying by 11%; and water-soluble OC (WSOC) constitutes 51% and 32% of OC, respectively. Elemental carbon (EC) for the USC METRO is approximately 2 times higher than the USC on-road campaign, which could be the result of reductions in EC due to the implementation of the recent Port of Los Angeles Clean Truck program that started in 2008. Total carbon (TC), which is the sum of EC and OC, is 4.5 and 3.6 μg m−3 for the USC METRO and USC on-road campaigns, respectively, thus varying by 20%. | ||
| Fig. 2 Comparison of major PM components at the USC reference site for the two campaigns to assess comparability of data. All bars presented in this study represent upper and lower data points (N = 2). *N = 1 for USC METRO data. | ||
A table of comparison of metals and trace element and PAH concentrations at USC during the two campaigns is shown in the ESI (Table S1†). The major difference observed between the two campaigns is the sulfur levels which are 2.4 times higher during the METRO campaign than during the on-road campaign, consistent with the higher and more variable levels of S observed at the light-rail and subway lines. This can be explained by the fact that the METRO campaign was conducted in the summertime, the photochemical period in Los Angeles. In Los Angeles, S in the particulate phase is mostly in the form of ammonium sulfate, which is primarily formed from gaseous precursors of SO2 in the presence of sunlight and reaches its highest levels in the summertime.28 Another important element, Na, which is influenced by sea salt, differs by 30% between the two campaigns. Otherwise, most of the other metals and trace elements differ by less than 20%. For PAHs, chrysene exhibits the highest concentration during the USC METRO campaign and is 3.6 times greater than during the on-road study. All other PAHs are generally less than 0.1 ng m−3.
In addition to PM species, meteorological parameters (temperature, relative humidity, wind direction and speed) and gaseous pollutants (NOx, CO, and O3) were compared during the two campaigns based on measurements at the SCAQMD monitoring site (Table S2†). Average temperatures are warmer during the METRO campaign since it is during the summertime while the on-road campaign is in the spring; average relative humidity (%) and wind speed are similar. NOx and CO concentrations are greater by about 20–25% on average during the on-road campaign because these species are emitted by traffic sources and are typically higher in the morning hours (6–9 AM) and decline throughout the day. On the other hand, O3, a secondary pollutant, is 12% higher during the METRO campaign because O3 levels are typically lower in the morning hours (6–9 AM). Overall, there is more variability in the meteorological parameters and gaseous pollutants due to the longer sampling period during the on-road campaign, but corresponding p-values demonstrate that none of the parameters are statistically significant between the two campaigns (p > 0.05). Overall, considering the differential sampling dates and times of the two campaigns and less a few PM species, the PM2.5 components and species do not vary substantially. As a result, concentrations (mass per m3 of air) will be presented in this manuscript as the metric of comparison among the five commute microenvironments.
3.2 Major PM components
Fig. 3 shows the concentrations of major PM components (OC, WSOC, TC, and EC) for the five microenvironments. The bars represent the upper and lower data points. Overall, the 710 exhibits the highest concentrations of OC and EC, and thus TC, while the two METRO lines have the lowest concentrations of OC and TC. The relatively higher levels observed on the 710 can be attributed to its higher volume of heavy-duty vehicles (HDVs), which are the greatest emitters of EC30 and OC31–33 in a traffic environment. For OC, the concentrations of the 110 and Wilshire/Sunset are comparable and are approximately 