Organoselenium-catalyzed synthesis of indoles through intramolecular C–H amination†
Xuelin
Zhang
,
Ruizhi
Guo
and
Xiaodan
Zhao
*
Institute of Organic Chemistry & MOE Key Laboratory of Bioinorganic and Synthetic Chemistry, School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou 510275, P. R. China. E-mail: zhaoxd3@mail.sysu.edu.cn; Tel: +86-20-84110418
First published on 29th July 2015
Abstract
A new and efficient route for organoselenium-catalyzed synthesis of indoles via intramolecular C–H amination has been developed. The reaction conditions were mild and the desired products were formed in up to 99% yield. When the method was applied in the reaction of a trisubstituted alkene, the corresponding indole was obtained in 99% yield via 1,2-phenyl migration.
Indoles are important and valuable heterocycles because their structural units are frequently found in pharmaceuticals and bioactive natural products.1 In the past few decades, many efforts have been devoted to their synthesis. Currently, a large number of protocols are available to prepare indoles via different pathways.2 One strategy is to use 2-alkenylanilines as the starting materials to give indoles via C–N bond coupling by only losing two hydrogen atoms.3,4 This kind of C–H amination is atom-economical and straightforward compared to other known methods. Based on the advantages of C–H amination, transition metals as catalysts are successfully applied in indole synthesis with 2-alkenylanilines.3 In contrast to metal catalysis, metal-free indole synthesis is greener, less toxic, and cheaper. Developing a metal-free C–H amination to prepare indoles is an attractive goal.
Recently, Muñiz reported a sulfonic acid-catalyzed synthesis of indoles with iodosobenzene and 2-vinylanilines as the substrates.4a In this particular transformation, only 2,3-nonsubstituted indoles could be achieved. Later on, Youn demonstrated an efficient metal-free C–H amination of 2-alkenylanilines using DDQ as an oxidant to afford various 2-aryl indoles.4b When they used an α-aryl-β-alkylalkene as the substrate, dihydroquinoline was generated in modest yield instead of the corresponding indole. Deng has described a NIS-mediated synthesis of indoles with 2-alkenylanilines under mild conditions.4c However, this method falls short of efficiency in the production of 2-long-chain alkyl indoles. Overall, for the above transformations with 2-alkenylanilines, the reaction efficiency was low to synthesize 2-long-chain alkyl indoles or 2,3-disubstituted indoles via functional group migration. Very recently, Breder reported a diselenide-catalyzed synthesis of 2-long-chain alkyl indoles.5 But the synthesis of 2,3-disubstituted indoles via functional group migration was not covered in their work. Thus, a general approach to synthesize various indoles via direct C–H amination is desirable.
Organoselenium compounds are low-cost in contrast to common metal complexes and belong to the non-metal category.6 They could mediate organic reactions through selenation–deselenation assisted by an oxidant.7 Thirty-five years ago, Sharpless first reported a diselenide-catalyzed chlorination of alkenes with NCS.8 After that, Tiecco and several other groups worked on this area and made important contributions.9 In the past decade, diselenide catalysis has been paid more and more attention.10 Different oxidants such as PhI(OOCF3)2, H2O2, and other peroxides were utilized. Although some progress has been made in this field, the study of diselenide catalysis is still in the infant stage and efficient reaction systems as well as new reactions need to be further developed. Our group is interested in organoselenium catalysis. We have demonstrated an efficient diselenide-catalyzed, hydroxy-controlled regio- and stereoselective amination of terminal alkenes with N-fluorobenzenesulfonimide (NFSI) as a nitrogen source.11 In order to diversify the nitrogen source, other amines were employed for this intermolecular C–H amination, but no desired aminated products were observed. We conceived that intramolecular transformations are easier than the intermolecular ones. Therefore, when 2-alkenylanilines were used as the starting materials, the desired indoles could be generated by diselenide-catalyzed intramolecular C–N coupling. The route might provide a broad substrate scope, overcoming the existing drawbacks in the known metal-free synthesis. Herein, we report our achievements in indole synthesis by diselenide-catalyzed intramolecular C–N amination.
