Potential of queen bee larvae as a dietary supplement for obesity management: modulating the gut microbiota and promoting liver lipid metabolism†
Zhuang
Li‡
abc,
Yiang
Chen‡
d,
Tengfei
Shi
abc,
Haiqun
Cao
abc,
Guijie
Chen
 *d and
Linsheng
Yu
*abc
*d and
Linsheng
Yu
*abc
aSchool of Plant Protection, Anhui Province Key Laboratory of Crop Integrated Pest Management, Hefei 230031, China. E-mail: yulinshengahau@163.com
bApiculture Research Institute, Anhui Agricultural University, Hefei 230031, China
cBiotechnology Center of Anhui Agriculture University, Hefei 230031, China
dNational Key Laboratory for Tea Plant Germplasm Innovation and Resource Utilization, School of Tea Science, Anhui Agricultural University, Hefei, 230036, China. E-mail: guijiechen@ahau.edu.cn
First published on 12th March 2025
Abstract
Queen bee larvae (QBL) have been consumed as both a traditional food and medicine in China for thousands of years; however, their specific benefits for human health, particularly their potential anti-obesity property, remain underexplored. This study investigated the anti-obesity effect of QBL freeze-dried powder (QBLF) on high-fat diet (HFD) induced obesity in mice and explored the underlying mechanisms. Our findings showed that QBLF effectively reduced body weight, fasting blood glucose levels, lipid accumulation, and inflammation in HFD mice. 16S rRNA sequencing revealed that QBLF significantly modulated the gut microbiota disrupted by an HFD, notably increasing the relative abundance of beneficial microbes such as Ileibacterium, Clostridium sensu stricto 1, Incertae sedis, Streptococcus, Lactococcus, Clostridia UCG-014, and Lachnospiraceae UCG-006, which were inversely associated with obesity-related phenotypes in the mice. RNA sequencing analysis further demonstrated that QBLF intervention upregulated the expression of genes involved in liver lipid metabolism, including Pck1, Cyp4a10, Cyp4a14, and G6pc, while downregulating genes associated with the inflammatory response, such as Cxcl10, Ccl2, Traf1, Mapk15, Lcn2, and Fosb. These results suggested that QBLF can ameliorate HFD-induced obesity through regulating the gut microbiota, promoting liver lipid metabolism, and reducing inflammatory response.
1 Introduction
Obesity is a chronic metabolic disorder characterized by an imbalance in energy intake and expenditure, resulting in excessive fat accumulation. This condition can lead to various complications including hypertension, type 2 diabetes (T2D), and cardiovascular diseases.1,2 Currently, approximately 20% of adults worldwide suffer from obesity, and with the prevalence increasing among both adults and children, it has emerged as a significant global public health challenge.3,4 The etiology of obesity includes both genetic factors, such as obesity-related and leptin genes, and environmental influences. Research has demonstrated that dietary supplements targeting the upregulation of genes involved in glucose and lipid metabolism and the downregulation of inflammation-related genes can effectively improve hyperlipidemia and prevent obesity.5–8 Furthermore, studies suggest that non-pharmacological measures such as dietary modifications and increased physical activity are more favorable than pharmacological and surgical interventions for weight management due to lower costs and risks.9 This underscores the importance of dietary interventions in providing a theoretical foundation for preventing obesity and crafting healthful eating strategies.Recent research has established a significant correlation between obesity and the gut microbiota. The gut microbiome plays a crucial role in influencing body weight by regulating host metabolism, appetite, and immune function.10 Predominantly, two beneficial bacterial groups, Bacteroidetes and Firmicutes, are crucial in the human gut. Obese individuals typically exhibit a lower proportion of Bacteroidetes and reduced levels of bifidobacteria compared to their lean counterparts, which impacts the diversity of the gut microbiota. Strategic modification of the microbial composition may aid in balancing the host's energy metabolism.11,12 Further studies illustrate that dietary changes can significantly affect the composition of the gut microbiome. For example, diets high in protein but low in carbohydrates, as well as probiotic supplements, have been shown to increase the prevalence of health-promoting gut bacteria, thereby aiding in weight loss and fat reduction.13,14 This underscores a profound link between dietary habits, gut microbiota interactions, and the metabolic processes associated with obesity. In recent decades, high-protein diets have been identified as microbiota-targeted options for preventing and treating obesity.15,16 However, they have garnered considerably less focus compared to other well-known prebiotics such as polysaccharides or polyphenols. Additionally, the type of protein in the diet differentially influences the gut microbiota and, consequently, lipid metabolism and triglyceride accumulation in high-fat diet (HFD) fed mice.16,17 Therefore, investigating suitable dietary proteins for the prevention and treatment of obesity remains a critical area of need.
Humans have been consuming insects for thousands of years, valuing them for their rich nutritional content and various bioactive compounds. Insects have traditionally been used not only to alleviate hunger but also for medicinal purposes. Immature insect larvae, in particular, are favored for their potential as alternative protein sources.18,19 Among these, queen bee larvae (QBL) are highly prized not only for their nutritional and medicinal properties but also for their potential as a resource in waste utilization.20 As a byproduct of royal jelly production, QBL offer a sustainable solution to repurpose apicultural waste into high-value products.21 QBL are abundant in proteins, fatty acids, and essential amino acids, making them a promising candidate for food and pharmaceutical applications. They have been used in traditional Chinese medicine for centuries, primarily for their immune-boosting properties.20,22 Previous research has identified that QBL contain significant amounts of major royal jelly proteins MRJP1 and MRJP2. MRJP1 is known to promote cell growth, aid wound healing, lower cholesterol levels, combat tumors, reduce blood pressure, and enhance immunity,23,24 while MRJP2 exhibits anticancer, antibacterial, and antioxidant properties.25,26 Additional studies suggest that QBL may improve sleep quality in mice by modulating the gut microbiota composition, which reverses changes caused by insomnia.21 However, there is a lack of research on whether QBL can prevent obesity through similar modulation of the gut microbiota.
In this study, QBL freeze-dried powder (QBLF) was administered to HFD fed mice to investigate the potential of QBLF in preventing obesity. Additionally, 16S rRNA sequencing and RNA sequencing were employed to analyze the impact of QBLF on the gut microbiota and liver genes of the mice, aiming to explore its anti-obesity effects and underlying molecular mechanisms.
