Photo-hydrogen production by Rhodopseudomonas faecalis RLD-53 immobilized on the surface of modified activated carbon fibers†
Guo-Jun
Xie
,
Bing-Feng
Liu
*,
De-Feng
Xing
,
Jun
Nan
,
Jie
Ding
,
Hong-Yu
Ren
,
Wan-Qian
Guo
and
Nan-Qi
Ren
*
State Key Laboratory of Urban Water Resource and Environment, School of Municipal and Environmental Engineering, Harbin Institute of Technology, P.O. Box 2614, 202 Haihe Road, Harbin, 150090, China. E-mail: rnq@hit.edu.cn; jianfeibio@yahoo.com.cn; Fax: +86 451 86282008; Tel: +86 451 86282110
First published on 2nd February 2012
Abstract
Surface modified activated carbon fibers were developed as a solid carrier to improve photo-fermentative hydrogen production. The surface morphology and functional groups of activated carbon fibers were modified by HNO3 oxidation followed by steam explosion, which greatly increased the immobilization capacity and allowed attachment of more photo-hydrogen producer on the surface. This allowed the photo-bioreactor to operate with a low hydraulic retention time and high organic loading rate.
Hydrogen gas has the potential to provide a clean and affordable energy supply that can minimize our dependence on oil and therefore enhance the global economy and reduce environmental pollution.1,2 Biological hydrogen production mediated by microorganisms has comparative advantages over chemical catalysis, such as low energy consumption and modest reaction conditions. Theoretically, bacteria are expected to convert 100% of carbohydrate into hydrogen. However, microbial fermentation must consume a significant fraction of carbohydrate for the production of microorganisms as biocatalysts. As a result, the potential yield of hydrogen is decreased.3 Moreover, because photo-fermentative bacteria flocculate poorly,4cells cannot be efficiently separated from the supernatant. So, photo-fermentative bacteria rush out continuously with effluents from a continuous reactor, leading to poor hydrogen production capacity. Hence, enhancing biomass retention in photo-bioreactors is an emerging issue to achieve higher hydrogen production efficiencies.
For better performance of photo-bioreactors, cell immobilization techniques have been applied to improve biomass retention and have been demonstrated to be suitable for continuous hydrogen production.5–7 Generally, there are two types of cell immobilization: entrapment8 and attachment.9Cell immobilization created by entrapment methods often suffers inefficient mass transfer10 and poor light penetration,11 not suitable for long-term operation. Recent studies have shown that addition of an appropriate amount of solid carrier into the photo-bioreactor could enhance cell growth and promote hydrogen production.12 Solid carriers can provide more surface attachment sites for attaching more bacteria and enhancing biofilm formation, so more hydrogen producer can easily be maintained in the reactor.6,13 In addition, solid carriers may also act as buffers under extreme conditions.14 Moreover, cell adhesion to solid carriers also decreases mass transfer limitation of substrates and products by direct contact of cells with the bulk liquid.15
Various solid carriers, such as granule activated carbon,13 optical fibers16 and porous glass, have been employed to immobilize photo-fermentative bacteria.9 However, most solid carriers, such as porous glass,9 expanded clay and granule activated carbon,13 settle at the bottom of the reactor, due to their high density. In this case, bacteria immobilized on a backlit surface could not receive light energy and just consumed the substrate without hydrogen production, even with some transparent solid matrices. Consequently, the shading effect of solid carriers greatly limits the hydrogen production performance of photo-bioreactors. In a previous study, activated carbon fibers (ACFs) with high specific surface area and adsorption capacity were found to be effective solid carriers to immobilize photo-fermentative bacteria (data not published). Different from conventional solid carriers, the ACFs were fluidized in the culture during the reactor operation and each bacterium immobilized on the ACFs could absorb the light energy well and convert the substrate into hydrogen.