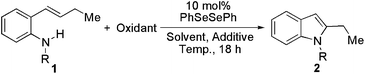
In the known metal-free processes with 2-alkenylanilines,4 the formation of 2-long-chain alkyl indoles is challenging. We initiated our investigation with N-Ts-2-(1-butenyl)aniline (1a) as the model substrate. Excitingly, when the reaction was carried out in acetonitrile at 30 °C for 18 h using NFSI as an oxidant, the desired product N-Ts-2-ethylindole (2a) was produced in 32% NMR yield (Table 1, entry 1). No (PhSO2)2N-functionalized alkene was observed by the direct reaction of 1a with NFSI.12 Different solvents were screened. Polar solvents, i.e. DMF and MeOH, were not effective resulting in almost no reaction (Table 1, entries 5 and 6). When THF was used as the solvent, product 2a was formed in 70% yield. The highest yield (79% isolated yield) was obtained when dioxane was replaced with THF as the solvent (Table 1, entry 10). When the reaction time was reduced from 18 h to 6 h, the transformation was not completed giving the product in 66% yield (Table 1, entry 11). The reaction temperature affected the reaction heavily. Only a trace amount of product was generated when the reaction was run at 0 °C (Table 1, entry 12). An increase in temperature did not result in higher yield (Table 1, entry 13). In order to increase the nucleophilicity of nitrogen, a base such as pyridine and NaHCO3 was added to the reaction which did not promote the reaction, and 2a was formed in lower yield (Table 1, entries 14 and 15). Other oxidants, e.g. Selectfluor and PhI(OOCCF3)2, were utilized for this transformation. To guarantee the efficiency of the oxidants, the reactions were conducted in suitable solvents. No better conversion was obtained (Table 1, entries 16 and 17). When 2-alkenylaniline was protected by other protecting groups, i.e., Cbz, Ac, and Boc, instead of the tosyl group, the corresponding indoles could not be produced. Probably, the nitrogen on the substrate was not nucleophilic enough to attack the intermediate seleniranium ion.13 However, when an electron-withdrawing sulfonyl protecting group such as 4-nitrobenzenesulfonyl (Ns) or ethylsulfonyl (Es) was immobilized on the nitrogen, the corresponding products (Ns-protected indole 2b and Es-protected indole 2c) were formed in acceptable yields.
| Entry | R | Oxidantb | Additive | Solvent | Temp. (°C) | Yieldc (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: alkenes 1, 1.05 equiv.; oxidant, 0.1 mmol; PhSeSePh, 10 mol%; solvent, 1 mL; additive, 0.2 equiv.; 18 h. b NFSI: N-fluorobenzenesulfonimide. c Refers to 1H NMR yield using benzyl benzoate as the internal standard; isolated yield is in the parenthesis. d Reaction time: 6 h instead of 18 h. | ||||||
| 1 | Ts | NFSI | — | CH3CN | 30 | 32 |
| 2 | Ts | NFSI | — | DCM | 30 | 32 |
| 3 | Ts | NFSI | — | DCE | 30 | 38 |
| 4 | Ts | NFSI | — | PhMe | 30 | 38 |
| 5 | Ts | NFSI | — | DMF | 30 | Trace |
| 6 | Ts | NFSI | — | MeOH | 30 | 0 |
| 7 | Ts | NFSI | — | AcOEt | 30 | 65 |
| 8 | Ts | NFSI | — | Et2O | 30 | 30 |
| 9 | Ts | NFSI | — | THF | 30 | 70 |
| 10 | Ts | NFSI | — | Dioxane | 30 | 79(79) |
| 11d | Ts | NFSI | — | Dioxane | 30 | 66 |
| 12 | Ts | NFSI | — | Dioxane | 0 | Trace |
| 13 | Ts | NFSI | — | Dioxane | 50 | 77 |
| 14 | Ts | NFSI | Pyridine | Dioxane | 30 | 34 |
| 15 | Ts | NFSI | NaHCO3 | Dioxane | 30 | 69 |
| 16 | Ts | Selectfluor | — | CH3CN | 30 | 70 |
