Comparative evaluation of impact of Zn and ZnO nanoparticles on brine shrimp (Artemia salina) larvae: effects of particle size and solubility on toxicity
Mehmet
Ates
a,
James
Daniels
a,
Zikri
Arslan
*a,
Ibrahim O.
Farah
b and
Hilsamar Félix
Rivera
c
aJackson State University, Department of Chemistry & Biochemistry, Jackson, MS, 39217 USA. E-mail: zikri.arslan@jsums.edu; Fax: +1 601 979 3674; Tel: +1 601 9791618
bJackson State University, Department of Biology, PO Box 18540, Jackson, MS 39217, USA
cDepartment of Chemistry, University of Puerto Rico-Mayaguez, PO Box 9000 Mayaguez, PR, 00681, USA
First published on 28th November 2012
Abstract
Brine shrimp (Artemia salina) larvae were exposed to different sizes of zinc (Zn) and zinc oxide (ZnO) nanoparticles (NPs) to evaluate their toxicity in marine aquatic ecosystems. Acute exposure was conducted in seawater with 10, 50 and 100 mg L−1 concentrations of the NPs for 24 h and 96 h. Phase contrast microscope images confirmed the accumulation of the NPs inside the guts. Artemia were unable to eliminate the ingested particles, which was thought to be due to the formation of massive particles in the guts. Although the suspensions of the NPs did not exhibit any significant acute toxicity within 24 h, mortalities increased remarkably in 96 h and escalated with increasing concentration of NP suspension to 42% for Zn NPs (40–60 nm) (LC50 ∼ 100 mg L−1) and to about 34% for ZnO NPs (10–30 nm) (LC50 > 100 mg L−1). The suspensions of Zn NPs were more toxic to Artemia than those of ZnO NPs under comparable regimes. This effect was attributed to higher Zn2+ levels (ca. up to 8.9 mg L−1) released to the medium from Zn NPs in comparison to that measured in the suspensions of ZnO NPs (ca. 5.5 mg L−1). In addition, the size of the nanopowders appeared to contribute to the observed toxicities. Although the suspensions possessed aggregates of comparable sizes, smaller Zn NPs (40–60 nm) were relatively more toxic than larger Zn NPs (80–100 nm). Likewise, the suspensions of 10–30 nm ZnO NPs caused higher toxicity than those of 200 nm ZnO NPs. Lipid peroxidation levels were substantially higher in 96 h (p < 0.05), indicating that the toxic effects were due to the oxidative stress.
Environmental impactNanoparticles of Zn and ZnO are used as additives in household and consumer products, such as bacterial disinfectants and sunscreens, to improve their effectiveness. As the concerns increase with the use of metal oxide nanomaterials in many products, this study provides important evidence about the safety of Zn and ZnO nanoparticles to environmental and human health using an aquatic model organism. The results show that Zn and ZnO nanoparticles are not totally benign. They exhibit toxicity and oxidative stress under long-term exposure. The release of Zn ions to the medium is responsible for this process as the nanoparticles disintegrate gradually. |
1. Introduction
Advances in nanotechnology offer numerous benefits with new formulations of nanoscale materials to meet the demands in various areas of human life spanning from health sciences to technology (e.g., communication and entertainment devices). Metal and metal oxide nanomaterials (e.g., TiO2 and ZnO) are manufactured in large scale today and products formulated with these nanomaterials have been available for household use and healthcare.1,2 Though the field of nanoscience is still in its early stages, the nanomaterials or particles released from commercial products have already been identified in environment and aquatic resources.3,4 Nonetheless, their potential effects on human health and environment are poorly understood. This is mainly because of the complexity of factors that influence the toxicological properties of nanomaterials, including size, shape, route of synthesis, elemental entities, surface coatings and the physicochemical properties of the medium.5–7Zinc oxide (ZnO) nanoparticles are among the most popular nanomaterials manufactured in industrial scale as they are widely used as additives in food, sunscreens and cosmetic products and in the manufacture of textiles, paint pigments, semiconductors, catalysts, and polishers.8–10 In addition, these NPs exhibit selective toxicity to bacteria and cancer cell lines, and thus are reported among the potential materials for water disinfection and chemotherapy.11,12 Because of these vast applications concerning human and environmental health, the ecotoxicological effects of ZnO NPs have been studied by a number of groups on bacteria,13–15 protozoa,16 zebra fish embryos,17 crustaceans,18,19 algae,20,21 and nematodes.22 In most studies, toxicity issues have been ascribed mainly to the zinc ions released from the nanoparticles,14–16,18,20,21 while several other studies have suggested that toxic effects were induced by both Zn ions and NPs.17,19 The Zn NPs are assumed to exhibit similar prospects of ZnO NPs in formulating consumer products, such as sunscreens and cosmetics. To date, however, there are no data available regarding their ecotoxicity and mode of action in aquatic species.
