Open Access Article
Open Access ArticlePlatinum-based nanoalloys for the oxygen reduction reaction: exposing the true active phase via in situ/operando techniques
Carlos A.
Campos-Roldán
 *,
Raphaël
Chattot
*,
Raphaël
Chattot
 ,
Pierre-Yves
Blanchard
,
Pierre-Yves
Blanchard
 ,
Deborah J.
Jones
and
Sara
Cavaliere
,
Deborah J.
Jones
and
Sara
Cavaliere
 *
*
ICGM, Univ. Montpellier, CNRS, ENSCM, 34095 Montpellier, cedex 5, France. E-mail: carlos-augusto.campos-roldan@umontpellier.fr; sara.cavaliere@umontpellier.fr
First published on 4th March 2025
Abstract
Platinum-based nanoalloys are efficient electrocatalysts for the oxygen reduction reaction (ORR). In situ/operando measurements have revealed that key properties including induced strain, chemical composition, coordination environment, evolve significantly during operation, which can hampertheir effective implementation in fuel cells. In fact, recent studies indicate that the impact of the early surface activation steps of Pt-based nanoalloys has been hitherto underestimated and is an important factor contributing to loss of their initial electroactivity. In this short perspective, we highlight the importance of in situ/operando characterization of Pt-based electrocatalysts during the initial operation steps in the ORR and discuss recent insights into their early degradation and evolution of their key properties during electrochemical characterization.
Introduction
The current interest in fuel cells (FCs) and electrolyzers stems from the versatility of hydrogen in sector coupling and its contribution to the transition to CO2 emission neutrality.1 Nevertheless, technical bottlenecks still remain,2,3 in particular the catalyst layer at the cathode of proton exchange membrane fuel cells (PEMFC),4 which requires a high Pt loading to drive the sluggish oxygen reduction reaction (ORR). The rational design of highly active and long-term stable Pt-based electrocatalysts for the ORR is, therefore, a research field of paramount importance, to reach industry targets of 0.1 gPGM kW−1 (platinum-group metal, PGM) for automotive and 0.3 gPGM kW−1 for heavy-duty transport applications.5 Within this context, exhaustive research has resulted in strong increase of the ORR activity, as determined using the thin-film rotating disk electrode (RDE) technique, by alloying Pt with late transition metals (usually Ni, Co, Fe, Cu, etc.), which also decreases simultaneously the amount of Pt.4 The RDE technique characterizes a half-cell reaction of a fuel cell in an idealized environment, and allows determination of the intrinsic ORR activity via the kinetic current evaluated at a given electrode potential (usually at 0.9 VRHE). However, strong discrepancy is frequently observed between the activity observed in the RDE and that in a PEMFC.6 Bridging the gap between these ex situ and in situ electrochemical results is crucial to advancing understanding of novel Pt-based nanostructures as PEMFC electrocatalysts. Along with the control of electrode structure,7 this calls for characterization of how key properties of the electrocatalysts evolve e.g., in terms of atomic-scale structure and surface chemistry under operation conditions to understand any loss in electrocatalytic activity and stability. In this context, in situ/operando characterization techniques play a crucial role in understanding the relationship between structure, activity, and stability in electrochemical environments. These methods not only allow the identification of the active phase in the system, often differing from that observed in ex situ studies, but also enable real-time tracking of structural changes during electrocatalyst operation.The development of in situ and/or operando techniques, such as X-ray diffraction/scattering (XRD),8 X-ray absorption spectroscopy (XAS),9,10 online inductively coupled plasma – mass spectrometry (ICP-MS),11 liquid phase electron microscopy,12,13etc. has allowed better understanding of electrocatalyst behavior in real conditions or electrochemical environments simulating those of the application device. The fundamentals advances of each technique are reviewed in the corresponding cited papers.
Quasi in situ techniques, such as identical-location transmission-electron microscopy (IL-TEM) coupled with advanced detectors and automatization approaches are also powerful tools to investigate structure–activity–stability relationships in Pt-based ORR catalysts.14,15 Insights from use of these in situ/operando techniques have revealed inevitable catalyst surface reconstruction in the particular environment of the PEMFC cathode on operation,9,16 and shown that the largely accepted fundamental reactivity descriptors (e.g., ligand, strain, and ensemble effects) of Pt-based electrocatalysts tailored and measured ex situ are not fully conserved in situ.17 These structural and activity changes can be so significant, that the as-synthesized materials have been referred to as “pre-catalysts” under non-reaction conditions, while the restructured components in actual reaction conditions are the “catalysts”.18,19 With the impressive development of modern in situ/operando techniques, few contributions (mainly recent, apart from that in 201320) have considered the role of the electrochemical activation steps on the catalyst properties. Within this context, it is of paramount importance to encourage the community to consider this emerging topic to improve understanding of mechanisms of reaction and degradation in electrocatalysis.