2 Materials and methods
2.1 Materials
Fresh 3–4 day-old QBL (Apis mellifera) were obtained from an apiary in Feixi County, Anhui Province, China. QBL samples were homogenized and freeze-dried to obtain QBLF according to the previously reported methods.21 The protein content of QBL was measured using the Kjeldahl method with a Kjeltec 8400 analyzer (FOSS, Denmark), following the protocols in Chinese National Standard GB5009.4-2016. The total amino acid (TAA), essential amino acid (EAA), and non-essential amino acid (NEAA) levels were determined using an L-8900 Amino Acid Analyzer (Hitachi, Japan) according to the protocols in Chinese National Standard GB5009.124-2016. The fat content in QBL was measured using the acid hydrolysis method in accordance with Chinese National Standard GB5009.6-2016. The mineral content in QBL was determined using an inductively coupled plasma emission spectrometer (ICP, iCAP 7400 Duo, Thermo Fisher, USA), in accordance with Chinese National Standard GB5009.268-2016.2.2 Animal experiments
The animal experiment protocol was approved by the Experimental Animal Welfare and Ethics Committee of Anhui Agricultural University (Approval No. AHAU2023071). Male C57BL/6J mice, 5 weeks old and Specific Pathogen Free (SPF), were purchased from GemPharmatech Co., Ltd (Nanjing, China). The mice were housed in an SPF environment (temperature 23 ± 2 °C, relative humidity 50 ± 5%) with a 12-hour light/dark cycle, where they had free access to food and water. After a one-week acclimation period, the mice were randomly divided into three groups (n = 10 per group): a low-fat diet (LFD) group, a high-fat diet (HFD) group, and a high-fat diet supplemented with QBLF (HFD + Q) group. The HFD + Q group was administered a daily gavage of 500 mg kg−1 QBLF;21,27 this dosage corresponds to approximately 2.43 g day−1 for a 60 kg adult. This amount is reasonable and aligns with typical daily consumption levels of similar dietary supplements in humans. Whereas the other groups received a daily gavage of an equivalent volume of sterile water, over a period of 10 weeks. The low-fat (D12450J) and high-fat (D12492) diets were purchased from Research Diets, Inc. (New Brunswick, NJ, USA), and the nutritional components of the diets are listed in Table S1.† Feed intake and body weight were recorded weekly. The energy intake was calculated based on feed intake and energy density of the diet according to the previous method.282.3 Oral glucose tolerance test (OGTT)
During the ninth week of the experiment, following a 12-hour fasting period, the mice were administered an oral glucose dose of 1.5 g kg−1. Blood samples were then collected from the tail vein at intervals of 0, 15, 30, 60, 90, and 120 minutes. Blood glucose levels were measured using a OneTouch UltraEasy glucometer (Johnson & Johnson, China). The area under the curve (AUC) was calculated to assess the glucose tolerance of the mice.2.4 Sample collection
At the end of the experiment, the mice were euthanized by intraperitoneal injection of pentobarbital. Blood was collected into anticoagulant tubes (BD Biosciences, USA) and plasma was then obtained by centrifugation at 3000 rpm for 10 minutes at 4 °C. The liver, inguinal fat, and epididymal fat were excised, weighed and photographed. A portion of liver and inguinal fat was fixed in a 4% paraformaldehyde solution, while the remaining samples were stored at −80 °C. Fresh colonic contents were collected into sterile 1.5 mL centrifuge tubes and stored at −80 °C for 16S rRNA sequencing analysis.2.5 Biochemical analysis
Plasma concentrations of triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and aspartate aminotransferase (AST), along with liver levels of TG, AST, and alanine aminotransferase (ALT), were quantified using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Additionally, levels of lipopolysaccharide (LPS), tumor necrosis factor alpha (TNF-α), and interleukin 6 (IL-6) were measured using ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).2.6 Histological analysis
Histological analysis of liver and inguinal adipose tissue was conducted using methods previously described.29 Tissues fixed in formaldehyde were embedded in paraffin and then sectioned into slices 5 μm in thickness. These sections were stained with hematoxylin and eosin (H&E) and imaged using a panoramic scanner (3DHISTECH Case Viewer, Hungary). The area of adipocytes (μm2) in the sections was quantitatively assessed using ImageJ software (version 1.8.0_172).2.7 Gut microbiota analysis
For the gut microbiota analysis, 5 mice were randomly selected from each group for sequencing to evaluate changes in gut microbiota. 16S rRNA sequencing was conducted by Genesky Biotechnologies Inc. (Shanghai, China). Total DNA was isolated from the colonic contents of mice (n = 5 per group) using a Fast DNA Spin Extraction Kit (MP Biomedicals, USA). The quality of the extracted DNA was assessed with a NanoDrop spectrophotometer (Thermo, USA). Amplification of the V3–V4 variable regions of the 16S rDNA was achieved using the forward primer 341F CCTACGGGNGGCWGCAG and the reverse primer 805R GACTACHVGGGTATCTAATCC. Agarose gel electrophoresis confirmed the specificity and singularity of the amplification products. These products were then purified using Agencourt AMPure XP magnetic beads (Beckman, USA). The size of the insert fragments in the sequencing library was evaluated using an Agilent 2100 Bioanalyzer (Agilent, USA) to ensure the absence of non-specific amplification and to confirm that sizes fell within the 120–200 bp range. The concentration of the sequencing library was accurately quantified. Sequencing was performed on a NovaSeq 6000 platform using the SP-Xp (PE250) paired-end strategy by Genesky Biotechnologies Inc. (Shanghai, China), and the data were subsequently subjected to bioinformatics analysis according to our previous work.30 Principal Coordinate Analysis (PCoA) was conducted using QIIME 2 (version 2023.5) based on a Bray–Curtis distance matrix.312.8 Transcriptome analysis
Total RNA was extracted from the livers of mice (n = 3 per group) using the FastPure Cell/Tissue Total RNA Isolation Kit V2 (Vazyme, China). The integrity and quantity of RNA were accurately assessed using the 2100 bioanalyzer (Agilent, USA). Library construction was carried out following the previously established methods.32 Initial quantification of the library was performed using the Qubit 2.0 Fluorometer (Thermo, USA), and the effective concentration was precisely quantified using RT-qPCR to ensure it exceeded 1.5 nM. Sequencing was conducted on an Illumina NovaSeq X Plus PE150 platform at Novogene Co., Ltd (Beijing, China). Differentially expressed genes (DEGs) were identified using fold change (FC) analysis comparing the LFD, HFD, and HFD + Q groups. DEGs were deemed significant if the fold change for HFD/LFD and HFD + Q/HFD was ≥1.5 or ≤0.75 with a p-value ≤0.05 according to the reported method.6,33 Subsequent Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed on the identified DEGs.2.9 Quantitative real-time PCR (RT-qPCR)
Total RNA was extracted from the liver using the FastPure Cell/Tissue Total RNA Isolation Kit V2 (Vazyme, China). The quality and quantity of RNA were evaluated with the 2100 bioanalyzer (Agilent, USA). RNA was reverse-transcribed to cDNA using the HiScript III RT SuperMix for qPCR (+gDNA wiper) (Vazyme, China). RT-qPCR was conducted using the 2× Universal SYBR Green Fast qPCR Mix (ABclonal, China) following the manufacturer's instructions. The expression levels of target genes were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and calculated using the 2−ΔΔCt method. Primers were synthesized by Sangon Biotech (Shanghai, China) and the details are provided in Table S2.†2.10 Statistical analysis
In this study, statistical analyses were performed using GraphPad Prism software (version 9.0.0). Data are presented as mean ± SEM. Throughout the study, results were analyzed using an unpaired two-tailed Student's t-test for comparisons between two groups and one-way analysis of variance (ANOVA) followed by Tukey's test for comparisons among multiple groups. The normality assumption for the data was rigorously assessed using SPSS 22 software (IBM, Armonk, NY, USA) through the Shapiro–Wilk test. The correlations were analyzed by Pearson correlation. A p-value <0.05 was considered statistically significant.3 Results
3.1 Nutrient composition analysis of QBL