Understanding the fate and bioavailability of NPs in aquatic systems is important to predict their impact on aquatic micro-organisms (e.g., zooplankton) and producers (e.g., algae), and transfer through the tropic food chain. Brine shrimp, Artemia species, are zooplanktons like copepods and daphnids and are commonly used to feed larval fish cultures.23 They play an important role in the energy flow of the food chain in marine environment. Artemia species have been used in testing acute toxicity of toxic materials, such as heavy metals and pesticides.24–26 They offer distinct advantages, including year-round availability, low cost, ease of culture, high offspring production, short life-cycle, and no feeding required during the assay that led to the development of a wide range of Artemia-based standard bioassays.24–28
The aim of this study is to evaluate the toxic effects of uncoated Zn and ZnO NPs comparatively on Artemia salina. Particle stability, accumulation and elimination patterns were examined under 24 h and 96 h exposure in seawater along a concentration gradient from 10 to 100 mg L−1. Also the effects of particle size and solubility were comparatively examined on the toxicity of Zn and ZnO NPs to Artemia salina. The total Zn content was determined by inductively coupled plasma mass spectrometry (ICP-MS) from the exposed cultures to elucidate temporal accumulation and elimination patterns. Oxidative stress levels were measured to elucidate the mechanism of toxicity.29
2. Materials and methods
2.1. Preparation of nanoparticle suspensions
Uncoated zinc (Zn) and zinc oxide (ZnO) nanoparticles were purchased from Skyspring Nanomaterials Inc., Houston, TX, USA. Physical properties are listed in Table 1. All NPs were spherical in morphology.| Nanoparticle | Average particle size/nm | Surface area/m2 g−1 | Appearance |
|---|---|---|---|
| Zn (99.9%) | 40–60 | 12 | Black |
| Zn (99.9%) | 80–100 | 6.5 | Black |
| ZnO (99.8%) | 10–30 | 30–50 | White-yellow |
| ZnO (99.8%) | 200 | na | White-yellow |
The NPs were stored at room temperature in the laboratory until the implementation of the experimental studies. The stock solutions of the NPs were prepared by suspending appropriate amounts of powders in deionized water (18.0 MΩ cm resistivity) at a stock concentration of 20% (w/v) separately. To homogenize the suspension, the contents were vortexed for 20 s at 2000 rpm and then exposed to ultrasound for 10 min for maximum dispersion in a sonicator bath. Appropriate volumes of the stock suspension were immediately transferred into the exposure containers that contained Artemia larvae in seawater.
2.2. Characterizations of NP suspensions
Particle size distribution of the NPs was characterized by transmission electron microscopy (TEM) using a JEOL-1011 TEM instrument providing a resolution of JEM-1011 of 0.2 nm lattice with magnification of 50 to 1 × 106 under the accelerating voltage of 40 to 100 kV. For TEM measurements, a drop of the colloidal solution of NPs was placed on a 50 Å thick carbon-coated copper grid and allowed to dry to record TEM images. Particle size distribution was determined by ImageJ software package. To estimate the mean particle size, approximately 100 NPs were measured in random fields of view of three images. Hydrodynamic (actual) size distribution of the NPs in stock solution and exposure medium was determined by dynamic light scattering (DLS) measurements by using a Nano ZS Zetasizer (Malvern Instruments). A portion of the suspension from stock and exposure medium was diluted to an appropriate range with water and briefly vortexed to homogenize the contents. Five DLS measurements were taken successively for each solution to estimate the particle size distribution in water.2.3. Preparation of Artemia salina larvae
The Artemia cysts were purchased from Artemia International LLC, Houston TX, and were kept at 4 °C in a refrigerator. The cysts were hatched in seawater (3% m/v). The seawater was prepared by dissolving an appropriate amount of Instant Ocean® salt in deionized water, stirred for 24 h under aeration and then filtered through 30 μm Millipore cellulose filters. Artemia were hatched by using the procedure described by Persoone et al.30 Briefly, encysted Artemia were first hydrated in distilled water at 4 °C for 12 h and then washed to separate the floating cysts. The sinking cysts were collected on a Buchner funnel and washed with cold deionized water. Approximately 3 g of the pre-cleaned cysts were incubated in 1.5 L seawater in a conical plastic container with graduations at 30 ± 1 °C. A 1500 lux day-light was provided continuously by a fluorescent lamp. Aeration was maintained by a small line extending to the bottom of the hatching device from an aquarium air-pump. Under these conditions, Artemia larvae hatched within 24 h.2.4. Exposure setup
Acute toxicity was conducted according to the Organization for Economic Cooperation and Development testing guidelines (OECD 202).31Artemia larvae were exposed to three different concentrations of the Zn and ZnO NPs (10, 50 and 100 mg L−1) for 24 h and 96 h. A control group was also set up without the test compound. Exposures were carried out in triplicate in 1.0 L conical plastic containers in 500 mL of seawater at 24 ± 2 °C. Slight aeration was provided through the bottom of the conical flask to prevent settling of NPs from the suspension. Total salinity of 2.9–3% m/v and a light regime of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 h light
8 h light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark were maintained. No food was provided during the course of the exposure. Details of the experimental conditions are summarized in Table 2.
dark were maintained. No food was provided during the course of the exposure. Details of the experimental conditions are summarized in Table 2.
| Exposure regime | NP concentration/mg L−1 | pH | Number of Artemia salina larvae (×103) | |||
|---|---|---|---|---|---|---|
| Zn NP (40–60 nm) | Zn NP (80–100 nm) | ZnO NP (10–30 nm) | ZnO NP (200 nm) | |||
| 24 h | 0 | 8.1–8.6 | 19.7 | 15.6 | 13.4 | 21.5 |
| 10 | 8.1–8.3 | 16.8 | 18.3 | 14.5 | 19.9 | |
| 50 | 8.2–8.4 | 18.4 | 17.3 | 12.3 | 15.8 | |
| 100 | 8.3–8.8 | 11.6 | 20.1 | 13.9 | 20.7 | |
| 96 h | 0 | 8.2–8.7 | 10.5 | 11.2 | 11.2 | 13.3 |
| 10 | 8.3–8.6 | 17.4 | 16.1 | 12.7 | 17.6 | |
| 50 | 8.3–8.8 | 16.3 | 14.9 | 12.2 | 21.7 | |
| 100 | 8.2–8.8 | 14.1 | 16.4 | 11.9 | 13.5 | |
In addition, the ability of Artemia to eliminate the ingested NPs was also investigated. At the end of the exposure, part of the Artemia from each group were washed with seawater and immediately transferred into a clean seawater bath. They were allowed to eliminate the ingested particles for a period of 24 h.