In this short perspective, we highlight the systematic need for in situ/operando characterization of Pt-based nanoalloys to unravel their structural self-reconstruction bridging precatalysts with true catalysts. We also discuss recent results and provide insights to the early degradation of Pt-based nanoalloys prior to ORR, which is critical for unambiguous evaluation of the intrinsic properties of the electrocatalysts.
In situ/operando characterization techniques
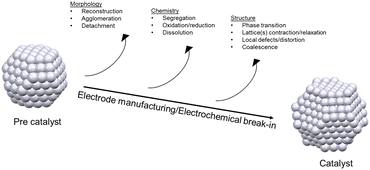
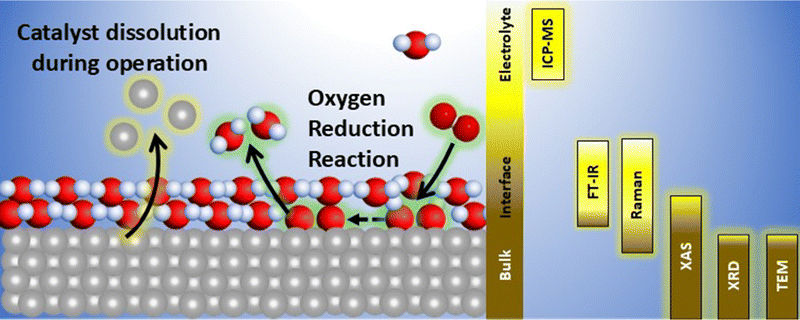
Fundamental understanding of the property–activity–stability relationships in electrocatalysis is crucial to the tailoring of advanced nanostructured materials for the electrochemical reactions of energy conversion devices, such as fuel cells and electrolyzers. Ex situ characterization of the electrocatalyst state before and after a specific reaction (e.g., ORR) is the most common method to deduce possible active sites or activity/stability descriptors. Nevertheless, stepwise reaction kinetics, structural/compositional transitions through surface reconstruction, the presence of short-lived reaction intermediates, etc., are clearly undetectable by ex situ techniques. Fig. 1 sketches the main properties of Pt-based nanostructures susceptible to evolve during the electrode manufacturing and/or the electrochemical operating steps, which are not captured by ex situ characterization techniques performed at the catalyst synthesis stage.In situ/operando characterization techniques offer the possibility of probing electrocatalyst–reactant interactions over multiple length scales (depending on the technique used and its inherent limitations, see Fig. 2), but most importantly, over all different steps of catalysts life. For instance, electrocatalyst crystalline structure (atomic long-range ordering), and the local electronic structure and coordination environment of the probe element may be elucidated by in situ/operando XRD and XAS, respectively. Synchrotron radiation is therefore needed for high flux and tunable energy and brilliance of the X-rays produced.21 Furthermore, their highly penetrating properties allow the investigation of the electrocatalysts in liquid–electrolyte and solid–electrolyte fuel cells under operation.22,23
Besides, the evolution of the electrocatalyst morphology can be observed (under certain limitations)12 by in situ liquid cell transmission electron microscopy (TEM).12,13 Complementarily, in situ/operando vibrational spectroscopic techniques (e.g., Fourier-transform infrared spectroscopy-FT-IR-, and Raman spectroscopy) have been applied in electrocatalysis research to track the formation/consumption of adsorbed intermediates at the electrode–electrolyte interface.24 Furthermore, electrocatalyst degradation can be probed by online detection of the species dissolved into the electrolyte by mass spectrometry (online ICP-MS).25 The latter was mostly restricted to liquid electrolyte environment but has been recently adapted to investigate electrocatalyst in gas diffusion electrode half-cell, possibly with polymeric electrolyte.26
Within this context and as it is shown in Fig. 1, in situ/operando techniques have clearly revealed that electrocatalyst properties undergo dynamic evolution during electrochemical operating conditions. Namely, the morphology (surface reconstruction and possible particle agglomeration/detachment), chemical composition (alloy segregation and dissolution) and structure (crystalline phase transitions, lattice contraction/relaxation, changes in local coordination) differ within the operating environment relative to the precatalysts. This evolution/degradation mechanism, however, is rather complex and it is dominated by several factors. Therefore, in-depth understanding of the corresponding property–activity–stability relationships through use of only a single in situ/operando approach is difficult,27 since each technique is specific to a length scale (specific probing mechanism/region). For instance, while high-energy X-ray and electron-based techniques can probe the bulk structure of the electrode, low-energy vibrational spectroscopies can provide insights regarding the electrode/electrolyte interface, and analysis of the electrolyte gives important information concerning catalyst dissolution, see Fig. 2. Moreover, each technique presents its own specific limitations, and thus provides information from within its specific scope (temporal/spatial resolution, detection limit, detection interferences, etc.). Combining information extracted from more than one in situ/operando technique and complementary approaches (e.g., ex situ characterizations, DFT calculations) can provide a reliable picture of property–activity–stability relationships. Indeed, since the ORR could be described by Sabatier's principle (activity volcano plots), combining in situ/operando measurements with high throughput machine learning-assisted theoretical calculations might provide an intuitive guide to catalyst design.
