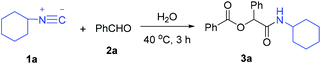Synthesis of Passerini adducts from aldehydes and isocyanides under the auxiliary of water†
Ming
Li
*,
Bin
Qiu
,
Xiang-Jing
Kong
and
Li-Rong
Wen
*
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, P. R. China. E-mail: liming928@qust.edu.cn; wenlirong@qust.edu.cn
First published on 23rd July 2015
Abstract
An efficient protocol for the synthesis of the Passerini adducts α-acyloxycarboxamides from aldehydes, isocyanides and water in a molecular ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was described. The possible mechanism for the reaction was discussed by employing cross condensation and H218O isotope labeling experiments. This method offers a straightforward access to α-acyloxycarboxamides bearing two identical functional groups in high yields under mild conditions.
3 was described. The possible mechanism for the reaction was discussed by employing cross condensation and H218O isotope labeling experiments. This method offers a straightforward access to α-acyloxycarboxamides bearing two identical functional groups in high yields under mild conditions.
Introduction
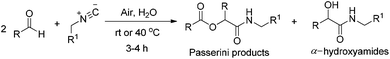
Isocyanides have long proved themselves to be irreplaceable building blocks in modern organic chemistry.1 Their application in pharmaceutical and academic research has undergone a dramatic increase over the past few years.2 Along with the Ugi reaction,3 the Passerini reaction is particularly attractive, and coupling of isocyanides with aldehydes and carboxylic acids represents the most important use of isocyanides in organic synthesis.4Water, as the sole medium for organic reactions, has advantages of being low cost, safe, and environmentally friendly that make it an ideal reaction medium in synthetic chemistry.5 The use of water could accelerate the rate of reaction and also lead to selectivity changes.6 Recently, reactions of water-insoluble organic compounds that take place in aqueous suspensions have received a great deal of attention because of their high efficiency and straightforward synthetic protocols.7 A. Vigalok8 reported a highly efficient aqueous three-component Passerini reaction (Scheme 1), where one of the components, carboxylic acid, is generated in situ via the aerobic oxidation of hydrophobic aldehydes upon stirring with water in the presence of air. However, the method often gave both the Passerini adducts, α-acyloxyamides and α-hydroxyamides, and the role of water is still unclear.
By taking note of Vigalok's report, we have two questions: (1) since the water-solubility of reactants, especially the aldehydes, could affect significantly the efficiency of the reaction, how about further decreasing the amount of water? (2) Could aldehydes be efficiently converted in situ into carboxylic acids that were successively utilized in the nucleophilic addition to the carbonyl carbon atom when the reaction takes place “in water”?
In order to answer these two questions, a series of experiments were designed and carried out by using 3 equiv. of water and simultaneously employing two different aldehydes in a reaction as well as a comparative oxidation of 4-fluorobenzaldehyde and 4-methoxybenzaldehyde. Based on our ongoing interest in IMCRs,9 herein, we present an efficient protocol to access the Passerini adducts α-acyloxycarboxamides in excellent yields from aldehydes, isocyanides and water in a molecular ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and another new possible mechanism for the reaction.
3, and another new possible mechanism for the reaction.
Results and discussion
To demonstrate the roles of water and oxygen or air, a series of experiments were designed and carried out by using the reaction of cyclohexyl isocyanide (1a) with benzaldehyde (2a) as the model reaction at 40 °C for 3 h (Table 1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Equiv. of 2a | Equiv. of H2O | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), 2a, and H2O (equiv. based on 1a). b Isolated yield based on 1a. c The reaction was performed under a N2 atmosphere. d The reaction was performed under an oxygen atmosphere. | |||
| 1 | 3 | 0 | 39 |
| 2 | 3 | 1 | 58 |
| 3 | 3 | 2 | 68 |
| 4 | 3 | 3 | 90 |
| 5 | 3 | 4 | 86 |
| 6 | 3 | 5 | 81 |
| 7 | 3 | 6 | 66 |
| 8 | 3 | 3 | 8c |
| 9 | 3 | 0 | 6c |
| 10 | 3 | 0 | 55d |
| 11 | 3 | 3 | 77d |
Excitingly, addition of water improved significantly the yield of 3a (Table 1, entries 1–7). A breakthrough result was achieved, the yield of 3a rose to 90% when 3 equiv. of water was employed (entry 4). Excessive water was unbeneficial for the reaction (entries 5–7). In addition, as a control experiment, the reactions of 1a, 2a, and H2O in a molecular ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 or without water addition were carried out under a nitrogen atmosphere for 3 h, and only less than 10% yield of 3a was generated (entries 8 and 9), which revealed that the presence of oxygen or air would be essential for the reaction. In the light of the importance of oxygen and water for the reaction, a comparative experiment was further carried out. Without water addition the reaction of 2a with 1a in a molecular ratio of 3
3 or without water addition were carried out under a nitrogen atmosphere for 3 h, and only less than 10% yield of 3a was generated (entries 8 and 9), which revealed that the presence of oxygen or air would be essential for the reaction. In the light of the importance of oxygen and water for the reaction, a comparative experiment was further carried out. Without water addition the reaction of 2a with 1a in a molecular ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 under an oxygen atmosphere provided only 55% yield of 3a (entry 10); in contrast, the reaction with 3 equiv. of H2O addition under the same reaction conditions afforded 3a in 77% yield (entry 11). These results indicated that oxidation of aldehydes is inevitable in the presence of oxygen, and water also plays an irreplaceable role.