2.5. ICP-MS analysis of Artemia samples
Artemia were sampled and thoroughly washed with water using a 40 μm plankton net. The cleaned samples were then filtered by 0.45 mm Whatman filter paper. To determine the NP accumulation, about 0.1 g of wet Artemia were weighed and digested in Teflon vessels in 2 mL of concentrated HNO3 (trace metal grade, Fisher Scientific) for 2 h by using a digestion block (DigiPrep MS, SCP Science) at 160 °C.32 The contents were diluted to 50 mL with deionized water and analyzed for Zn by ICP-MS using a Varian 820-MS ICP-MS instrument (Varian, Australia). Total Zn concentration obtained from ICP-MS analysis was used to determine the concentration of Zn and ZnO NPs. Similarly, Artemia samples that were kept in clean seawater for NP elimination were digested in nitric acid and analyzed by ICP-MS. The Zn concentration measured from the samples was used to estimate the NP depuration rate.2.6. Toxicity assay
Thiobarbituric acid-reactive substances (TBARS) were measured to determine the lipid peroxidation products as a measure of oxidative stress induced by the NPs of Zn and ZnO. The values were expressed as total malondialdehyde (MDA) concentration per gram of Artemia. The MDA concentration was measured as described by Van Ye et al.33 At the end of exposure, 0.1 g Artemia were washed with cold water and then assayed using the MDA kit (Northwest Life Science, LLC, Vancouver, WA). Samples were homogenized in 2 mL of phosphate buffer solution (pH 7.2) by an ultrasonic homogenizer and then centrifuged at 6000 rpm for 10 min. The resulting supernatant was used for biochemical assay immediately. Briefly, 10 μL of butylated hydroxytoluene (BHT), 0.25 mL of sample supernatant, 0.25 mL of phosphoric acid (1.0 M), and 0.25 mL of TBA were added to a vial. A set of MDA standards were freshly prepared from tetramethoxypropane in a concentration range of 0 to 10 μM. All samples and standards were incubated at 90 °C for one hour, then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min to separate the suspending tissue. The absorbance of the supernatant (reaction mixture) was measured at 532 nm. Measurements were performed in triplicate for all experimental groups.
000 rpm for 15 min to separate the suspending tissue. The absorbance of the supernatant (reaction mixture) was measured at 532 nm. Measurements were performed in triplicate for all experimental groups.
2.7. Statistical analysis
The data were recorded as the mean and standard deviation. One-way analysis of variance (ANOVA) with Tukey's multiple comparisons was used to detect significant differences in mortality, accumulation and elimination rates, and toxicity among the controls and treatments. A p-value of <0.05 was considered statistically significant.3. Results and discussion
3.1. Particle size distribution of NP suspensions
The TEM data in Table 3 demonstrate that the NPs in the stock suspensions possessed a narrow size distribution which is within the range of the reported values. The TEM images shown in Fig. 1a and c are also consistent with the TEM data for the stocks. Surface charge is critical to maintain the stability of NPs in solution through electrostatic repulsions. As indicated in Table 3, NPs of Zn and ZnO possessed negatively and positively charged surfaces, respectively (see zeta potentials). These values were relatively small. Though it was minimal, particles aggregated in water indicating that the surface charge was not sufficient to prevent the aggregation of the NPs in the water even in the absence of counter ions. On the other hand, the TEM images of the suspensions from the exposure medium contained micrometer size aggregates of NPs due to the loss of electrostatic repulsion in the saltwater (Fig. 1b and d). These results were consistent with the data reported previously.13–21 | ||
| Fig. 1 TEM images of Zn and ZnO NPs from stock NP suspensions and exposure medium. (a) Zn NPs (40–60 nm) in water, (b) ZnO NPs (10–30 nm) in water, (c) Zn NPs (40–60 nm) in seawater, and (d) ZnO NPs (10–30 nm) in seawater. Nanoparticles in exposure medium (b and d) rapidly aggregated into micrometer size particles. | ||
As expected the hydrodynamic diameters of Zn and ZnO NPs in exposure medium were substantially larger than those estimated by TEM (see DLS data, Table 3). The mean particle size ranged between 1620 and 2203 nm, which was due to the hydration of NP surfaces and reduction of electrostatic repulsion in the medium.18–21,34,35 It is interesting to note that hydrodynamic sizes were slightly larger for the smaller NPs, Zn (40–60 nm) and ZnO (10–30 nm), which attributed to the increasing surface area with decreasing particle size (see Table 1). Nonetheless, both TEM and DLS results verify that aggregation or agglomeration of Zn and ZnO NPs in aqueous solution is inevitable, suggesting that Artemia were indeed exposed to aggregates of Zn and ZnO NPs rather than the NPs.
3.2. Accumulation of NPs
Artemia are non-selective filter-feeders like daphnids, and thus can ingest fine particles smaller than 50 μm readily.36 At the termination of the exposure, larvae from each group were examined under a phase contrast microscope (Micromaster model 12-575-252, Fisher Scientific) equipped with a digital camera to acquire a visual view of the ingested NPs. Images were captured by Micron Imaging software package from live Artemia in Petri dishes.As shown in Fig. 2, the guts for the control were empty (transparent), whereas they were entirely filled with NPs (e.g., aggregates of the NPs) in the treatment. The images also verify that Artemia larvae were unable to eliminate the ingested NPs. The same accumulation pattern was observed regardless of the size of the NPs, indicating that Artemia did not show any discrimination to differences in NP size. This is attributed to the fact that the aggregates (Table 3) were still significantly smaller compared with the large particles that Artemia can assimilate (e.g., 50 μm).