The electrochemical testing protocol of an ORR electrocatalyst usually starts with surface conditioning, i.e., electrochemical activation (ECA), to remove any surface contaminants and reach a defined initial surface state. The ECA significantly contributes to self-reconstruction of the electrocatalyst surface, with not insignificant impact on activity and stability of Pt-based nanoalloys. However, this process is often under-investigated and electrocatalytic performance attributed to the precatalyst characteristics. Although the properties determined in situ describe the beginning of life state of the electrocatalyst, detailed study of the evolution of properties of Pt-based nanoalloys during the ECA is rare. Hereafter we will discuss recent progress in this field, focusing on how Pt-based nanoalloys evolve in terms of their composition and structure in the earliest steps when used as ORR catalysts, which is of paramount importance when rationalizing the BoL state of ORR electrocatalysts in property–activity–stability relationships.
Pt-based nanoalloy properties during early operation steps
The use of Pt-based alloys at the nanoscale has been adopted as a reliable strategy for practical application in fuel cells since they can offer higher electrocatalytic performance than pure Pt. Ligand, strain, and ensemble effects are recognized as fundamental reactivity descriptors by which the electrocatalytic activity can be increased and activation overpotential can be reduced.28 However, such electrocatalysts still have long-term stability challenges that must be overcome.29 Therefore, using in situ/operando techniques for understanding how these nanostructures degrade is highly desirable. Although several in situ/operando studies can be found in the literature, systematic and detailed monitoring of the degradation of Pt-based electrocatalysts that occurs during electrode preparation and/or during the first electrochemical operation steps is seldom studied.The first study on this topic was reported by Tuaev et al.,20 who investigated the chemical and atomic-scale structural evolution of two different Pt–Ni nanostructured catalysts under electrochemical potential cycling using in situ anomalous small-angle X-ray scattering (ASAXS). During such activation a selective surface Ni dissolution occurs, followed by a spontaneous electrochemically induced transition from disordered alloy phases (solid solutions) to ordered Au3Cu-type alloy structures. While core@shell structures were not formed using the Ni-richer nanoparticles (PtNi6), disordered PtNi3 nanoparticles formed Pt-rich core@shell structures due to the faster Ni dissolution, where compressive strain was related to the high ORR activity.
By using in situ X-ray diffraction, Ronovský et al. have recently reported that hot-pressing the membrane-electrode assembly (MEA) during preparation induces compositional changes of PtCo/C and PtNi/C electrocatalysts, revealing that dissolution is primarily driven by temperature.30
In another study, Gatalo et al.31 have explored the effect of the applied ECA protocol on metal dissolution of a commercial Pt–Co/C and a synthesized Pt–Cu/C nanocatalyst using electrochemical online ICP-MS. The authors employed two different ECA protocols: one consisting of potential cycling (200 cycles between 0.05 and 1.2 VRHE at 300 mV s−1), and a second one consisting of a potential hold (0.6 VRHE for 30 min). For Pt–Co/C and Pt–Cu/C nanocatalysts, both ECA protocols induced significant dissolution of Co and Cu, respectively. In the case of Pt–Cu/C, nevertheless, the potential hold protocol leads to about an order of magnitude more Cu dissolution relative to the potential cycling protocol.31 Such Cu dissolution caused the ORR activity to diminish, which demonstrates the influence of the ECA protocol on the ORR electrocatalytic activity. Alekseenko et al.32 observed dependence of ORR specific and mass activity on the upper potential limit (UPL) used in the ECA protocol by cyclic voltammetry: electrocatalysts activated by cycling in the potential range of 0.04–1.00 VRHE showed 1.5–2 times higher ORR specific and mass activity compared to similar samples that were activated in the potential range from 0.04–1.20 VRHE.32 This dependence was attributed to the loss of compressive strain/ligand effect due to the Cu loss. Similar results were observed by Danisman et al.,33,34 who have recently reported significant activity changes of PtNiMo/C depending on the initial ECA protocol.