In this study, the nutritional composition of QBL, including proteins, fats, amino acids, and minerals, was quantitatively analyzed. Moreover, the nutrient composition of QBL was compared with those of various other types of meat including mutton leg, veal leg, pork shoulder, beef sirloin, duck carcass, chicken breast, and chicken drumstick referencing previous research,34 as detailed in Tables 1, S3 and S4.† The protein content in QBL was higher than that in duck carcass, whereas, its fat content was only higher than those in veal leg and chicken breast. Notably, QBL had the highest ratios of EAA/TAA and EAA/NEAA. Thus, QBL are considered a high-quality protein source according to the ideal protein model proposed by the Food and Agriculture Organization/World Health Organization.35 Among the minerals, the contents of Ca, Mg, Cu, Fe, and Mn in QBL were higher than those in other meats. Its K content was only lower than that in beef sirloin and chicken breast, while it had the lowest Na content. The Zn content was only lower than those in mutton leg and beef sirloin, and the P content was moderate. In summary, QBL derived from A. mellifera emerge as a high-quality protein source rich in amino acids and minerals and low in fat, presenting high potential for development and utilization. Future studies could expand this research by exploring other bee species as alternative insect-based food sources, offering further insights into their nutritional profiles and therapeutic applications.| Basic nutrient species | Protein (g per 100 g) | Fat (g per 100 g) | TAA (g per 100 g) | EAA (g per 100 g) | NEAA (g per 100 g) | EAA/TAA | EAA/NEAA |
|---|---|---|---|---|---|---|---|
| TAA: total amino acid; EAA: essential amino acid; NEAA: non-essential amino acid; the units are expressed as g per 100 g wet weight; the nutrient content in QBL was measured in this study, whereas the concentrations for the other groups were sourced from ref. 34. | |||||||
| QBL | 13.42 ± 0.03 | 3.48 ± 0.04 | 11.33 ± 0.02 | 4.91 ± 0.00 | 6.42 ± 0.02 | 0.43 | 0.76 |
| Mutton leg | 15.12 | 15.12 | 14.20 | 5.02 | 9.18 | 0.35 | 0.55 |
| Veal leg | 15.72 | 2.45 | 14.81 | 5.39 | 9.41 | 0.36 | 0.57 |
| Pork shoulder | 16.89 | 7.05 | 16.00 | 5.85 | 10.15 | 0.37 | 0.58 |
| Beef sirloin | 20.10 | 3.50 | 19.09 | 7.03 | 12.06 | 0.37 | 0.58 |
| Duck carcass | 8.64 | 18.30 | 8.11 | 2.71 | 5.40 | 0.33 | 0.50 |
| Chicken breast | 21.50 | 1.30 | 20.11 | 7.60 | 12.51 | 0.38 | 0.61 |
| Chicken drumstick | 17.80 | 6.00 | 16.71 | 5.97 | 10.74 | 0.36 | 0.56 |
3.2 QBLF supplementation alleviated the features of obesity and glucose tolerance in HFD mice
In this study, QBLF was administered orally at a dosage of 500 mg kg−1 day−1 to HFD-fed mice for 10 weeks to assess its therapeutic effects on diet-induced obesity. As delineated in Fig. 1A–E, the body weight, body weight gain, liver weight, epididymal fat weight, and inguinal fat weight in the HFD group were significantly elevated compared to those in the LFD group at the end of the experiment. Notably, the treatment with QBLF markedly mitigated these increases, thus effectively preventing the progression of HFD-induced obesity. Morphological analyses revealed substantially smaller liver, epididymal fat, and inguinal fat sizes in the HFD + Q group compared to the HFD group, as shown in Fig. 1I–K. As shown in Fig. 1G, the glucose levels remained elevated in the HFD group throughout the study period compared to the LFD group. Whereas, QBLF intervention significantly improved glucose tolerance, evidenced by reduced glucose levels at all measured time points and a decreased AUC during OGTT analysis (Fig. 1H). Energy intake was lower in the HFD + Q group compared to the HFD group, although this difference was not statistically significant (Fig. 1F), suggesting that the anti-obesity effects of QBLF were not attributable to reduced energy consumption. In summary, QBLF effectively ameliorates the obese phenotype, enhances glucose regulation, and improves glucose tolerance in HFD-fed mice, independent of changes in energy intake.3.3 QBLF reduced lipid accumulation and inflammation and ameliorated hepatic impairment in HFD mice
The effects of QBLF on plasma lipid profiles and liver function in HFD-fed mice are depicted in Fig. 2A–E. The levels of plasma LDL-C and liver TG were significantly elevated in the HFD group compared to the LFD group, whereas, these levels decreased after QBLF treatment. However, QBLF showed a limited effect on TG, TC, or HDL-C levels compared to the HFD group (Fig. S1†). Numerous studies have shown that an HFD can lead to hepatic impairment;36,37 therefore, the levels of AST and ALT were assessed to evaluate the protective effect of QBLF on the liver. As indicated in Fig. 2B–E, the levels of plasma AST, liver AST, and liver ALT in the HFD + Q group were significantly lower than those in the HFD group. Furthermore, histological analyses of inguinal fat and liver tissues revealed that, compared to the LFD group, the HFD group exhibited enlarged inguinal fat cells, increased liver fat droplets, and severe hepatic steatosis. In contrast, the HFD + Q group showed smaller inguinal fat cells, reduced liver fat droplets, and improved hepatic steatosis, as shown in Fig. 2J–L. Therefore, QBLF effectively prevents HFD-induced lipid accumulation and hepatic impairment. It has been previously reported that increased pro-inflammatory cytokines can contribute to obesity in HFD-fed mice.38,39 As illustrated in Fig. 2F–I, the HFD remarkably triggered systemic inflammation; however, QBLF treatment significantly reduced levels of LPS and inflammatory markers including IL-6, IL-1β, and TNF-α in these mice.3.4 QBLF supplementation modulated the gut microbiota in HFD mice
Previous studies have demonstrated that dysbiosis of the gut microbiota in both humans and mice can lead to obesity, insulin resistance, and impaired glucose tolerance.40,41 Consequently, 16S rRNA amplicon sequencing was employed to assess the impact of gut microbiota on the prevention of obesity by QBLF. Alpha diversity indices, including Chao1, Shannon, and Simpson, were utilized to measure the richness and diversity of the gut microbiota. As illustrated in Fig. 3A–C, the HFD significantly decreased the Chao1 and Shannon indices, while supplementation with QBLF effectively countered these reductions in microbial richness and diversity. The HFD and QBLF showed a limited effect on the Simpson index (Fig. 3C). Beta diversity measures, such as Principal Coordinate Analysis (PCoA), highlighted distinct clustering differences among the LFD, HFD, and HFD + Q groups. In line with previous research,42 PCoA indicated that an HFD intervention dramatically altered the structure of the gut microbiota. As expected, QBLF treatment also significantly influenced the structure and composition of the gut microbiota (Fig. 3D).Further analysis at the class level revealed that the gut microbiota of the three groups predominantly comprised Bacilli, Clostridia, Verrucomicrobiae, Desulfovibrionia, Coriobacteriia, and Actinobacteria (Fig. 3E). Notably, Clostridia, often referred to as a “weight-loss bacteria”,43 exhibited a significant increase in the HFD + Q group compared to the HFD group (Fig. 3F). At the genus level (Fig. 3G–O), compared to the LFD group, the HFD group demonstrated a significant decrease in the relative abundance of Ileibacterium, Clostridium_sensu_stricto_1, Clostridia_UCG-014_genus, and Incertae_sedis and a significant increase in Ligilactobacillus. The addition of QBLF reversed these trends and significantly increased the relative abundance of Streptococcus, Lactococcus, and Lachnospiraceae_UCG-006 and markedly decreased the relative abundance of Ligilactobacillus. Furthermore, Linear Discriminant Analysis Effect Size (LEfSe) was utilized to identify the key gut microbiota in each group (Fig. 4A and B). Specifically, Verrucomicrobiae and Coriobacteriia were significantly predominant in the LFD group, Bacilli were notably enriched in the HFD group, and Clostridia were significantly enriched in the HFD + Q group. The associations between genus-level differential bacteria and phenotypic indices of obese mice, along with biochemical markers and inflammation levels, were examined using the Pearson correlation analysis (Fig. S2†). The analysis revealed that the relative abundance of Ileibacterium, Clostridium_sensu_stricto_1, Clostridia_UCG-014_genus, and Incertae_sedis exhibited a negative correlation with several parameters including mouse body weight, liver weight, inguinal and epididymal fat weights, fasting blood glucose, liver TG, plasma LDL-C, inflammatory markers (TNF-α, IL-1β, IL-6), plasma LPS, and liver ALT and AST. Conversely, the Ligilactobacillus demonstrated an opposite correlation pattern. Additionally, the relative abundance of Lachnospiraceae_UCG-006 was negatively correlated with mouse liver weight, inguinal fat weight, liver TG, plasma inflammatory markers (TNF-α, IL-1β, IL-6), plasma LPS, and liver ALT and AST. In contrast, Streptococcus showed a negative correlation only with the plasma inflammatory marker IL-6.