 | ||
| Fig. 2 Phase contrast microscope images of live Artemia larvae showing the accumulated NPs. The guts are empty in controls. Ingested NPs are visible as a dark strip inside the guts of the treatment. | ||
The accumulation profiles of Zn and ZnO NPs are illustrated in Fig. 3 and 4, respectively. The concentrations are based on the wet weight of Artemia, reflecting the total body burden across the concentration gradient from 10 to 100 mg NP L−1. All controls showed about 9 to 13 μg g−1 Zn which was thought to be derived from the medium (e.g., seawater). Average Zn concentration resulting from exposure to the suspensions of 40–60 nm Zn NPs ranged from 56 to 128 μg g−1 and 65 to 198 μg g−1 for 24 and 96 h, respectively. For relatively larger Zn NPs (80–60 nm), total Zn in the guts ranged from 53 to 135 μg g−1 in 24 h and 86 to 220 μg g−1 in 96 h. Differences between the two sizes of Zn NPs were not significant within the same exposure regime (e.g., 24 h) (p > 0.05), presumably because of the fact that the aggregates in water had almost the same hydrodynamic sizes (2110–1952 nm) that were also too small to affect Artemia's filtration rate. Conversely, accumulation was more influenced by the NP concentration and the duration of the exposure such that the guts exhibited the highest Zn concentration in 96 h in 100 mg L−1 Zn NPs.
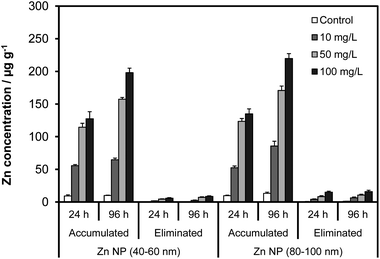 | ||
| Fig. 3 The effects of NP size, concentration and exposure time on the accumulation and elimination of Zn NPs by Artemia larvae. | ||
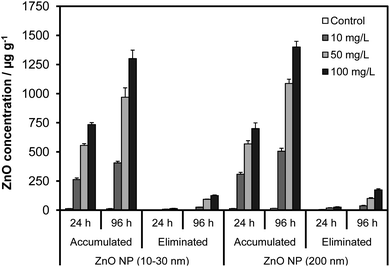 | ||
| Fig. 4 The effects of NP size, concentration and exposure time on the accumulation and elimination of ZnO NPs by Artemia larvae. | ||
The particle accumulation from the suspensions of the ZnO NPs followed a similar trend and was not influenced by the size of the NPs as observed for the Zn NPs (p > 0.05). For 10–30 nm ZnO NPs, the total ZnO concentration in the guts ranged from 264 to 735 μg g−1 in 24 h and 406 to 1301 μg g−1 in 96 h. Exposure to 200 nm ZnO NPs resulted in comparable levels of ZnO in the guts that varied from 309 to 700 μg g−1 and 506 to 1400 μg g−1 within 24 h and 96 h, respectively. Differently from the Zn NPs, however, Artemia accumulated substantially higher amounts of ZnO NPs (ca. 6-fold higher). The Zn NPs tended to attach to the walls of the exposure tank as black deposits and fall out of the solution. In contrast, the ZnO NP suspensions did not exhibit any such instability, suggesting that large accumulation was presumably associated with better stability of the ZnO suspensions compared with those of Zn NPs.
3.3. Elimination rate of ingested NPs
The elimination profiles for the accumulated Zn and ZnO NPs are illustrated in Fig. 3 and 4, respectively, when exposed Artemia are kept in nanoparticle-free seawater. The elimination exhibited first order kinetics where the rate of depuration increased with time as well as the concentration in the guts. Nevertheless, the loss in the concentration of ingested NPs was significantly lower than the accumulation for all the NPs. As expected the highest loss occurred for the groups exposed to 100 mg L−1 levels for which the average concentration of NPs eliminated in 24 h was about 6.0 and 15.2 μg g−1 for Zn (40–60 nm) and Zn (80–100 nm) NPs, respectively. These values increased to about 15.6 and 25.0 μg g−1, respectively, in 96 h. In the case of ZnO, the concentration of NPs eliminated in 24 h was 32 and 52 μg g−1 for ZnO (10–30 nm) and ZnO (200 nm), respectively. For a 96 h depuration period, the elimination was about 126 μg g−1 and 174 μg g−1 for the same size NPs, respectively. Compared with the accumulated levels, the values reflect a reduction of about 7.9% (Zn NP 40–60 nm), 11.4% (Zn NP 80–100 nm), 9.7% (ZnO 10–30 nm) and 12.4% (ZnO NP 200 nm) in 96 h. The removal of the ingested particles was significantly slow, indicating that Artemia were unable to eliminate the ingested particles as readily as they accumulated. This phenomenon could be due to the formation of massive aggregates inside the guts after accumulation.3.4. Dissolution of Zn and ZnO NPs in the medium
Zinc is an essential trace element for biological organisms, but it is also known to cause cellular damage at high concentrations. Regarding the toxic effects of ZnO NPs, some studies concerning D. magna,14 protozoa,16 and microalgae20 attributed the toxicity to the Zn ions (Zn2+) released from the NPs into the solution. On the other hand, the findings of other studies with zebra fish (Danio rerio) embryos,17D. magna,19 and marine copepod (Tigriopus japonicus)37 imply that Zn2+ cannot account entirely for the toxicity of ZnO NPs.In order to elucidate the extent of dissolution of Zn and ZnO NPs and its contribution to the toxic effects, the concentrations of Zn2+ in experimental suspensions were determined by ultrafiltration. In this procedure, 2 mL from each suspension were taken and centrifuged for 1 h to separate the suspending particulates and NP aggregates. Then, 0.5 mL of the supernatant solution was passed through ultra-filtration filters (VWR International) with a molecular cutoff of 3000 Da to separate the Zn ions from the particles. This filter rejects particles greater than 1.3 nm; therefore, the filtrate is assumed to contain Zn2+ predominantly and all NPs and aggregates greater than 1.3 nm are retained on the filter. The filtrate was then analyzed by ICP-MS for Zn in the solution. The results showed that Zn2+ levels released from the Zn NPs were significantly higher than those released from the ZnO NPs (p < 0.05) (see Fig. 5).