Surface properties of solid carriers play important roles in the immobilization process, as bacterial attachment mainly occurs at interfaces. Many researchers have investigated the effects of surface characteristics of a support on immobilization and subsequent biofilm formation.17–20 And surface modification has caused conforming changes in the concentration of surface-active sites and surface functionalities that has enhanced the performance of carriers.21–23 Here, we firstly applied the surface modified ACFs as solid carriers to immobilize photo-fermentative bacteria for further enhancement of hydrogen production. The surface of the ACFs was modified by HNO3 oxidation under different concentrations (0, 2, 4 and 6 mol l−1) and steam explosion (1.21 MPa, 15 min). The modified ACFs were defined as ACFs-0, ACFs-2, ACFs-4, ACFs-6, according to the concentrations of HNO3. After the HNO3 oxidation, the surface morphology of the modified ACFs was investigated using scanning electron microscopy (SEM) (Fig. 1).
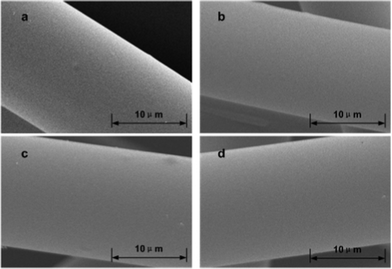 | ||
| Fig. 1 Scanning electron micrographs of ACFs after HNO3 oxidation: (a), ACFs-0; (b), ACFs-2; (c), ACFs-4; (d), ACFs-6. | ||
ACFs-0 had an extremely smooth morphology, free of any specific features (Fig. 1a). Following HNO3 oxidation treatments, it was observed that the general morphology of the fibers was almost unaltered (Fig. 1b, c, d). Their smooth appearance was maintained after the oxidation treatment. This was consistent with a previous report,24 which showed that HNO3 oxidation could increase the concentration of surface oxygen functionalities, but did not affect significantly the surface morphology of the fibers.
After HNO3 oxidation, steam explosion was carried out. After steam explosion, the surface morphology of the ACFs became rough or pitted (Fig. 2). The surface of the ACFs-0 was still quite smooth, as shown in Fig. 2a, compared with that of previous single treatment (Fig. 1a). This suggested that only steam explosion without HNO3 oxidation could not modify the surface morphology of the ACFs. Fig. 2b showed an SEM micrograph of ACFs obtained at a HNO3 concentration of 2 mol l−1 after steam explosion. Small grains have formed on the surfaces. More grains were on the surfaces of ACFs-4 than ACFs-2 (Fig. 2c). The results suggested that the amount of grains increased with an increase of HNO3 concentration. When the HNO3 concentration was further increased, coalescence occurred (Fig. 2d). This was because the oxidizing ability increased when the HNO3 was at high concentration. It has been generally accepted that oxidization of HNO3 causes the formation of a great number of oxygen-containing groups on the surfaces of solid carriers.25HNO3 oxidation disrupted the binding forces of the surface structure and reduced the surface structure stability. Increasing the concentration of nitric acid led to more severe attack on the ACFs structure and weakened the structural integrity. Although the surface morphology of ACFs was almost unaltered by only HNO3 oxidation, the corrosion of the surface structure of ACFs increased with an increasing concentration of HNO3. Therefore, the surface morphology of modified ACFs showed distinct differences after the same steam explosion conditions. It is worth noting that the smooth surface of ACFs became full of grains after steam explosion, resulting in the increase of surface roughness. This was favorable for further attachment of bacteria to the surfaces of ACFs and improved photo-hydrogen production.