 | ||
| Fig. 4 LEfSe analysis of the gut microbiota. (A) Only taxa with an LDA significance threshold greater than 4. (B) LEfSe branching diagram of significantly discrepant species. | ||
3.5 QBLF treatment regulated lipid metabolism and inflammatory response signaling pathways
To investigate the mechanisms by which QBLF counteracts obesity, RNA sequencing (RNA-seq) was performed on liver samples from mice. Initial quality control of raw sequencing data yielded clean reads, which were aligned to the reference genome using the HISAT2 software. This process achieved high-quality mapping, with all groups showing a Q30 (%) above 97.28%, a GC content around 50%, a total mapping rate over 88.89%, and a unique mapping rate over 82.21% (Tables S5 and S6†).44 These metrics confirmed that the RNA-seq data were of suitable quality for further analysis.Differential gene expression analysis indicated significant changes after HFD and QBLF treatment. The HFD group exhibited 677 upregulated and 791 downregulated DEGs compared to the LFD group (Fig. 5A), and the HFD + Q group showed 312 upregulated and 332 downregulated DEGs compared to the HFD group (Fig. 5B). GO and KEGG pathway analyses were conducted to explore the roles of these DEGs in the prevention of obesity. GO analysis showed that DEGs in the HFD versus LFD mice were enriched in biological processes mainly related to lipid metabolism, such as the fatty acid metabolic process, steroid metabolic process, triglyceride metabolic process, neutral lipid metabolic process, lipid biosynthetic process, alcohol metabolic process, cholesterol metabolic process, secondary alcohol metabolic process, sterol metabolic process, steroid biosynthetic process, and regulation of the lipid metabolism (Fig. S3A†). Similarly, DEGs in the HFD + Q vs. HFD mice were also enriched in lipid metabolism-related functions, such as the cholesterol biosynthetic process, sterol metabolic process, steroid metabolism, and cholesterol metabolism (Fig. S3B†). KEGG analysis indicated that DEGs in the HFD vs. LFD mice were enriched in metabolic pathways related to lipid metabolism and inflammatory response signaling pathways, including steroid biosynthesis, the PPAR signaling pathway, fatty acid elongation, biosynthesis of unsaturated fatty acids, and the IL-17 signaling pathway (Fig. 5C). In the HFD + Q vs. HFD mice, DEGs were enriched in metabolic pathways related to lipid metabolism and inflammatory response signaling pathways, such as the PPAR signaling pathway, AMPK signaling pathway, TNF signaling pathway, and IL-17 signaling pathway (Fig. 5D).
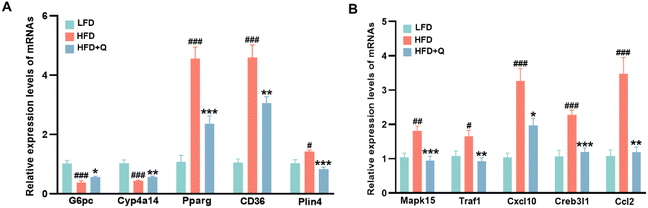
Based on these experimental results, an analysis of DEGs in lipid metabolism and inflammatory response signaling pathways was conducted to identify potential functional genes that might contribute to the lipid-lowering and anti-inflammatory effects of QBLF. Fourteen DEGs were enriched in the PPAR and AMPK signaling pathways involved in lipid metabolism (Fig. 5E). Compared to the HFD group, key genes such as Pck1, Cyp4a10, Cyp4a14, Cyp4a31, G6pc, Pfkfb2, Irs2, and Lepr were upregulated in the HFD + Q group, while Cd36, Plin4, Pparg, Scd1, Creb3l2, and Creb3l1 were downregulated. Additionally, nine DEGs were identified in the TNF and IL-17 inflammatory response signaling pathways (Fig. 5F). The key genes such as Cxcl10, Tnfaip3, Ccl2, Creb3l2, Traf1, Creb3l1, Mapk15, Lcn2, and Fosb were all downregulated in the HFD + Q group compared to the HFD group. Then, five selected genes related to lipid metabolism (G6pc, Cyp4a10, Pparg, Cd36, and Plin4) and five genes related to inflammation (Mapk15, Traf1, Cxcl10, Creb3l1, and Ccl2) were further validated using RT-qPCR. Specifically, G6pc is involved in the AMPK signaling pathway, while Cyp4a10, Pparg, Cd36, and Plin4 are associated with the PPAR signaling pathway. Among the inflammation-related genes, Cxcl10 is part of the IL-17 signaling pathway, and Mapk15, Traf1, Cxcl10, Creb3l1, and Ccl2 are implicated in the TNF signaling pathway. The selected genes were chosen based on their significant enrichment in key pathways (PPAR, AMPK, TNF, and IL-17) and their well-established roles in lipid metabolism and inflammation. The results showed that HFD-induced decreases of G6pc and Cyp4a10 and increases of Pparg, Cd36, Plin4, Mapk15, Traf1, Cxcl10, Creb3l1, and Ccl2 were significantly reversed by QBLF, consistent with the RNA-seq results (Fig. 6A and B).
3.6 Correlation between key DEGs and the phenotypic index, biochemical markers and inflammation levels
The Pearson correlation analysis was employed to evaluate the associations between key DEGs and phenotypic indices, biochemical markers, and inflammation levels in HFD mice after QBLF treatment, as depicted in Fig. S4A.† The analysis revealed that the expression of genes involved in lipid metabolism, including Pck1, Cyp4a10, Cyp4a14, Cyp4a31, G6pc, Lepr, Irs2, and Pfkfb2, was predominantly negatively correlated with mouse phenotypic indices, biochemical markers, and inflammation levels. Conversely, genes such as Cd36, Plin4, Pparg, and Creb3l1 exhibited largely positive correlations with these parameters. Notably, Creb3l1 expression was uniquely positively correlated with liver weight alone. Furthermore, the expression of genes associated with inflammatory responses demonstrated an almost universally positive correlation with mouse phenotypic indices, biochemical markers, and plasma inflammation levels (Fig. S4B†). These findings suggest that dietary modifications influence the expression of genes associated with lipid metabolism and inflammatory responses. Such alterations in gene expression potentially contribute to the observed reductions in lipid accumulation and inflammatory responses in mice, thereby playing a pivotal role in obesity resistance.4 Discussion
Obesity is a well-documented risk factor for T2D, cardiovascular diseases, and cancer and is often accompanied by low-grade inflammation.45,46 Prior studies have highlighted the benefits of increasing high-quality protein intake, which enhances satiety and helps control weight gain and triglyceride and cholesterol levels.47–49 Additionally, research on insect-based proteins indicates that they not only meet the amino acid requirements of mice but also play a role in regulating energy metabolism. For example, incorporating mealworms into the diet has been shown to modify the expression of genes associated with glucose and lipid metabolism, as well as inflammatory responses in the liver, subsequently reducing mouse weight, blood lipid levels, and inflammation.50 In this study, QBL were characterized as a high-quality protein source, rich in EAAs and minerals, while being low in fat. The high ratios of EAA/TAA and EAA/NEAA further highlight their nutritional value. However, the direct impact of QBL on obesity has yet to be fully understood. Therefore, this research focused on the effects of QBLF on HFD-induced obesity. The results indicated that QBLF significantly suppressed HFD-induced body weight gain, reduced fasting blood glucose, improved glucose tolerance, decreased lipid accumulation, and ameliorated liver damage, while maintaining the same caloric intake. These effects align with the known benefits of high-protein diets and insect-based proteins in modulating human health.15,51 The high-protein content of QBL, combined with their favorable amino acid profile and low-fat composition, makes them a promising dietary supplement for obesity management. Our findings suggest that QBL not only meet the nutritional requirements but also exert significant metabolic benefits. Future studies will further explore the potential application of QBL in the food industry.It is well-recognized that an HFD can compromise intestinal integrity, allowing endotoxin LPS from intestinal Gram-negative bacteria to enter the bloodstream, thereby inducing chronic low-grade inflammation, which is closely associated with metabolic diseases.52,53 Pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α, which are linked to the development of chronic inflammation and obesity in HFD-fed mice, were notably reduced by QBLF.54,55 Previous studies corroborate that mitigating chronic inflammation through dietary interventions can significantly attenuate HFD-induced obesity.39,56
The gut microbiome is intricately linked to metabolic diseases, with disruptions in the microbial community potentially leading to obesity and chronic inflammation.57 Consequently, the gut microbiota has been recognized as a potential target for both the prevention and treatment of obesity.58,59 Recent studies underscore the significant impact of both short-term and long-term diets on shaping the composition, functionality, and diversity of the gut microbiome, highlighting the influential role of diet on human health.60 While the benefits of anti-obesogenic diets on metabolism and obesity have been supported by mechanistic studies and clinical trials, the precise mechanisms are still unknown. The interaction between diet and the gut microbiota to regulate body weight homeostasis may be one of the key mechanisms.61 In this study, 16S rRNA amplicon sequencing was utilized to evaluate the effects of QBLF on the gut microbiota. Previous research has demonstrated that higher microbial richness and diversity, as reflected by Chao1 and Shannon indexes, are inversely associated with obesity-related traits. Accordingly, QBLF was found to enhance the richness and diversity of the gut microbiota, contributing to its obesity prevention effects. PCoA revealed significant alterations in the gut microbiota of HFD mice compared to the LFD group. The introduction of QBLF not only partially restored these changes but also increased the abundance of some beneficial bacteria.