 | ||
| Fig. 5 Temporal dissolution patterns of Zn and ZnO NPs, and the variation of Zn ion concentration in the suspensions as a function of NP concentration and size. | ||
Eventually, the dissolution of the NPs was higher at low test concentrations (ca. 25% for Zn NPs and 16% ZnO NPs in 10 mg L−1 suspensions). However, a large fraction of the NPs was still intact (e.g., undissolved) in the medium. This phenomenon can be ascribed to the higher pH of the suspensions (pH 8.1–8.8). Studies utilizing sensory bacteria to measure the free Zn ions reported that ZnO NPs were almost fully dissolved in the medium and were available to the sensor bacteria.14,16,18 However, it should be noted that the pH of the medium in bacterial biosensor assays is usually lower (e.g., pH 6.5–7.2) and could facilitate the dissolution of the NPs.
Poynton et al.19 determined much lower levels of Zn2+ (about 0.4 mg L−1) released from uncoated ZnO NPs (20 nm) in the exposure of D. magna. These levels were almost identical with Zn2+ levels (0.4–0.5 mg L−1) that we determined from 10 mg L−1 suspensions of similar size uncoated ZnO NPs (10–30 nm). In their determination, they used ultrafiltration devices similar to those we utilized here and the pH of the exposure medium was alkaline (pH 8.2) as for the Artemia larvae (pH 8.1–8.8, Table 2). It is evident from these results that the dissolution of ZnO and Zn NPs depends on the pH of the medium, and thus, the effects could vary greatly if the toxicity is mediated by Zn2+ only. In addition, they found that the Zn concentration in the medium was independent of the exposure time and the NP concentration. Our results for ZnO NPs (Fig. 5) suggest that free Zn2+ concentration in the exposure medium was not influenced by the exposure time (p > 0.05) but the concentration of the suspension and the size of the NPs (p < 0.05). Larger ZnO NPs (e.g., 200 nm) appeared to dissolve to a greater extent compared with 10–30 nm ZnO NPs. The average Zn2+ concentration in the 10 mg L−1 suspensions of 10–30 nm ZnO NPs was about 0.4–0.5 mg L−1 while it ranged from 1.3 to 1.6 mg L−1 for 200 nm ZnO NPs (see Fig. 5). The 50 and 100 μg mL−1 suspensions of the 200 nm size ZnO NPs also exhibited a similar profile and contained significantly higher Zn2+, up to 4.4 and 5.5 mg L−1, respectively, in comparison to the suspensions of 10–30 nm ZnO NPs (2.8 and 3.7 mg L−1, respectively).
Suspensions of Zn NPs also contained high levels of Zn2+ in the test medium (Fig. 5). Apparently, the results imply that under similar physicochemical conditions (e.g., pH, salinity, and temperature), Zn NPs dissolved to a greater extent (ca. about a factor of 1.5) than ZnO NPs. The concentration of Zn2+ was as high as 1.6, 4.8 and 5.9 mg L−1 in 24 h and increased to 2.6, 6.9 and 8.9 mg L−1 in 96 h for the 10, 50 and 100 mg L−1 Zn NP suspensions, respectively. Dissolution of the NPs did increase proportionately with the NP concentration and the exposure time but was not influenced by the variation in particle size unlike that occurred for the ZnO NPs (p > 0.05). This is presumably due to the fact that the differences in mean particle size for Zn NPs were not as large as for the ZnO NPs. As a result, the suspensions with identical NP concentration possessed almost identical levels of Zn2+ regardless of the size of the NPs (Fig. 5).
3.5. Acute toxicity of Zn and ZnO NP suspensions
The mortality rates are illustrated in Fig. 6 for the Zn and ZnO NPs. The controls showed about 3 to 5% mortality in 24 h and 96 h, which were not statistically significant (p > 0.05). As pointed out above, the exposures were conducted in the absence of food. The experimental mortalities for the controls clearly demonstrate that deprivation from food did not induce any lethal effects on Artemia larvae even up to 96 h. In the treatments, the mortalities increased with increasing NP concentration and time (p < 0.05). In 24 h, the average mortality ranged from 5% (10 mg L−1) to 12% (100 mg L−1) for Zn NPs (40–60 nm), and from 4% (10 μg mL−1) to 10% (100 mg L−1) for Zn NPs (80–100 nm) (LC50 > 100 mg L−1). These results demonstrated that Zn NPs were relatively benign to Artemia larvae during 24 h exposure, even at the highest test concentration. The lethal effects recorded for 96 h exposure were more prominent. The average mortality was about 24% in 10 μg mL−1 suspensions of the Zn NPs (40–60 nm) and increased to 42% in 100 mg L−1 suspensions, indicating that the LC50 concentration was around 100 mg L−1. Likewise, the mortality for the relatively larger Zn NPs (80–100 nm) was 18% in 10 mg L−1 suspensions and increased to 34% in 100 mg L−1 suspensions (LC50 > 100 mg L−1). Although the effects did not differ significantly in 24 h (p > 0.05), it should be noted that smaller Zn NPs (40–60 nm) induced higher lethal effects than relatively larger 80–100 nm size NPs in 96 h (p < 0.05) despite the fact that hydrodynamic sizes did not differ significantly. This effect could be indicative of size-dependent toxicity of the NPs. | ||
| Fig. 6 Percent mortality rates for Artemia salina larvae from exposure to Zn and ZnO NPs for 24 h and 96 h. | ||
