Open Access Article
Open Access ArticleFlame retardance-donated lignocellulose nanofibers (LCNFs) by the Mannich reaction with (amino-1,3,5-triazinyl)phosphoramidates and their properties†
Fumiaki Ono g,
Takumi Okihara
g,
Takumi Okihara b,
Noboru Osaka
b,
Noboru Osaka c,
Noriyuki Nagaoka
c,
Noriyuki Nagaoka d,
Yuji Kameokae,
Akira Ishikawae,
Hironari Ookif,
Takumi Itof,
Daisuke Todomec,
Shinya Uemotog,
Mitsuaki Furutanig,
Tsutomu Inokuchi
d,
Yuji Kameokae,
Akira Ishikawae,
Hironari Ookif,
Takumi Itof,
Daisuke Todomec,
Shinya Uemotog,
Mitsuaki Furutanig,
Tsutomu Inokuchi *bg and
Kenji Okada
*bg and
Kenji Okada *ag
*ag
aDepartment of Life Science, Kurashiki University of Science & the Arts, 2640, Nishinoura, Tsurajima, Kurashiki 712-8505, Japan. E-mail: okada.kenji205@gmail.com
bGraduate School of Natural Science and Technology, Okayama University, 3-1-1, Tsushima-naka, Kita-Ku, Okayama 700-8530, Japan. E-mail: inokuchi@cc.okayama-u.ac.jp
cFaculty of Science, Okayama University of Science, 1-1, Ridaicho, Kita-ku, Okayama 700-0005, Japan
dAdvanced Research Center for Oral and Craniofacial Science, Okayama University Dental School, 2-5-1, Shikata-cho, Kita-ku, Okayama 700-8558, Japan
eMarubishi Oil Chemical Co., Ltd, 1-4-16, Dojimahama, Kita-ku, Osaka 530-0004, Japan
fGen Gen Corporation, 74, Nakano Ori, Kamori, Tsushima, Aichi 496-0005, Japan
gOkayama Biomass Innovation Creative Center, 5301, Haga, Kita-ku, Okayama 701-1221, Japan
First published on 26th January 2022
Abstract
Nitrogen/phosphorus-containing melamines (NPCM), a durable flame-retardant, were prepared by the successive treatment of ArOH (Ar = BrnC6H5−n, n = 0, 1, 2, and 3) with POCl3 and melamine monomer. The prepared flame-retardants were grafted through the CH2 unit to lignocellulose nanofibers (LCNFs) by the Mannich reaction. The resulting three-component products were characterized using FT-IR (ATR) and EA. The thermal behavior of the NPCM-treated LCNF fabric samples was determined using TGA and DSC analyses, and their flammability resistances were evaluated by measuring their Limited Oxygen Index (LOI) and the UL-94V test. A multitude of flame retardant elements in the fabric samples increased the LOI values as much as 45 from 20 of the untreated LCNFs. Moreover, the morphology of both the NPCM-treated LCNFs and their burnt fabrics was studied with a scanning electron microscope (SEM). The heat release lowering effect of the LCNF fabric against the water-based paint was observed with a cone calorimeter. Furthermore, the mechanical properties represented as the tensile strength of the NPCM-treated LCNF fabrics revealed that the increase of the NPCM content in the PP-composites led to an increased bending strength with enhancing the flame-retardance.
1. Introduction
In recent years, electronic devices, such as electric vehicles, personal computers, and mobile phones, have been actively developed, and the plasticization rate of the devices has been increasing due to the pursuit of weight reduction and higher strength. Therefore, high flame retardance is essential from the perspective of user protection. Generally, if the content of the flame retardant is high in the incorporated devices, the flame retardance becomes high, which may cause the deterioration of dynamic physical properties of the resin and bleed the flame retardant. At the same time, the removal of hazardous chemicals due to the increasing rate of electronic waste is becoming more and more important.1 Under these circumstances, the development of bio-renewable polymer-based environmentally friendly materials is of intensive interest.2 Lignin is a macromolecule of biological origin and expected to be a promising flame retardant,3–5 and its application through a chemical modification into nanocomposites is currently a hot research topic.6Lignin, together with cellulose and hemicellulose, constitutes the main components of the tissue cells of woody plants and functions to bind them. The structure of lignin cannot be specified due to the polymeric structure containing various 4-propylphenol units. When coniferous trees are used as raw materials of manufacturing, lignin mainly consists of the dehydrated condensate of coniferyl alcohol (guacinyl lignin), and occupies 20 to 35% of the cell wall.7–10
Because of the advantages of being inexpensive, renewable, available in abundant quantities, and containing various functional groups, such as benzenols,11 the modification of lignin for improving its functional capacity is currently being actively investigated. For example, the Mannich reaction was used to graft with amines,12–14 and applied to improve the absorbance of toxic metal ions, such as Pb and Cu, as reported by Li.15,16
Furthermore, lignin is expected to have the potential to be used as the raw material for flame-retardants, because of its unique aromatic structure and high charring capability,17 compared to other biomass-derived natural macromolecules such as cotton. Recently, the preparation, modification and application of lignin-derived flame retardants for polymeric materials were summarized in a review article, which focuses on the flame-retardant effects of pristine lignin by chemical modification by introducing the retardant elements, such as phosphorus, nitrogen, silicon, etc., as well as their synergistic effects with existing flame-retardant additives.4,6
Lignocellulose nanofibers (LCNFs) were manufactured by the wet-disk milling of wood flour,18 and were subsequently used as the reinforcing filler for a polypropylene (PP) polymer matrix.19 This material possesses two characteristics, i.e., chemical reactivity of lignin and physical strength of cellulose as a filler of polymers.
Accordingly, in this study, we designed amines II bearing flame-retardant elements, such as phosphorus,20 nitrogen,21 and both22,23 and then examined the Mannich reaction in order to graft with the lignin sites of the LCNFs (I), leading to III–V (Scheme 1). We observed changes in the surface using IR and checked the morphology by SEM. We also tested the flame retardance of the resulting Mannich adducts III–V using the LOI and UL testing methods and examined the heat release lowering effect of the flame retardant-treated LCNF fabrics against the water-based paint using a cone calorimeter. The compatibility of the Mannich products with polyolefin, such as PP, and their mechanical properties for fillers were also evaluated.
 | ||
| Scheme 1 Mannich reaction of (amino-1,3,5-triazinyl)mono- or diphosphoramidates (II) with LCNFs (I) and HCHO, leading to flame-retardant fabrics III–V. | ||
2. Experimental section
2.1. Materials and chemicals
The LCNFs (CellFim L-45™) manufactured from cypress, fiber width of 50–300 nm and fiber length of 45 μm or less, were provided as an aqueous 90–95% dispersion by the Cellulose Development Division of Mori Machinery Corp. (Okayama, Japan). Polypropylene pellets (MA3, 0.5 mm or under) from Japan Polypropylene Co. were used.2.2. Characterization
Preparation of (amino-1,3,5-triazinyl)mono- or diphosphoramidates 4 and 5, and their Mannich reactions with LCNFs are described in the ESI.†The melting point (mp) was determined by Differential scanning calorimetry (DSC) using an SII EXSTAR6000 at a heating rate of 10 °C min−1, and the data are shown in the ESI.† TGA measurements were carried out using a Thermo Plus TG 8120, Rigaku Corp. The FT-IR (ATR method) was performed with a PerkinElmer spotlight 300. 1H, 13C NMR, and 31P NMR were recorded with a JEOL LNM-ECS-400. EA was performed with a PE 2400 II (PerkinElmer) elemental analyzer. SEM was performed with an FE-SEM (JSM-6701F, JEOL) operated at 5 kV, in which specimens were coated with a thin layer of osmium (Neoc-STB, Meiwafosis).
LOI (Limited Oxygen Index) measurements were achieved using a Suga flammability test device ON-1. The test pieces were prepared as follows: the aqueous dispersions of the Mannich adducts, obtained by the reaction of the LCNFs (CellFim L-45™) with phosphoramidated melamines 4 or 5 at varying ratio (mmol g−1), were molded using the appropriate amount into a small vessel (100 × 15 × 15 mm) and shaped to a thin plate by air-drying followed by vacuum drying.
UL94V test method: The Laboplast mill melts PP resin (prime polymer homo PP/prime polypro J-106), calcium stearate (used as an acid adsorbent and dispersant), and antioxidant at 200 °C, and then the flame retardant sample of the Mannich adduct was gradually kneaded for 5 min at 50 rpm. The obtained compound was placed in a hot press to form a sheet having a predetermined thickness. The UL94V test pieces were cut from the sheet and evaluated for their flame-retardance. Cone calorimeter combustion experiments were performed with a Toyo Seiki Seisakusho, Cone calorimeter C3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 and blended using an extreme mill (MX-1100 XTS manufactured by WARING). This mixture was subjected to a solid phase shearing treatment using a kneader (Roller Mixer R60 manufactured by Toyo Seiki Seisakusho, Ltd.). Subsequently, 10% of the maleic acid-modified PP (Kayabrid 002PP manufactured by Kayaku Akzo Chemical Co., Ltd.) was added to the total amount of the recovered mixture, and after a thorough stirring, melting was performed using a twin-screw extruder (2D 15 W made by Toyo Seiki Seisaku-sho, Ltd.), and then kneading was carried out. The obtained strand was pelletized using a pelletizer and a test piece for the strength test was prepared using an injection-molding machine (Babyplast 6/10 P made by Rambaldi). The test piece was allowed to stand at room temperature for 1 week and sufficiently crystallized before being used in the strength test.
90 and blended using an extreme mill (MX-1100 XTS manufactured by WARING). This mixture was subjected to a solid phase shearing treatment using a kneader (Roller Mixer R60 manufactured by Toyo Seiki Seisakusho, Ltd.). Subsequently, 10% of the maleic acid-modified PP (Kayabrid 002PP manufactured by Kayaku Akzo Chemical Co., Ltd.) was added to the total amount of the recovered mixture, and after a thorough stirring, melting was performed using a twin-screw extruder (2D 15 W made by Toyo Seiki Seisaku-sho, Ltd.), and then kneading was carried out. The obtained strand was pelletized using a pelletizer and a test piece for the strength test was prepared using an injection-molding machine (Babyplast 6/10 P made by Rambaldi). The test piece was allowed to stand at room temperature for 1 week and sufficiently crystallized before being used in the strength test.Dispensability of the PP/flame retardant LCNF composite. The pellets prepared by the above procedure were formed into a film by a press machine and observed under natural light and polarized light (crossed Nicol). As a result, aggregates derived from the LCNFs and bubbles due to poor interfacial adhesion (compatibility) were not visually confirmed, suggesting that the dispensability of the flame retardant LCNFs in the resin was relatively good.
For the measurement of the tensile strength and bending strength, AGS-5 kNG made by Shimadzu was used. For the Izod impact strength measurement, no. 258-D manufactured by Yasuda Seiki Seisakusho was used.
3. Results and discussion
3.1. Synthesis of grafting agents for flame-retardance
According to our plan shown in Scheme 2, linking of the phosphorus atom(s) to the melamine monomer 3 (2,4,6-triamino-1,3,5-triazine) scaffolds is a primary task; hence, we surveyed the literature about the methods affording the corresponding diphosphoramidates 5. The productions of N,N′-disubstituted triazine, namely, tetraphenyl(6-amino-1,3,5-triazine-2,4-diyl)diphosphoramidate (5a), a durable flame retardant monomer, have been achieved by the reaction of diphenyl chlorophosphate (2a) with melamine 3 at elevated temperature, i.e., 150 °C for 2 h and 180 °C for 2 h (ref. 24) or at room temperature in pyridine.25 In addition to these examples, the tetraethyl analog was obtained from diethyl chlorophosphate.26 However, these reported methods have relied on the use of commercially available diphenyl and diethyl chlorophosphates, and the diaryl chlorophosphates bearing substituents, such as Br(s) on the aryl group (Ar), remained untouched, though they are potent as precursors of the corresponding 4,6-diamino(1,3,5-triazin-2-yl) 4a–c or 6-amino(1,3,5-triazin-2,4-diyl)phosphoramidates 5a,d. | ||
| Scheme 2 One-pot sequence to bis- or tetrakis(aryl) (1,3,5-triazinyl)phosphoramidates 4 or 5 from ArOH (Ar = BrnC6H5−n, n = 0, 1–3) (1), using POCl3 and melamine monomer (3). | ||
We next examined a successive one-pot procedure that involves phosphorylation of ArOH (Ar = BrnC6H5−n, n = 0, 1–3) with POCl3, followed by N-phosphoramidation of the melamine monomer 3 with the resulting 2, giving the bis(aryl)monophoramidates 4 or tetra(aryl)diphosphoramidates 5 (Scheme 2). Thus, a solution of 2,4,6-tribromophenol (1d, 4 equiv. against 3) dissolved in THF was dropped into a mixture of POCl3 (2 equiv.), MgCl2 (catalytic), and pyridine in THF with cooling and the resulting di(2,4,6-tribromophenyl) chlorophosphate (2d) was reacted in situ with the melamine monomer (3, 1 equiv.) in the presence of pyridine, giving the desired tetra(2,4,6-tribromophenyl) (6-amino-1,3,5-triazine-2,4-diyl)diphosphoramidate (5d) in 55% yield based on 3. A catalytic amount of MgCl2 was added to enhance the reaction of the tribromophenol 1d.27,28 A small amount of tris(2,4,6-tribromophenyl) phosphate was found as one of the by-products (∼1%), which can easily be removed by washing with hot MeOH–AcOEt.
Similarly, tetraphenyl (6-amino-1,3,5-triazine-2,4-diyl)diphosphoramidate (5a) was prepared from phenol (1a) by the reaction with POCl3 followed by melamine 3 in the absence of MgCl2 in 36% yield (based on 3). The same 5a can be prepared in 76% yield by the reaction of the commercially available 2a (two equivalents) with 3.
This reaction procedure can successfully be applied to the preparation of bis(4-bromophenyl)- 4b and bis(2,4-dibromophenyl) (6-amino-1,3,5-triazine-2,4-diyl)diphosphoramidate 4c from 4-bromophenol (1b) and 2,4-dibromophenol (1c) in 50 and 60% yields, respectively, using excess melamine monomer 3 in the absence of MgCl2. Furthermore, the addition mode by the dropping of ArOH 1b or 1c into POCl3 without MgCl2 is crucial for the predominant formation of the desired mono-substituted diarylphosphoramidates 4b,c. On the contrary, a reverse addition by the dropping of POCl3 into 4-bromophenol (1b) at 0–4 °C in the presence of MgCl2 (catalytic) caused the formation of the undesired tri(4-bromophenyl) phosphate as a formidable by-product, and the ratio of 4b/PO(OC6H4Br-4)3 was estimated to be ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by 1H NMR analysis. The NMR and the EA data of the products are recorded in the ESI.†
1 by 1H NMR analysis. The NMR and the EA data of the products are recorded in the ESI.†
3.2. Mannich reactions of LCNFs with amino-1,3,5-triazines
We initially investigated the reactivity of the LCNFs for a three-component-combining (Mannich) reaction with the flame-retardant element-installed melamines 3–5 and formalin in water. Our initial attempt to confirm the Mannich reaction of the LCNFs was started using the melamine monomer 3 in the presence of tetraalkylammonium halide as a surfactant (Scheme 3). | ||
| Scheme 3 Mannich reactions of LCNFs with melamine monomer 3 and its phosphorylated derivatives 4a–c and 5a,d, leading to III, IVa–c, and Va,d. | ||
A heterogeneous mixture of the melamine monomer 3 (760 mg), LCNFs (5.2 g), hexadecyltrimethylammonium bromide (126 mg), and formalin (5 mL) in water (total volume: 500 mL) was heated at 55 °C for 24 h with mechanical agitation. The Mannich product III (5.8 g) obtained by filtration and successive washing of the reaction products with acetone followed by hot water (70–80 °C) amounted to almost the weight of the loaded substrates. Elemental analysis (EA) of the product denotes the formation of a composite incorporating melamine units as a nitrogen source (C, 48.49%, H, 6.34%, and N, 3.24%).
On the other hand, in spite of our careful attempt to determine the intrinsic polymerization of the melamine monomer 3 with formalin during the Mannich reaction, an insoluble material, which can be ascribed to the melamine polymerization, was not observed. However, we found that when melamine and formalin were heated at 50–65 °C without the LCNFs, an insoluble liquid that solidified upon standing was formed, ascribable to the melamine oligomer or polymer.
Furthermore, we attempted to confirm the involvement of formalin in the Mannich reaction by a test reaction with and without formalin. Thus, the reaction of the LCNFs (1.07 g) and 5d (Ar = 2,4,6-Br3C6H2, 690 mg) in the absence of formalin under a heated condition resulted in the recovery of the LCNFs (1.03 g) and 5d (690 mg, contaminated with impurity), while a similar reaction of the LCNFs and 5d (485 mg) in the presence of formalin produced the Mannich adducts Vd (1.29 g) along with contamination of the by-products (102 mg) from the acetone washing layer.
The next question is to estimate the quantitative ability of the LCNFs to react with the melamine phosphoramidates. We searched a profile of the Mannich reactions through the product amount for varying ratios of 5a (Ar = Ph) vs. LCNFs. As indicated in Fig. 1, the Mannich adducts increase in proportion to the increase of 5a and reached a plateau when the ratio of 5a vs. the LCNFs (mmol g−1) was about 2.2. Thus, 5a can be incorporated as high as ca. 2.2 mmol g−1 into the LCNF surface.
A similar trend of reactivity in the Mannich reaction of the LCNFs with the melamine monomer 3 and formalin under increasing the 3/LCNF ratio is described in the ESI.†
3.3. EA, IR, and SEM measurements
Elemental analysis of the Mannich adducts was performed to quantify the uptaken amount of the N elements and thus to confirm that (amino-1,3,5-triazinyl)phosphoroamidate 5a had been grafted to the LCNFs. The hydrogen contents of the Mannich product Va gradually decrease by comparison with the original LCNFs, while the nitrogen contents distinctly increase in proportion to the ratio of 5a vs. the LCNFs, and reach 5.37% at 2.2 mmol g−1 (see ESI†).The IR spectrum of the Mannich adduct Va is shown in Fig. 2 and compared to those of the precursors, i.e., the untreated LCNFs and 5a, respectively.
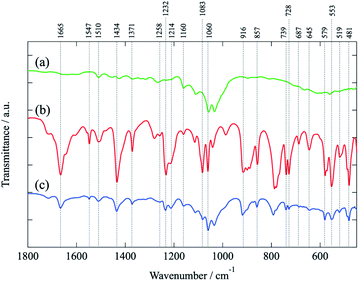 | ||
| Fig. 2 FT-IR (ATR) spectra of (a) untreated LCNFs, (b) phosphoramidate 5a, and (c) Mannich adducts Va. | ||
The IR (a) of the freeze-dried non-treated LCNFs mainly shows broad absorptions in the fingerprint region due to their polymeric structures composed of unspecifiable building residues. On the other hand, the IR (b) of 5a shows sharp absorptions in the same region,29 and most of those peaks are related to the IR (c) of the Mannich products Va, without big displacement of the peak positions. Thus, these results suggest that (6-amino-1,3,5-triazine-2,4-diyl)diphosphoramidate 5a was successfully grafted on the LCNF scaffold through a CH2 unit by the Mannich reaction under the heated conditions.
Iwamoto et al. reported that the lignin contained in the LCNFs is not separated from the cellulose fibers, and most of it remained coated on the fibrillated cellulose fibers.19 This property would be beneficial to the chemical reaction in the lignin part of the LCNFs with the retardant materials in aqueous media (Scheme 1). In this regard, the comparison of the morphology of the LCNFs before and after the chemical modification should be helpful to determine the property of the LCNFs as a fiber.
Fig. 3a shows the SEM images of the freeze-dried samples of the LCNFs, and those of the chemically modified LCNFs by the Mannich reaction with the melamine monomer 3 (b), and with the diphosphoramidate 5d (c). The non-treated LCNFs (a) can be seen as amorphous plates. The SEM images (b) and (c) of the Mannich adducts from 3 and 5d indicate a similar morphology to (a), and that the plate size of the LCNF fibers was maintained after the chemical modification.
3.4. Mass change by TG/DTA
The thermal analysis curve of the treated LCNFs with 5d, i.e., the fabric Vd, is presented in Fig. 4a, demonstrating two stages of thermal degradation. For comparison, the TG/DTA curves of the untreated LCNF sample are shown in Fig. 4b. During the initial stage of thermal degradation of the TG curve, in the temperature range of 221–280 °C, the weight loss is 57% (see Fig. 4a). The peak of the heat generation reaction is seen in the DTA curve. In this stage, the decomposition and dehydration of hemicellulose dominate, which is very fast and significant. The second pyrolysis stage occurs in the temperature range of 280–602 °C, and the weight loss is 34%. The residue is completely burned at 602 °C (Fig. 4a). On the other hand, the second stage of the untreated LCNFs is in the temperature range between 338 and 443 °C. At 443 °C, the amount of residue is completely burned at 443 °C (see Fig. 4b). It can be seen that the thermal decomposition of the second stage of the treated LCNF sample Vd is suppressed in comparison to that of the untreated LCNF sample. Prieur et al. reported that the introduction of phosphorus to the lignin promotes dehydration and decarboxylation reactions, thus increasing the amount of the carbonaceous residue, which is more stable at the higher temperature.303.5. Flame-retardance performance of the Mannich fabrics by LOI
With these results in hand, we examined the Mannich reaction of the LCNFs with the mono- and bis-phosphoramidates 4 and 5 at their varying ratios against the LCNFs, and the flame-retardance of the resulting Mannich adducts was evaluated by the LOI testing (Table 1).Based on a typical Mannich reaction procedure, the fabrics from the LCNFs and (1,3,5-triazine-2,4-diyl) mono-, and diphosphoramidates 4, 5, and the melamine monomer 3 were prepared with varying ratios of these reactants vs. the LCNFs (mmol g−1). The obtained test pieces were submitted to the LOI testing,31 and the results are tabulated in Table 1. The LOI testing results are aligned in accordance with the increasing ratio of the melamine monomer 3, phosphoramidates 4, or 5 vs. LCNFs, and then listed in order with the increasing Br atoms on the Ar groups in every one of the phosphoramidates (see ESI†).
The attachment of melamine to the LCNFs is expected to be effective for the LOI value due to its closed char layer formation, which may involve condensation of the melamine to form melam, melem, and related products during burning.32 However, the LOI value of the melamine–LCNF fabric was 21.0 (run 2), whose data are not significant compared to that of the non-treated virgin LCNFs (19.5, run 1). When (1,3,5-triaziniyl)monophosphoramidate 4a was linked to the LCNFs, the LOI value of the resulting fabric was improved to 27.5 due to the combined effect of the phosphorus-based charring33 along with the char layer with the melamine unit. The synergistic effect was prominently observed in the case of (6-amino-1,3,5-triazine-2,4-diyl)diphosphoramidate 5a, the LOI of which was improved to 30.5 at the point of the 5a/LCNFs 1.35 (run 4).
To our surprise, the installation of Br atom(s) to the flame-retardant agents containing N and P atoms induced a significant increase in the LOI values. Namely, the Mannich adducts Vd obtained by the reaction of diphosphoramidate 5d (Ar = 2,4,6-Br3C6H2) and LCNFs showed an LOI value of 46 (run 7, combustion tests by ignition of the Mannich adducts Vd and LCNFs are shown in Fig. 5 and the ESI†); hence, we examined the use of these flame retardant-treated LCNF fabrics as a filler of plastics.
The combustion test of the untreated LCNFs and their modified materials is shown in Fig. 5 regarding their stability to fire. In the case of the untreated LCNFs (Fig. 5a), the flame burned throughout and the specimen mostly burned after ignition. In the case of the treated Va and Vd, after ignition, a char layer was promptly formed, and the test piece hardly burned (Fig. 5b and c).
 | ||
| Fig. 5 (a) The untreated LCNFs, (b) Va (5a/LCNFs = 1. 3 mmol g−1), and (c) Vd (5d/LCNFs = 0.9 mmol g−1). | ||
Moreover, the morphology of the burnt fabrics of either the untreated or treated ones was studied by scanning electron microscopy (SEM) and is shown in the ESI.†
3.6. UL-94V flame retardant evaluation
Flame retardance of 5d itself and Mannich adducts Va of LCNFs with 5a and formalin was homogenized using PP and tested by the UL-94V methods.34The UL94V test results of 5d are listed in Table 2. The 10% and 20% kneaded samples had a good dripping property which significantly reduced after the flame time regardless of the thickness of the test piece. However, the dipped flame almost certainly ignited the cotton, so it was judged as V-2. The 30% kneaded sample occasionally fell into drip failure, and in that case, the fire was hard to extinguish (the fire was strong). It could be seen that the flame-retardant with a high melting point tended to slightly carbonize and became like a core material and difficult to drip. The tendency was quite remarkable in the 1.5 mm test piece. These results are shown in Table 2.
| 3.0 mm test piece | 1.5 mm test piece | ||
|---|---|---|---|
| Flame retardant added (wt%) | Evaluation | Flame retardant added (wt%) | Evaluation |
| 10 | V-2 | 10 | V-2 |
| 20 | V-2 | 20 | V-2 |
| 30 | Failure | 30 | Failure |
The UL-94V flame retardance evaluation of the Va fabric (the ratio of 5a/LCNFs: 1.2 mmol g−1) was performed using the freeze-dried sample. For the testing, antimony trioxide (Sb2O3) was added in order to improve the non-flammability.
| Composition | UL94V | LOI | |||
|---|---|---|---|---|---|
| Sample A | Sample B | Sb2O3 | [3.0 mm] | [1.5 mm] | |
| a Sample A: powder PP/flame retardant LCNFs/maleic acid-modified PP = 80/20/10. Sample B: PP powder/flame retardant LCNFs = 20/80. | |||||
| 100 | — | 1.83 | V-2 | Failed | 21.5 |
| — | 100 | 1.83 | Failed | Failed | 21.5 |
3.7. Combustion test of a blended water-based paint with Vd by a cone calorimeter
Aqueous LCNF fabric was blended with a water-based paint composed of acrylic urethane emulsion, 34.3% (Gen Gen Corporation, Japan, SC-91™) by mixing an equal amount of SC-91™ (100 g) and aqueous 3.87% (non-volatile content) NPCM-treated LCNF fabric Vd (5d/untreated LCNFs = 0.63 mmol g−1, 100 g). The SC-91™ and the SC-91™/Vd blended samples were separately sprayed on an iron plate (10 × 10 cm2) to 30 μm (thin film when dried) and air-dried.35In cone calorimeter combustion experiments, each specimen is wrapped in aluminum foil and exposed horizontally to the external heat flux, e.g. 35 kW m−2. Total calorific values of SC-91 and the blended SC-91™-Vd are given in Table 4.
In this test, the total calorific value of the water-based paint composed of acrylic urethane emulsion was significantly lowered due to the addition of flame retardant-treated LCNF fabric Vd.
3.8. Mechanical properties
A powdery flame retardant fabric Vd derived from 5d/LCNFs (0.63 mmol g−1, freeze-dried) was used. The appearance of the PP/flame retardant LCNF composite film is shown in Fig. 6.Three types of strength tests evaluated the mechanical properties: tensile strength, bending strength, and Izod impact strength test. The results are shown in Fig. 7a and b.
From Fig. 7a, as a result of the tensile test, the elastic modulus was improved by 1.1 times, but the strength hardly changed. Although the elongation rate decreased to about 1/2, it was maintained at about 400%.
From Fig. 7b, as a result of the bending test and the impact test, the elastic modulus was increased by 1.2 times, the strength by 1.1 times, and the impact strength by 1.4 times. No deterioration of the mechanical properties of the resin due to the addition of the flame retardant LCNF fabric was observed, and the improvement of the mechanical properties due to the resin reinforcing effect of the CNF contained in LCNFs was confirmed, especially in the bending test.36,37
4. Conclusions
Development of value-added materials from the abundant LCNFs, which are a raw material from woody plants and mainly composed of two different polymeric substances, namely cellulose and lignin, providing flame-retardance to the lignin scaffold using the Mannich reaction was achieved. The nitrogen/phosphorus containing (NPC) aryl (amino-1,3,5-triazinyl)phosphoramidate monomers, being a durable flame-retardant, as a counterpart of the Mannich reaction, were designed via a one-pot, three-step sequence starting from ArOH (Ar = BrnC6H5−n, n = 0, 1, 2, and 3). The IR spectra and SEM analysis of the grafted LCNFs indicated that the surface is smoothly coated with functionalized amino-1,3,5-triazine compounds, keeping the original lignin–cellulose bio-polymeric structures. As a result of the LOI testing of the flame retardant-grafted LCNFs, a wide spectrum of flame-retardance is achieved by the choice of the flame-retardant compounds and their amount in the Mannich reactions. The LCNF fabric significantly lowered the total calorific value of acrylic urethane emulsion paint as a result of blending. Grafted LCNFs can be kneaded with plastics such as PP and the mechanical properties are about 10% improved compared to the single PP component due to the inherent strength of the cellulose in the LCNFs. Thus, the present flame-retardant functionalized-LCNFs provide a promising alternative for the environmentally friendly and value-added utilization of abundant lignin and sustainable supply of raw materials for flame retardant PP production. Importantly, this functionalized-lignin could also be used in other fields, such as flame retardant natural rubbers, epoxy resins and polyurethanes.Author contributions
Kenji Okada: investigation, funding acquisition, project administration, validation, and writing. Tsutomu Inokuchi: investigation, methodology, formal analysis, data curation, validation, and draft writing. Takumi Okihara, Noboru Osaka, and Noriyuki Nagaoka: investigation, formal analysis, validation, and writing. Fumiaki Ono: methodology, investigation, formal analysis, data curation, and writing. Daisuke Todome, Shinya Uemoto, and Mitsuaki Furutani: data curation and analysis. Yuji Kameoka, Akira Ishikawa, Hironari Ooki, and Takumi Ito: data curation, formal analysis, validation, and writing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are greatly grateful to Okayama prefecture officials for the opportunity and support of research activities under the Okayama Green-Bio Project, and to MEXT under the Social system reform program aimed at creating a new society that responds to climate change, i.e. “SMART factory model demonstration in which forests and people coexist”. We are thankful to Mori Machinery Corp. (Okayama, Japan) for the generous donation of LCNFs (CellFim L-45™), and Manac Incorporated (Fukuyama, Japan) for the gift of 2,4,6-tribromophenol and the determination of Br content. We also thank Prof. Takashi Endo (National Institute of Advanced Industrial Science and Technology: Hiroshima, Japan), Prof. Tadashi Okamoto (Kinki University), Prof. Shigetoshi Amiya (Shinshu University), Dr Koji Kawabata (Industrial Technology Centre of Okayama prefecture), and Dr Takayuki Kato (Toyota Central R&D Labs., Inc.) for valuable discussions in the course of the study. We are indebted to Prof. Manabu Kuroboshi (Okayama University) for NMR and Prof. Li-Jian Ma (Sichuan University) for MALDI-TOF Mass.Notes and references
- H. Millet, P. Vangheluwe, C. Block, A. Sevenster, L. Garcia and R. Antonopoulos, The Nature of Plastics and Their Societal Usage, in “Plastics and the Environment” (Issues in Environmental Science and Technology, No. 47), ed. R. E. Hester and R. M. Harrison, The Royal Society of Chemistry, 2018, pp. 10–20 Search PubMed
.
- F. H. Isikgor and C. R. Becer, Polym. Chem., 2015, 6, 4497–4559 RSC
.
- C. R. Correa, M. Stollovsky, T. Hehr, Y. Rauscher, B. Rolli and A. Kruse, ACS Sustainable Chem. Eng., 2017, 5, 8222–8233 CrossRef
.
- H. Yang, B. Yu, X. Xu, S. Bourbigot, H. Wang and P. Song, Green Chem., 2020, 22, 2129–2161 RSC
.
- H. Zhu, Z. Peng, Y. Chen, G. Li, L. Wang, Y. Tang, R. Pang, Z. U. H. Khan and P. Wan, RSC Adv., 2014, 4, 55271–55279 RSC
.
- V. K. Thakur, M. K. Thakur, P. Raghavan and M. R. Kessler, ACS Sustainable Chem. Eng., 2014, 2, 1072–1092 CrossRef CAS
.
- A. Lourenço and H. Pereira, in “Lignin – Trends and Applications”, ed. M. Poletto, IntechOpen, 2018, ch. 3, pp. 65–98 Search PubMed
.
- A. Lourenço and H. Pereira, in “Methods in Lignin Chemistry”, ed. C. W. Dence and S. Y. Lin, 2018, pp. 3–19 Search PubMed
.
- T. Takano, J. Network Polym., Jpn., 2010, 31, 213–223 CAS
.
- J. D. Gargulak and S. E. Lebo, Lignin: Historical, Biological, and Materials Perspectives, ACS Symp. Ser., 1999, 742, 304–320 CrossRef
.
- H. Mikawa, K. Sato, C. Takasaki and K. Ebisawa, Bull. Chem. Soc. Jpn., 1956, 29, 259–265 CrossRef CAS
.
- X. Du, J. Li and M. E. Lindström, Ind. Crops Prod., 2014, 52, 729–735 CrossRef CAS
.
- G. J. Jiao, P. Peng, S. L. Sun, Z. C. Geng and D. She, Int. J. Biol. Macromol., 2019, 127, 544–554 CrossRef CAS PubMed
.
- B. Wang, T. Y. Chen, H. M. Wang, H. Y. Li, C. F. Liu and J. L. Wen, Int. J. Biol. Macromol., 2018, 107, 426–435 CrossRef CAS PubMed
.
- Y. Ge and Z. Li, ACS Sustainable Chem. Eng., 2018, 6, 7181–7192 CrossRef CAS
.
- Y. Ge, Q. Song and Z. Li, J. Ind. Eng. Chem., 2015, 23, 228–234 CrossRef CAS
.
- N. Mandlekar, A. Cayla, F. Rault, S. Giraud, F. Salaün, G. Malucelli and J. Guan, Ind. Eng. Chem. Res., 2017, 56(46), 13704–13714 CrossRef CAS
.
- S. Iwamoto, A. N. Nakagaito and H. Yano, Appl. Phys. A: Mater. Sci. Process., 2007, 89, 461–466 CrossRef CAS
.
- S. Iwamoto, S. Yamamoto, S.-H. Lee and T. Endo, Cellulose, 2014, 21, 1573–1580 CrossRef CAS
.
- B. Schartel, Materials, 2010, 3, 4710–4745 CrossRef CAS PubMed
.
- H. Horacek and R. Grabner, Polym. Degrad. Stab., 1996, 54, 205–215 CrossRef CAS
.
- L. Costes, F. Laoutid, M. Aguedo, A. Richel, S. Brohez, C. Delvosalle and P. Dubois, Eur. Polym. J., 2016, 84, 652–667 CrossRef CAS
.
- Y. Matsushita, D. Hirano, D. Aoki, S. Yagami, Y. Takagi and K. Fukushima, Adv. Sustainable Syst., 2017, 1, 1700073 CrossRef
.
- K. Matsubara, T. Katsumata, Jpn. Kokai Tokkyo Koho, Jan 16 1996, Japense Patent, JP 08012692 A 19960116 Search PubMed
.
- N. Zhang, C.-M. Cheng and R.-J. Wang, Chin. J. Struct. Chem., 2012, 31, 1042–1046 CAS
.
- W. Cho, Y. Wan, W. Seo, Faming Zhuanli Shenqing, 1997, Chinese Patent, CN 1155601 A 19970730 Search PubMed
.
- L. T. Gunkel and J. Crosby, Eur. Pat. Appl., EP 278353 A2 19880817, 1988
.
- E. Kudo and H. Kouchi, Jpn. Kokai Tokkyo Koho, 1975, JP 500479 Search PubMed
.
- L. W. Daasch and D. C. Smith, Anal. Chem., 1951, 23, 853–868 CrossRef CAS
.
- B. Prieur, M. Meub, M. Wittemann, R. Kleinb, S. Bellayer, G. Fontaine and S. Bourbigot, RSC Adv., 2017, 7, 16866–16877 RSC
.
- J. J. Willard and R. E. Wondra, Text. Res. J., 1970, 40, 203–210 CrossRef CAS
.
- A. König, U. Fehrenbacher, E. Kroke and T. Hirth, J. Fire Sci., 2009, 27, 187–211 CrossRef
.
- B. Schartel, Materials, 2010, 3, 4710–4745 CrossRef CAS PubMed
.
- C. G. McCoy and S. I. Stoliarov, Combust. Flame, 2021, 225, 214–227 CrossRef CAS
.
- C. Réti, M. Casetta, S. Duquesne, S. Bourbigot and R. Delobel, Polym. Adv. Technol., 2008, 19, 628–635 CrossRef
.
- A. Ito, T. Semba, K. Kitagawa, H. Okumura and H. Yano, J. Cell. Plast., 2019, 55, 385–400 CrossRef CAS
.
- L. Qi, L. Wu, R. He, H. Cheng, B. Liu and X. He, RSC Adv., 2019, 9, 23994–24002 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: General information on materials synthesis, DSC, IR, NMR, MALDI-TOF mass, and EA data of compounds, SEM, the LOI, UL, and cone calorimeter testings of the LCNFs fabric samples, and optimization of the polymer composites. See DOI: 10.1039/d1ra08716a |
| This journal is © The Royal Society of Chemistry 2022 |