At the class level, QBLF significantly elevated the relative abundance of the “weight-reducing” bacteria Clostridia, known for its obesity-preventive properties.43 Furthermore, diversity within Clostridia is negatively correlated with human obesity.62 At the genus level, QBLF reversed the diminished relative abundances of beneficial gut microbiota such as Ileibacterium, Clostridium_sensu_stricto_1, Clostridia_UCG-014_genus, and Incertae_sedis, which were reduced by an HFD. Additionally, QBLF promoted the growth of Lactococcus and Lachnospiraceae_UCG-006. Pearson correlation analysis indicated a significant negative correlation between changes in the relative abundance of these bacteria and the phenotypic and physiological biochemical markers of obesity in mice. Studies have shown that the relative abundance of Ileibacterium is significantly negatively associated with plasma ALT and AST and liver lipid levels in non-alcoholic fatty liver disease (NAFLD) models,63 aligning with our observations. Clostridium_sensu_stricto_1 is known to enhance immunity in humans by reducing pro-inflammatory Proteobacteria64 and protecting the intestinal barrier, thereby improving microbial homeostasis and alleviating colitis in a dextran sulfate sodium (DSS)-induced mouse model.65 The genus Clostridia_UCG-014 has been reported to protect against liver damage induced by D-galactosamine in rats.66 Both Lactococcus lactis and Lactococcus chungangensis CAU 28 have been demonstrated to reduce body weight, lipid accumulation, and chronic inflammation in obese mice.67,68 Additionally, the presence of Lachnospiraceae_UCG-006 has been significantly negatively correlated with blood lipid levels in obese mice.69 Regarding the enrichment of Streptococcus in the HFD + Q group, it is important to highlight that this genus comprises both pathogenic bacteria (e.g., Streptococcus pneumoniae, Streptococcus mutans, Streptococcus pyogenes) and beneficial bacteria (e.g., Streptococcus thermophilus).70 However, 16S rRNA sequencing provides only genus-level resolution, making it difficult to precisely identify microbial species or distinguish between beneficial and harmful strains within the same genus. Therefore, further investigation using species-level identification methods, such as metagenomic sequencing or strain-specific qPCR, is essential to determine the exact composition and potential roles of Streptococcus in this context. These results suggest that QBLF may reduce lipid accumulation and inflammation by fostering the growth of beneficial bacteria within the gut of HFD mice, thus addressing gut microbiome dysbiosis. However, 5 mice were randomly selected from each group for sequencing to evaluate changes in the gut microbiota. Therefore, further studies with larger sample sizes are strongly recommended to validate our findings.
The liver plays a pivotal role in lipogenesis and lipid metabolism. Numerous anti-obesogenic candidates have been shown to modulate gene expression levels in the liver, thereby preventing HFD-induced obesity.71 It has been documented that dietary supplementation with an HFD induces differential expression of genes related to lipid metabolism and inflammation. These DEGs are predominantly involved in signaling pathways associated with carbohydrate and lipid metabolism, as well as inflammatory responses.1,6 In this study, GO analysis revealed that DEGs were significantly enriched in biological processes primarily associated with lipid metabolism following HFD treatment, aligning with previously reported findings. More critically, disorders in lipid metabolism induced by the HFD, such as processes related to cholesterol biosynthesis, sterol metabolism, steroid metabolism, and cholesterol metabolism, were ameliorated after QBLF intervention (Fig. S2B†). Specifically, QBLF upregulated genes involved in lipid metabolism such as Pck1, Cyp4a10, Cyp4a14, and G6pc in the liver of HFD mice, while downregulating genes including Cd36, Pparg, and Scd1. Phosphoenolpyruvate carboxykinase 1 (PCK1) deficiency is known to enhance the expression of lipogenic genes and lipid synthesis, leading to lipid disorders and liver damage in healthy mice and to fibrosis and exacerbated inflammation in HFD mice.72 The cytochrome P450 4A (CYP4A) family, through lipid ω-hydroxylation, metabolizes medium and long-chain fatty acids, providing an alternative pathway of fatty acid metabolism. Upregulation of Cyp4a10 and Cyp4a14 has been shown to diminish the severity of steatosis and inflammation in mice with non-alcoholic steatohepatitis (NASH).73,74 Glucose-6-phosphatase catalytic subunit (G6PC), a crucial enzyme in carbohydrate metabolism, is involved in gluconeogenesis and glycogenolysis. Downregulation or deficiency of G6PC can lead to increased glucose storage and subsequently glycogen storage disease.75,76 Moreover, studies indicated that the expression levels of genes such as Cd36, Pparg, and Scd1, which are integral to the AMPK and PPAR signaling pathways, correlate positively with lipid accumulation and blood lipid levels in obese mice. Activation of the PPAR signaling pathway has been demonstrated to mitigate obesity induced by an HFD in mice.1 Our experimental findings corroborated these established mechanisms of lipid metabolism; thus, we hypothesized that QBLF may reduce fat accumulation and body weight in HFD mice by modulating their lipid metabolism processes.
Increasing evidence underscores the critical role of chronic and low-grade inflammation in the development of obesity and metabolic disorders.77 Furthermore, an HFD has been implicated in triggering hepatic inflammation. In this study, QBLF was observed to downregulate the expression of genes associated with the inflammatory response in the liver of HFD mice, including Cxcl10, Ccl2, Traf1, Mapk15, Lcn2, and Fosb. Research has demonstrated that Cxcl10 acts as a pro-inflammatory factor that exacerbates obesity-related metabolic dysfunctions, with its gene expression positively correlating with the prevalence of obesity and T2D.78,79 The intraperitoneal administration of a CCL2–CCR2 signaling antagonist has been shown to ameliorate inflammation in adipose tissue and enhance systemic glucose homeostasis in obese mice.80Traf1, a signaling adaptor, is known to activate the ASK1/JNK pathway, thereby promoting liver injury.81 Additionally, 7-methylmethoxyflavone has been reported to downregulate the expression of inflammation-related genes such as Ccl2, Ccl4, and Cxcl10 in rats on an HFD, effectively preventing obesity and hyperlipidemia.8 Our experimental findings align with these studies, indicating that QBLF may reduce lipid accumulation and plasma inflammation levels in the liver of HFD mice by downregulating the expression of genes associated with inflammatory factors.
Based on the results discussed above, QBLF significantly mitigated HFD-induced obesity and modulated the gut microbiota. Although the current study cannot definitively confirm that QBLF prevents obesity through modulation of the gut microbiota, our data indicated a strong association between QBLF-induced changes in the gut microbiota and obesity in HFD mice. Consequently, we hypothesized that the mechanisms underlying this effect involved QBLF-regulated microbiota alterations. This hypothesis should be further investigated using germ-free mouse models and fecal microbiota transplantation. Recent studies increasingly support the role of prebiotics, a microbiota-management tool, in inhibiting harmful pathogens and promoting the proliferation of beneficial bacteria, thus preventing HFD-induced obesity.62,71 Additionally, high-protein diets have been extensively studied as potential prebiotics for the prevention and treatment of obesity.82,83 However, proteins, as microbiota-directed foods, have received considerably less attention compared to other dietary components such as polysaccharides or polyphenols. Thus, our findings provided compelling evidence that proteins could serve as novel prebiotics, supplementing controlled diets for individuals with obesity. This opens new avenues for exploring dietary strategies in the management of obesity, emphasizing the therapeutic potential of protein-enriched diets.