The temporal patterns of lethal effects from the ZnO NPs were similar to those of Zn NPs. The mortalities recorded within 24 h ranged from 5 to 9% for ZnO (10–30 nm) and 5 to 6% for ZnO (200 nm) within the same concentration gradient (e.g., 10 to 100 mg L−1). A 10-fold increase in the NP concentration induced only moderate effects on Artemia (LC50 > 100 mg L−1). However, the same suspensions exhibited elevated toxicity in 96 h as it occurred for the Zn NPs. The average mortality was about 14% (10 mg L−1) and 26% (100 mg L−1) for 10–30 nm ZnO NPs, and 11% (10 mg L−1) and 18% (100 mg L−1) for 200 nm ZnO NPs. Apparently, the smaller ZnO NPs (10–30 nm) induced higher mortalities than larger ZnO NPs (200 nm) as it occurred for the Zn NPs (p < 0.05). Yet, the 96 h LC50 levels were still well above 100 mg L−1. These results clearly demonstrated that the ZnO NPs were not acutely toxic to Artemia. In contrast, studies conducted on D. magna, a freshwater crustacean, reported significant toxicity for uncoated ZnO NPs from different suppliers.13,14,16,18 The LC50 values ranged from 2.1 (ref. 18) to 22 mg L−1.19 In comparison to D. magna, Artemia are more tolerant to toxic effects of heavy metals;24–26 therefore, the LC50 values reported for D. magna may not reflect the toxicity to Artemia salina. In addition, the effects could be dependent on the formulations of the NPs since manufacturers utilize different routes of synthesis. Consequently, the physicochemical and toxicological properties of ZnO NPs could vary substantially even if NPs possess similar size distribution.
3.6. Effect of NP dissolution on mortality
Our results point to the fact that both Zn and ZnO NPs exhibited moderate toxicity to Artemia larvae in 24 h regardless of their size and concentration. However, the suspensions of Zn NPs were more toxic during 96 h and induced significantly higher mortalities than ZnO NPs (see Fig. 6, p < 0.05). The results also substantiate that NPs did release a significant amount of Zn2+ into the suspension during the prolonged exposure (Fig. 5). This phenomenon clearly implies that Artemia were exposed to Zn2+ and aggregates of the NPs. Namely, the sources of the toxic effects were two-fold viz. total body burden of NPs and free Zn2+ in the medium. In previous studies concerning the toxicity of ZnO NPs on D. magna, the LC50 values for ZnO NPs and Zn2+ were found to be similar (ca. 1.4–4.9 mg L−1).14,16,38 Thus, the lethal effects were attributed to Zn2+ from the dissolution of NPs. Poynton et al.,19 on the other hand, found that the concentration of Zn2+ was small (0.4 mg L−1) though the LC50 values were similar (1.3 mg L−1), and thus concluded that the toxicity of the ZnO NPs was mediated by NPs and Zn2+.Artemia salina are relatively resistant to heavy metal toxicity and can tolerate wide ranges of metal concentration.24,26,39 Typical LC50 values for Zn2+ for Artemia salina larvae were reported to be around 17.8 and 12.3 mg L−1 in 24 and 48 h.24 Apparently, Zn2+ concentrations measured from the suspensions of ZnO NPs in 24 h were all lower than the 24 h LC50 value, ranging from 0.4 to 3.7 mg L−1 for 10–30 nm ZnO NPs and 1.3 to 5.5 mg L−1 for 200 nm ZnO NPs. Accordingly, the mortality rates were lower, 5–9% (10–30 nm ZnO NPs) and 5–6% (200 nm ZnO NPs). In 96 h, Zn2+ levels in the suspensions of ZnO NPs did not change significantly (p > 0.05, Fig. 5) but the mortalities increased substantially to 14–26% (10–30 nm ZnO NPs) and 11–18% (200 nm ZnO NPs). At first, this effect could be attributed to the prolonged exposure in the presence of elevated levels of Zn2+. Interestingly, however, 96 h mortalities were higher for the suspensions of 10–30 nm ZnO NPs despite that they contained significantly lower Zn2+ levels. This result supports the findings of Poynton et al.19 that toxic effects were not totally due to Zn2+ in the solution; other factors viz. particle toxicity, body burden (accumulation) and size effects should also be considered. As can be seen in Fig. 4, total body burden of the ZnO NPs was not affected from the size differences, but the mortalities (Fig. 6), within same exposure regime (e.g., 96 h exposure in 100 mg L−1 suspensions). This result implies that toxic effects of suspensions of ZnO NPs could vary with the size of their nanopowders even if their hydrodynamic sizes are similar. This result also supports our assumption above (Section 3.4) that the elevated toxicity observed in suspensions of smaller ZnO NPs (10–30 nm) was indeed associated with the size.
The scenario was similar for the suspensions of Zn NPs. Total body burden had no significant influence on toxic effects. The suspensions did induce similar mortalities as those of ZnO NPs within 24 h during which the free Zn2+ levels (1.6–5.9 mg L−1) were all well below the 24 h LC50 value (17.8 mg L−1). With increasing Zn2+ concentration (ca. 8.9 mg L−1) in 96 h, the suspensions of Zn NPs became more toxic to Artemia than those of ZnO NPs. This result reveals that the increasing toxicity was due to the prolonged exposure to elevated Zn2+ levels. Likewise, 96 h mortalities were higher for the suspensions of smaller Zn NPs (40–60 nm) than 80–100 nm Zn NPs (24% vs. 18% in 10 μg mL−1 and 42% vs. 34% in 100 mg L−1 Zn NP suspension). This result was also consistent with those observed for ZnO NPs, confirming that relative toxicity of Zn NPs increased with decreasing size of nanopowder.