In recent work, we have investigated the evolution of Pt–Nd/C nanoalloys during ECA by means of combined operando wide angle synchrotron X-ray scattering (WAXS)35 and electrochemical online ICP-MS,36cf.Fig. 3. Ex situ characterization of the as-prepared Pt–Nd/C electrocatalyst revealed that the crystal structure corresponded to the hexagonal Pt5Nd phase, with an induced compressive strain of approximately −3% relative to the Pt reference. Further, the electrocatalyst presented a core@shell structure, which comprises the Pt5Nd alloy surrounded by a thin smooth Pt overlayer. Notwithstanding, during the ECA by cycling voltammetry (Fig. 3a), the first cathodic scan from the open circuit potential (OCP) to 0.05 VRHE induced a structural modification, as observed by operando WAXS measurements. The refined microstructural parameters derived from the operando WAXS patterns (Fig. 3b) indicated a weight fraction decrease of the hexagonal Pt5Nd phase from ca. 100 wt% to 80 wt%. Simultaneously, the presence of a Pt fcc component increased to 20 wt%. Both values remained almost constant on further cycling.35 The specific dissolution profiles (Fig. 3c) indicate that the first potential transition from the OCP to 0.05 VRHE induced metal dissolution, a sharp dissolution peak for Pt and Nd being observed, with the signal being stable afterwards. Interestingly, the specific dissolution profiles also revealed that Pt dissolution started slightly before that of Nd dissolution. Combining the extracted information from the operando WAXS and online ICP-MS experiments (see Fig. 3d), we proposed that the first potential excursion from the OCP to 0.05 VRHE triggers the partial or total dissolution of the thin Pt shell produced ex situ by the electrochemical reduction of the already formed oxides. This Pt dissolution exposes the Nd atoms to the electrolyte, which are quickly oxidized into Nd3+ that dissolve once in contact with the acidic electrolyte, thereby giving a strong thermodynamic driving force for Nd segregation from the bulk of the alloy towards the surface. This process can continue until Pt is sufficiently available to protect the Nd atoms, inducing surface reconstruction and the expected thickening of a protective Pt-rich shell, which stabilizes the surface.36 However, the structural properties determined ex situ are, eventually, modified by this surface reconstruction during the conditioning step, i.e., the ECA. Indeed, in situ XAS measurements indicated that the average local Pt–Pt interatomic distances (RPt–Pt) increased after the ECA, starting from 2.66 Å (ex situ measurement before ECA) to 2.67 Å (in situ measurement after the ECA), namely, the ex situ induced compressive strain is relaxed due to the surface reconstruction after the ECA, from −3.7% to −2.9%. These results clearly suggest that an overestimated ex situ strain magnitude might not properly rationalize the measured ORR electrochemical activity since the induced strain evolves during the activation reaction. Similar trends have been observed during the electrochemical activation of carbon-supported Pt–Y nanoalloys.37
that dissolve once in contact with the acidic electrolyte, thereby giving a strong thermodynamic driving force for Nd segregation from the bulk of the alloy towards the surface. This process can continue until Pt is sufficiently available to protect the Nd atoms, inducing surface reconstruction and the expected thickening of a protective Pt-rich shell, which stabilizes the surface.36 However, the structural properties determined ex situ are, eventually, modified by this surface reconstruction during the conditioning step, i.e., the ECA. Indeed, in situ XAS measurements indicated that the average local Pt–Pt interatomic distances (RPt–Pt) increased after the ECA, starting from 2.66 Å (ex situ measurement before ECA) to 2.67 Å (in situ measurement after the ECA), namely, the ex situ induced compressive strain is relaxed due to the surface reconstruction after the ECA, from −3.7% to −2.9%. These results clearly suggest that an overestimated ex situ strain magnitude might not properly rationalize the measured ORR electrochemical activity since the induced strain evolves during the activation reaction. Similar trends have been observed during the electrochemical activation of carbon-supported Pt–Y nanoalloys.37
 | ||
| Fig. 3 Evolution of electrochemical and structural properties during electrochemical activation. (a) Electrochemical activation via cyclic voltammetry; (b) metallic phase weight fraction evolution, derived from refined operando WAXS patterns, during electrochemical activation; (c) specific dissolution profiles recorded via online ICP-MS during electrochemical activation of Pt–Nd/C. (d) Schematic representation of the evolution of Pt–Nd/C nanoparticles during electrochemical activation. Reproduced with permission from ref. 35 and 36 Copyright 2023, American Chemistry Society. | ||
In another contribution, using in situ IR spectroscopy and the RDE technique, Danisman et al.34 have explored the number of cycles and the optimal scan rate required to achieve the first constant steady state ORR activity of trimetallic PtNiMo/C catalysts during the ECA. The authors observed that while the conventionally used fast cyclic voltammetry scans of 500 mV s−1 results in a lower activity (0.82 mA cm−2 at 0.9 VRHE), a slower scan rate of 20 mV s−1 results in significantly higher initial activity (1.25 mA cm−2 at 0.9 VRHE), demonstrating the important impact of the preconditioning conditions on Pt-based alloy catalysts for the ORR.