One of the limitations of this study is the use of only a single dose of QBLF in the experimental design, which limited our ability to determine the optimal dosage for achieving the best anti-obesity effects. Comparing multiple doses of QBLF could provide more comprehensive insights into its dose-dependent effects and help identify the most effective dosage for reducing obesity. This limitation will be addressed in future studies by incorporating a range of QBLF doses to better understand its efficacy and safety profile.
5. Conclusion
In conclusion, this study demonstrated that QBLF is a high-quality protein source, abundant in essential amino acids and minerals, while being low in fat. This research has established that QBLF can effectively regulate body weight, blood glucose, lipid levels, and inflammation in HFD mice. In addition, QBLF intervention could modulate gut microbiota homeostasis, promote liver lipid metabolism, and reduce the expression of genes related to inflammatory responses in HFD mice. Our findings highlight the significant medicinal and nutritional value of QBLF, providing a scientific basis for its further development and utilization as a functional food.Author contributions
Zhuang Li: writing – review & editing, writing – original draft, and data curation. Yiang Chen: investigation and data curation. Tengfei Shi: investigation. Haiqun Cao: writing – review & editing, project administration, and funding acquisition. Guijie Chen: writing – review & editing, supervision, and project administration. Linsheng Yu: conceptualization, writing – review, & editing, project administration, and funding acquisition.Data availability
All data needed to evaluate the conclusions in the paper are presented in the paper and the ESI.† Additional data related to this paper may be requested from the corresponding authors.Conflicts of interest
The authors declare no conflicting financial interests.Acknowledgements
This research was funded by the Earmarked Fund for Modern Agro-Industry Technology Research System (No. CARS-45-KXJ10), the National Natural Science Foundation of China (32372611 and 32102604), and the Stabilization Project of Talent Project of Anhui Agricultural University (rc352220).References
- W. Ke, K. J. Flay, X. Huang, X. Hu, F. Chen and C. Li, et al., Polysaccharides from Platycodon grandiflorus attenuates high-fat diet induced obesity in mice through targeting gut microbiota, Biomed. Pharmacother., 2023, 166, 115318 CrossRef CAS PubMed.
- S. Nepali, J. Y. Cha, H. H. Ki, H. Y. Lee, Y. H. Kim and D. K. Kim, et al., Chrysanthemum indicum Inhibits Adipogenesis, Activates the AMPK Pathway in High-Fat-Diet-Induced Obese Mice, Am. J. Chin. Med., 2018, 46, 119–136 CrossRef CAS PubMed.
- G. Wu, H. Cheng, H. Guo, Z. Li, D. Li and Z. Xie, Tea polyphenol EGCG ameliorates obesity-related complications by regulating lipidomic pathway in leptin receptor knockout rats, J. Nutr. Biochem., 2023, 118, 109349 CrossRef CAS PubMed.
- D. A. Williamson, Fifty Years of Behavioral/Lifestyle Interventions for Overweight and Obesity: Where Have We Been and Where Are We Going, Obesity, 2017, 25, 1867–1875 CrossRef PubMed.
- M. Pigeyre, F. T. Yazdi, Y. Kaur and D. Meyre, Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity, Clin. Sci., 2016, 130, 943–986 CrossRef CAS PubMed.
- X. Wu, T. Guo, B. Li, S. Han, Z. Hu and Y. Luo, et al., Parboiled rice supplementation alleviates high-fat diet-induced hyperlipidemia by regulating genes and gut microbiota in mice, Food Sci. Hum. Wellness, 2024, 13, 1422–1438 CrossRef CAS.
- Y. Nie, F. Luo, L. Wang, T. Yang, L. Shi and X. Li, et al., Anti-hyperlipidemic effect of rice bran polysaccharide and its potential mechanism in high-fat diet mice, Food Funct., 2017, 8, 4028–4041 RSC.
- K. Feng, X. Zhu, T. Chen, B. Peng, M. Lu and H. Zheng, et al., Prevention of Obesity and Hyperlipidemia by Heptamethoxyflavone in High-fat Diet-induced Rats, J. Agric. Food Chem., 2019, 67, 2476–2489 CrossRef CAS PubMed.
- S. B. Heymsfield and T. A. Wadden, Mechanisms, Pathophysiology, and Management of Obesity, N. Engl. J. Med., 2017, 376, 254–266 CrossRef CAS PubMed.
- M. Van Hul and P. D. Cani, The gut microbiota in obesity and weight management: microbes as friends or foe, Nat. Rev. Endocrinol., 2023, 19, 258–271 CrossRef PubMed.
- R. E. Ley, P. J. Turnbaugh, S. Klein and J. I. Gordon, Microbial ecology: human gut microbes associated with obesity, Nature, 2006, 444, 1022–1023 CrossRef CAS PubMed.
- R. E. Ley, F. Bäckhed, P. Turnbaugh, C. A. Lozupone, R. D. Knight and J. I. Gordon, Obesity alters gut microbial ecology, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11070–11075 CrossRef CAS PubMed.
- H. C. Wastyk, G. K. Fragiadakis, D. Perelman, D. Dahan, B. D. Merrill and F. B. Yu, et al., Gut-microbiota-targeted diets modulate human immune status, Cell, 2021, 184, 4137–4153 CrossRef CAS PubMed.
- I. N. Sergeev, T. Aljutaily, G. Walton and E. Huarte, Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity, Nutrients, 2020, 12, 222 CrossRef CAS PubMed.
- W. Zhong, H. Wang, Y. Yang, Y. Zhang, H. Lai and Y. Cheng, et al., High-protein diet prevents fat mass increase after dieting by counteracting Lactobacillus-enhanced lipid absorption, Nat. Metab., 2022, 4, 1713–1731 CrossRef CAS PubMed.
- M. Zhang, S. Song, D. Zhao, J. Shi, X. Xu and G. Zhou, et al., High intake of chicken and pork proteins aggravates high-fat-diet-induced inflammation and disorder of hippocampal glutamatergic system, J. Nutr. Biochem., 2020, 85, 108487 CrossRef CAS PubMed.
- F. Zhao, G. Zhou, X. Liu, S. Song, X. Xu and G. Hooiveld, et al., Dietary Protein Sources Differentially Affect the Growth of Akkermansia muciniphila and Maintenance of the Gut Mucus Barrier in Mice, Mol. Nutr. Food Res., 2019, 63, e1900589 CrossRef PubMed.
- Y. Zhou, D. Wang, S. Zhou, H. Duan, J. Guo and W. Yan, Nutritional Composition, Health Benefits, and Application Value of Edible Insects: A Review, Foods, 2022, 11, 3961 CrossRef CAS PubMed.
- C. Teixeira, C. Villa, J. Costa, I. Ferreira and I. Mafra, Edible Insects as a Novel Source of Bioactive Peptides: A Systematic Review, Foods, 2023, 12, 2026 CrossRef CAS PubMed.
- D. Y. Dong, M. Y. Dong, K. M. Liu, Y. Lu and B. Yu, Antioxidant activity of queen bee larvae processed by enzymatic hydrolysis, J. Food Process. Preserv., 2018, 42, e13461 CrossRef.
- Q. H. Tang, J. Xiong, J. X. Wang, Z. Cao, S. Q. Liao and Y. Xiao, et al., Queen bee larva consumption improves sleep disorder and regulates gut microbiota in mice with PCPA-induced insomnia, Food Biosci., 2021, 43, 101256 CrossRef CAS.
- T. Zhao, L. Wu, F. Fan, Y. Yang and X. Xue, Supplementation with Queen Bee Larva Powder Extended the Longevity of Caenorhabditis elegans, Nutrients, 2022, 14, 3976 CrossRef CAS PubMed.
- X. Xu and Y. X. Gao, Isolation and characterization of proteins and lipids from honeybee (Apis mellifera L.) queen larvae and royal jelly, Food Res. Int., 2013, 54, 330–337 CrossRef CAS.
- W. Tian, M. Li, H. Guo, W. Peng, X. Xue and Y. Hu, et al., Architecture of the native major royal jelly protein 1 oligomer, Nat. Commun., 2018, 9, 3373 CrossRef PubMed.