1 under an oxygen atmosphere provided only 55% yield of 3a (entry 10); in contrast, the reaction with 3 equiv. of H2O addition under the same reaction conditions afforded 3a in 77% yield (entry 11). These results indicated that oxidation of aldehydes is inevitable in the presence of oxygen, and water also plays an irreplaceable role.
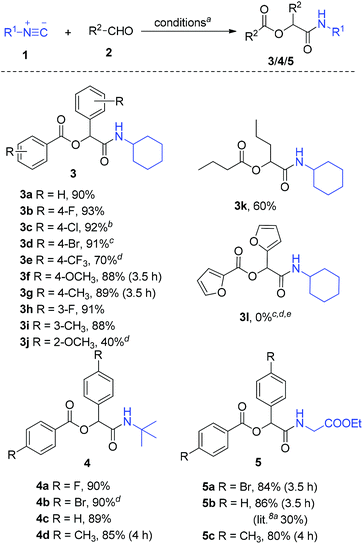
In view of limited number of the Passerini adducts reported before, we next expanded the scope of the reaction mainly using various aromatic aldehydes under the above standard conditions (1/2/H2O in a molecular ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 at 40 °C in air), and the results are shown in Scheme 2. All aromatic aldehydes were well tolerated to afford the expected products 3a–j.
3 at 40 °C in air), and the results are shown in Scheme 2. All aromatic aldehydes were well tolerated to afford the expected products 3a–j.
The aromatic aldehydes with electron-withdrawing groups (F, Cl, Br, CF3) reacted faster than those with electron-donating groups (CH3, OCH3), while the yields do not have evident differences except for 3e (Scheme 2, 3b–g). However, o-methoxybenzaldehyde gave a low yield of 40% and needed a long reaction time of 4 h (Scheme 2, 3j), which is subjected to the steric effect. Aliphatic aldehydes such as n-butylaldehyde also could participate in the reaction though it gave a relatively low yield of 60% (Scheme 2, 3k). Unfortunately, when a heteroaromatic aldehyde such as furan-2-carbaldehyde was used, no reaction occurred (Scheme 2, 3l). Next, we employed tert-butyl isocyanide (1b) and ethyl 2-isocyanoacetate (1c) instead of 1a in this IMCR, and it was found that the reactions involving 1b/1c also proceeded well to afford the corresponding products 4/5 in excellent yields. It is worth mentioning that the less reactive ethyl isocyanoacetate (1c) also gave yields of 80–86% (Scheme 2, 5a–c), while only 30% yield of 5b was obtained in the previous report.8a
The structures of 3/4/5 were confirmed by the X-ray diffraction analysis of 3g (Fig. S1 in the ESI†).
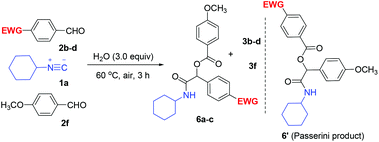
The success of the above reactions promoted us to gain deep insight into the reaction mechanism with regard to aldehyde oxidation by applying two different aromatic aldehydes: bearing an electron-withdrawing group such as 2b–d and bearing an electron-donating group such as 4-methoxybenzaldehyde (2f), to react with 1a (for details, see Table S1 in the ESI†). To our great delight, new “hetero” products 6a–c that are different from the Passerini adducts were obtained besides “homo” products 3b–d and 3f (Table 2). Obviously, according to the Passerini reaction mechanism, 6′ should be obtained due to these aromatic aldehydes bearing an electron-withdrawing group which is more susceptible to be oxidized to the corresponding carboxylic acid. A comparative oxidation experiment with 4-fluorobenzaldehyde (2b) and 4-methoxybenzaldehyde (2f) authenticated this clear-cut fact.10 As expected, a small amount of 6a′ was also observed along with 6a in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 by the 1H NMR spectrum. The above experimental results implied that two mechanisms for the present reaction may exist at the same time.
10 by the 1H NMR spectrum. The above experimental results implied that two mechanisms for the present reaction may exist at the same time.