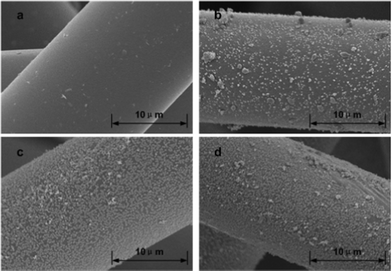 | ||
| Fig. 2 Scanning electron micrographs of ACFs after HNO3 oxidation and steam explosion: (a), ACFs-0; (b), ACFs-2; (c), ACFs-4; (d), ACFs-6. | ||
The surface chemical properties of modified ACFs samples were studied by X-ray photoelectron spectroscopy (XPS),26 which revealed the composition of the external surfaces of the ACFs. The major peaks in the spectra identified by XPS were the C 1s and O 1s, and a smaller N 1s peak was also discernible. Table 1 showed the elemental compositions on the surfaces of modified ACFs samples, which indicated an obvious change of elemental content in the samples. It was found that the surface carbon concentrations decreased significantly from 97.47% to 75.33% with increasing concentrations of HNO3. Lower surface carbon concentrations in modified ACFs compared to that in ACFs-0 could be attributed to bonding of oxygen on the carbon surface produced by the HNO3 oxidation process. Consequently, the oxygen content of ACFs samples was significantly increased, from 2.53 to 22.07%. This result clearly showed that the formation of surface oxygen functional groups resulted from the surface modification.
| Samples | C 1s | O 1s | N 1s | O/C |
|---|---|---|---|---|
| ACFs-0 | 97.47 | 2.53 | 0 | 2.59 |
| ACFs-2 | 81 | 17.58 | 1.42 | 21.70 |
| ACFs-4 | 77.68 | 21.14 | 1.18 | 27.21 |
| ACFs-6 | 75.33 | 22.07 | 2.6 | 29.30 |
XPS was also used to further analyze the functional groups on the carbon fiber surfaces27 and the high-resolution XPS spectra of the C 1s region were illustrated in Fig. 3. C 1s spectra were curve-fitted using five individual component groups that represented graphitic carbon (CG1, 284.5 eV), and carbon present in C–OH (CG2, 286.2 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (CG3, 287.3 eV), COOH (CG4, 289.1 eV) and carbonate groups (CG5, 290.6 eV).28 The percentages of surface functional groups were determined from the XPS peak areas after subtraction of a linear background (Table 2). The total surface oxygen-containing functions included CG2, CG3, CG4, and CG5 groups.28,29 In addition to graphitic carbon, carbonyl groups, and phenolic were the most predominant functional groups on the surfaces of modified ACFs. The most abundant oxygen-containing groups on the surfaces of ACFs-0 were C–OH (22.49%), with high intensities, while no C
O (CG3, 287.3 eV), COOH (CG4, 289.1 eV) and carbonate groups (CG5, 290.6 eV).28 The percentages of surface functional groups were determined from the XPS peak areas after subtraction of a linear background (Table 2). The total surface oxygen-containing functions included CG2, CG3, CG4, and CG5 groups.28,29 In addition to graphitic carbon, carbonyl groups, and phenolic were the most predominant functional groups on the surfaces of modified ACFs. The most abundant oxygen-containing groups on the surfaces of ACFs-0 were C–OH (22.49%), with high intensities, while no C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups were resolved. However, the content of C–OH groups decreased with HNO3 concentration, while C
O groups were resolved. However, the content of C–OH groups decreased with HNO3 concentration, while C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups significantly increased from 0 to 19.71% with increasing HNO3 concentration. This result indicated that severe oxidation led to a decrease in the ratio of C–OH, resulting in an increase in that of C
O groups significantly increased from 0 to 19.71% with increasing HNO3 concentration. This result indicated that severe oxidation led to a decrease in the ratio of C–OH, resulting in an increase in that of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.
O.