| 17 | Ts | PhI(OOCCF3)2 | — | DCM | 30 | 10 |
| 18 | Cbz | NFSI | — | Dioxane | 30 | 0 |
| 19 | Ac | NFSI | — | Dioxane | 30 | 0 |
| 20 | Boc | NFSI | — | Dioxane | 30 | 0 |
| 21 | Ns | NFSI | — | Dioxane | 30 | 59(61) |
| 22 | Es | NFSI | — | Dioxane | 30 | 56(54) |
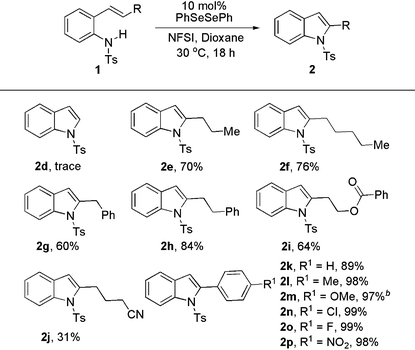
Next, we explored the substrate scope under the optimal conditions (Table 2). N-Ts-2-vinylaniline gave a trace amount of product 2d. The 2,3-group-free indole could be accessed in good yield by Muñiz's method.4a When our method was applied in the synthesis of 2-long-chain alkyl indoles, the desired products were obtained in good yield (2e–i, 60–84%). The ester group could be tolerated in the reaction. However, nitrile-containing aniline only gave the product 2j in 31% yield. It is likely that the nitrile group participated in the transformation to slow down the formation of the desired product 2j. This method is quite efficient for the transformation of 2-arylindoles as well. The corresponding products were achieved in excellent yields (2k–p, 89–99%).
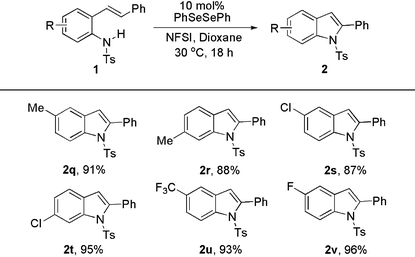
When different substituents were put on the aromatic ring of substrates to vary the nitrogen nucleophilicity, the C–H amination was slightly affected. All the desired products were generated in high yields (Table 3). For example, substrates with electron-neutral Me– and electron-deficient CF3– at the same position underwent C–N coupling to give the corresponding products in similar yields (2q, 91%; 2u, 93%).
| a Reaction conditions: alkenes 1, 1.05 equiv.; NFSI, 0.1 mmol; PhSeSePh, 10 mol%; dioxane, 1 mL; 30 °C, 18 h. |
|---|
|
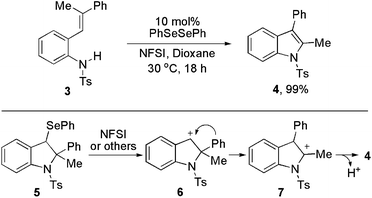
To further extend the generality of our method, trisubstituted alkene 3 was employed as the substrate. Surprisingly, only 2-methyl-3-phenyl indole 4 was formed in excellent yield of 99% via 1,2-phenyl migration (Scheme 1). No methyl migration product was observed. In the literature, there has been no report on the efficient preparation of the same product via phenyl migration.3e,4b,c For the formation of 4, the possible mechanism might involve the generation of intermediate 5. The PhSe group was removed by the oxidation of NFSI or other active species to form 6. Then phenyl group migrates from the 2-position to give intermediate 7. By proton elimination, the indole 4 is produced.
In summary, we have demonstrated an efficient approach for the synthesis of indoles via intramolecular C–H amination. The particular reactions were accomplished by organoselenium catalysis and the reaction conditions were mild. When the method was applied in the reaction of a trisubstituted alkene, the corresponding indole was obtained in 99% yield via 1,2-phenyl migration. In the field of diselenide catalysis, our discovery provides a possibility to develop more new organoselenium-catalyzed reactions. The mechanistic study is ongoing in our laboratory.
Acknowledgements
We thank Sun Yat-Sen University, the “One Thousand Youth Talents” Program of China and the Natural Science Foundation of Guangdong Province (Grant No. 2014A030312018) for financial support.Notes and references
- (a) R. J. Sundberg, Indoles, Academic Press, San Diego, CA, 1996 Search PubMed; (b) T. Kawasaki and K. Higuchi, Nat. Prod. Rep., 2005, 22, 761 RSC; (c) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489 CrossRef CAS PubMed.