Within this context, the effect of the upper potential limit (UPL) during the ECA on the structure, chemical composition, and ORR performance of faceted Pt–Ni/C and sponge-like Pt–Ni/C nanocatalysts was studied using operando WAXS, electrochemical online ICP-MS and the rotating disk electrode (RDE) techniques.23 To this end, the different Pt–Ni nanocatalysts were sequentially subjected to two electrochemical steps each one consisting in a ECA, cyclic voltammetry at 20 mV s−1, and a ORR measurement. Thus, two different protocols were compared: (i) for protocol 1, the ECA was performed using 50 potential cycles at 100 mV s−1 in O2-free 0.1 M HClO4, with lower potential limit (LPL) and UPL respectively of 0.05 VRHE and 1.0 VRHE. After that, three cyclic voltammograms were acquired between 0.05–1.0 VRHE at 20 mV s−1. Finally, three cyclic voltammograms were acquired in the quasi-stationary state between 0.05–1.0 VRHE at 5 mV s−1. (ii) Protocol 2 is similar to protocol 1 with the difference of the UPL during the ECA, namely, the ECA was performed using 50 potential cycles at 500 mV s−1 in O2-free 0.1 M HClO4 being the LPL and UPL, respectively, 0.05 VRHE and 1.23 VRHE. Fig. 4 shows the acquired cyclic voltammograms, the potential-resolved lattice strain dynamics and specific metal dissolution profiles of the Pt–Ni catalysts, and those of the Pt/C benchmark. Looking at the CVs of the three materials under comparison, cf.Fig. 4(a)–(c), although the signals using protocol 1 and protocol 2 are reproducible, their shapes are slightly different by changing the UPL during the ECA. Namely, a potential downshift of ca. 30 mV on the onset of surface oxide formation is observed, which is associated with a pronounced tailing in their subsequent electrochemical reduction to a potential as low as 0.4 VRHE. This feature suggests that an ECA with higher UPL leads to a more oxophilic surface in average. Interestingly, the variations of the lattice constant or electrochemical strain dynamics (derived from the operando WAXS patterns, Fig. 4(d)–(f)) during the cyclic voltammograms are strongly modified by the UPL during the ECA: using protocol 1 (UPL = 1 VRHE) the average lattice strain presents higher degree of compression than during the protocol 2 (UPL = 1.23 VRHE), which suggests a greater loss of the alloying effect benefits regarding weakened adsorption of hydrogen and oxide species. Meanwhile, the potential-resolved metal dissolution profiles, Fig. 4(g)–(l), clearly show that the electrochemical dissolution trends are affected by the UPL used during the ECA, since high metal leaching in the faceted PtNi/C using the protocol 1 is observed, which is decreased by increasing the UPL (protocol 2). Even though the trends of the sponge PtNi/C are qualitatively similar to the faceted PtNi/C, the magnitude of the metal dissolution is quantitatively lower for the former, clearly indicating that the early degradation trends depend on the catalyst structure. In fact, by using an UPL = 1.0 VRHE, activity enhancement factors (relative to the Pt/C benchmark) of 14–16 (faceted PtNi/C) and 10–11 (sponge PtNi/C) were observed; meanwhile by using an UPL = 1.23 VRHE such activity enhancement factors decreased to 4–6 (faceted PtNi/C) and 8–9 (sponge PtNi/C). In conclusion, regardless of the catalyst structure, the electrochemical stabilization is accompanied by significant structural and chemical transformations for the PtNi/C nanomaterials, which eventually govern the ORR activity–stability performance.