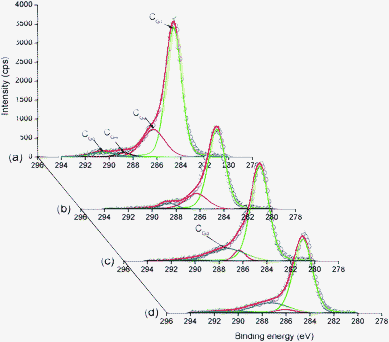 | ||
| Fig. 3 High-resolution fitted C 1s spectra of modified ACFs: (a), ACFs-0; (b), ACFs-2; (c), ACFs-4; (d), ACFs-6. | ||
| Samples | Binding energy (eV) | ||||
|---|---|---|---|---|---|
| 284.5 | 286.2 | 287.3 | 289.1 | 290.6 | |
| (CG1) | (CG2) | (CG3) | (CG4) | (CG5) | |
| C–C/C–H | C–OH | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
CO32−, CO, CO2 | |
| ACFs-0 | 73.42 | 22.49 | 0.00 | 2.16 | 4.22 |
| ACFs-2 | 77.08 | 19.15 | 5.21 | 0.33 | 0.91 |
| ACFs-4 | 76.05 | 5.61 | 19.53 | 0.16 | 1.42 |
| ACFs-6 | 75.51 | 3.55 | 19.71 | 0.09 | 4.38 |
The modified ACFs samples of 0.8 g l−1 with length of 1 mm were added into the photo-bioreactor. Hydrogen production by R. faecalis RLD-53 immobilized on the modified ACFs is shown in Fig. 4, and the control was a suspension culture without any carrier. The hydrogen yield increased with the concentration of HNO3 from 0 to 4 mol l−1, and then reached a steady state on further increasing the concentration of HNO3 from 4 to 6 mol l−1 (Fig. 4b). R. faecalis RLD-53 immobilized on ACFs-0 produced hydrogen rapidly and reached the yield of 3.01 mol H2/mol acetate (Fig. 4). In comparison with ACFs-0, R. faecalis RLD-53 immobilized on ACFs-6 obtained a maximum cumulative hydrogen volume of 3697 ml l−1 culture and a hydrogen yield of 3.30 mol H2/mol acetate (Fig. 4). The differences in hydrogen production performance might result from the different bacterial adhesion on ACFs caused by modification.
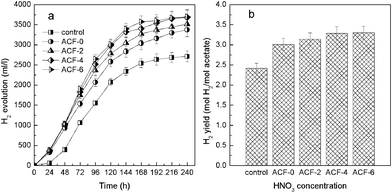 | ||
| Fig. 4 Hydrogen production by R. faecalis RLD-53 immobilized on surface modified ACFs: (a) cumulative hydrogen production; (b) hydrogen yield. | ||
In order to investigate bacterial adhesion, the bacterial morphology on the ACFs surfaces was monitored by SEM. As shown in Fig. 5, the bacterial density on the surfaces of the ACFs increased gradually from ACFs-0 to ACFs-6 and it also indicated that bacterial adhesion capability was enhanced with an increase in the treatment concentration of the HNO3. This phenomenon may be attributed to the change of surface roughness and functional groups. The ACFs-0 exhibited low bacterial adhesion capability due to the smooth surface and poor surface functional groups. And a small number of bacteria were immobilized on the ACFs, leading to low hydrogen yield. When bacterial cells were immobilized on the surfaces of the ACFs-6, it showed a good capability for attaching bacteria because of its high surface roughness (Fig. 2d). The increase of surface roughness corresponded to the accentuation of the peaks and valleys on the surface, which formally resulted in an augmentation of the area available for microbial settlement, and the rough surfaces provided more shielding from shear forces.30 Thus, increasing surface roughness increased both initial microbial adhesion and subsequent colonization of surfaces.31 A rougher matt finish stainless steel surface had 1.4 times more microorganisms than electro polished steel in biofilm development.32 Consequently, in this study, the number of immobilized photo-fermentative bacteria increased with the surface roughness of the modified ACFs (Fig. 5). More importantly, bacterial adhesion onto a surface was not only influenced by surface roughness, but also by solid carrier's surface chemistry, including the elemental composition and surface functional groups. Bacteria interact with a solid carrier surface through cell surface-active compounds in the bacterial adhesion process.33 In this work, abundant surface functional groups were introduced on the surfaces of ACFs by HNO3 oxidation followed by steam explosion. This might greatly enhance adhesion force between photo-fermentative bacteria and the surfaces of ACFs. The number of adherent cells on the carrier surfaces have been demonstrated to be directly correlated with the bacterial adhesion force.34 As a result, many more photo-fermentative bacteria were immobilized on the surfaces of modified ACFs (Fig. 5d).