3.7. Effect of Zn and ZnO NPs on oxidative stress
Oxidative stress induced by the suspensions of the Zn and ZnO NPs was measured as total malondialdehyde (MDA) concentration, a natural bi-product of lipid peroxidation. The results are summarized in Table 4. The data were consistent with the mortality rates for 24 and 96 h exposure in different concentrations of Zn and ZnO NP suspensions. MDA levels in controls increased marginally (p = 0.042) in 96 h, which could be attributed to the food deprivation, though this effect was not reflected in their mortalities (3–5%). Eventually, the MDA levels confirm that both Zn and ZnO NPs induced oxidative stress even in 24 h, especially at higher concentrations of NPs (e.g., 100 mg L−1) where mortalities were moderate (about 9–12%). Presumably, Artemia salina were still acclimating to the effects of NPs and increasing Zn2+ levels in the medium.| Groups | Zn (40–60 nm) | Zn (80–100 nm) | ZnO (10–30 nm) | ZnO (200 nm) | ||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 96 h | 24 h | 96 h | 24 h | 96 h | 24 h | 96 h | |
| Control | 11.4 ± 0.6 | 15.5 ± 2.5 | 11.3 ± 1.4 | 14.2 ± 1.3 | 11.3 ± 1.4 | 16.2 ± 1.3 | 12.1 ± 0.8 | 16.5 ± 1.4 |
| 10 mg L−1 | 16.5 ± 2.5 | 44.9 ± 2.4 | 12.8 ± 1.8 | 37.1 ± 1.1 | 12.8 ± 1.8 | 31.1 ± 1.1 | 14.9 ± 2.2 | 28.1 ± 2.0 |
| 50 mg L−1 | 20.3 ± 2.1 | 50.2 ± 2.1 | 15.4 ± 1.7 | 39.3 ± 1.2 | 14.4 ± 1.7 | 35.3 ± 1.2 | 16.8 ± 1.5 | 32.6 ± 0.5 |
| 100 mg L−1 | 23.3 ± 2.6 | 53.0 ± 0.9 | 19.2 ± 1.6 | 40.4 ± 1.5 | 19.2 ± 1.6 | 40.4 ± 1.5 | 21.9 ± 2.5 | 36.4 ± 2.5 |
The MDA concentrations substantially increased in 96 h compared with 24 h (p < 0.05). Further, 96 h mortalities correlated with the MDA levels for appropriate NPs (0.98 ≥ r ≥ 0.92) suggesting that toxicity effects of Zn and ZnO NPs were mediated by oxidative stress. These results are supported by previous reports.17,40 It should also be noted that the 96 h MDA levels for the suspensions of Zn NPs were higher than those of ZnO NPs, especially for smaller size NPs. This result confirms that Zn NPs were more toxic than ZnO NPs. Additionally, the 96 h MDA levels showed differences with the size of the NPs. The suspensions of smaller Zn NPs (10–60 nm) and ZnO NPs (10–30 nm) induced higher oxidative stress compared to the suspensions of relatively larger NPs. This result is also coherent with higher mortalities observed in these suspensions, confirming that the size of the Zn and ZnO NPs influences the toxicity of their suspensions.
4. Conclusion
In this study, we evaluated the stability and solubility of Zn and ZnO NPs in seawater, and the toxic effects of their suspensions on Artemia salina larvae to elucidate the chemical and toxicological impact to marine micro-organisms. The results pointed to the fact that suspensions of Zn and ZnO NPs were not acutely toxic to Artemia at environmentally feasible levels. However, prolonged exposure to the same suspensions induced significant toxicity and oxidative stress resulting in increased lipid peroxidation levels. Moreover, the suspensions of Zn NPs induced more toxicity than ZnO NPs under similar exposure regimes. We concluded that this effect was associated with elevated free Zn2+ released from Zn NPs into exposure medium.The results reveal that Zn and ZnO NPs aggregate in seawater to micrometer particles. This process would ultimately reduce the toxic properties of the NPs. Nevertheless, both Zn and ZnO NPs showed differences in toxic effects depending on the size of their nanopowders, viz., the suspensions of smaller size NPs were more toxic. These results suggest that the size of the nanopowders could contribute to the observed toxicity, besides Zn2+, even if the NPs aggregate to similar hydrodynamic sizes in the solution. In future studies more attention should be given to the formulations of Zn and ZnO NPs to better understand their toxicological properties since both surface properties and ion release kinetics change with underlying manufacturing processes.
Acknowledgements
This project is funded in part by grants from the National Institutes of Health (NIH) through Research Centers in Minority Institutions (RCMI) Program (grant no: G12RR013459) and the U.S. Department of Defense (DOD) through the Engineer, Research and Development Center (Vicksburg, MS) (contract #W912HZ-10-2-0045). The views expressed herein are those of the authors and do not necessarily represent the official views of the funding agencies, and any of their sub-agencies. The authors thank Jackson State University, Biostatistical Support Unit for assistance in statistical analysis.Notes and references
- R. Chatterjee, Environ. Sci. Technol., 2008, 42, 339 CrossRef CAS.
- J. Y. Choi, G. Ramachandran and M. Kandlikar, Environ. Sci. Technol., 2009, 43, 3030 CrossRef CAS.
- T. M. Benn and P. Westerhoff, Environ. Sci. Technol., 2008, 42, 4133 CrossRef CAS.
- L. Geranio, M. Heuberger and B. Nowack, Environ. Sci. Technol., 2009, 43, 8113 CrossRef CAS.
- A. Nel, T. Xia, L. Mädler and N. Li, Science, 2006, 3, 622 CrossRef.
- L. Reijnders, J. Ind. Ecol., 2008, 12, 297 CrossRef CAS.