 | ||
| Fig. 4 (a)–(c) Cyclic voltammograms recorded at 20 mV s−1 using the RDE technique; (d)–(f) lattice strain dynamics measured using operando WAXS; specific dissolution profiles for (g)–(i) Pt and (j)–(l) Ni measured using online ICP-MS after protocol 1 (cyan, dark cyan, and blue traces) and protocol 1 + 2 (green, orange, and red traces) for Pt/C, faceted PtNi/C, and sponges PtNi/C catalysts. Reproduced with permission from ref. 23 Copyright 2022, American Chemistry Society. | ||
Beyond in situ/operando measurements mimicking RDE conditions, the effect of a fast conditioning protocol by using operando WAXS in a single cell PEMFC (5 cm2 MEA with a Nafion 115 membrane, targeting 0.4 mgPt cm−2 at the cathode, operating temperature of 80 °C and 100% relative humidity, anode and cathode sides fed with 104 sccm H2 and 250 sccm O2, respectively, with a 0.5 bar back pressure) was investigated using the above-discussed faceted Pt–Ni/C and sponge-like Pt–Ni/C nanocatalysts.23 The conditioning protocol comprised five repetitions of a potential cycle of 2 min at open circuit voltage (OCV), followed by 3 min at a cell voltage of 0.85 V and 10 min at 0.65 V. The lattice expansion was measured by operando WAXS, relative to that of their initial structure measured by ex situ WAXS, after different steps of surface conditioning in a liquid cell (mimicking the RDE conditions) or in a PEMFC cell for both faceted Pt–Ni/C and sponge-like Pt–Ni/C nanocatalysts. For the faceted PtNi/C material, a lattice expansion of ca. 0.3% was observed to occur for the catalyst in the MEA at the very beginning of the experiment, even before the ECA was performed, this being greater than the lattice expansion observed for measurements in the liquid cell. This result suggests that the steps involved in preparing the MEA (ink formulation, sonication, deposition, hot pressing, etc.) led to greater catalyst degradation (equivalent to 3 Ni at% loss) than the potential cycling performed during the ECA in the liquid cell, as recently confirmed by Ronovský et al.30 After the ECA of the PEMFC, the faceted PtNi/C catalyst underwent a lattice expansion of 0.45% (equivalent to a loss of 4 Ni at% from the PtNi alloy), which is more than twice that observed in the liquid cell. These observations strongly suggest that this class of shape-controlled Ni-rich catalysts are quite sensitive to the MEA preparation steps and to the PEMFC operating conditions. In the case of the sponge-like PtNi/C material, the catalyst in the MEA underwent a lattice strain relaxation of 0.16%. In fact, for this class of Ni-poor nanocatalysts, the ECA in the liquid cell and conditioning in the PEMFC both led to a lattice expansion of 0.31% (i.e., 2.8% Ni at% loss).23
At this point and according to the discussed studies, it is clear that building up structure–activity–stability relationships using ex situ characterization would not ex situ characterization describe the nature of the true catalyst under operating conditions.
Conclusions and outlook
In this short perspective, we have highlighted the need for in situ/operando characterization of Pt-based nanoalloys during the first operation steps of the oxygen reduction reaction. Although such studies are scarce, solid evidence for the evolution of the key electrocatalytic properties (structure, chemical composition, local coordination environment, morphology, etc.) of Pt-based electrocatalysts during the early conditioning steps is available, which challenges the usefulness of properties measured ex situ as exact activity/stability descriptors in the property–activity–stability relationships.Synchrotron-based techniques are powerful and valuable approaches for in situ/operando measurements, but lab-facility techniques, such as online ICP-MS, IR and Raman spectroscopy are also crucial in this regard. Complementary approaches including identical-location transmission-electron microscopy, machine learning-assisted theoretical calculations, and post mortem ARTEM provide further insights to the understanding of electrocatalyst properties.
Greater focus is required on the proper description of the in situ beginning of life catalyst state and not only on the ex situ catalyst state to rationalize the property–activity–stability relationships of Pt-based catalysts towards the oxygen reduction reaction,38 including new insights at MEA level.39 Indeed, this approach has been currently debated in the literature for oxygen evolution reaction (OER)27,40 and CO2 reduction reaction (CO2RR)41 electrocatalysts, evoking the need to understand the actual properties of any electrocatalyst for any reaction after its activation step.
Author contributions
Carlos A. Campos-Roldán: conceptualization, writing – original draft; Raphaël Chattot: conceptualization, writing – review & editing; Pierre-Yves Blanchard: supervision, writing – review & editing; Deborah J. Jones: funding acquisition, supervision, writing – review & editing; Sara Cavaliere: conceptualization, supervision, writing – review & editing.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this perspective paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research leading to these results has received funding from the HIGHLANDER and IMMORTAL projects, which receive funding from the Clean Hydrogen Partnership under grant agreement numbers 101101346 and 101006641, respectively. This Joint Undertaking receives support from the European Union's Horizon Europe research and innovation programme, Hydrogen Europe, and Hydrogen Europe Research. R. C. received financial support from the French National Research Agency through the HOLYCAT project (grant no. ANR-22-CE05-0007).References
- M. van der Spek, C. Banet, C. Bauer, P. Gabrielli, W. Goldthorpe, M. Mazzotti, S. T. Munkejord, N. A. Røkke, N. Shah, N. Sunny, D. Sutter, J. M. Trusler and M. Gazzani, Energy Environ. Sci., 2022, 15, 1034–1077 RSC.
- K. Kodama, T. Nagai, A. Kuwaki, R. Jinnouchi and Y. Morimoto, Nat. Nanotechnol., 2021, 16, 140–147 CrossRef PubMed.
- K. Jiao, J. Xuan, Q. Du, Z. Bao, B. Xie, B. Wang, Y. Zhao, L. Fan, H. Wang, Z. Hou, S. Huo, N. P. Brandon, Y. Yin and M. D. Guiver, Nature, 2021, 595, 361–369 CrossRef PubMed.
- C. Y. Ahn, J. E. Park, S. Kim, O. H. Kim, W. Hwang, M. Her, S. Y. Kang, S. Park, O. J. Kwon, H. S. Park, Y. H. Cho and Y. E. Sung, Chem. Rev., 2021, 121, 15075–15140 CrossRef PubMed.
- D. A. Cullen, K. C. Neyerlin, R. K. Ahluwalia, R. Mukundan, K. L. More, R. L. Borup, A. Z. Weber, D. J. Myers and A. Kusoglu, Nat. Energy, 2021, 6, 462–474 CrossRef.
- J. Fan, M. Chen, Z. Zhao, Z. Zhang, S. Ye, S. Xu, H. Wang and H. Li, Nat. Energy, 2021, 6, 475–486 CrossRef.
- C. Lee, W. J. M. Kort-Kamp, H. Yu, D. A. Cullen, B. M. Patterson, T. A. Arman, S. Komini Babu, R. Mukundan, R. L. Borup and J. S. Spendelow, Nat. Energy, 2023, 8, 685–694 CrossRef.
- O. M. Magnussen, J. Drnec, C. Qiu, I. Martens, J. J. Huang, R. Chattot and A. Singer, Chem. Rev., 2024, 124, 629–721 CrossRef PubMed.
- J. Timoshenko and B. R. Cuenya, Chem. Rev., 2021, 121, 882–961 CrossRef PubMed.
- C. A. Campos-Roldán, R. Chattot, F. Pailloux, A. Zitolo, J. Rozière, D. J. Jones and S. Cavaliere, J. Mater. Chem. A, 2024, 12, 1253–1258 RSC.
- A. A. Topalov, S. Cherevko, A. R. Zeradjanin, J. C. Meier, I. Katsounaros and K. J. J. Mayrhofer, Chem. Sci., 2014, 5, 631–638 RSC.
- N. Hodnik, G. Dehm and K. J. Mayrhofer, Acc. Chem. Res., 2016, 49, 2015–2022 CrossRef PubMed.
- T.-H. Shen, R. Girod and V. Tileli, Acc. Chem. Res., 2023, 56, 3023–3032 CrossRef CAS PubMed.
- M. Bele, G. K. Podborsek, A. Loncar, P. Jovanovic, A. Hrnjic, Z. Marinko, J. Kovac, A. K. Surca, A. R. Kamsek, G. Drazic, N. Hodnik and L. Suhadolnik, ACS Appl. Nano Mater., 2023, 6, 10421–10430 CrossRef CAS PubMed.
- A. R. Kamšek, F. Ruiz-Zepeda, A. Pavlišič, A. Hrnjić and N. Hodnik, Curr. Opin. Electrochem., 2022, 35, 101052 CrossRef.
- T. Fuchs, J. Drnec, F. Calle-Vallejo, N. Stubb, D. Sandbeck, M. Ruge, S. Cherevko, D. Harrington and O. Magnussen, Nat. Catal., 2020, 3, 754–761 CrossRef CAS.
- Y. Wang, T. Gong, M. Lee and A. S. Hall, Curr. Opin Electrochem., 2021, 30, 100796 CrossRef CAS.
- M. Ma, L. Shen, Z. Zhao, P. Guo, J. Liu, B. Xu, Z. Zhang, Y. Zhang, L. Zhao and Z. Wang, eScience, 2024, 100254, DOI:10.1016/j.esci.2024.100254.
- H. Jiang, Q. He, Y. Zhang and L. Song, Acc. Chem. Res., 2018, 51, 2968–2977 CrossRef CAS PubMed.
- X. Tuaev, S. Rudi, V. Petkov, A. Hoell and P. Strasser, ACS Nano, 2013, 7, 5666–5674 CrossRef CAS PubMed.
- M. Fehse, A. Iadecola, L. Simonelli, A. Longo and L. Stievano, Phys. Chem. Chem. Phys., 2021, 23, 23445–23465 RSC.
- M. Ronovsky, L. Pan, M. Klingenhof, I. Martens, L. Fusek, P. Kus, R. Chattot, M. Mirolo, F. Dionigi, H. Burdett, J. Sharman, P. Strasser, A. M. Bonastre and J. Drnec, ACS Appl. Energy Mater., 2023, 6, 8660–8665 CrossRef.
- R. Chattot, C. Roiron, K. Kumar, V. Martin, C. A. Campos Roldan, M. Mirolo, I. Martens, L. Castanheira, A. Viola, R. Bacabe, S. Cavaliere, P.-Y. Blanchard, L. Dubau, F. Maillard and J. Drnec, ACS Catal., 2022, 12, 15675–15685 CrossRef.
- J. Li and J. Gong, Energy Environ. Sci., 2020, 13, 3748–3779 RSC.
- O. Kasian, S. Geiger, K. J. J. Mayrhofer and S. Cherevko, Chem. Rec., 2019, 19, 2130–2142 CrossRef PubMed.
- K. Ehelebe, J. Knoppel, M. Bierling, B. Mayerhofer, T. Bohm, N. Kulyk, S. Thiele, K. J. J. Mayrhofer and S. Cherevko, Angew. Chem., Int. Ed., 2021, 60, 8882–8888 CrossRef.
- Y. Zhu, J. Wang, H. Chu, Y.-C. Chu and H. M. Chen, ACS Energy Lett., 2020, 5, 1281–1291 CrossRef.
- C. Lim, A. R. Fairhurst, B. J. Ransom, D. Haering and V. R. Stamenkovic, ACS Catal., 2023, 13, 14874–14893 CrossRef.
- C. A. Campos-Roldán, D. J. Jones, J. Rozière and S. Cavaliere, ChemCatChem, 2022, 14, e202200334 CrossRef PubMed.
- M. Ronovský, O. Dunseath, T. Hrbek, P. Kúš, M. Gatalo, S. Polani, J. Kubát, D. Götz, H. Nedumkulam, A. Sartori, E. Petrucco, F. Ruiz-Zepeda, N. Hodnik, A. M. Bonastre, P. Strasser and J. Drnec, ACS Energy Lett., 2024, 9, 5251–5258 CrossRef.
- M. Gatalo, P. Jovanovič, U. Petek, M. Šala, V. S. Šelih, F. Ruiz-Zepeda, M. Bele, N. Hodnik and M. Gaberšček, ACS Appl. Energy Mater., 2019, 2, 3131–3141 CrossRef.
- A. A. Alekseenko, A. S. Pavlets, S. V. Belenov, O. I. Safronenko, I. V. Pankov and V. E. Guterman, Appl. Surf. Sci., 2022, 595, 153533 CrossRef.
- B. Danisman, G. R. Zhang, A. F. Baumunk, J. Yang, O. Brummel, P. Darge, K. J. J. Mayrhofer, J. Libuda, M. Ledendecker and B. J. M. Etzold, ChemElectroChem, 2023, 10, e202300109 CrossRef.
- B. Danisman, G. R. Zhang, A. F. Baumunk, J. Yang, O. Brummel, P. Darge, D. Dworschak, K. J. J. Mayrhofer, J. Libuda, X. Zhou, M. Wu, E. Spiecker, M. Ledendecker and B. J. M. Etzold, ChemElectroChem, 2024, 11, e202400070 CrossRef.
- C. A. Campos-Roldán, R. Chattot, J.-S. Filhol, H. Guesmi, F. Pailloux, R. Bacabe, P.-Y. Blanchard, A. Zitolo, J. Drnec, D. J. Jones and S. Cavaliere, ACS Catal., 2023, 13, 7417–7427 CrossRef.
- C. A. Campos-Roldán, J.-S. Filhol, H. Guesmi, M. Bigot, R. Chattot, A. Zitolo, P.-Y. Blanchard, J. Rozière, D. J. Jones and S. Cavaliere, ACS Catal., 2023, 13, 13319–13324 CrossRef.
- C. A. Campos-Roldán, R. Chattot, J.-S. Filhol, H. Guesmi, N. Romero, R. Bacabe, P.-Y. Blanchard, V. Vinci, J. Drnec, J. Rozière, D. J. Jones and S. Cavaliere, ACS Catal., 2024, 14, 11941–11948 CrossRef.
- A. S. Mule, K. Tran, A. M. Aleman, Y. E. Cornejo-Carrillo, G. A. Kamat, M. B. Stevens and T. F. Jaramillo, Adv. Energy Mater., 2024, 14, 2401939, DOI:10.1002/aenm.202401939.
- C. A. Campos-Roldán, A. Gasmi, M. Ennaji, M. Stodel, I. Martens, J. Filhol, P. Blanchard, S. Cavaliere, D. Jones, J. Drnec and R. Chattot, Nat. Commun., 2025, 16, 936, DOI:10.1038/s41467-024-55299-3.
- S. Chen, L. Ma, Z. Huang, G. Liang and C. Zhi, Cell Rep. Phys. Sci., 2022, 3, 100729 CrossRef.
- W. van der Stam, Chem. Mater., 2023, 35, 386–394 CrossRef.
| This journal is © the Owner Societies 2025 |