Significantly, the previous reports have not investigated the crossing reactions with two different aldehydes.
The structures of 6a–c were confirmed by the X-ray diffraction analysis of the representative compound 6b (Fig. S2 in the ESI†).
To further investigate the role of water, an isotope labeling experiment was conducted. The reaction of 4-fluorobenzaldehyde (2b), cyclohexyl isocyanide (1a) (0.1 mmol), and H218O in a molecular ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for 2b/1a/H218O was carried out at 40 °C for 30 min. HRMS (ESI-TOF, [M + H]+) analysis exhibited that besides the ion peak of 374.1572 for 2[16O]-3b, other two ion peaks at m/z 376.1591 and 378.1650 in a ratio of 7
3 for 2b/1a/H218O was carried out at 40 °C for 30 min. HRMS (ESI-TOF, [M + H]+) analysis exhibited that besides the ion peak of 374.1572 for 2[16O]-3b, other two ion peaks at m/z 376.1591 and 378.1650 in a ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 also were observed, which should be relative to [18O]-3b and 2[18O]-3b, respectively (Fig. S3 in the ESI†). This observation is in agreement with the previous report.8a In addition, we have noticed that in A. Vigalok's report, the reaction with nonlabeled 1-octanal, 1-octanoic acid, and ethyl isocyanoacetate or pentyl isocyanide in H218O, gave the Passerini adduct with one incorporated 18O as the major product, which also reveals that the present reaction mechanism should be different from the classic Passerini reaction.
6 also were observed, which should be relative to [18O]-3b and 2[18O]-3b, respectively (Fig. S3 in the ESI†). This observation is in agreement with the previous report.8a In addition, we have noticed that in A. Vigalok's report, the reaction with nonlabeled 1-octanal, 1-octanoic acid, and ethyl isocyanoacetate or pentyl isocyanide in H218O, gave the Passerini adduct with one incorporated 18O as the major product, which also reveals that the present reaction mechanism should be different from the classic Passerini reaction.
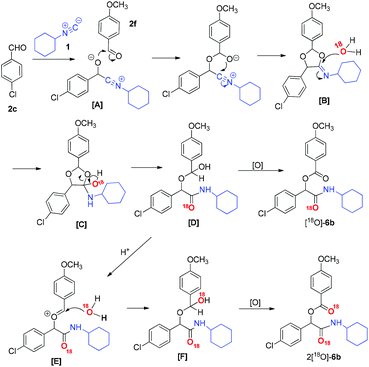
On the basis of the above experimental results, a plausible mechanism for this reaction is proposed and shown in Scheme 3. A more reactive 4-chlorobenzaldehyde (2c) first reacts with isocyanide 1a to generate an intermediate [A], which then reacts with another less reactive aldehyde such as 4-methoxybenzaldehyde (2f) leading to 1,3-dioxolamine [B].11 Next, [B] reacts with water to form [C], which undergoes a ring-opening reaction to give [D]. Successively, an oxidation process gives the final product 6b. In addition, a small amount of aldehydes are oxidized to the corresponding acid in the presence of air. Under such acidic conditions, a part of [D] would lose hydroxyl to form an intermediate [E], which reacts with another molecule of H2O giving [F], followed by an oxidation process to form the final product 6b. This mechanism could explain reasonably why in the isotope labeling experiments there are both products with one labeled 18O ([18O]-3b) and products with two 18O (2[18O]-3b) incorporated in the structures.
Conclusions
In conclusion, we have demonstrated a rapid and efficient approach to access the Passerini adducts α-acyloxycarboxamides using aldehydes, isocyanides, and water in a molecular ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 rather than needing carboxylic acids. The striking feature of the method is to use 3 equiv. of H2O in the reaction system to give good to excellent yields under mild reaction conditions. The experiments of isotope labeling of H218O and crossing reactions by using two different aldehydes clearly indicated that the reaction mechanism is different from the previous report.8a This research also provided a convenient and green method to access α-acyloxycarboxamides with two identical functional groups in high yields.
3 rather than needing carboxylic acids. The striking feature of the method is to use 3 equiv. of H2O in the reaction system to give good to excellent yields under mild reaction conditions. The experiments of isotope labeling of H218O and crossing reactions by using two different aldehydes clearly indicated that the reaction mechanism is different from the previous report.8a This research also provided a convenient and green method to access α-acyloxycarboxamides with two identical functional groups in high yields.