20% higher than the METRO lines even though reference OC levels at USC were 11% higher during the METRO campaign. It is also important to note that EC is largely from primary emissions while OC has a primary and secondary component,30 and is thus less influenced by primary emissions. As discussed by Kam et al. (2011),22 the source of OC in the METRO red line is primarily from the entrance of ambient air through the ventilation system. For WSOC, the concentrations of the microenvironments range from 1.2–2.0 μg m−3, with the METRO red line exhibiting the lowest concentration and Wilshire/Sunset exhibiting the highest. For EC, the 710 concentrations are 2.0–3.3 times higher than the other commute microenvironments. Similar to OC, the main source of EC for the METRO lines is the influence of ambient air. Although Wilshire/Sunset is a highly trafficked roadway environment, its low levels of EC can be explained by its negligible HDV volumes, designating it as a primarily LDV roadway. Moreover, Geller et al. (2005)31 showed that the fuel-based emission factors (mg of pollutant emitted per kg of fuel burned) for HDVs and LDVs for PM2.5 are 710 and 29 mg kg−1 of fuel, respectively, which means HDVs emit almost 25 times more EC than LDVs per kg of fuel burned.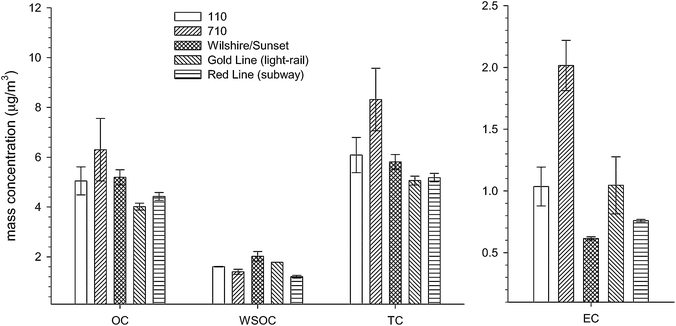 | ||
| Fig. 3 Comparison of major PM components (OC, WSOC, EC, and TC) for the five commute microenvironments. EC appears on a separate axis to highlight differences. METRO data have been published by Kam et al. (2011).22 | ||
3.3 Metals and trace elements
A number of metals and trace elements that have been quantified in this study are identified as hazardous air pollutants under the U.S. EPA Clean Air Act Amendments of 1990, which includes Sb, Cd, Cr, Co, Pb, Mn, and Ni compounds. The U.S. EPA states that these airborne pollutants are known to cause or possibly cause cancer or other serious health effects, including birth defects, further emphasizing the importance of understanding the exposure of metals and trace elements for public and private commuters as well as residents who are in the vicinity of major roadways. Fig. 4 and Table S3† show the concentrations (ng m−3) of total metals and trace elements for the commute microenvironments, and are presented based on concentration levels. Overall, the METRO red line (subway) exhibits the highest concentrations for Fe, Mn, Mo, Ba, Cr, Co, Ni, and Cd. For Fe, the red line is approximately 10 times higher than the 110 and 710, 15 times higher than Wilshire/Sunset, and 20 times higher than the gold line (light-rail). The sources of these enriched species have been discussed extensively by Kam et al. (2011),22 thus only a brief summary follows. In the enclosed underground environment, subway dust is generated mainly from the abrasion processes between the rail, wheels, and brakes as well as by the mechanical wearing of the parts. The rail tracks and the main body of the train are composed of stainless steel, an Fe-based alloy mixed with other metallic elements to enhance its properties. The same study also demonstrated that Fe is strongly correlated with Cr, Mn, Co, Ni, Mo, Cd, and Eu (R2 > 0.9, p < 0.05), indicating that these species may be components of stainless steel. In addition, PM may be resuspended due to train and passenger movement. Although the gold line (light-rail) exhibits relatively low concentrations of these steel-associated elements, the study determined that based on crustal enrichment factors,34 of which values close to 1 indicate crustal origins and greater values indicate anthropogenic sources, these elements have enrichment factors that are 2–3 times greater than the USC reference site. This suggests that these elements have indeed been influenced by additional sources (i.e. steel abrasion, wear of rail parts, etc.) that do not influence USC. Regardless of its additional source, the concentrations of metals and trace elements on the gold line (light-rail) are the lowest of the five commute environments, meaning commuters of this light-rail line are exposed to the lowest amounts of these airborne toxics. | ||