- For selected reviews, see: (a) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed; (b) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS PubMed; (c) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608 CrossRef CAS PubMed; (d) S. Cacchi and G. Fabrizi, Chem. Rev., 2011, 111, PR215 CrossRef PubMed; (e) M. Shiri, Chem. Rev., 2012, 112, 3508 CrossRef CAS PubMed; (f) M. Inman and C. J. Moody, Chem. Sci., 2013, 4, 29 RSC.
- For transition metal-catalyzed C–H amination with 2-alkenylanilines, see: (a) L. S. Hegedus, G. F. Allen, J. J. Bozell and E. L. Waterman, J. Am. Chem. Soc., 1978, 100, 5800 CrossRef CAS; (b) P. J. Harrington, L. S. Hegedus and K. F. McDaniel, J. Am. Chem. Soc., 1987, 109, 4335 CrossRef CAS; (c) D. Tsvelikhovsky and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 14048 CrossRef CAS PubMed; (d) S. W. Youn, J. H. Bihn and B. S. Kim, Org. Lett., 2011, 13, 3738 CrossRef CAS PubMed; (e) S. Maity and N. Zheng, Angew. Chem., Int. Ed., 2012, 51, 9562 CrossRef CAS PubMed; (f) T. W. Liwosz and S. R. Chemler, Chem. – Eur. J., 2013, 19, 12771 CrossRef CAS PubMed.
- For metal-free C–H amination with 2-alkenylanilines, see: (a) L. Fra, A. Millán, J. A. Souto and K. Muñiz, Angew. Chem., Int. Ed., 2014, 53, 7349 CrossRef CAS PubMed; (b) H. J. Jang and S. W. Youn, Org. Lett., 2014, 16, 3720 CrossRef PubMed; (c) Y.-L. Li, J. Li, A.-L. Ma, Y.-N. Huang and J. Deng, J. Org. Chem., 2015, 80, 3841 CrossRef CAS PubMed.
- When our manuscript was in preparation, a piece of similar work was published online by Breder. See: S. Ortgies and A. Breder, Org. Lett., 2015, 17, 2748 CrossRef CAS PubMed.
- (a) G. Mugesh, W.-W. du Mont and H. Helmut Sies, Chem. Rev., 2001, 101, 2125 CrossRef CAS PubMed; (b) C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255 CrossRef CAS PubMed.
- For reviews, see: (a) D. Liotta, Acc. Chem. Res., 1984, 17, 28 CrossRef CAS; (b) Organoselenium Chemistry: Synthesis and Reactions, ed. T. Wirth, Wiley-VCH, Weinheim, 2011 Search PubMed; (c) D. M. Freudendahl, S. A. Shahzad and T. Wirth, Eur. J. Org. Chem., 2009, 1649 CrossRef CAS PubMed; (d) D. M. Freudendahl, S. Santoro, S. A. Shahzad, C. Santi and T. Wirth, Angew. Chem., Int. Ed., 2009, 48, 8409 CrossRef CAS PubMed; (e) C. Santi, S. Santoro and B. Battistelli, Curr. Org. Chem., 2010, 14, 2442 CrossRef CAS; (f) S. Santoro, J. B. Azeredo, V. Nascimento, L. Sancineto, A. L. Braga and C. Santi, RSC Adv., 2014, 4, 31521 RSC; (g) A. Breder and S. Ortgies, Tetrahedron Lett., 2015, 56, 2843 CrossRef CAS PubMed.
- (a) T. Hori and K. B. Sharpless, J. Org. Chem., 1979, 44, 4208 CrossRef CAS; (b) B. Chabaud and K. B. Sharpless, J. Org. Chem., 1979, 44, 4204 CrossRef.