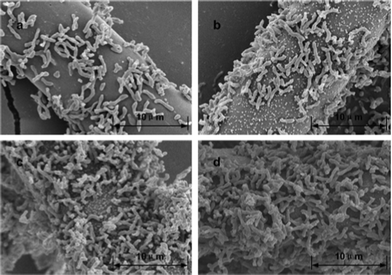 | ||
| Fig. 5 Scanning electron micrographs of surface modified ACFs after bacterial immobilization: (a), ACFs-0; (b), ACFs-2; (c), ACFs-4; (d), ACFs-6. | ||
Therefore, the above results indicate that the surface morphology and chemistry changes of the ACFs caused by HNO3 oxidation and steam explosion could greatly increase the immobilization capacity of the ACFs enabling attachment of more bacteria on the surfaces of the ACFs. Consequently, high biomass concentration was retained in the photo-bioreactor. This enabled the photo-fermentative hydrogen production process to be operated significantly at a low hydraulic retention time and with a high organic loading rate.
In conclusion, surface modified ACFs were used to immobilize R. faecalis RLD-53 for photo-hydrogen production. ACFs were treated with HNO3 of different concentrations (0, 2, 4 and 6 mol l−1) followed by steam explosion (1.21 MPa, 15 min). The surfaces of the ACFs became full of grains after surface modification, which resulted in an increase of surface roughness. XPS analysis showed that the oxidation of ACFs increased the concentration of oxygen functional groups on the external surfaces and modified their distribution in terms of function type. Consequently, the number of immobilized photo-fermentative bacteria increased due to increasing surface roughness and abundant surface functional groups. A maximum cumulative hydrogen volume of 3697 ml l−1 culture and a hydrogen yield of 3.30 mol H2/mol acetate were obtained by R. faecalisRLD-53 immobilization on the surface modified ACFs.
Acknowledgements
This research was supported by the Fundamental Research Funds for the Central Universities (Grant No. HIT. NSRIF. 201186), the National Natural Science Foundation of China (Grant No. 30870037, Grant No. 51106040), the China Postdoctoral Science Foundation (Grant No. 20110491054), the Major Program of National Natural Science Foundation of China (Grant No. 50638020), and the Key Laboratory of water/soil toxic pollutants control and bioremediation of Guangdong Higher Education Institutes, Jinan University.References
- R. M. Navarro, M. C. Sanchez-Sanchez, M. C. Alvarez-Galvan, F. del Valle and J. L. G. Fierro, Energy Environ. Sci., 2009, 2, 35–54 CAS.
- G. Wang, H. Wang, W. Li and J. Bai, RSC Adv., 2011, 1, 1585–1592 RSC.
- W. D. Huang and Y. H. Percival Zhang, Energy Environ. Sci., 2011, 4, 784–792 CAS.
- X. M. Liu, G. P. Sheng and H. Q. Yu, Environ. Sci. Technol., 2007, 41, 4620–4625 CrossRef CAS.
- S. Y. Wu, C. N. Lin and J. S. Chang, Biotechnol. Prog., 2003, 19, 828–832 CrossRef CAS.
- G. J. Xie, B. F. Liu, J. Ding, H. Y. Ren, D. F. Xing and N. Q. Ren, Int. J. Hydrogen Energy, 2011, 36, 13991–13996 CrossRef CAS.
- X. Tian, Q. Liao, W. Liu, Y. Z. Wang, X. Zhu, J. Li and H. Wang, Int. J. Hydrogen Energy, 2009, 34, 4708–4717 CrossRef CAS.
- B. F. Liu, G. J. Xie, W. Q. Guo, J. Ding and N. Q. Ren, Natural Resources, 2011, 2, 1–7 CrossRef CAS.
- X. Tian, Q. Liao, X. Zhu, Y. Z. Wang, P. Zhang, J. Li and H. Wang, Bioresour. Technol., 2010, 101, 977–983 CrossRef CAS.