| Fig. 4 Comparison of concentrations of total metals and trace elements for the five commute environments. Results of the METRO lines have been published by Kam et al. (2011).22 | ||
The differential microenvironments play a major role in the contribution of various sources of metals and trace elements presented in this study. For example, Na may be part of soil dust, but because Los Angeles is largely affected by the southwesterly onshore breeze from the Pacific Ocean, sea salt is a substantial source of Na in the Basin. The higher Na concentrations observed on the three roadways relative to the METRO lines are expected in light of its major sea salt influence. Moreover, the higher levels of Na observed at Wilshire/Sunset relative to the 110 and 710 (average of 33% higher) can be explained by the proximity of the Wilshire/Sunset route to the coast, while the 110 and 710 routes are located more inland, and thus less influenced by sea salt. The average concentrations of Mg, Al, and K for the three roadways are 96.3 ± 8.8, 183.3 ± 13.6, and 102.6 ± 14.2 ng m−3, respectively, and are all higher than average corresponding concentrations on both METRO lines (47.2 ± 23.4, 106.2 ± 63.0, and 59.9 ± 3.2 ng m−3). This is consistent with previous studies which determined that these species are primarily of crustal origin and derived from resuspension rather than from vehicular sources.35
Studies have shown that Fe, Al, Ca, Mg, K, Na are abundant in crustal materials, but road dust may be enriched with some of these elements, indicating anthropogenic sources.36,37 While the focus of this study is not to quantify the enrichment of these species relative to a reference site, our data are consistent with earlier roadside studies for elements which have a contribution from traffic sources (i.e. Cu, Ba, Pb, Fe, Ca, Sb, etc.). The most obvious observations are the elements that are associated with vehicular traffic but not rail abrasion or wear, which are Ca, Ti, Sn, Sb, and Pb. These elements are primarily derived from vehicular wear processes such as brake and tire wear (Ti, Sb, and Pb) and motor oil additives (Ca).37,38 The average concentrations for the three roadways are 208.3 ± 13.0 ng m−3 for Ca, 36.3 ± 9.4 ng m−3 for Ti, 6.51 ± 2.1 ng m−3 for Sn, 8.0 ± 2.2 ng m−3 for Sb, and 4.6 ± 0.5 ng m−3 for Pb, while corresponding averages for the two METRO lines are 131.9 ± 81.6, 10.6 ± 1.7, 3.7 ± 0.5, 2.7 ± 1.4, and 2.6 ± 0.4 ng m−3. HDVs are known to be greater emitters of elements associated with brake wear due to the greater mechanical force required to decelerate relative to LDVs.35 However, our results found no statistically significant differences for Ca, Ti, Sn, Sb, and Pb between the 110, which is composed of 3.9% HDVs, and 710, which is composed of 11.3% HDVs (p = 0.42).
Elements associated with both steel and vehicular sources include Fe, Ba, Cu, and Zn. Traffic sources for these elements include engine wear (Fe), brake wear (Ba and Cu), and tire wear (Zn).35,39 BaSO4 is known to be commonly used in brake linings.40 The overall trend for these four elements is substantially higher concentrations in the red line (subway) relative to the other environments except for Zn, which exhibit comparable levels for the five environments. For Fe, Ba, and Cu, concentrations on the three roadways vary within 50% of each other (with averages of 829.4 ± 147.8 ng m−3 for Fe, 69.6 ± 20.0 ng m−3 for Ba, and 46.8 ± 12.2 ng m−3 for Cu), and are generally higher than the gold line (490.5 ng m−3 for Fe, 18.4 ng m−3 for Ba, and 37.5 ng m−3 for Cu).