Experimental section
General experimental details
The benzaldehyde (2a) used for the condition test was produced by Acros Organics (99.5+%). Cyclohexyl isocyanide (1a) for the condition test was dried with anhydrous MgSO4 before use. All other isocyanides and aldehydes were used as purchased commercially without the drying process. Water used was common running water and untreated. All reagents were weighed and handled in air at room temperature. Melting points were recorded on RY-1 microscopic melting apparatus and uncorrected. NMR spectra were recorded on a Bruker Avance 500 spectrometer at 500 MHz for 1H and 125 MHz for 13C in DMSO-d6 or CDCl3. Chemical shifts δ were relative to TMS as the internal standard. The IR spectra were recorded on a Nicolet iS10 FT-IR spectrometer and only major peaks are reported in cm−1. High-resolution mass spectra (HRMS) were recorded on an Ultima Global spectrometer. The X-ray single-crystal diffraction was performed on a Saturn 724+ instrument.General procedure for the synthesis of 3/4/5
A mixture of isocyanides 1 (0.5 mmol), aldehydes 2 (1.5 mmol), and water (1.5 mmol) was stirred at 40 °C in a 25 mL round-bottomed flask for an indicated time in air until 1 was completely consumed. The solid mixture was isolated by filtration and washing with petroleum ether (3 × 5 mL), and then the pure products 3/4/5 were obtained.General procedure for the preparation of 6
A mixture of the aromatic aldehydes with electron-withdrawing groups 2b–d (1 mmol), 1a (0.5 mmol), 2f (0.5 mmol), and H2O (1.5 mmol) was stirred at 60 °C in a 25 mL round-bottomed flask for 30 min in air until 1a was completely consumed. The solid obtained by filtration was isolated by silica gel column chromatography, and the pure products 6a–c were obtained.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the desired product 3e (165.6 mg, 70%) as a white powder. Mp: 178–179 °C; IR (KBr, cm−1) 3288, 2936, 2857, 1736, 1661, 1560, 1413, 1327, 1129, 755. 1H NMR (500 MHz, CDCl3) 1.10–1.25 (m, 3H, CH), 1.33–1.37 (m, 2H, CH), 1.60–1.72 (m, 3H, CH), 1.89–1.96 (m, 2H, CH), 3.79–3.82 (m, 1H, CH), 6.01 (d, 1H, J = 7.8 Hz, NH), 6.32 (s, 1H, CH), 7.67 (s, 4H, ArH), 7.77 (d, J = 8.2 Hz, 2H, ArH), 8.21 (d, J = 8.1 Hz, 2H, ArH); 13C NMR (125 MHz, CDCl3) 25.2, 25.9, 33.4, 49.1, 122.8, 123.2, 125.0, 125.4, 126.4, 128.3, 130.8, 131.8 (2J = 32.4 Hz), 132.7, 135.8 (2J = 32.9 Hz), 139.6, 164.3, 166.7. HRMS (ESI-TOF, [M + H]+): calcd for C23H22F6NO3, 474.1504, found 474.1515.
1), the desired product 3e (165.6 mg, 70%) as a white powder. Mp: 178–179 °C; IR (KBr, cm−1) 3288, 2936, 2857, 1736, 1661, 1560, 1413, 1327, 1129, 755. 1H NMR (500 MHz, CDCl3) 1.10–1.25 (m, 3H, CH), 1.33–1.37 (m, 2H, CH), 1.60–1.72 (m, 3H, CH), 1.89–1.96 (m, 2H, CH), 3.79–3.82 (m, 1H, CH), 6.01 (d, 1H, J = 7.8 Hz, NH), 6.32 (s, 1H, CH), 7.67 (s, 4H, ArH), 7.77 (d, J = 8.2 Hz, 2H, ArH), 8.21 (d, J = 8.1 Hz, 2H, ArH); 13C NMR (125 MHz, CDCl3) 25.2, 25.9, 33.4, 49.1, 122.8, 123.2, 125.0, 125.4, 126.4, 128.3, 130.8, 131.8 (2J = 32.4 Hz), 132.7, 135.8 (2J = 32.9 Hz), 139.6, 164.3, 166.7. HRMS (ESI-TOF, [M + H]+): calcd for C23H22F6NO3, 474.1504, found 474.1515.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the desired product 3j (79.4 mg, 40%) as a white powder. Mp: 150–152 °C; IR (KBr, cm−1) 3361, 3074, 2931, 2851, 1701, 1675, 1602, 1536, 1492, 1467, 766. 1H NMR (500 MHz, CDCl3) 1.12–1.72 (m, 10H, CH), 3.84–3.86 (m, 1H, CH), 3.87 (s, 3H, CH), 3.96 (s, 3H, CH), 6.63 (s, 1H, CH), 6.82 (d, J = 8.0 Hz, 1H, NH), 6.89–7.87 (m, 8H, ArH); 13C NMR (125 MHz, CDCl3) 24.8, 25.6, 33.0, 33.4, 47.9, 55.7, 56.2, 71.2, 111.2, 112.3, 119.9, 120.7, 120.8, 124.9, 129.4, 130.0, 132.4, 133.9, 157.3, 159.0, 165.0, 168.0. HRMS (ESI-TOF, [M + H]+): calcd for C23H28NO5, 398.1967, found 398.1975.
1), the desired product 3j (79.4 mg, 40%) as a white powder. Mp: 150–152 °C; IR (KBr, cm−1) 3361, 3074, 2931, 2851, 1701, 1675, 1602, 1536, 1492, 1467, 766. 1H NMR (500 MHz, CDCl3) 1.12–1.72 (m, 10H, CH), 3.84–3.86 (m, 1H, CH), 3.87 (s, 3H, CH), 3.96 (s, 3H, CH), 6.63 (s, 1H, CH), 6.82 (d, J = 8.0 Hz, 1H, NH), 6.89–7.87 (m, 8H, ArH); 13C NMR (125 MHz, CDCl3) 24.8, 25.6, 33.0, 33.4, 47.9, 55.7, 56.2, 71.2, 111.2, 112.3, 119.9, 120.7, 120.8, 124.9, 129.4, 130.0, 132.4, 133.9, 157.3, 159.0, 165.0, 168.0. HRMS (ESI-TOF, [M + H]+): calcd for C23H28NO5, 398.1967, found 398.1975.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the desired product 4b (210.2 mg, 90%) as a white powder. Mp: 173–175 °C; IR (KBr, cm−1) 3308, 3087, 2974, 2927, 1727, 1659, 1591, 1557, 1490, 1453. 1H NMR (500 MHz, CDCl3) 1.36(s, 9H, CH), 5.86 (s, 1H, NH), 6.12 (s, 1H, CH), 7.37–7.93 (m, 8H, ArH); 13C NMR (125 MHz, CDCl3) 28.4, 28.6, 51.8, 75.6, 123.3, 128.1, 129.0, 129.1, 131.2, 132.0, 132.1, 134.7, 164.2, 166.6. HRMS (ESI-TOF, [M + H]+): calcd for C19H20Br2NO3, 467.9810, found 467.9805.
1), the desired product 4b (210.2 mg, 90%) as a white powder. Mp: 173–175 °C; IR (KBr, cm−1) 3308, 3087, 2974, 2927, 1727, 1659, 1591, 1557, 1490, 1453. 1H NMR (500 MHz, CDCl3) 1.36(s, 9H, CH), 5.86 (s, 1H, NH), 6.12 (s, 1H, CH), 7.37–7.93 (m, 8H, ArH); 13C NMR (125 MHz, CDCl3) 28.4, 28.6, 51.8, 75.6, 123.3, 128.1, 129.0, 129.1, 131.2, 132.0, 132.1, 134.7, 164.2, 166.6. HRMS (ESI-TOF, [M + H]+): calcd for C19H20Br2NO3, 467.9810, found 467.9805.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the desired product 6a (71.3 mg, 37%) as a white powder. Mp: 205–207 °C; IR (KBr, cm−1) 3276, 3088, 2934, 2853, 1709, 1653, 1608, 1413, 1452, 1266, 770. 1H NMR (500 MHz, CDCl3) 1.12–1.23 (m, 3H, CH), 1.35–1.50 (m, 2H, CH), 1.61–1.68 (m, 3H, CH), 1.89–1.95 (m, 2H, CH), 3.73–3.81 (m, 1H, CH), 3.89 (s, 3H, CH), 6.09 (d, J = 7.5 Hz, 1H, NH), 6.26 (s, 1H, CH), 6.91–7.51 (m, 6H, ArH), 8.02–8.04 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3) 24.7, 25.4, 32.9, 33.0, 48.2, 55.5, 74.8, 114.0, 114.2, 115.7 (d, 2JC–F = 21.6 Hz), 121.4, 129.3 (d, 3JC–F = 7.4 Hz), 131.8, 132.3, 161.9, 164.0, 164.5, 167.3. HRMS (ESI-TOF, [M + H]+): calcd for C22H25FNO4, 386.1768, found 386.1772.
1), the desired product 6a (71.3 mg, 37%) as a white powder. Mp: 205–207 °C; IR (KBr, cm−1) 3276, 3088, 2934, 2853, 1709, 1653, 1608, 1413, 1452, 1266, 770. 1H NMR (500 MHz, CDCl3) 1.12–1.23 (m, 3H, CH), 1.35–1.50 (m, 2H, CH), 1.61–1.68 (m, 3H, CH), 1.89–1.95 (m, 2H, CH), 3.73–3.81 (m, 1H, CH), 3.89 (s, 3H, CH), 6.09 (d, J = 7.5 Hz, 1H, NH), 6.26 (s, 1H, CH), 6.91–7.51 (m, 6H, ArH), 8.02–8.04 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3) 24.7, 25.4, 32.9, 33.0, 48.2, 55.5, 74.8, 114.0, 114.2, 115.7 (d, 2JC–F = 21.6 Hz), 121.4, 129.3 (d, 3JC–F = 7.4 Hz), 131.8, 132.3, 161.9, 164.0, 164.5, 167.3. HRMS (ESI-TOF, [M + H]+): calcd for C22H25FNO4, 386.1768, found 386.1772.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the desired product 6b (80.4 mg, 40%) as a white powder. Mp: 195–197 °C (lit. 184–186 °C); IR (KBr, cm−1) 3283, 3087, 2929, 2852, 1713, 1654, 1607, 1595, 1494, 1448, 1265, 772. 1H NMR (500 MHz, CDCl3) 1.14–1.21 (m, 3H, CH), 1.36–1.39 (m, 2H, CH), 1.58–1.74 (m, 3H, CH), 1.87–1.97 (m, 2H, CH), 3.80–3.84 (m, 1H, CH), 3.89 (s, 3H, CH), 6.07 (d, J = 7.5 Hz, 1H, NH), 6.26 (s, 1H, CH), 7.36–7.47 (m, 6H, ArH), 8.02–8.05 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3) 24.7, 25.4, 32.9, 33.0, 48.2, 55.5, 74.8, 114.0, 121.4, 128.7, 128.9, 131.8, 134.5, 134.8, 164.0, 164.4, 167.1. HRMS (ESI-TOF, [M + H]+): calcd for C22H25ClNO4, 402.1472, found 402.1481.
1), the desired product 6b (80.4 mg, 40%) as a white powder. Mp: 195–197 °C (lit. 184–186 °C); IR (KBr, cm−1) 3283, 3087, 2929, 2852, 1713, 1654, 1607, 1595, 1494, 1448, 1265, 772. 1H NMR (500 MHz, CDCl3) 1.14–1.21 (m, 3H, CH), 1.36–1.39 (m, 2H, CH), 1.58–1.74 (m, 3H, CH), 1.87–1.97 (m, 2H, CH), 3.80–3.84 (m, 1H, CH), 3.89 (s, 3H, CH), 6.07 (d, J = 7.5 Hz, 1H, NH), 6.26 (s, 1H, CH), 7.36–7.47 (m, 6H, ArH), 8.02–8.05 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3) 24.7, 25.4, 32.9, 33.0, 48.2, 55.5, 74.8, 114.0, 121.4, 128.7, 128.9, 131.8, 134.5, 134.8, 164.0, 164.4, 167.1. HRMS (ESI-TOF, [M + H]+): calcd for C22H25ClNO4, 402.1472, found 402.1481.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the desired product 6c (78.1 mg, 35%) as a white powder. Mp: 185–186 °C; IR (KBr, cm−1) 3288, 3082, 2934, 2854, 1715, 1655, 1607, 1580, 1511, 1452, 1262, 770. 1H NMR (500 MHz, CDCl3) 1.10–1.26 (m, 3H, CH), 1.32–1.40 (m, 2H, CH), 1.59–1.68 (m, 3H, CH), 1.89–1.94 (m, 2H, CH), 3.78–3.84 (m, 1H, CH), 3.89 (s, 3H, CH), 6.06 (d, J = 7.5 Hz, 1H, NH), 6.23 (s, H, CH), 6.90–7.61 (m, 6H, ArH), 8.02–8.04 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3) 24.7, 25.5, 33.0, 33.7, 48.3, 55.6, 75.0, 114.1, 121.4, 123.0, 129.0, 131.0, 131.3, 131.9, 135.1, 164.1, 164.4, 167.0. HRMS (ESI-TOF, [M + H]+): calcd for C22H25BrNO4, 446.0967, found 446.0973.