- G. Oberdorster, E. Oberdorster and J. Oberdorster, Environ. Health Perspect., 2005, 113, 823 CrossRef CAS.
- B. Nowack and T. D. Bucheli, Environ. Pollut., 2007, 71, 632 Search PubMed.
- A. Becheri, M. Dürr, P. L. Nostro and P. Baglioni, J. Nanopart. Res., 2008, 10, 679 CrossRef CAS.
- N. Serpone, D. Dondi and A. Albini, Inorg. Chim. Acta, 2007, 360, 794 CrossRef CAS.
- D. Lin and B. Xing, Environ. Sci. Technol., 2008, 42, 5580 CrossRef CAS.
- C. Hanley, J. Layne, A. Punnoose, K. M. Reddy, I. Coombs, A. Coombs, K. Feris and D. Wingett, Nanotechnology, 2008, 19, 5103 CrossRef.
- L. K. Adams, D. Y. Lyon and P. J. J. Alvarez, Water Res., 2006, 40, 3527 CrossRef CAS.
- M. Heinlann, A. Ivask, I. Blinova, H. C. Dubourguier and A. Kahru, Chemosphere, 2008, 71, 1308 CrossRef.
- Z. Huang, X. Zheng, D. Yan, G. Yin, X. Liao, Y. Kang, Y. Yao, D. Huang and B. Hao, Langmuir, 2008, 24, 4140 CrossRef CAS.
- M. Mortimer, K. Kasamets and A. Kahru, Toxicology, 2010, 69, 182 CrossRef.
- X. Zhu, J. Wang, X. Zhang, Y. Chang and Y. Chen, Nanotechnology, 2009, 20, 5103 Search PubMed.
- I. Blinova, A. Ivask, M. Heinlann, M. Mortimer and A. Kahru, Environ. Pollut., 2010, 158, 41–47 CrossRef CAS.
- H. C. Poynton, J. M. Lazorchack, C. A. Impellitteri, M. E. Smith, K. Rogers, M. Patra, K. A. Hammer, H. J. Allen and C. D. Vulpe, Environ. Sci. Technol., 2011, 45, 762 CrossRef CAS.
- N. M. Franklin, N. J. Rogers, S. C. Apte, G. E. Batley, G. E. Gadd and P. S. Casey, Environ. Sci. Technol., 2007, 41, 8484 CrossRef CAS.
- V. Aruoja, H. C. Dubourguier, K. Kasemets and A. Kahru, Sci. Total Environ., 2009, 407, 1461 CrossRef CAS.
- S. W. Wong, P. T. Leung, A. B. Djurisic and K. M. Leung, Anal. Bioanal. Chem., 2010, 396, 609 CrossRef CAS.
- P. Sorgeloos, Mar. Ecol.: Prog. Ser., 1980, 3, 363 CrossRef.
- S. N. Gajbhiye and R. Hirota, J. Indian Fish. Assoc., 1990, 20(43), 50 Search PubMed.
- B. S. Nunes, F. D. Carvalho, L. M. Guilhermino and G. Van Stappen, Environ. Pollut., 2006, 144, 453 CrossRef CAS.
- V. Kokkali, I. Katramados and J. D. Newman, Biosensors, 2011, 1(36), 45 Search PubMed.
- P. Vanhaecke, G. Persoone, C. Claus and P. Sorgeloos, Ecotoxicol. Environ. Saf., 1981, 5, 392 CrossRef.
- F. Sanchez, F. Sanz, A. Santa-Maria, J. Ros, M. De Vicente, M. Encinas and E. Vinagre, Bull. Environ. Contam. Toxicol., 1997, 59, 445 CrossRef.
- I. Sayeed, S. Parvez, S. Pandey, B. Bin-Hafeez and S. Raisuddin, Ecotoxicol. Environ. Saf., 2003, 56, 295 CrossRef CAS.
- G. Persoone, A. Van de Vell, M. Van Steertegem and B. Nayer, Aquat. Toxicol., 1989, 14, 149 CrossRef CAS.
- OECD, Guideline for the Testing of Chemicals (Part 202), Organization for Economic Co-operation and Development (OECD), 2004 Search PubMed.
- Z. Arslan, M. Ates, W. McDuffy, M. S. Agachan, I. O. Farah, W. W. Yu and A. J. Bednar, J. Hazard. Mater., 2011, 192, 192 CAS.
- T. M. Van Ye, A. M. Roza and G. M. Pieper, J. Surg. Res., 1993, 55, 553 CrossRef CAS.
- T. Y. Peng, H. J. Lv, P. Zeng and X. H. Zhang, Chin. J. Chem. Phys., 2011, 24, 464 CrossRef CAS.
- R. J. Miller, H. S. Lenihan, E. B. Muller, N. Tseng, S. K. Hanna and A. A. Keller, Environ. Sci. Technol., 2010, 44, 7329 CrossRef CAS.
- K. Hund-Rinke and M. Simon, Environ. Sci. Pollut. Res., 2006, 13, 225 CrossRef CAS.
- H. Wang, R. L. Wick and B. Xing, Environ. Pollut., 2009, 157, 1171 CrossRef CAS.
- K. Wiench, W. Wohlleben, V. Hisgen, K. Radtke, E. Salinas, S. Zok and R. Landsiedel, Chemosphere, 2009, 76, 1356 CrossRef CAS.
- A. Kissa, M. Moraitou-Apostolopoulou and V. Kiortsis, Arch. Hydrobiol., 1984, 102, 255 Search PubMed.
- J. Valanta, D. Drobnea, K. Sepcic, A. Jemec, K. Kogej and R. Kostanjsek, J. Hazard. Mater., 2009, 179, 160 CrossRef.
| This journal is © The Royal Society of Chemistry 2013 |