Sulfur, which is the predominant element in all environments, shows unexpectedly high concentrations for the METRO lines relative to the three roadways. As discussed earlier, since S is in the form of ammonium sulfate in the Los Angeles Basin,41 this is most likely explained by the warmer temperatures observed during the METRO campaign, which would lead to greater formation of sulfate from the increase in photochemical reactions. A previous study in the basin also found that sulfate levels were higher in the summer than in the winter.28 Another source of S is from fuel, which is known to be emitted at higher rates from HDVs due to higher S content in diesel fuel,42 or S can be emitted from fuel, motor oil, and additives such as zinc dithiophosphate.37
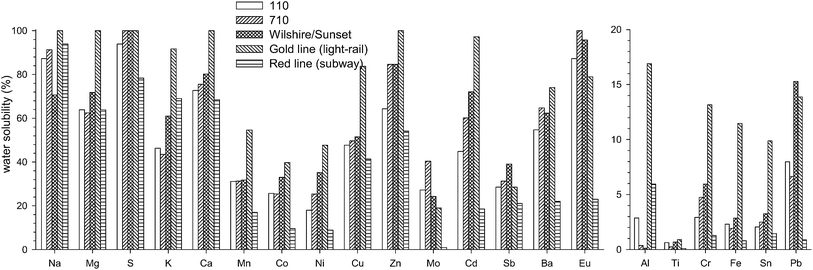 | ||
| Fig. 5 Comparison of water-solubility (%) of metals and trace elements for the five microenvironments separated into high and low solubility species. | ||
3.4 Polycyclic aromatic hydrocarbons (PAHs)
The U.S. EPA classifies 16 PAHs as priority pollutants based on their carcinogenicity and mutagenicity. Many PAHs and their derivatives are identified as probable (group 2A) or possible (group 2B) carcinogens as defined by the International Agency for Research on Cancer (IARC). Therefore, it is essential to identify and quantify the concentrations of PAHs to which public and private commuters are exposed on a daily basis. Fig. 6a and b and Table S3† show the average and range of concentrations of 11 PAHs (ng m−3) and total PAH concentrations for the five commute environments. It is important to note that the temporal and seasonal differences in sampling times may affect PAH concentrations to a certain degree. Total PAH concentrations are substantially higher on the roadway environments than on the two METRO lines. Specifically, the 710, 110, and Wilshire/Sunset roadways are 4.2, 2.8, and 2.2 times higher than the average of the two METRO lines, respectively. For most of the individual PAH species, the 710 levels are generally 2–3 times higher than the other two roadway environments, except for pyrene, where levels are 4–8 times higher. Although the 710 has the highest total PAH concentrations, statistical analysis using a t-test showed that the concentrations of all the PAHs (N = 11) on the 710 were not significantly different from those of the 110 (p = 0.37) or Wilshire/Sunset (p = 0.18). PAH concentrations for the two METRO lines are consistently lower than those of the roadways, which is expected considering the main source of PAHs in the subway and light-rail environment is most likely entrainment from ambient air. Pairwise multiple comparison tests (Tukey test) were performed to further investigate statistical significance, which determined that only two pairs (710 and red line, 710 and gold line) were significantly different (p < 0.05).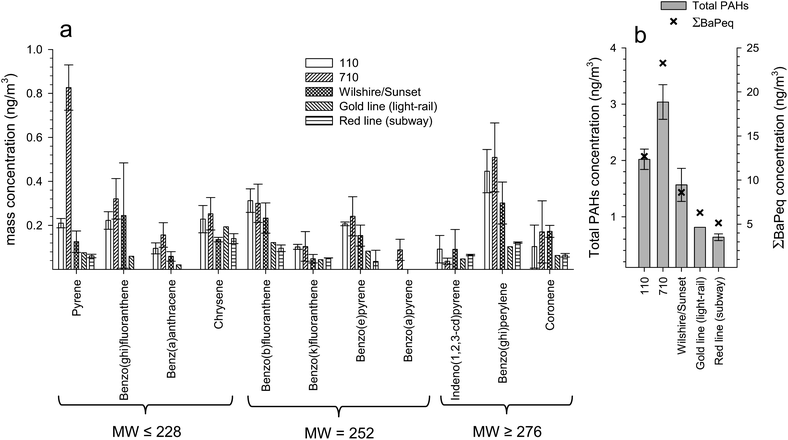 | ||
| Fig. 6 (a) Concentrations of 11 PAHs and (b) sum of PAHs concentrations and ΣBaPeq for the five commute environments. | ||
In urban environments, the main source of PAHs is from fuel and combustion processes.45 Both LDVs and HDVs emit PAHs, but HDVs emit PAHs at substantially higher amounts than LDVs.46,47 Numerous studies which have apportioned PAH emission factors (μg of pollutant per kg of fuel burned) for HDVs and LDVs through tunnel studies47 and dynamometer studies26,27 have found that HDVs can emit up to 50 times more PAHs than LDVs. The same studies also found that low molecular weight (MW) (Mw ≤ 228) PAHs (i.e. pyrene) are primarily emitted by HDVs and high MW (Mw ≥ 276) PAHs (i.e. benzo(ghi)perylene, indeno(1,2,3-cd)pyrene) are emitted by both HDVs and LDVs, which is consistent with the results of our current study. Although the 110 has high total traffic flows but low HDV flows (6378 vehicles per hour and 243 trucks per hour) and the 710 has a lower total traffic flow but higher truck flow (4247 vehicles per hour and 470 trucks per hour), the near 2-fold difference in truck volumes present on the 710 is most likely responsible for the higher concentrations of light MW PAHs.
An important property of PAHs is their semi-volatile nature. PAHs can be found in the urban environment in the gaseous phase or adsorbed onto particles in the solid phase based on its vapor pressure. Ambient temperatures can also play a role in the presence of particle-bound PAHs.48 Generally, high MW PAHs have lower vapor pressures than low MW PAHs. Thus, pyrene (Mw = 202) can partition between the gas and particle phase depending on ambient temperatures, while indeno(1,2,3-cd)pyrene (Mw = 276) and coronene (Mw = 300) are found almost entirely in the particle phase. This is consistent with a previous study which found that emission factors for high MW PAH based on roadside sampling at the 110 and 710 were comparable to the reconstructed LDV and HDV emission factors based on tunnel sampling in spite of the different temperatures and dilution conditions, while low MW PAH emission factors differed by over two times.46 For the current study, the ambient temperatures for the two campaigns varied from 17 to 24 °C (Table 1). Of the 3 roadway environments, Wilshire/Sunset exhibited the lowest PAH levels, consistent with the higher observed temperatures. Although the METRO campaign also had higher average ambient temperatures, the PAH levels for the red and gold lines are also influenced by the particle removal efficiency of the subway and train ventilation systems and thus particle penetration into the train.
3.5 Lung cancer risk for commuters
As mentioned earlier, PAHs are a major public health concern due to their carcinogenicity, and more specifically, to lung cancer risk. A number of PAHs identified in this study are classified as priority pollutants under the U.S. EPA. According to the IARC, benzo(a)pyrene, or BaP, is classified as a probable carcinogen (group 2A). Since BaP has been studied and its cancer potency values have been well established, it is commonly used as the index compound to which the potency activity of other PAH compounds and their derivatives are compared. Relative potency values are referred to as potency equivalent factors (PEFs), for which BaP has a PEF of 1. Benz(a)anthracene has a PEF of 0.1, meaning it has 1/10th the potency of BaP. PEFs for other PAH compounds are from OEHHA. The calculation of lung cancer risk follows the method of Sauvain et al. (2003),49 and a brief summary follows. The PEFs are multiplied by the corresponding PAH concentration to determine the BaP equivalent concentrations (BaPeq). The sum of the individual BaPeq (ΣBaPeq) is subsequently multiplied by its unit risk factor (μg m−3)−1, which has been determined based on rodent or epidemiology studies.49–51 Unit risk factors based on rodent and epidemiology studies are 1.1 × 10−4 and 2.1 × 10−3 (μg m−3)−1, respectively. Fig. 6b shows ΣBaPeq as a marker for each of the 5 microenvironments. Since the unit risk factors of Sauvain et al. (2003)49 are based on occupational continuous exposures (45 years for 8 hours per day), the current unit risk factors are determined by a multiplication factor of 0.18 to account for the lower risk for a commuter's lifetime on the road, which is considered to be 45 years for 5 days per week for 2 hours per day.Table 2 shows the concentrations of ΣBaPeq and corresponding unit risk factors and lung cancer risk based on earlier rodent and epidemiology studies. Note that the unit risk factors between the rodent and epidemiology studies differ by approximately 19 times, yielding cancer risk values that differ by the same factor. Overall, the 710 exhibits the highest cancer risk. The 710 is greater than the two METRO lines by an average of 4.2 times, and is greater than the 110 and Wilshire/Sunset by 1.9 and 2.7 times, respectively. Although the results offer meaningful insight into the cancer risk that various commuters face on a daily basis, the authors acknowledge that there are relatively large uncertainties associated with the results as seen with the differences in unit risk factors between the rodent and epidemiology studies. In addition, concentrations of PAHs may exhibit temporal variation. For example, a previous study in Wilmington, CA, which is located between the 110 and 710 and in the proximity of the Ports of Los Angeles and Long Beach, found that lung cancer risk is highest during rush hour traffic (around 8:00 AM) and lowest in the late afternoon (around 5:00 PM),15 whereas the current study represents results based on time-integrated samples from 6:00 AM to 5:00 PM. Nonetheless, results from this study are substantive in assessing the lung cancer risk for commuters on the light-rail, subway, freeway, and surface street environments in Los Angeles.
| ΣBaPeq (ng m−3) | Lung cancer risk (×10−6) | ||
|---|---|---|---|
| Rodent | Epidemiology | ||
| 110 | 12.7 | 1.4 | 27.1 |
| 710 | 23.3 | 2.7 | 49.7 |
| Wilshire/Sunset | 8.6 | 1.0 | 18.4 |
| Gold line (light-rail) | 6.3 | 0.7 | 13.5 |
| Red line (subway) | 5.1 | 0.6 | 11.0 |
4 Concluding remarks
This study compares the major PM components (EC, OC, WSOC), metals and trace elements, and PAHs in PM2.5 for public and private commuters in five differential environments: subway (METRO red line), light-rail (METRO gold line), surface street (Wilshire/Sunset), and two freeways which represent the highest (710) and lowest (110) truck compositions in Los Angeles. The 710 exhibited the highest EC and OC levels, most likely due to its higher volume of HDVs, while the two METRO lines had the lowest EC and OC levels. Metals and trace elements quantified in this study are derived from a variety of sources depending on the commute environment. Substantially high levels of Fe and other steel-associated elements (Mn, Mo, Ba, Cr, Co, Ni, and Cd) were observed on the red line (subway), and substantially low levels were observed on the gold line (light-rail). Major sources in the rail environment are steel abrasion and wear of parts. Another group of elements (Ca, Ti, Sn, Sb, and Pb) was identified to be associated with urban traffic sources only, which are generated from vehicular wear processes and emitted from motor oil additives. Additionally, a number of the elements are primarily of crustal (i.e. Mg, Al) or sea salt (Na) origins, and not influenced by rail or traffic sources. In the roadway environment, PAHs are primarily derived from vehicular emissions, and total PAHs were found to be substantially higher on the 710, consistent with earlier studies which found HDVs to be significantly greater emitters of PAHs than LDVs.46,47 Lastly, lung cancer risk was estimated and the 710 was determined to have the greatest cancer risk, while the two METRO lines had the lowest risk. Since the gold line (light-rail) was observed to have low concentrations of both PAHs and metals and trace elements, this suggests that commuting on a light-rail may have potential health benefits due to lower PM exposure levels as opposed to driving on freeways and major roadways. Results from this study are especially important for understanding PM2.5 exposure not only for daily commuters but also for residents and pedestrians who are in the proximity of roadways and can be subjected to these pollutants and its inherent health risks.Acknowledgements
The authors of this study would like to acknowledge Dr Kalam Cheung, Nancy Daher, Jimmy Liacos, Dr Vishal Verma, Dr Payam Pakbin, and Dr Zhi Ning for their contributions in the data collection for the two major campaigns in this study. The on-road campaign was funded by the California Air Resources Board through contract number #07-310 and by the Southern California Particle Center (SCPC), funded by EPA under the STAR program through Grant RD-8324-1301-0 to the University of Southern California, and the South Coast Air Quality Management District (SCAQMD) through award #11527. The METRO campaign was supported by METRANS Transportation Center through grant 53-4507-5382. The research described herein has not been subjected to the agency's required peer and policy review and therefore does not necessarily reflect the views of the agency, and no official endorsement should be inferred. In addition, we would like to thank Drs Martin M. Shafer and Jeff DeMinter for their assistance in the chemical analysis.References
- L. Y. Chan, W. L. Lau, S. C. Lee and C. Y. Chan, Atmos. Environ., 2002, 36, 3363–3373 CrossRef CAS.
- M. Zuurbier, G. Hoek, M. Oldenwening, V. Lenters, K. Meliefste, P. van den Haze and B. Brunekreef, Environ. Health Perspect., 2010, 118, 783–789 CrossRef.
- C. A. Pope and D. W. Dockery, J. Air Waste Manage. Assoc., 2006, 56, 709–742 CAS.
- M. C. Turner, D. Krewski, C. A. Pope, Y. Chen, S. M. Gapstur and M. J. Thun, Am. J. Respir. Crit. Care Med., 2011, 184, 1374–1381 CrossRef.
- P. Vineis, F. Forastiere, G. Hoek and M. Lipsett, Int. J. Cancer, 2004, 111, 647–652 CrossRef CAS.
- R. D. Brook, S. Rajagopalan, C. A. Pope, J. R. Brook, A. Bhatnagar, A. V. Diez-Roux, F. Holguin, Y. L. Hong, R. V. Luepker, M. A. Mittleman, A. Peters, D. Siscovick, S. C. Smith, L. Whitsel and J. D. Kaufman, Circulation, 2010, 121, 2331–2378 CrossRef CAS.