1), the desired product 6c (78.1 mg, 35%) as a white powder. Mp: 185–186 °C; IR (KBr, cm−1) 3288, 3082, 2934, 2854, 1715, 1655, 1607, 1580, 1511, 1452, 1262, 770. 1H NMR (500 MHz, CDCl3) 1.10–1.26 (m, 3H, CH), 1.32–1.40 (m, 2H, CH), 1.59–1.68 (m, 3H, CH), 1.89–1.94 (m, 2H, CH), 3.78–3.84 (m, 1H, CH), 3.89 (s, 3H, CH), 6.06 (d, J = 7.5 Hz, 1H, NH), 6.23 (s, H, CH), 6.90–7.61 (m, 6H, ArH), 8.02–8.04 (m, 2H, ArH); 13C NMR (125 MHz, CDCl3) 24.7, 25.5, 33.0, 33.7, 48.3, 55.6, 75.0, 114.1, 121.4, 123.0, 129.0, 131.0, 131.3, 131.9, 135.1, 164.1, 164.4, 167.0. HRMS (ESI-TOF, [M + H]+): calcd for C22H25BrNO4, 446.0967, found 446.0973.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21372137 and 21072110) and the Natural Science Foundation of Shandong Province (ZR2012BM003 and ZR2014BM006).References
- (a) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef PubMed; (b) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef; (c) R. M. Wilson, J. L. Stockdill, X.-G. Wu, X.-C. Li, P. A. Vadola, P. K. Park, P. Wang and S. J. Danishefsky, Angew. Chem., Int. Ed., 2012, 51, 2834 CrossRef CAS PubMed; (d) S. Chakrabarty, S. Choudhary, A. Doshi, F.-Q. Liu, R. Mohan, M. P. Ravindra, D. Shah, X. Yang and F. F. Flemingb, Adv. Synth. Catal., 2014, 356, 2135 CrossRef CAS PubMed; (e) T. Vlaar, E. Ruijter, B. U. W. Maes and R. V. A. Orru, Angew. Chem., Int. Ed., 2013, 52, 7084 CrossRef CAS PubMed; (f) A. V. Lygin and A. Meijere, Angew. Chem., Int. Ed., 2010, 49, 9094 CrossRef CAS PubMed; (g) S. Lang, Chem. Soc. Rev., 2013, 42, 4867 RSC; (h) G. Y. S. Qiu, Q.-P. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC; (i) T. Kaicharla, S. R. Yetra, T. Roy and A. T. Biju, Green Chem., 2013, 15, 1608 RSC.
- (a) V. Nair, R. S. Menon, A. R. Sreekanth, N. Abhilash and A. T. Biju, Acc. Chem. Res., 2006, 39, 520 CrossRef CAS PubMed; (b) T. Ngouansavanh and J.-P. Zhu, Angew. Chem., Int. Ed., 2006, 45, 3495 CrossRef CAS PubMed; (c) Y. Odabachian, S. Tong, Q. Wang, M.-X. Wang and J.-P. Zhu, Angew. Chem., Int. Ed., 2013, 52, 10878 CrossRef CAS PubMed; (d) J. Brioche, G. Masson and J. P. Zhu, Org. Lett., 2010, 12, 1432 CrossRef CAS PubMed; (e) B. O. Okandeji and J. K. Sello, J. Org. Chem., 2009, 74, 5067 CrossRef CAS PubMed; (f) X.-C. Li and S. J. Danishefsky, J. Am. Chem. Soc., 2008, 130, 5446 CrossRef CAS PubMed; (g) B.-F. Liu, Y.-B. Li, M.-Z. Yin, W.-Q. Wu and H.-F. Jiang, Chem. Commun., 2012, 48, 11446 RSC; (h) G. Y. S. Qiu, G. Liu, S.-Z. Pu and J. Wu, Chem. Commun., 2012, 48, 2903 RSC; (i) J. Li, Y.-J. Liu, C.-J. Li and X.-S. Jia, Chem. – Eur. J., 2011, 17, 7409 CrossRef CAS PubMed; (j) J. Li, Y.-J. Liu, C.-J. Li, H.-H. Jie and X.-S. Jia, Green Chem., 2012, 14, 1314 RSC; (k) X.-X. Xu, L.-J. Zhang, X.-Q. Liu, L. Pan and Q. Liu, Angew. Chem., Int. Ed., 2013, 52, 9271 CrossRef CAS PubMed; (l) J.-Q. Liu, Z.-X. Fang, Q. Zhang, Q. Liu and X.-H. Bi, Angew. Chem., Int. Ed., 2013, 52, 6953 CrossRef CAS PubMed; (m) X. Wang, S.-Y. Wang and S.-J. Ji, Org. Lett., 2013, 15, 4246 CrossRef CAS PubMed; (n) T.-H. Zhu, S.-Y. Wang, Y.-Q. Tao, T.-Q. Wei and S.-J. Ji, Org. Lett., 2014, 16, 1260 CrossRef CAS PubMed; (o) G.-H. Ma, B. Jiang, X.-J. Tu, Y. Ning, S.-J. Tu and G.-G. Li, Org. Lett., 2014, 16, 4504 CrossRef CAS PubMed; (p) C.-H. Lei, L. Zhao, D.-X. Wang, J.-P. Zhu and M.-X. Wang, Org. Chem. Front., 2014, 1, 909 RSC; (q) C.-H. Lei, D.-X. Wang, L. Zhao, J. Zhu and M.-X. Wang, J. Am. Chem. Soc., 2013, 135, 4708 CrossRef CAS PubMed; (r) S. Tong, D.-X. Wang, L. Zhao, J. Zhu and M.-X. Wang, Angew. Chem., Int. Ed., 2012, 51, 4417 CrossRef CAS PubMed; (s) L.-Y. Lyu, H. Xie, H.-X. Mu, Q.-J. He, Z. Bian and J. Wang, Org. Chem. Front., 2015, 2, 815 RSC.
- (a) I. Ugi, R. Meyr, U. Fetzer and C. Steinbrückner, Angew. Chem., 1959, 71, 386 Search PubMed; (b) I. Ugi and C. Steinbrückner, Angew. Chem., Int. Ed. Engl., 1960, 72, 267 CrossRef CAS PubMed.
- (a) M. Passerini, Gazz. Chim. Ital., 1921, 51, 126 CAS; (b) M. Passerini, Gazz. Chim. Ital., 1922, 52, 432 CAS; (c) L. Banfi and R. Riva, in Organic Reactions, ed. A. B. Charette, Wiley, New York, 2005, vol. 65, pp. 1–140 Search PubMed.
- (a) S. Minakata and M. Komatsu, Chem. Rev., 2009, 109, 711 CrossRef CAS PubMed; (b) A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 CrossRef CAS PubMed; (c) F.-Q. Shi, X. Li, Y.-Z. Xia, L.-M. Zhang and Z.-X. Yu, J. Am. Chem. Soc., 2007, 129, 15503 CrossRef CAS PubMed; (d) L.-P. Yang, D.-X. Shi, S. Chen, H.-X. Chai, D.-F. Huang, Q. Zhang and J.-R. Li, Green Chem., 2012, 14, 945 RSC; (e) M. Sengoden and T. Punniyamurthy, Angew. Chem., Int. Ed., 2013, 2, 600 CrossRef PubMed.
- (a) R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302 CrossRef CAS PubMed; (b) M. B. Gawande, V. D. B. Bonifácio, R. Luque, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522 RSC; (c) O. H. Kwon and O. F. Mohammed, Phys. Chem. Chem. Phys., 2012, 14, 8974 RSC; (d) H.-Y. Yuan, Y.-Y. Zheng, Z.-X. Fang, X.-H. Bi and J.-P. Zhang, Green Chem., 2014, 16, 2653 RSC.
- (a) S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275 CrossRef CAS PubMed; (b) B. K. Price and J. M. Tour, J. Am. Chem. Soc., 2006, 128, 12899 CrossRef CAS PubMed; (c) M. C. Pirrung and K. D. Sarma, J. Am. Chem. Soc., 2004, 126, 444 CrossRef CAS PubMed; (d) A. K. Chakraborti, S. Rudrawar, K. B. Jadhav, G. Kaur and S. V. Chankeshwara, Green Chem., 2007, 9, 1335 RSC; (e) M. Rogozińska, A. Adamkiewicz and J. Mlynarski, Green Chem., 2011, 13, 1155 RSC; (f) T. Sela and A. Vigalok, Org. Lett., 2014, 16, 1964 CrossRef CAS PubMed.
- (a) N. Shapiro and A. Vigalok, Angew. Chem., Int. Ed., 2008, 120, 2891 CrossRef PubMed; (b) T. Sela and A. Vigalok, Adv. Synth. Catal., 2012, 354, 2407 CrossRef CAS PubMed.
- (a) M. Li, X.-L. Lv, L.-R. Wen and Z.-Q. Hu, Org. Lett., 2013, 15, 1262 CrossRef CAS PubMed; (b) L.-R. Wen, M.-C. Lan, W.-K. Yuan and M. Li, Org. Biomol. Chem., 2014, 12, 4628 RSC; (c) M. Li, W. Kong, L.-R. Wen and F.-H. Liu, Tetrahedron, 2012, 68, 4838 CrossRef CAS PubMed; (d) M. Li, Z.-X. Qiu, L.-R. Wen and Z.-M. Zhou, Tetrahedron, 2011, 67, 3638 CrossRef CAS PubMed.
- 4-Fluorobenzaldehyde (2b) (1.5 mmol, 0.186 g) and 4-methoxybenzaldehyde (2f) (1.5 mmol, 0.204 g) were separately added to two Schlenk tubes, which were evacuated and recharged with pure O2 three times, then stirred at 60 °C for 1 h. Then the mixtures were dissolved with EtOAc. Evaporation of the solvent followed by purification on silica gel (petroleum ether/ethyl acetate = 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) provided the unconverted aldehydes. The conversion ratio of 2b was 85% and 2f was 15%.
1, v/v) provided the unconverted aldehydes. The conversion ratio of 2b was 85% and 2f was 15%. - M. Bayat and S. Nasri, Helv. Chim. Acta, 2011, 94, 1657 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: X-Ray crystal structures of 3g and 6b, 1H NMR, 13C NMR spectra of new compounds, and HRMS spectra of isotope labeled 3b. CCDC 891438 and 900082. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00202h |
| This journal is © the Partner Organisations 2015 |