- (a) S. Torii, K. Uneyama, M. Ono and T. Bannou, J. Am. Chem. Soc., 1981, 103, 4606 CrossRef CAS; (b) T. Inokuchi, M. Kusumoto and S. Torii, J. Org. Chem., 1990, 55, 1548 CrossRef CAS; (c) M. Tiecco, L. Testaferri, M. Tingoli, D. Chianelli and D. Bartoli, J. Org. Chem., 1991, 56, 4529 CrossRef CAS; (d) S.-I. Fukuzawa, K. Takahashi, H. Kato and H. Yamazaki, J. Org. Chem., 1997, 62, 7711 CrossRef CAS; (e) T. Wirth, S. Häuptli and M. Leuenberger, Tetrahedron: Asymmetry, 1998, 9, 547 CrossRef CAS; (f) M. Tiecco, L. Testaferri, F. Marini, C. Santi, L. Bagnoli and A. Temperini, Tetrahedron: Asymmetry, 1999, 10, 747 CrossRef CAS; (g) D. Crich, S. Neelamkavil and F. Sartillo-Piscil, Org. Lett., 2000, 2, 4029 CrossRef CAS PubMed; (h) G.-J. Ten Brink, J.-M. Vis, I. W. C. E. Arends and R. A. Sheldon, J. Org. Chem., 2001, 66, 2429 CrossRef CAS PubMed.
- (a) S. R. Mellegaard and J. A. Tunge, J. Org. Chem., 2004, 69, 8979 CrossRef CAS PubMed; (b) J. A. Tunge and S. R. Mellegaard, Org. Lett., 2004, 6, 1205 CrossRef CAS PubMed; (c) H. Ichikawa, Y. Usami and M. Arimoto, Tetrahedron Lett., 2005, 46, 8665 CrossRef CAS PubMed; (d) D. M. Browne, O. Niyomura and T. Wirth, Org. Lett., 2007, 9, 3169 CrossRef CAS PubMed; (e) S. Santoro, C. Santi, M. Sabatini, L. Testaferri and M. Tiecco, Adv. Synth. Catal., 2008, 350, 2881 CrossRef CAS PubMed; (f) J. C. van der Toorn, G. Kemperman, R. A. Sheldon and I. W. Arends, J. Org. Chem., 2009, 74, 3085 CrossRef CAS PubMed; (g) F. V. Singh and T. Wirth, Org. Lett., 2011, 13, 6504 CrossRef CAS PubMed; (h) S. P. Curran and S. J. Connon, Org. Lett., 2012, 14, 1074 CrossRef CAS PubMed; (i) J. Trenner, C. Depken, T. Weber and A. Breder, Angew. Chem., Int. Ed., 2013, 52, 8952 CrossRef CAS PubMed; (j) L. Yu, H. Li, X. Zhang, J. Ye, J. Liu, Q. Xu and M. Lautens, Org. Lett., 2014, 16, 1346 CrossRef CAS PubMed; (k) L. Yu, Y.-L. Wu, H.-E. Cao, X. Zhang, X.-K. Shi, J. Luan, T. Chen, Y. Pan and Q. Xu, Green Chem., 2014, 16, 287 RSC; (l) L. Yu, J. Wang, T. Chen, Y. Wang and Q. Xu, Appl. Organomet. Chem., 2014, 28, 652 CrossRef CAS PubMed; (m) A. J. Cresswell, S. T.-C. Eey and S. E. Denmark, Nat. Chem., 2015, 7, 148 CrossRef PubMed; (n) F. Krätzschmar, M. Kaßel, D. Delony and A. Breder, Chem. – Eur. J., 2015, 21, 7030 CrossRef PubMed; (o) L. Yu, H. Li, X. Zhang, J. Ye, J. Liu, Q. Xu and M. Lautens, Adv. Synth. Catal., 2015, 357, 955 CrossRef PubMed. Recently, selenide catalysis has been paid attention as well. See: (p) D. W. Tay, I. T. Tsoi, J. C. Er, G. Y. C. Leung and Y.-Y. Yeung, Org. Lett., 2013, 15, 1310 CrossRef CAS PubMed; (q) F. Chen, C. K. Tan and Y.-Y. Yeung, J. Am. Chem. Soc., 2013, 135, 1232 CrossRef CAS PubMed.
- Z. Deng, J. Wei, L. Liao, H. Huang and X. Zhao, Org. Lett., 2015, 17, 1834 CrossRef CAS PubMed.
- In the reaction of styrenes with NFSI catalyzed by PhSeSePh, (PhSO2)2N-functionalized products were formed. See ref. 10i and 11.
- The possible reaction mechanism might involve a seleniranium ion. For the relevant proposed mechanism on diselenide catalysis, see ref. 5, 10d and 11.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qo00179j |
| This journal is © the Partner Organisations 2015 |