- A. Campbell, Ann. N. Y. Acad. Sci., 2004, 1035, 117–132 CrossRef CAS.
- T. E. Morgan, D. A. Davis, N. Iwata, J. A. Tanner, D. Snyder, Z. Ning, W. Kam, Y. T. Hsu, J. W. Winkler, J. C. Chen, N. A. Petasis, M. Baudry, C. Sioutas and C. E. Finch, Environ. Health Perspect., 2011, 119, 1003–1009 CrossRef CAS.
- S. Fruin, D. Westerdahl, T. Sax, C. Sioutas and P. M. Fine, Atmos. Environ., 2008, 42, 207–219 CrossRef CAS.
- K. Cheung, M. M. Shafer, J. J. Schauer and C. Sioutas, Environ. Sci. Technol., 2012, 46, 3779–3787 CrossRef CAS.
- V. Verma, M. M. Shafer, J. J. Schauer and C. Sioutas, Atmos. Environ., 2010, 44, 5165–5173 CrossRef CAS.
- L. Risom, P. Moller and S. Loft, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2005, 592, 119–137 CrossRef CAS.
- D. L. Costa and K. L. Dreher, Environ. Health Perspect., 1997, 105, 1053–1060 Search PubMed.
- L. C. Marr, L. A. Grogan, H. Wohrnschimmel, L. T. Molina, M. J. Molina, T. J. Smith and E. Garshick, Environ. Sci. Technol., 2004, 38, 2584–2592 CrossRef CAS.
- A. Polidori, S. Hu, S. Biswas, R. J. Delfino and C. Sioutas, Atmos. Chem. Phys., 2008, 8, 1277–1291 CrossRef CAS.
- K. F. Moore, Z. Ning, L. Ntziachristos, J. J. Schauer and C. Sioutas, Atmos. Environ., 2007, 41, 8633–8646 CrossRef CAS.
- Z. Ning, M. D. Geller, K. F. Moore, R. Sheesley, J. J. Schauer and C. Sioutas, Environ. Sci. Technol., 2007, 41, 6000–6006 CrossRef CAS.
- C. Misra, M. Singh, S. Shen, C. Sioutas and P. A. Hall, J. Aerosol Sci., 2002, 33, 1027–1047 CrossRef CAS.
- M. Singh, C. Misra and C. Sioutas, Atmos. Environ., 2003, 37, 4781–4793 CrossRef CAS.
- W. C. Hinds, Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles, Wiley-Interscience, New York, NY, 2nd edn, 1999 Search PubMed.
- W. Kam, J. W. Liacos, J. J. Schauer, R. J. Delfino and C. Sioutas, Atmos. Environ., 2012, 55, 90–97 CrossRef CAS.
- W. Kam, Z. Ning, M. M. Shafer, J. J. Schauer and C. Sioutas, Environ. Sci. Technol., 2011, 45, 6769–6776 CrossRef CAS.
- Y. X. Zhang, J. J. Schauer, M. M. Shafer, M. P. Hannigan and S. J. Dutton, Environ. Sci. Technol., 2008, 42, 7502–7509 CrossRef CAS.
- M. E. Birch and R. A. Cary, Aerosol Sci. Technol., 1996, 25, 221–241 CrossRef CAS.
- M. A. Mazurek, B. R. T. Simoneit, G. R. Cass and H. A. Gray, Int. J. Environ. Anal. Chem., 1987, 29, 119–139 CrossRef CAS.
- J. J. Schauer, M. J. Kleeman, G. R. Cass and B. R. T. Simoneit, Environ. Sci. Technol., 1999, 33, 1578–1587 CrossRef CAS.
- J. J. Schauer, M. J. Kleeman, G. R. Cass and B. R. T. Simoneit, Environ. Sci. Technol., 2002, 36, 1169–1180 CrossRef CAS.
- S. B. Sardar, P. M. Fine and C. Sioutas, J. Geophys. Res., [Atmos.], 2005, 110, D07S08 CrossRef.
- B. J. Turpin and H. J. Lim, Aerosol Sci. Technol., 2001, 35, 602–610 CAS.
- J. J. Schauer, J. Exposure Anal. Environ. Epidemiol., 2003, 13, 443–453 CrossRef CAS.
- V. D. Geller, S. B. Sardar, H. Phuleria, P. N. Fine and C. Sioutas, Environ. Sci. Technol., 2005, 39, 8653–8663 CrossRef.