- K. J. Wu and J. S. Chang, Process Biochem., 2007, 42, 279–284 CrossRef CAS.
- E. Nakada, Y. Asada, T. Arai and J. Miyake, J. Ferment. Bioeng., 1995, 80, 53–57 CrossRef CAS.
- C. Y. Chen, C. H. Liu, Y. C. Lo and J. S. Chang, Bioresour. Technol., 2011, 102, 8484–8492 CrossRef CAS.
- C. Y. Chen and J. S. Chang, Process Biochem., 2006, 41, 2041–2049 CrossRef CAS.
- K. K. Jefferson, FEMS Microbiol. Lett., 2004, 236, 163–173 CAS.
- T. Brányik, A. Vicente, R. Oliveira and J. Teixeira, Biotechnol. Bioeng., 2004, 88, 84–93 CrossRef.
- A. Yamada, H. Takano, J. Burgess and T. Matsunaga, J. Mar. Biotechnol., 1996, 4, 23–27 CAS.
- M. A. Ginsburg, K. Penev and D. Karamanev, Miner. Eng., 2009, 22, 140–148 CrossRef CAS.
- M. A. Basile, L. Dipasquale, A. Gambacorta, M. F. Vella, A. Calarco, P. Cerruti, M. Malinconico and G. Gomez d'Ayala, Bioresour. Technol., 2010, 101, 4386–4394 CrossRef CAS.
- M. A. Ghannoum, J. Chandra, J. D. Patel, J. Li, G. Y. Zhou, P. K. Mukherjee, T. S. McCormick and J. M. Anderson, Appl. Environ. Microbiol., 2005, 71, 8795–8801 CrossRef.
- G. He, Y. Gu, S. He, U. Schröder, S. Chen and H. Hou, Bioresour. Technol., 2011, 102, 10763–10766 CrossRef CAS.
- W. Shen, H. Wang, R. Guan and Z. Li, Colloids Surf., A, 2008, 331, 263–267 CrossRef CAS.
- J. S. Moon, K. K. Park, J. H. Kim and G. Seo, Appl. Catal., A, 2000, 201, 81–89 CrossRef CAS.
- N. Zhu, X. Chen, T. Zhang, P. Wu, P. Li and J. Wu, Bioresour. Technol., 2011, 102, 422–426 CrossRef CAS.
- P. Serp, J. L. Figueiredo, P. Bertrand and J. P. Issi, Carbon, 1998, 36, 1791–1799 CrossRef CAS.
- X. Song, H. Liu, L. Cheng and Y. Qu, Desalination, 2010, 255, 78–83 CrossRef CAS.
- I. Seo and S. W. Martin, RSC Adv., 2011, 1, 511–517 RSC.
- J. Gaume, P. Wong-Wah-Chung, A. Rivaton, S. Therias and J.-L. Gardette, RSC Adv., 2011, 1, 1471–1481 RSC.
- Y. C. Chiang, C. Y. Lee and H. C. Lee, Mater. Chem. Phys., 2007, 101, 199–210 CrossRef CAS.
- C. U. Pittman, W. Jiang, G. R. He and S. D. Gardner, Carbon, 1998, 36, 25–37 CrossRef CAS.
- L. Pons, M. L. Délia and A. Bergel, Bioresour. Technol., 2011, 102, 2678–2683 CrossRef CAS.
- S. L. Percival, J. S. Knapp, D. S. Wales and R. G. J. Edyvean, J. Ind. Microbiol. Biotechnol., 1999, 22, 152–159 CrossRef CAS.
- K. Pedersen, Water Res., 1990, 24, 239–243 CrossRef CAS.
- T. R. Neu, Microbiol. Mol. Biol. Rev., 1996, 60, 151 CAS.
- C. Tedjo, K. G. Neoh, E. T. Kang, N. Fang and V. Chan, J. Biomed. Mater. Res., Part A, 2007, 82A, 479–491 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section. See DOI: 10.1039/c2ra01075e |
| This journal is © The Royal Society of Chemistry 2012 |