- M. J. Park, B. Y. Kim, H. G. Park, Y. Deng, H. J. Yoon and Y. S. Choi, et al., Major royal jelly protein 2 acts as an antimicrobial agent and antioxidant in royal jelly, J. Asia-Pac. Entomol., 2019, 22, 684–689 CrossRef.
- M. M. Abu-Serie and N. H. Habashy, Two purified proteins from royal jelly with in vitro dual anti-hepatic damage potency: Major royal jelly protein 2 and its novel isoform X1, Int. J. Biol. Macromol., 2019, 128, 782–795 CAS.
- B. P. Kilari, P. Mudgil, S. Azimullah, N. Bansal, S. Ojha and S. Maqsood, Effect of camel milk protein hydrolysates against hyperglycemia, hyperlipidemia, and associated oxidative stress in streptozotocin (STZ)-induced diabetic rats, J. Dairy Sci., 2021, 104, 1304–1317 CAS.
- K. D. Hall, J. Guo, A. B. Courville, J. Boring, R. Brychta and K. Y. Chen, et al., Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake, Nat. Med., 2021, 27, 344–353 CAS.
- G. Wu, X. Sun, H. Cheng, S. Xu, D. Li and Z. Xie, Large Yellow Tea Extract Ameliorates Metabolic Syndrome by Suppressing Lipogenesis through SIRT6/SREBP1 Pathway and Modulating Microbiota in Leptin Receptor Knockout Rats, Foods, 2022, 11, 1638 CrossRef CAS PubMed.
- S. Li, H. Peng, Y. Sun, J. Yang, J. Wang and F. Bai, et al., Yeast β-glucan attenuates dextran sulfate sodium-induced colitis: Involvement of gut microbiota and short-chain fatty acids, Int. J. Biol. Macromol., 2024, 280, 135846 CAS.
- E. Bolyen, J. R. Rideout, M. R. Dillon, N. A. Bokulich, C. C. Abnet and G. A. Al-Ghalith, et al., Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2, Nat. Biotechnol., 2019, 37, 852–857 CAS.
- D. Parkhomchuk, T. Borodina, V. Amstislavskiy, M. Banaru, L. Hallen and S. Krobitsch, et al., Transcriptome analysis by strand-specific sequencing of complementary DNA, Nucleic Acids Res., 2009, 37, e123 Search PubMed.
- S. Vickovic, G. Eraslan, F. Salmén, J. Klughammer, L. Stenbeck and D. Schapiro, et al., High-definition spatial transcriptomics for in situ tissue profiling, Nat. Methods, 2019, 16, 987–990 CAS.
- A. Orkusz, Edible Insects versus Meat-Nutritional Comparison: Knowledge of Their Composition Is the Key to Good Health, Nutrients, 2021, 13, 1207 CAS.
- S. Y. Yan, Q. Xia, Z. Q. Cui, G. L. Ren, X. Y. Li and H. X. Ge, et al., Environmental adaptation, growth performance and nutrient content of the clam Cyclina sinensis from different geographic locations, Front. Mar. Sci., 2024, 11, 1397324 Search PubMed.
- H. Li, X. K. Wang, M. Tang, L. Lei, J. R. Li and H. Sun, et al., Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism, Gut Microbes, 2024, 16, 2304159 Search PubMed.
- Q. Nie, X. Luo, K. Wang, Y. Ding, S. Jia and Q. Zhao, et al., Gut symbionts alleviate MASH through a secondary bile acid biosynthetic pathway, Cell, 2024, 187, 2717–2734 CAS.
- S. Quesada-Vázquez, A. Castells-Nobau, J. Latorre, N. Oliveras-Cañellas, I. Puig-Parnau and N. Tejera, et al., Potential therapeutic implications of histidine catabolism by the gut microbiota in NAFLD patients with morbid obesity, Cell Rep. Med., 2023, 4, 101341 Search PubMed.
- D. Y. Kim, J. Y. Park and H. Y. Gee, Lactobacillus plantarum ameliorates NASH-related inflammation by upregulating L-arginine production, Exp. Mol. Med., 2023, 55, 2332–2345 CAS.
- E. Schéle, L. Grahnemo, F. Anesten, A. Hallén, F. Bäckhed and J. O. Jansson, The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system, Endocrinology, 2013, 154, 3643–3651 Search PubMed.
- P. D. Cani, Human gut microbiome: hopes, threats and promises, Gut, 2018, 67, 1716–1725 CAS.
- G. Chen, D. Chen, W. Zhou, Y. Peng, C. Chen and W. Shen, et al., Improvement of Metabolic Syndrome in High-Fat Diet-Induced Mice by Yeast β-Glucan Is Linked to Inhibited Proliferation of Lactobacillus and Lactococcus in Gut Microbiota, J. Agric. Food Chem., 2021, 69, 7581–7592 CAS.
- C. Petersen, R. Bell, K. A. Klag, S. H. Lee, R. Soto and A. Ghazaryan, et al., T cell-mediated regulation of the microbiota protects against obesity, Science, 2019, 365, eaat9351 Search PubMed.
- A. Mortazavi, B. A. Williams, K. McCue, L. Schaeffer and B. Wold, Mapping and quantifying mammalian transcriptomes by RNA-Seq, Nat. Methods, 2008, 5, 621–628 CAS.
- S. Sarma, S. Sockalingam and S. Dash, Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications, Diabetes, Obes. Metab., 2021, 23(Suppl 1), 3–16 CAS.
- F. M. Wensveen, S. Valentić, M. Šestan, T. Turk Wensveen and B. Polić, The “Big Bang” in obese fat: Events initiating obesity-induced adipose tissue inflammation, Eur. J. Immunol., 2015, 45, 2446–2456 Search PubMed.
- S. M. Phillips, S. Chevalier and H. J. Leidy, Protein “requirements” beyond the RDA: implications for optimizing health, Appl. Physiol., Nutr., Metab., 2016, 41, 565–572 CAS.
- S. H. Ley, O. Hamdy, V. Mohan and F. B. Hu, Prevention and management of type 2 diabetes: dietary components and nutritional strategies, Lancet, 2014, 383, 1999–2007 CrossRef CAS PubMed.
- S. C. Garcia Caraballo, T. M. Comhair, S. M. Houten, C. H. Dejong, W. H. Lamers and S. E. Koehler, High-protein diets prevent steatosis and induce hepatic accumulation of monomethyl branched-chain fatty acids, J. Nutr. Biochem., 2014, 25, 1263–1274 CrossRef CAS PubMed.
- Y. Kang, C. C. Applegate, F. He, P. M. Oba, M. D. Vieson and L. Sánchez-Sánchez, et al., Yellow Mealworm (Tenebrio molitor) and Lesser Mealworm (Alphitobius diaperinus) Proteins Slowed Weight Gain and Improved Metabolism of Diet-Induced Obesity Mice, J. Nutr., 2023, 153, 2237–2248 CrossRef PubMed.
- Y. Yoshinari, T. Nishimura, T. Yoshii, S. Kondo, H. Tanimoto and T. Kobayashi, et al., A high-protein diet-responsive gut hormone regulates behavioral and metabolic optimization in Drosophila melanogaster, Nat. Commun., 2024, 15, 10819 CrossRef CAS PubMed.
- C. J. Chang, C. S. Lin, C. C. Lu, J. Martel, Y. F. Ko and D. M. Ojcius, et al., Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota, Nat. Commun., 2015, 6, 7489 CrossRef CAS.
- G. Chen, Y. Peng, Y. Huang, M. Xie, Z. Dai and H. Cai, et al., Fluoride induced leaky gut and bloom of Erysipelatoclostridium ramosum mediate the exacerbation of obesity in high-fat-diet fed mice, J. Adv. Res., 2023, 50, 35–54 CrossRef CAS PubMed.
- J. Kuang, J. Wang, Y. Li, M. Li, M. Zhao and K. Ge, et al., Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis, Cell Metab., 2023, 35, 1752–1766 CrossRef CAS PubMed.