- L. Ntziachristos, Z. Ning, M. D. Geller, R. J. Sheesley, J. J. Schauer and C. Sioutas, Atmos. Environ., 2007, 41, 5684–5696 CrossRef CAS.
- H. C. Phuleria, R. J. Sheesley, J. J. Schauer, P. M. Fine and C. Sioutas, Atmos. Environ., 2007, 41, 4653–4671 CrossRef CAS.
- S. R. Taylor and S. M. McLennan, The Continental Crust: Its Composition and Evolution, Blackwell Scientific Publications, Oxford, Boston, Palo Alto, Victoria, 1985 Search PubMed.
- J. Sternbeck, A. Sjodin and K. Andreasson, Atmos. Environ., 2002, 36, 4735–4744 CrossRef CAS.
- R. M. Harrison, R. Tilling, M. S. C. Romero, S. Harrad and K. Jarvis, Atmos. Environ., 2003, 37, 2391–2402 CrossRef CAS.
- G. C. Lough, J. J. Schauer, J. S. Park, M. M. Shafer, J. T. Deminter and J. P. Weinstein, Environ. Sci. Technol., 2005, 39, 826–836 CrossRef CAS.
- A. P. Grieshop, E. M. Lipsky, N. J. Pekney, S. Takahama and A. L. Robinson, Atmos. Environ., 2006, 40, S287–S298 CrossRef CAS.
- P. G. Sanders, N. Xu, T. M. Dalka and M. M. Maricq, Environ. Sci. Technol., 2003, 37, 4060–4069 CrossRef CAS.
- B. D. Garg, S. H. Cadle, P. A. Mulawa, P. J. Groblicki, C. Laroo and G. A. Parr, Environ. Sci. Technol., 2000, 34, 4463–4469 CrossRef CAS.
- L. S. Hughes, J. O. Allen, P. Bhave, M. J. Kleeman, G. R. Cass, D. Y. Liu, D. F. Fergenson, B. D. Morrical and K. A. Prather, Environ. Sci. Technol., 2000, 34, 3058–3068 CrossRef CAS.
- D. H. Lowenthal, B. Zielinska, J. C. Chow, J. G. Watson, M. Gautam, D. H. Ferguson, G. R. Neuroth and K. D. Stevens, Atmos. Environ., 1994, 28, 731–743 CrossRef CAS.
- S. Loft, C. Stevns-Hansen, A. T. Saber, L. Risom, P. Hoegh-Cristensen, J. Kjaergaard, E. Vaclavic, P. Moller, U. Vogel and H. Wallin, Free Radical Res., 2005, 39, S26 Search PubMed.
- J. C. Chow, J. G. Watson, E. M. Fujita, Z. Q. Lu, D. R. Lawson and L. L. Ashbaugh, Atmos. Environ., 1994, 28, 2061–2080 CrossRef CAS.
- A. H. Miguel, T. W. Kirchstetter, R. A. Harley and S. V. Hering, Environ. Sci. Technol., 1998, 32, 450–455 CrossRef CAS.
- Z. Ning, A. Polidori, J. J. Schauer and C. Sioutas, Atmos. Environ., 2008, 42, 3099–3114 CrossRef CAS.
- H. C. Phuleria, M. D. Geller, P. M. Fine and C. Sioutas, Environ. Sci. Technol., 2006, 40, 4109–4118 CrossRef CAS.
- A. Eiguren-Fernandez and A. H. Miguel, Environ. Sci. Technol., 2012, 46, 2607–2615 CrossRef CAS.
- J. J. Sauvain, T. V. Duc and M. Guillemin, Int. Arch. Occup. Environ. Health, 2003, 76, 443–455 CrossRef CAS.
- C. E. Bostrom, P. Gerde, A. Hanberg, B. Jernstrom, C. Johansson, T. Kyrklund, A. Rannug, M. Tornqvist, K. Victorin and R. Westerholm, Environ. Health Perspect., 2002, 110, 451–488 CrossRef CAS.
- J. F. Collins, J. P. Brown, G. V. Alexeeff and A. G. Salmon, Regul. Toxicol. Pharmacol., 1998, 28, 45–54 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2em30495c |
| This journal is © The Royal Society of Chemistry 2013 |
