Facile fabrication of hierarchically nanoporous SBA-1 nanoparticles†
Na
Li
,
Jin-Gui
Wang
,
Hui-Jing
Zhou
,
Ping-Chuan
Sun
and
Tie-Hong
Chen
*
Institute of New Catalytic Materials Science, Key Laboratory of Advanced Energy Materials Chemistry (MOE), College of Chemistry, Nankai University, Tianjin 300071, PR China. E-mail: chenth@nankai.edu.cn
First published on 6th February 2012
Abstract
Mesoporous silica nanoparticles are drawing increasing attention in biomedical applications. In this work, hierarchically nanoporous SBA-1 single-crystal-like nanoparticles, with a size of 50–300 nm, were synthesized using a cationic surfactant and anionic polymer poly(acrylic acid) (PAA) mesomorphous complexes as a template. The obtained mesoporous silica nanoparticles showed a well ordered cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/i_char_0033_0304.gif) n mesostructure, large pore volume and high BET surface area. The materials possess biomodal nanopores corresponding to well-ordered mesopores and secondary interstitial nanopores.
n mesostructure, large pore volume and high BET surface area. The materials possess biomodal nanopores corresponding to well-ordered mesopores and secondary interstitial nanopores.
Uniform and colloidal stable nanoparticles have received much attention in practical fields, such as catalysis, adsorption, separation and biomedicine applications,1,2 because of their unique chemical, magnetic and optical properties.3–6 Recently, mesoporous silica nanoparticles (MSNs) have been considered as excellent candidates in nanomedicine system due to their controllable morphologies, mesostructures, porosities, high level of biocompatibility and ease of functionalization.7–10 Until now, MSNs have been intensively used as bio-markers,11–12 delivery carriers,13 biosensors14 and in controlled drug release.15–17 It has been demonstrated that the control of structure, morphology, size, and surface properties of MSNs is important for their biomedical applications. A variety of methods have been developed to fabricate MSNs, including co-solvent methods,18,19 a fluorocarbon-surfactant-mediated method,20 a dilute solution method,21 a phosphate buffer medium method22etc. However, most MSNs are limited by their relatively small pore size, which restrict their application for loading and delivery of large molecules such as DNA, proteins and enzymes.9 The synthesis of MSNs with both nanoscale particle size and large pore diameter or multimodal pores is highly desired.
Recently, hierarchically nanoporous silica materials with multimodal pore systems have been intensively studied in the catalysis of bulky molecules, adsorption systems and biomedical applications, due to their unique hierarchically porous structure and superior performance.23–27 However, the reported hierarchically nanoporous silica materials often have relative large particle sizes (micrometre or sub-micrometre). The synthesis of MSNs with hierarchical structure is therefore currently attracting significant interest. For example, Shi et al. have successfully synthesized core-shell structured dual-mesoporous silica nanospheres which possess smaller pores in the shells and larger tunable pores in the core by utilizing an amphiphilic block copolymer (polystyrene-b-poly (acrylic acid), PS-b-PAA) and cetyltrimethyl ammonium bromide (CTAB) as a co-template.28 This material may provide a useful platform for multidrug-combined therapy.9
In our previous work, we have prepared single-crystal-like, hierarchically nanoporous silica with particle size from submicrometer to micrometre, and the morphology invovled from spherical to “hexagonal drum-like polyhedron”.29 In this work, this simple one step route was empolyed to synthesize hierarchically structured single-crystal mesoporous silica SBA-1 nanoparticles (HMSNs) by using a cationic surfactant and anionic polymer poly(acrylic acid) (PAA) mesomorphous complexes as a template. To the best of our knowledge, there are no reports on the synthesis of single-crystal mesoporous nanoparticles with a hierarchically nanoporous structure.
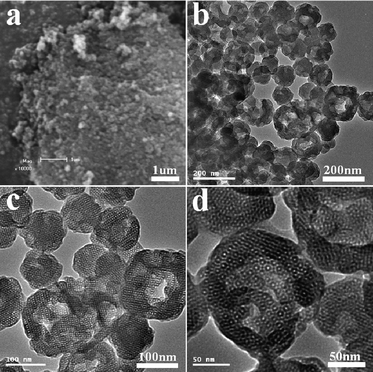
When cetyltrimethylammonium bromide (CTAB) was used in the synthesis (for details see ESI†) the product was named HMSN-1. The small angle X-ray scattering (SAXS) pattern of the calcined HMSN-1 sample (Fig. 1) indicated three well-resolved diffraction peaks, which were indexed to the (200), (210) and (211) characteristic diffraction peaks of the cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/i_char_0033_0304.gif) n mesostructure of SBA-1. Scanning electron microscopy (SEM) (Fig. 2a) and transmission electron microscopy (TEM) (Fig. 2b) images showed that the samples were generally nanometre-sized particles with a particle size range of 50–200 nm. The TEM image also showed that the pore structure of the synthesized nanoparticles containing both the mesopores and the secondary interstitial nanopores, as seen from the different contrast inside the nanoparticles (Fig. 2c). The generation of the mesopores and the secondary interstitial nanopores was due to the surfactant micelles and the phase-separated PAA, respectively. It is interesting to note that large cavities in the range of 40∼80 nm could be observed in some particles (Fig. 2c, d). The formation of these large cavities may partially due to the phase-separated PAA with relatively larger domain size. High-resolution TEM images (Fig. 2d) revealed that the mesopores of the HMSN-1 possessed a high degree of periodicity within the whole particle, indicating that the HMSN-1 exhibited a single-crystal-like feature.
n mesostructure of SBA-1. Scanning electron microscopy (SEM) (Fig. 2a) and transmission electron microscopy (TEM) (Fig. 2b) images showed that the samples were generally nanometre-sized particles with a particle size range of 50–200 nm. The TEM image also showed that the pore structure of the synthesized nanoparticles containing both the mesopores and the secondary interstitial nanopores, as seen from the different contrast inside the nanoparticles (Fig. 2c). The generation of the mesopores and the secondary interstitial nanopores was due to the surfactant micelles and the phase-separated PAA, respectively. It is interesting to note that large cavities in the range of 40∼80 nm could be observed in some particles (Fig. 2c, d). The formation of these large cavities may partially due to the phase-separated PAA with relatively larger domain size. High-resolution TEM images (Fig. 2d) revealed that the mesopores of the HMSN-1 possessed a high degree of periodicity within the whole particle, indicating that the HMSN-1 exhibited a single-crystal-like feature.
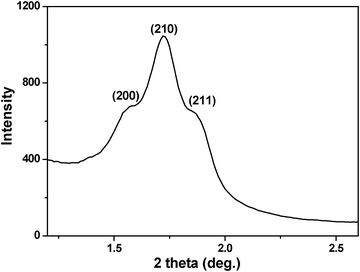 | ||
| Fig. 1 SAXS pattern of the calcined HMSN-1. | ||
The nitrogen adsorption–desorption isotherms of the HMSN-1 exhibited type IV isotherms with three distinct adsorption steps at the relative pressure of 0.3–0.5, 0.75–0.95 and 0.95–0.99, respectively (Fig. 3A). The first step corresponded to nitrogen capillary condensation in the cagelike mesopores of SBA-1, and resulted in a relatively narrow peak (∼3.2 nm) in the pore size distribution curve. The second adsorption step, where relative pressure p/p0 = 0.75–0.95, resulted in a broad pore size distribution centered at about 62 nm, corresponding to the secondary interstitial nanopores and large cavities, as observed in the TEM images. The third adsorption step, where relative pressure p/p0 = 0.95–0.99, was due to the voids of the aggregated nanoparticles. The BET surface area and total pore volume were 543 m2 g−1 and 1.57 cm3 g−1.
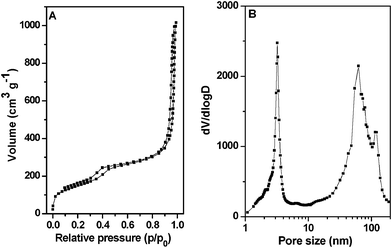 | ||
| Fig. 3 (A) Nitrogen adsorption–desorption isotherms and (B) pore size distribution curve of the calcined HMSN-1. | ||
Hierarchically nanoporous SBA-1 nanoparticles could also be prepared using hexadecyl pyridinium chloride (CPC) and PAA mesomorphous complexes as template (named HMSN-2). By changing the cationic surfactant in the synthesis, the morphology and the interior structure of the nanoparticles could be adjusted. The X-ray diffraction (XRD) pattern of the calcined sample (Fig. 4) displayed three distinct diffraction peaks indexed to (200), (210) and (211) diffractions respectively, according to the cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/i_char_0033_0304.gif) n mesostructure of SBA-1.
n mesostructure of SBA-1.
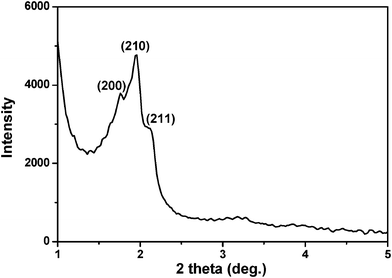 | ||
| Fig. 4 XRD pattern of the calcined HMSN-2. | ||
SEM (Fig. 5a) and TEM (Fig. 5b) images showed that the HMSN-2 sample consisted of spherical particles and the particle sizes ranged from 100 to 300 nm. TEM images at different magnifications revealed that there were both ordered mesopores and secondary interstitial nanopores inside the HMSN-2 sample (Fig. 5b–d). Furthermore, the alignment of the mesopores kept long-range order within the entire nanoparticle as shown in Fig. 5d, indicating that the presence of the secondary interstitial nanopores did not disturb the single-crystal characteristic of the HMSN-2 nanoparticles.
Fig. 6 showed the nitrogen adsorption–desorption isotherms and the pore size distribution curve of the calcined HMSN-2 sample. Type IV isotherm with three capillary condensation steps at relative pressure p/p0 of 0.3–0.5, 0.75–0.95 and 0.95–0.99 corresponding to the ordered mesopore (2.6 nm), secondary interstitial nanopores (around 48 nm) and aggregated voids between the particles, respectively, were clearly observed. These confirmed that the HMSN-2 possessed a hierarchically nanoporous structure. The BET surface area and pore volume of the HMSN-2 was 702 m2 g−1 and 1.55 cm3 g−1, respectively.
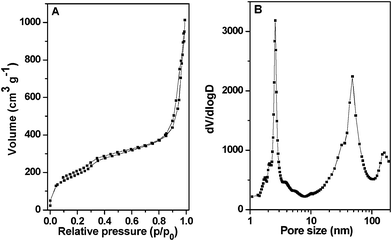 | ||
| Fig. 6 (A) Nitrogen adsorption–desorption isotherms and (B) pore size distribution curve of the calcined HMSN-2. | ||
In summary, hierarchically nanoporous single-crystal silica nanoparticles with size of 50–300 nm could be fabricated by using mesomorphous polyelectrolyte-surfactant complexes as template. Notably, the nanoparticles could retain single-crystal characteristics of SBA-1 in spite of the presence of secondary interstitial nanopores. In addition, this synthesis strategy is facile and the polymer and surfactants used in the synthesis are largely produced on an industrial scale. It is expected that the HMSNs will be applicable in different fields, such as catalysis, environment protection, optics and nanomedicine.
Acknowledgements
This work was supported by National Science Foundation of China (Grants 20873070, 20973095), National Basic Research Program of China (2009CB623502), and NCET of Ministry of Education (NCET-07-0448) and MOE (IRT-0927).References
- H. Goesmann and C. Feldmann, Angew. Chem. Int. Ed., 2010, 49, 1362–1395 CAS.
- T. Pellegrino, S. Kudera, T. Liedl, A. M. Javier, L. Manna and W. J. Parak, Small, 2005, 1, 48–63 CrossRef CAS.
- C. Burda, X. Chen, R. Narayanan and M. A. EI-Sayed, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS.
- A. H. Lu, E. L. Salabas and F. Schüth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS.
- J. Gao, H. Gu and B. Xu, Acc. Chem. Res., 2009, 42, 1097–1107 CrossRef CAS.
- V. Biju, T. Itoh and M. Ishikawa, Chem. Soc. Rev., 2010, 39, 3031–3056 RSC.
- Q. He, J. Shi, J. Zhao, Y. Chen and F. Chen, J. Mater. Chem., 2009, 19, 6498–6503 RSC.
- B. G. Trewyn, S. Giri, I. I. Slowing and V. S. -Y. Lin, Chem. Commun., 2007, 31, 3236–3245 RSC.
- Q. He and J. Shi, J. Mater. Chem., 2011, 21, 5845–5855 RSC.
- I. I. Slowing, J. L. Vivero-Escoto, B. G. Trewyn and V. S.-Y. Lin, J. Mater. Chem., 2010, 20, 7924–7937 RSC.
- C. W. Lu, Y. Hung, J. K. Hsiao, M. Yao, T. H. Chung, Y. S. Lin, S. H. Wu, S. C. Hsu, H. M. Liu, C. Y. Mou, C. S. Yang, D. M. Huang and Y. C. Chen, Nano Lett., 2007, 7, 149–154 CrossRef CAS.
- C. P. Tsai, Y. Hung, Y. H. Chou, D. M. Huang, J. K. Hsiao, C. Chang, Y. C. Chen and C. Y. Mou, Small, 2008, 4, 186–191 CrossRef CAS.
- M. Manzano and M. Vallet-Regí, J. Mater. Chem., 2010, 20, 5593–5604 RSC.
- I. I. Slowing, B. G. Trewyn, S. Giri and V. S. Y. Lin, Adv. Funct. Mater., 2007, 17, 1225–1236 CrossRef CAS.
- A. García, M. Colilla, I. I. Barba and M. Vallet-Regí, Chem. Mater., 2009, 21, 4135–4145 CrossRef.
- I. I. Slowing, C. W. Wu, J. L. Vivero-Escoto and V. S.-Y. Lin, Small, 2009, 5, 57–62 CrossRef CAS.
- Y. S. Lin and C. L. Haynes, J. Am. Chem. Soc., 2010, 132, 4834–4842 CrossRef CAS.
- Y. Yamada and K. Yano, Microporous Mesoporous Mater., 2006, 93, 190–198 CrossRef CAS.
- K. Möller, J. Kobler and T. Bein, Adv. Funct. Mater., 2007, 17, 605–612 CrossRef.
- Y. Han and J. Y. Ying, Angew. Chem., Int. Ed., 2005, 44, 288–292 CrossRef CAS.
- C. E. Fowler, D. Khushalani, B. Lebeau and S. Mann, Adv. Mater., 2001, 13, 649–652 CrossRef CAS.
- Q. He, X. Cui, F. Cui, L. Guo and J. Shi, Microporous Mesoporous Mater., 2009, 117, 609–616 CrossRef CAS.
- B. Zhao and M. M. Collinson, Chem. Mater., 2010, 22, 4312–4319 CrossRef CAS.
- X. Guo, Y. Deng, B. Tu and D. Zhao, Langmuir, 2010, 26, 702 CrossRef CAS.
- Y. Li, W. Cai, B. Cao, G. Duan, F. Sun, C. Li and L. Jia, Nanotechnology, 2006, 17, 238–243 CrossRef CAS.
- Y. Li, T. Sasaki, Y. Shimizu and N. Koshizaki, Small, 2008, 4, 2286–2291 CrossRef CAS.
- Y. Li, T. Sasaki, Y. Shimizu and N. Koshizaki, J. Am. Chem. Soc., 2008, 130, 14755–14762 CrossRef CAS.
- D. Niu, Z. Ma, Y. Li and J. Shi, J. Am. Chem. Soc., 2010, 132, 15144–15147 CrossRef CAS.
- N. Li, J. G. Wang, H. J. Zhou, P. C. Sun and T. H. Chen, Chem. Mater., 2011, 23, 4241–4249 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra01116f |
| This journal is © The Royal Society of Chemistry 2012 |