- W. Wei, C. C. Wong, Z. Jia, W. Liu, C. Liu and F. Ji, et al., Parabacteroides distasonis uses dietary inulin to suppress NASH via its metabolite pentadecanoic acid, Nat. Microbiol., 2023, 8, 1534–1548 CrossRef CAS.
- W. L. Sun, S. Hua, X. Y. Li, L. Shen, H. Wu and H. F. Ji, Microbially produced vitamin B12 contributes to the lipid-lowering effect of silymarin, Nat. Commun., 2023, 14, 477 Search PubMed.
- A. C. Gomes, C. Hoffmann and J. F. Mota, The human gut microbiota: Metabolism and perspective in obesity, Gut Microbes, 2018, 9, 308–325 CAS.
- D. F. de Wit, N. Hanssen, K. Wortelboer, H. Herrema, E. Rampanelli and M. Nieuwdorp, Evidence for the contribution of the gut microbiome to obesity and its reversal, Sci. Transl. Med., 2023, 15, eadg2773 CrossRef CAS PubMed.
- P. D. Cani and M. Van Hul, Gut microbiota in overweight and obesity: crosstalk with adipose tissue, Nat. Rev. Gastroenterol. Hepatol., 2024, 21, 164–183 CrossRef CAS PubMed.
- F. C. Ross, D. Patangia, G. Grimaud, A. Lavelle, E. M. Dempsey and R. P. Ross, et al., The interplay between diet and the gut microbiome: implications for health and disease, Nat. Rev. Microbiol., 2024, 22, 671–686 CrossRef CAS PubMed.
- E. C. Deehan, V. Mocanu and K. L. Madsen, Effects of dietary fibre on metabolic health and obesity, Nat. Rev. Gastroenterol. Hepatol., 2024, 21, 301–318 Search PubMed.
- L. Salazar-Jaramillo, J. de la Cuesta-Zuluaga, L. A. Chica, M. Cadavid, R. E. Ley and A. Reyes, et al., Gut microbiome diversity within Clostridia is negatively associated with human obesity, mSystems, 2024, e0062724 Search PubMed.
- J. Qiu, L. Chen, L. Zhang, F. Xu, C. Zhang and G. Ren, et al., Xie Zhuo Tiao Zhi formula modulates intestinal microbiota and liver purine metabolism to suppress hepatic steatosis and pyroptosis in NAFLD therapy, Phytomedicine, 2023, 121, 155111 Search PubMed.
- X. Li, S. Hu, J. Yin, X. Peng, L. King and L. Li, et al., Effect of synbiotic supplementation on immune parameters and gut microbiota in healthy adults: a double-blind randomized controlled trial, Gut Microbes, 2023, 15, 2247025 Search PubMed.
- L. Ma, Q. Shen, W. Lyu, L. Lv, W. Wang and M. Yu, et al., Clostridium butyricum, and Its Derived Extracellular Vesicles Modulate Gut Homeostasis and Ameliorate Acute Experimental Colitis, Microbiol. Spectrum, 2022, 10, e0136822 CrossRef PubMed.
- H. Zha, G. Si, C. Wang, H. Zhang and L. Li, Multiple Intestinal Bacteria Associated with the Better Protective Effect of Bifidobacterium pseudocatenulatum LI09 against Rat Liver Injury, BioMed Res. Int., 2022, 2022, 8647483 Search PubMed.
- Q. Zhang, J. H. Kim, Y. Kim and W. Kim, Lactococcus chungangensis CAU 28 alleviates diet-induced obesity and adipose tissue metabolism in vitro and in mice fed a high-fat diet, J. Dairy Sci., 2020, 103, 9803–9814 CrossRef CAS PubMed.
- C. R. Naudin, K. Maner-Smith, J. A. Owens, G. M. Wynn, B. S. Robinson and J. D. Matthews, et al., Lactococcus lactis Subspecies cremoris Elicits Protection Against Metabolic Changes Induced by a Western-Style Diet, Gastroenterol., 2020, 159, 639–651 Search PubMed.
- A. Li, N. Wang, N. Li, B. Li, F. Yan and Y. Song, et al., Modulation effect of chenpi extract on gut microbiota in high-fat diet-induced obese C57BL/6 mice, J. Food Biochem., 2021, 45, e13541 Search PubMed.
- Q. Li, W. Hu, W. X. Liu, L. Y. Zhao, D. Huang and X. D. Liu, et al., Streptococcus thermophilus Inhibits Colorectal Tumorigenesis Through Secreting β-Galactosidase, Gastroenterology, 2021, 160, 1179–1193 Search PubMed.
- G. Chen, M. Xie, P. Wan, D. Chen, Z. Dai and H. Ye, et al., Fuzhuan Brick Tea Polysaccharides Attenuate Metabolic Syndrome in High-Fat Diet Induced Mice in Association with Modulation in the Gut Microbiota, J. Agric. Food Chem., 2018, 66, 2783–2795 Search PubMed.
- Q. Ye, Y. Liu, G. Zhang, H. Deng, X. Wang and L. Tuo, et al., Deficiency of gluconeogenic enzyme PCK1 promotes metabolic-associated fatty liver disease through PI3K/AKT/PDGF axis activation in male mice, Nat. Commun., 2023, 14, 1402 Search PubMed.
- B. Xu, M. Jiang, Y. Chu, W. Wang, D. Chen and X. Li, et al., Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice, J. Hepatol., 2018, 68, 773–782 Search PubMed.
- Z. Yang, R. V. Smalling, Y. Huang, Y. Jiang, P. Kusumanchi and W. Bogaert, et al., The role of SHP/REV-ERBα/CYP4A axis in the pathogenesis of alcohol-associated liver disease, JCI Insight, 2021, 6, e140687 Search PubMed.
- Q. Liu, J. Li, W. Zhang, C. Xiao, S. Zhang and C. Nian, et al., Glycogen accumulation and phase separation drives liver tumor initiation, Cell, 2021, 184, 5559–5576 Search PubMed.
- K. Zhu, C. Deng, P. Du, T. Liu, J. Piao and Y. Piao, et al., G6PC indicated poor prognosis in cervical cancer and promoted cervical carcinogenesis in vitro and in vivo, Reprod. Biol. Endocrinol., 2022, 20, 50 Search PubMed.
- Y. Wu and Y. Ma, CCL2-CCR2 signaling axis in obesity and metabolic diseases, J. Cell. Physiol., 2024, 239, e31192 Search PubMed.
- Y. H. Tseng, Adipose tissue in communication: within and without, Nat. Rev. Endocrinol., 2023, 19, 70–71 Search PubMed.
- X. Zhang, J. Shen, K. Man, E. S. Chu, T. O. Yau and J. C. Sung, et al., CXCL10 plays a key role as an inflammatory mediator and a non-invasive biomarker of non-alcoholic steatohepatitis, J. Hepatol., 2014, 61, 1365–1375 CrossRef CAS PubMed.
- X. Li, Y. Su, Y. Xu, T. Hu, X. Lu and J. Sun, et al., Adipocyte-Specific Hnrnpa1 Knockout Aggravates Obesity-Induced Metabolic Dysfunction via Upregulation of CCL2, Diabetes, 2024, 73, 713–727 Search PubMed.
- X. F. Zhang, R. Zhang, L. Huang, P. X. Wang, Y. Zhang and D. S. Jiang, et al., TRAF1 is a key mediator for hepatic ischemia/reperfusion injury, Cell Death Discovery, 2014, 5, e1467 Search PubMed.
- M. E. Sanders, D. J. Merenstein, G. Reid, G. R. Gibson and R. A. Rastall, Probiotics and prebiotics in intestinal health and disease: from biology to the clinic, Nat. Rev. Gastroenterol. Hepatol., 2019, 16, 605–616 Search PubMed.
- M. Cunningham, M. A. Azcarate-Peril, A. Barnard, V. Benoit, R. Grimaldi and D. Guyonnet, et al., Shaping the Future of Probiotics and Prebiotics, Trends Microbiol., 2021, 29, 667–685 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5fo00166h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |