Open Access Article
Open Access ArticleVolatile components in Yinchenzhufu decoction and their pharmacokinetics after oral administration in rats†
Bin Zana,
Yuanyuan Lia,
Xiaoshu Suna,
Tianming Wanga,
Rong Shi*b and
Yueming Ma *ac
*ac
aDepartment of Pharmacology, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. E-mail: mayueming@shutcm.edu.cn; Fax: +86-21-51322386; Tel: +86-21-51322200
bScience and Technology Experimental Center, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. E-mail: rongshi56@126.com; Tel: +86-21-51322386
cShanghai Key Laboratory of Compound Chinese Medicines, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, China
First published on 24th January 2022
Abstract
In China, Yinchenzhufu decoction (YCZFD) has been used to treat cholestatic liver disease in clinical practice for hundreds of years. Nonvolatile components in YCZFD, their composition, components absorbed in blood, and pharmacokinetic characteristics have been clarified. However, information about its volatile components is limited. The aim of the present study was to identify the components of the volatile oil (VO) of YCZFD, quantify the major volatile components in YCZFD, and reveal their pharmacokinetic characteristics. In YCZFD, 85 components representing 95.36% of the total oil composition were identified by gas chromatography-mass spectrometry. Next, 11 highly abundant components were quantified in YCZFD and YCZFD VO. Finally, a sensitive headspace solid-phase dynamic extraction-chromatography-quadruple mass spectrometry method for determining 8 volatile components in rat plasma was established and applied to compare the pharmacokinetics of YCZFD and YCZFD VO after oral administration in rats. These volatile components were rapidly absorbed and eliminated, and they presented highly different exposure levels. The area under the concentration–time curves of some volatile components in YCZFD was higher than that in YCZFD VO. The results showed that the water extract of YCZFD increased the exposure of volatile components. Our study provides valuable information for understanding the potential effective components of YCZFD.
1. Introduction
Yinchenzhufu decoction (YCZFD) consists of Artemisia capillaris Thunb. (ACT), Rhizoma Atractylodes Macrocephala (RAM), Radix Glycyrrhizae (RG), Radix Aconiti Lateralis Preparata (RALP), Rhizoma Zingiberis (RZ), and Cinnamomum cassia Presl (CCP). It is a classic Chinese herbal prescription that has been used to treat cholestatic liver disease since the Qing Dynasty (18th century CE).1 In China, YCZFD is still widely used to treat chronic liver failure with jaundice in modern clinical practice.2 Experimental studies have shown that YCZFD decreases jaundice, improves liver function, and alleviates liver damage in animal models.3,4 However, to date, the effective components associated with the effect of YCZFD against liver injury have not been very clear in vivo.In our previous studies, nonvolatile components of YCZFD were quantified1 and their pharmacokinetics were elucidated,5 providing information about the potential effective components in YCZFD. However, information pertaining to the volatile components in YCZFD and their pharmacokinetics is limited. Volatile components extracted from plants are often mixed in volatile oil (VO), and they are characterized by various biological activities. The essential oil of A. capillaris exhibits anti-inflammatory effect.6 Moreover, the essential oil and capillin from A. capillaris exhibit antibacterial activity.7,8 The essential oil of Rhizoma Zingiberis shows antimicrobial,9 anti-oxidant,10 and anti-inflammatory activities.11 The essential oils and atractylon from Rhizoma Atractylodes Macrocephala exert anti-inflammatory activity.12 The essential oil of C. cassia shows antibacterial13,14 and anti-inflammatory activities.15,16 The activities of these volatile components may contribute to the effect of YCZFD against liver injury. Therefore, it is necessary to identify the volatile components in YCZFD and clarify their pharmacokinetic characteristics.
Therefore, in this study, we aimed to identify the volatile components in the VO of YCZFD, quantify the principal volatile components in YCZFD, elucidate their pharmacokinetic characteristics, and furtherly to explore the effect of water extract (WE) of YCZFD on the pharmacokinetics of volatile components. The findings of the present study would help better understand the potential effective constituents in YCZFD.
2 Material and methods
2.1 Chemicals, reagents, and materials
α-Pinene, β-phellandrene, copaene, zingiberene, and curcumene were supplied by Shanghai ZZBio Co., Ltd (Shanghai, China). Camphene, eucalyptol, borneol, and naphthalene (internal standard, IS) were provided by Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China). Caryophyllene and trans-cinnamaldehyde were purchased from the National Institutes for Food and Drug Control (Beijing, China). Atractylon was supplied by Nanjing Spring & Autumn Biological Engineering Co., Ltd (Nanjing, China). The chemical structures of these analytes are shown in Fig. 1. C7–C30 saturated alkanes and dichloromethane of GC grade were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Methyl tert-butyl ether was obtained from TCI (Shanghai) Development Co., Ltd (Shanghai, China). The crude herbs ACT, RAM, RG, and CCP were obtained from Shanghai Dehua Pharmaceutical Co., Ltd (Shanghai, China). RALP and RZ were purchased from Shanghai Kangqiao Chinese Medicine Tablet Co., Ltd (Shanghai, China). Sodium sulfate anhydrous was supplied by Sinopharm Chemical Regent Co., Ltd.2.2 Instrument
The gas chromatography-tandem mass spectrometry (GC-MS/MS) system consisted of an Agilent 7890A gas chromatograph combined with a 7000B triple-quadrupole mass spectrometry detector (Agilent, Palo Alto, USA).2.3 Preparation of extracts
2.4 Identification of components in the VO of YCZFD
The GC-MS/MS system with an HP-5 MS 5% phenyl methyl siloxane column (30 m × 0.25 mm, 0.25 μm) and NIST11 library was used to identify volatile components in the VO of YCZFD. The carrier gas was helium applied at a velocity of 1.0 mL min−1, the oven temperature program was initial at 40 °C, then raised to 250 °C at 3 °C min−1, and finally held at 250 °C for 5 min. The injection was in split mode at the split ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 with the injection volume of 1 μL and the injector temperature of 250 °C. A mass spectrometer was used in the electron impact (EI) mode, in which a filament attached to the source body emits electrons into the ionization chamber through the guidance of a magnetic field, with ionization potential set at 70 eV, ionization current at 150 μA, and mass range at 50–500. The YCZFD VO/C7–C30 saturated alkanes were serially diluted with n-hexane and the supernatant was analyzed after centrifuging at 4832 × g for 10 min. According to Kovats method,18 the linear retention index (RI) was calculated for all components in the YCZFD VO sample using the retention time (RT) of a homologous series of n-alkanes (C7–C30) injected in the same conditions as the reference. The components in the YCZFD VO were identified based on RI relative to that of n-alkanes, computer matching with those in the NIST11 library, and comparisons of the fragmentation pattern of the mass spectra with the data in the database. In addition, 11 standard compounds were used for comparison and final confirmation.
20 with the injection volume of 1 μL and the injector temperature of 250 °C. A mass spectrometer was used in the electron impact (EI) mode, in which a filament attached to the source body emits electrons into the ionization chamber through the guidance of a magnetic field, with ionization potential set at 70 eV, ionization current at 150 μA, and mass range at 50–500. The YCZFD VO/C7–C30 saturated alkanes were serially diluted with n-hexane and the supernatant was analyzed after centrifuging at 4832 × g for 10 min. According to Kovats method,18 the linear retention index (RI) was calculated for all components in the YCZFD VO sample using the retention time (RT) of a homologous series of n-alkanes (C7–C30) injected in the same conditions as the reference. The components in the YCZFD VO were identified based on RI relative to that of n-alkanes, computer matching with those in the NIST11 library, and comparisons of the fragmentation pattern of the mass spectra with the data in the database. In addition, 11 standard compounds were used for comparison and final confirmation.
2.5 Quantification of 11 volatile components in YCZFD
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 with the injection volume of 1 μL. Mass spectrometer was used in the EI source multiple reaction monitoring (MRM) mode with ionization potential set at 70 eV. The MRM parameters for the 11 volatile components and ISs are listed in Table 1. The temperature of the injector and ion source was 250 °C and 230 °C, respectively. The carrier gas was helium applied at a velocity of 1.0 mL min−1, and collision cell gases were helium and nitrogen at a velocity of 2.25 and 1.5 mL min−1, respectively.
15 with the injection volume of 1 μL. Mass spectrometer was used in the EI source multiple reaction monitoring (MRM) mode with ionization potential set at 70 eV. The MRM parameters for the 11 volatile components and ISs are listed in Table 1. The temperature of the injector and ion source was 250 °C and 230 °C, respectively. The carrier gas was helium applied at a velocity of 1.0 mL min−1, and collision cell gases were helium and nitrogen at a velocity of 2.25 and 1.5 mL min−1, respectively.
| Components | Precursor ion | Product ion | Dwell time (ms) | CE (V) | RT (min) |
|---|---|---|---|---|---|
| α-Pinene | 136 | 93 | 50 | 8 | 7.2 |
| Camphene | 136 | 93 | 50 | 8 | 8.6 |
| β-Phellandrene | 136 | 93 | 50 | 8 | 15.1 |
| Eucalyptol | 154 | 139 | 50 | 2 | 14.9 |
| Copaene | 204 | 161 | 50 | 10 | 31.3 |
| Caryophyllene | 189 | 105 | 50 | 22 | 37.2 |
| Borneol | 95 | 67 | 50 | 15 | 43.2 |
| Zingiberene | 204 | 119 | 50 | 8 | 44.5 |
| Curcumene | 202 | 132 | 50 | 10 | 46.8 |
| trans-Cinnamaldehyde | 131 | 77 | 50 | 30 | 51.1 |
| Atractylon | 216 | 108 | 50 | 20 | 52.0 |
| Naphthalene (IS) | 128 | 102 | 50 | 30 | 44.7 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) and centrifuged at 4832 × g for 10 min. Next, 90 μL of the supernatant was mixed with 10 μL of IS solution. One microliter of sample was injected into the GC-MS/MS system to determine the content of 11 volatile components.
50, v/v) and centrifuged at 4832 × g for 10 min. Next, 90 μL of the supernatant was mixed with 10 μL of IS solution. One microliter of sample was injected into the GC-MS/MS system to determine the content of 11 volatile components.2.6 Quantitation of the 8 volatile components of YCZFD in rat plasma
The GC-MS/MS conditions, including capillary column, column temperature program, injector temperature, ion source temperature, carrier gas, collision cell gases, and MRM parameters, were the same as those in Section 2.5.1. The sample was injected in the splitless mode. The multiplier voltage was 1375 V.
The SPDE method has been previously reported;18 here, it was partially modified as follows: the SPDE casing was inserted through the diaphragm to a depth of 20 mm, 40 times each time, and the volume of sampling headspace volume was 500 μL, and the extraction speed was maintained at 100 μL s−1. The sample was injected at 20 μL s−1. A blank run was appended to show the absence of carryover effects. To achieve the highest extraction efficiency, some parameters affecting the extraction rate were optimized, such as the type of SPDE sorbent coating, salting-out effect, concentration and volume of HCl added to the sample, number of extraction cycles, extraction temperature, preincubation time, desorption volume, and desorption flow speed.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) and stored at −20 °C. Next, the 8 volatile analyte stock solutions were mixed and diluted with tert-butyl ether:dichloromethane (50
50, v/v) and stored at −20 °C. Next, the 8 volatile analyte stock solutions were mixed and diluted with tert-butyl ether:dichloromethane (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) to prepare a mixed working solution. The mixed working solution was serially diluted with blank rat plasma to prepare standard and QC samples.
50, v/v) to prepare a mixed working solution. The mixed working solution was serially diluted with blank rat plasma to prepare standard and QC samples.The pharmacokinetic parameters of the volatile components were calculated using non-compartmental methods with Phoenix WinNonlin 6.1 (Pharsight, Mountain View, CA, USA) software. The observed value of Cmax was obtained from the observed data and the observed value of AUC0–t was calculated using the trapezoidal rule. Cmax/dose and AUC0–t/dose (the dose-normalized values) were calculated by dividing the observed values of Cmax and AUC0–t by the dose in each extract. Data are expressed as mean ± standard deviation (SD). The statistical differences between groups were determined using t-test, and p < 0.05 were considered statistically significant.
3. Results and discussion
3.1 Identification of multiple volatile components in the VO of YCZFD
In order to improve the reliability of the qualitative analysis of volatile components, we used the automated mass spectral deconvolution and identification system for purification of the chromatographic peak; the relatively “pure” mass spectrogram was compared and matched with that in Wiley libraries (NIST11). Furthermore, values were confirmed by calculating linear retention indices (RI) of the test compounds to n-alkanes (C7–C30) under the same conditions as those of the reference. Furthermore, the RI was compared with values in the literature to accurately identify similar compounds that matched NIST11 well. In addition, we also used as many commercially available standard compounds as possible for comparison and final confirmation. Based on these three approaches, the components in YCZFD VO were identified.A typical chromatographic profile of the VO of YCZFD is presented in Fig. 2. The volatile components in the VO of YCZFD are listed in the order of their elution time in Table 2. Eighty-five volatile components were identified in the VO, which accounted for 95.4% of the total oil composition by normalization of whole chromatographic peak area. Sesquiterpene hydrocarbons (39.5%) and oxygenated sesquiterpene (30.5%) were the most abundant components. In this study, the composition of volatile components in VO of YCZFD was clarified for the first time.
 | ||
| Fig. 2 Total ion compound (TIC) chromatogram of (A) the volatile oil of Yinchenzhufu decoctionand; (B) N-alkanes containing 9 to 17 carbons. | ||
| No. | Components | % | RI | RI lit. | Identif. |
|---|---|---|---|---|---|
| a RI: retention index; RI lit.: retention index of target compound in literature from database; MS: identify by comparing the mass spectrum fragments from the NIST11 database; S: identify by comparing with the standards. | |||||
| 1 | 2-Heptanol | 0.03 | 901 | 886 | RI, MS |
| 2 | Cyclene | 0.04 | 919 | 918 | RI, MS |
| 3 | a-piene | 1.24 | 930 | 931 | RI, MS, S |
| 4 | Camphene | 2.97 | 946 | 943 | RI, MS, S |
| 5 | Benzaldehyde | 0.29 | 956 | 929 | RI, MS |
| 6 | β-Pinene | 0.17 | 973 | 970 | RI, MS |
| 7 | Sulcatone | 0.10 | 982 | 960 | RI, MS |
| 8 | β-Myrcene | 0.34 | 988 | 981 | RI, MS |
| 9 | Pseudolimonen | 0.01 | 1001 | 993 | RI, MS |
| 10 | α-Phellandrene | 0.30 | 1003 | 1007 | RI, MS |
| 11 | α-Terpinene | 0.06 | 1014 | 1017 | RI,MS |
| 12 | β-Cymene | 0.10 | 1022 | 1013 | RI, MS |
| 13 | β-Phellandrene | 7.19 | 1028 | 1030 | RI, MS, S |
| 14 | Eucalyptol | 2.56 | 1029 | 1023 | RI,MS,S |
| 15 | γ-Terpinene | 0.08 | 1055 | 1053 | RI, MS |
| 16 | α-Terpinolene | 0.15 | 1082 | 1080 | RI, MS |
| 17 | 2-Nonanone | 0.08 | 1089 | 1074 | RI, MS |
| 18 | 2,3-Epoxypinane | 0.06 | 1094 | 1095 | RI, MS |
| 19 | β-Linalool | 0.42 | 1098 | 1082 | RI, MS |
| 20 | β-Fenchol | 0.02 | 1115 | 1112 | RI, MS |
| 21 | cis-p-Menth-2-en-1-ol | 0.05 | 1121 | 1118 | RI, MS |
| 22 | trans-p-Ment-2-en-1-ol | 0.04 | 1139 | 1138 | RI, MS |
| 23 | (−)-Camphor | 0.07 | 1142 | 1139 | RI, MS |
| 24 | 2-Norbornanol | 0.04 | 1150 | 1142 | RI, MS |
| 25 | Benzenepropanal | 0.19 | 1157 | 1123 | RI, MS |
| 26 | endo-Borneol | 1.78 | 1168 | 1148 | RI, MS, S |
| 27 | Isomenthol | 0.02 | 1174 | 1174 | RI, MS |
| 28 | (−)-4-Terpineol | 0.19 | 1177 | 1175 | RI, MS |
| 29 | α-Terpineol | 0.60 | 1191 | 1172 | RI, MS |
| 30 | cis-Piperitol | 0.07 | 1204 | 1190 | RI, MS |
| 31 | (R)-(+)-β-Citronellol | 0.29 | 1225 | 1220 | RI, MS |
| 32 | Citral | 0.86 | 1235 | 1241 | RI, MS |
| 33 | Geraniol | 0.25 | 1248 | 1238 | RI, MS |
| 34 | α-Citral | 1.23 | 1266 | 1250 | RI, MS |
| 35 | trans-Cinnamaldehyde | 2.63 | 1268 | 1243 | RI, MS, S |
| 36 | Phellandral | 0.03 | 1273 | 1252 | RI, MS |
| 37 | (−)-Bornyl acetate | 0.22 | 1281 | 1273 | RI,MS |
| 38 | 2-Undecanone | 0.35 | 1291 | 1274 | RI, MS |
| 39 | 2-Undecanol | 0.03 | 1301 | 1294 | RI, MS |
| 40 | Myrtenyl acetate | 0.01 | 1319 | 1306 | RI, MS |
| 41 | δ-EIemene | 0.05 | 1332 | 1334 | RI, MS |
| 42 | Cephreine | 0.15 | 1348 | 1331 | RI, MS |
| 43 | Ylangene | 0.08 | 1363 | 1360 | RI, MS |
| 44 | Copaene | 2.26 | 1371 | 1376 | RI, MS, S |
| 45 | Berkheyaradulene | 0.22 | 1382 | 1416 | RI, MS |
| 46 | β-Elemene | 0.38 | 1385 | 1387 | RI, MS |
| 47 | Cyperene | 0.14 | 1397 | 1390 | RI, MS |
| 48 | Caryophyllene | 1.24 | 1414 | 1421 | RI, MS, S |
| 49 | γ-Elemene | 2.17 | 1426 | 1425 | RI, MS |
| 50 | Humulene | 0.53 | 1449 | 1454 | RI, MS |
| 51 | α-Guaiene | 0.08 | 1454 | 1440 | RI, MS |
| 52 | Calarene | 0.16 | 1468 | 1463 | RI, MS |
| 53 | γ-Muurolene | 0.92 | 1475 | 1471 | RI, M S |
| 54 | Curcumene | 2.86 | 1478 | 1472 | RI, MS, S |
| 55 | β-Selinene | 1.09 | 1483 | 1483 | RI, MS |
| 56 | Zingiberene | 13.96 | 1494 | 1492 | RI,MS,S |
| 57 | α-Farnesene | 1.34 | 1503 | 1499 | RI, MS |
| 58 | β-Bisabolene | 1.30 | 1505 | 1500 | RI, MS |
| 59 | δ-Cadinene | 1.20 | 1514 | 1514 | RI, MS |
| 60 | Calamenene | 0.42 | 1516 | 1517 | RI, MS |
| 61 | β-Sesquiphellandrene | 2.68 | 1522 | 1516 | RI, MS |
| 62 | Ledene | 0.16 | 1524 | 1520 | RI, MS |
| 63 | Selina-3,7(11)-diene | 2.83 | 1532 | 1533 | RI, MS |
| 64 | γ-Selinene | 0.67 | 1536 | 1531 | RI, MS |
| 65 | α-Copaen-11-ol | 0.74 | 1542 | 1541 | RI, MS |
| 66 | Germacrene B | 2.75 | 1553 | 1554 | RI, MS |
| 67 | trans-Nerolidol | 0.42 | 1558 | 1555 | RI, MS |
| 68 | Caryophyllenyl alcohol | 0.08 | 1568 | 1569 | RI, MS |
| 69 | Spathulenol | 0.19 | 1569 | 1569 | RI, MS |
| 70 | Caryophyllene oxide | 0.56 | 1574 | 1575 | RI, MS |
| 71 | Carotol | 0.21 | 1585 | 1594 | RI, MS |
| 72 | Isoaromadendrene epoxide | 0.02 | 1594 | 1590 | RI, MS |
| 73 | Viridiflorol | 0.06 | 1598 | 1594 | RI, MS |
| 74 | Humulane-1,6-dien-3-ol | 0.09 | 1601 | 1606 | RI, MS |
| 75 | α-acorenol | 0.30 | 1610 | 1598 | RI, MS |
| 76 | γ-eudesmol | 0.15 | 1614 | 1626 | RI, MS |
| 77 | α-eudesmol | 0.23 | 1619 | 1637 | RI, MS |
| 78 | (−)-Spathulenol | 1.39 | 1622 | 1619 | RI, MS |
| 79 | Atractylone | 22.28 | 1656 | 1652 | RI, MS, S |
| 80 | Bulnesol | 0.29 | 1660 | 1652 | RI, MS |
| 81 | β-bisabolol | 0.62 | 1665 | 1619 | RI, MS |
| 82 | α-Bisabolol | 0.58 | 1681 | 1683 | RI, MS |
| 83 | Eudesm-7(11)-en-4-ol | 0.56 | 1689 | 1681 | RI, MS |
| 84 | 2,2,7,7-Tetramethyltricyclo[6.2.1.0(1,6)]undec-4-en-3-one | 1.63 | 1724 | 1730 | RI, MS |
| 85 | 2-(4a,8-Dimethyl-1,2,3,4,4a,5,6,7-octahydro-naphthalen-2-yl)-prop-2-en-1-ol | 0.15 | 1756 | 1732 | RI, MS |
| Total | 95.36 | ||||
| Hydrocarbon monoterpenes | 12.65 | ||||
| Oxygenated monoterpenes | 8.27 | ||||
| Sesquiterpene hydrocarbon | 39.51 | ||||
| Oxygenated sesquiterpene | 30.54 | ||||
| Others | 4.39 | ||||
3.2. Quantitation of the 11 major components in YCZFD
The GC-MS/MS method established had good specificity. The calibration curves for all analytes showed good linearity (r2 > 0.9962) and the variation in intra- and inter-batch precisions for all analytes was less than 6.92%. The recovery rate varied from 92.1% to 105%. The corresponding RSDs did not exceed 11.1%. The repeatability (RSD < 3.87%) and stability (RSD < 9.65%) were also within the acceptable limits. The results indicated that the analytical method was sensitive, and reliable for the quantification of the 11 volatile constituents in YCZFD (details are presented in the ESI†).
| Content (μg g−1) | |||||
|---|---|---|---|---|---|
| Components | Batch no. 180611 | Batch no. 180612 | Batch no. 180613 | Batch no. 180614 | Batch no. 180615 |
| a “n = 3”mean that three different samples were analyzed. | |||||
| α-Pinene | 13.8 ± 0.4 | 14.3 ± 0.3 | 13.5 ± 0.5 | 13.5 ± 0.4 | 13.3 ± 0.4 |
| Camphene | 48.6 ± 3.0 | 47.7 ± 2.3 | 47.3 ± 1.0 | 49.4 ± 3.3 | 50.1 ± 2.3 |
| β-Phellandrene | 117 ± 6 | 128 ± 5 | 121 ± 2 | 108 ± 2 | 129 ± 5 |
| Eucalyptol | 23.0 ± 0.5 | 24.6 ± 1.2 | 23.4 ± 2.3 | 21.2 ± 1.8 | 23.7 ± 1.0 |
| Copaene | 11.9 ± 0.5 | 12.8 ± 0.4 | 12.3 ± 1.0 | 11.9 ± 0.6 | 15.1 ± 0.4 |
| Caryophyllene | 13.0 ± 0.4 | 14.2 ± 0.4 | 13.9 ± 0.3 | 12.9 ± 0.2 | 15.2 ± 0.4 |
| Borneol | 21.6 ± 0.9 | 23.9 ± 0.5 | 24.3 ± 1.4 | 22.8 ± 0.9 | 25.4 ± 0.7 |
| Zingiberene | 90.1 ± 2.9 | 112 ± 1 | 109 ± 2 | 85.3 ± 1.3 | 106 ± 1 |
| Curcumene | 24.7 ± 1.0 | 27.2 ± 0.7 | 26.3 ± 0.8 | 23.0 ± 1.2 | 26.8 ± 0.8 |
| trans-Cinnamaldehyde | 67.7 ± 2.1 | 70.3 ± 3.3 | 72.2 ± 1.7 | 68.1 ± 3.3 | 71.6 ± 2.3 |
| Atractylon | 458 ± 18 | 489 ± 18 | 434 ± 21 | 416 ± 17 | 504 ± 20 |
| Content (μg g−1) | |||||
|---|---|---|---|---|---|
| Components | Batch no. 180611 | Batch no. 180612 | Batch no. 180613 | Batch no. 180614 | Batch no. 180615 |
| a “n = 3” mean that three different samples were analyzed. | |||||
| α-Pinene | 14.3 ± 0.4 | 14.8 ± 0.1 | 13.3 ± 0.4 | 13.7 ± 0.4 | 12.8 ± 0.3 |
| Camphene | 47.5 ± 1.3 | 50.1 ± 0.4 | 49.1 ± 0.5 | 48.5 ± 3.1 | 48.9 ± 1.3 |
| β-Phellandrene | 121 ± 4 | 131 ± 1 | 120 ± 2 | 104 ± 2 | 124 ± 4 |
| Eucalyptol | 22.6 ± 0.7 | 24.2 ± 1.6 | 23.8 ± 0.5 | 22.2 ± 1.0 | 23.1 ± 0.4 |
| Copaene | 11.7 ± 0.5 | 12.9 ± 0.3 | 12.0 ± 0.5 | 11.6 ± 0.2 | 14.7 ± 0.1 |
| Caryophyllene | 13.4 ± 0.3 | 13.8 ± 0.7 | 13.9 ± 0.3 | 12.6 ± 0.2 | 15.9 ± 0.9 |
| Borneol | 20.9 ± 0.7 | 24.7 ± 0.4 | 23.5 ± 0.9 | 22.4 ± 0.5 | 26.0 ± 0.7 |
| Zingiberene | 89.1 ± 1.3 | 114 ± 1 | 106 ± 1 | 90.9 ± 0.5 | 107 ± 1 |
| Curcumene | 23.7 ± 0.4 | 28.6 ± 1.8 | 26.3 ± 1.7 | 23.5 ± 0.8 | 27.4 ± 1.0 |
| trans-Cinnamaldehyde | 69.7 ± 1.0 | 72.2 ± 1.4 | 74.9 ± 2.7 | 70.3 ± 1.3 | 76.7 ± 0.4 |
| Atractylon | 463 ± 16 | 504 ± 17 | 421 ± 10 | 438 ± 22 | 519 ± 9 |
3.3. Quantification of the 8 volatile components in YCZFD in rat plasma
3.3.1.1 Stability of zingiberene and curcumene. Zingiberene and curcumene are susceptible to oxidation in rat plasma. To prevent oxidation, ascorbic acid at different concentrations were added as an antioxidant agent under dark conditions. The results showed that the addition of 5 μL of 2 mg mL−1 aqueous ascorbic acid solution to 100 μL of plasma could maintain stable levels of zingiberene and curcumene under various storage and treatment conditions. To prevent the potential degradation in the sample collection stage, 10 μL of 8 mg mL−1 aqueous ascorbic acid solution was immediately added to 200 μL of fresh blood samples, which were then placed on ice and immediately centrifuged to separate the plasma. In addition, all sample preparation procedures were performed under dark conditions.
3.3.1.2 Optimization of the SPDE conditions. The following types of coatings for solid phase dynamic microextraction were tested: the WAX coating (polyethylene glycol), CT-225 coating (25% cyanopropyl/25% phenylpolysiloxane/50% methylpolysiloxane), CT-1701 coating (14% cyanopropyl/86% dimethylpolysiloxane), CT-5 coating (15% diphenyl/95% dimethylpolysiloxane), PDMS coating (PDMS), and PDMS/AC coating (PDMS + 10% active charcoal). Our results showed that PDMS coating achieved the best extraction rate.
Because the salting-out effect usually results in an increase in recovery,19 different amounts of NaCl were added to 100 μL of the spiked plasma standard to optimize recovery. The maximum salting-out effect was achieved with 40 mg of NaCl per 100 μL of plasma.
We also investigated the effect of pH on the extraction efficiency at five pH levels: initial plasma pH, two acidic pH values (achieved by adding 0.1 M HCl or 0.01 M HCl), and two basic pH values (achieved by adding 0.1 M NaOH or 0.01 M NaOH). As a result, the pH had no significant effect on the extraction efficiency of the other 8 components, and the acidic pH (by adding 0.1 M HCl) was used in the study.
According to the peak response, the number of extraction cycles of 50 was considered when the number of cycles ranged between 20 and 70 (Fig. 3A). The extraction temperature of 90 °C was chosen when the temperature ranged between 60 and 100 °C (Fig. 3B). The optimal preincubation time of 5 min was determined when preincubation time ranged between 5 and 20 min (Fig. 3C). The optimum desorption gas volume was considered to be 1 mL when desorption gas volume ranged between 0.25 and 1.00 mL (Fig. 3D), and the optimal desorption gas flow speed was 20 μL s−1 when desorption gas flow speed ranged between 20 and 100 μL s−1 (Fig. 3E).
3.3.2.1 Specificity. Typical MRM chromatograms are shown in Fig. 4. The chromatogram of the blank plasma sample showed no interference peak for both the analytes and IS, and the analytical method displayed good specificity.
3.3.2.2 Linearity, accuracy, and precision. The calibration curves of the analytes showed good linearity (1/x2 weighted regression model) in rat plasma (Table 5). The intra- and inter-batch precision and accuracy are summarized in Table 6. The RE% was within the acceptable criteria of ±15% and the RSD% was less than 15%.
| Components | Calibration curve | r2 | Linear range (ng mL−1) | LLOQ (ng mL−1) |
|---|---|---|---|---|
| Camphene | Y = 0.000248 × X + 1.52 × 10−5 | 0.9913 | 1.00–500 | 1.00 |
| β-Phellandrene | Y = 0.00105 × X + 4.83 × 10−5 | 0.9940 | 1.00–500 | 1.00 |
| Eucalyptol | Y = 0.000171 × X + 4.8 × 10−6 | 0.9943 | 1.00–500 | 1.00 |
| Copaene | Y = 0.000195 × X + 4.16 × 10−7 | 0.9969 | 1.00–500 | 1.00 |
| Borneol | Y = 0.0074 × X + 8.79 × 10−5 | 0.9943 | 0.500–250 | 0.500 |
| Zingiberene | Y = 0.000225 × X − 3E − 05 | 0.9962 | 1.00–500 | 1.00 |
| Curcumene | Y = 0.000334 × X − 2.4E − 06 | 0.9945 | 1.00–500 | 1.00 |
| Atractylone | Y = 0.000485 × X − 0.00057 | 0.9922 | 8.00–4000 | 8.00 |
| Components | Conc. (ng mL−1) | Intra-day (n = 6) | Inter-day (n = 18) | ||
|---|---|---|---|---|---|
| Mean (ng mL−1) | RSD (%) | Mean (ng mL−1) | RSD (%) | ||
| Camphene | 3.00 | 3.17 ± 0.22 | 7.0 | 3.04 ± 0.22 | 7.3 |
| 30.0 | 30.5 ± 2.3 | 7.7 | 30.1 ± 2.2 | 7.4 | |
| 400 | 377 ± 29 | 7.8 | 386 ± 27 | 6.9 | |
| β-Phellandrene | 3.00 | 2.84 ± 0.08 | 2.9 | 2.91 ± 0.18 | 6.1 |
| 30.0 | 28.8 ± 2.2 | 7.5 | 29.5 ± 2.1 | 7.2 | |
| 400 | 380 ± 28 | 7.5 | 380 ± 20 | 5.2 | |
| Eucalyptol | 3.00 | 2.90 ± 0.18 | 6.3 | 2.94 ± 0.20 | 6.9 |
| 30.0 | 30.5 ± 2.3 | 7.6 | 29.9 ± 2.0 | 6.5 | |
| 400 | 383 ± 15 | 4.0 | 388 ± 22 | 5.6 | |
| Copaene | 3.00 | 3.23 ± 0.09 | 2.8 | 3.09 ± 0.21 | 6.8 |
| 30.0 | 28.9 ± 1.8 | 6.1 | 29.2 ± 1.6 | 5.5 | |
| 400 | 404 ± 20 | 5.0 | 396 ± 21 | 5.3 | |
| Borneol | 1.50 | 1.47 ± 0.13 | 9.2 | 1.51 ± 0.11 | 7.3 |
| 15.0 | 14.3 ± 1.2 | 8.4 | 14.6 ± 1.0 | 6.7 | |
| 200 | 195 ± 17 | 8.7 | 199 ± 14 | 6.9 | |
| Zingiberene | 3.00 | 2.86 ± 0.21 | 7.5 | 2.92 ± 0.21 | 7.1 |
| 30.0 | 29.2 ± 1.5 | 5.2 | 30.2 ± 1.7 | 5.7 | |
| 400 | 386 ± 27 | 7.1 | 389 ± 27 | 6.9 | |
| Curcumene | 3.00 | 3.14 ± 0.28 | 8.8 | 2.97 ± 0.25 | 8.6 |
| 30.0 | 29.5 ± 2.0 | 6.6 | 30.3 ± 2.1 | 6.8 | |
| 400 | 423 ± 29 | 6.9 | 404 ± 30 | 7.3 | |
| Atractylone | 24.0 | 25.2 ± 1.6 | 6.3 | 24.6 ± 1.6 | 6.5 |
| 240 | 228 ± 18 | 8.1 | 238 ± 16 | 6.7 | |
| 3200 | 3110 ± 240 | 7.8 | 3080 ± 170 | 5.6 | |
3.3.2.3 Recovery. The mean extraction recoveries of all analytes from rat plasma were 88.2–107% at three QC levels, and the RSD% was less than 15%.
3.3.2.4 Sample stability. All analytes in the rat plasma were stable at room temperature for 6 h, in an autosampler vial for 24 h, at −80 °C for 31 days, and after three freeze–thaw cycles (the RE% of all the analytes ranged from −10.7% to 4.11% and the RSD% was within 11.4%) (Table 7).
| Components | Nominal conc. (ng mL−1) | Room temperature for 6 h | In autosampler vials for 24 h | Three freeze–thaw cycles | −80 °C for 31 days | ||||
|---|---|---|---|---|---|---|---|---|---|
| RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | ||
| Camphene | 3.00 | 10.6 | −0.9 | 4.3 | −2.9 | 4.0 | −0.1 | 6.6 | −4.4 |
| 400 | 4.1 | −4.1 | 7.1 | −1.8 | 2.5 | −2.2 | 7.2 | 1.5 | |
| β-Phellandrene | 3.00 | 7.3 | −6.3 | 7.6 | −3.9 | 6.9 | −5.4 | 2.1 | −7.6 |
| 400 | 2.9 | −9.2 | 9.3 | −2.4 | 2.0 | −6.8 | 7.3 | −3.6 | |
| Eucalyptol | 3.00 | 7.0 | −2.4 | 10.1 | 0.0 | 2.7 | 0.3 | 11.4 | −2.2 |
| 400 | 2.9 | −6.6 | 7.6 | −3.9 | 9.1 | −4.2 | 2.0 | −0.3 | |
| Copaene | 3.00 | 5.1 | −6.9 | 5.4 | −5.5 | 8.8 | −5.0 | 3.3 | −7.9 |
| 400 | 2.4 | −9.8 | 9.4 | −2.7 | 6.6 | 0.1 | 7.3 | −3.7 | |
| Borneol | 1.50 | 6.6 | −6.0 | 3.4 | −6.2 | 5.9 | −3.8 | 3.3 | 2.9 |
| 200 | 5.3 | −8.0 | 7.9 | −6.0 | 4.6 | −6.4 | 6.7 | 2.7 | |
| Zingiberene | 3.00 | 3.9 | −0.7 | 11.0 | −0.3 | 7.5 | −1.3 | 7.4 | 2.1 |
| 400 | 5.7 | −2.2 | 3.1 | −5.3 | 3.3 | −3.0 | 8.6 | −0.5 | |
| Curcumene | 3.00 | 2.6 | −8.8 | 5.6 | −10.7 | 9.7 | −4.8 | 3.7 | −5.5 |
| 400 | 2.8 | −9.2 | 3.0 | −9.4 | 2.9 | −4.5 | 7.0 | −3.4 | |
| Atractylone | 24.0 | 6.0 | −2.8 | 5.7 | −5.6 | 5.8 | 4.1 | 4.0 | −2.2 |
| 3200 | 3.5 | −6.0 | 4.3 | −3.9 | 3.1 | −2.2 | 6.5 | −3.3 | |
The above results showed that the established quantitative method of 8 volatile components from YCZFD in rat plasma met the requirements for biological sample determination.
In the present study, the simultaneous quantitation of eight volatile components in a traditional Chinese medicine formula in plasma using the GC-MS/MS method was reported for the first time. Of these eight components, five components (camphene, β-phellandrene, copaene, zingiberene, and curcumene) had not previously been quantified in the plasma. In the methods reported in the literature, only a small number of components such as borneol,20 atractylon,21 and eucalyptol22 were determined. Compared to the methods reported in the literature, this paper provided a sensitive and reliable method for the simultaneous determination of more volatile components in plasma with a simple, automatic SPDE.
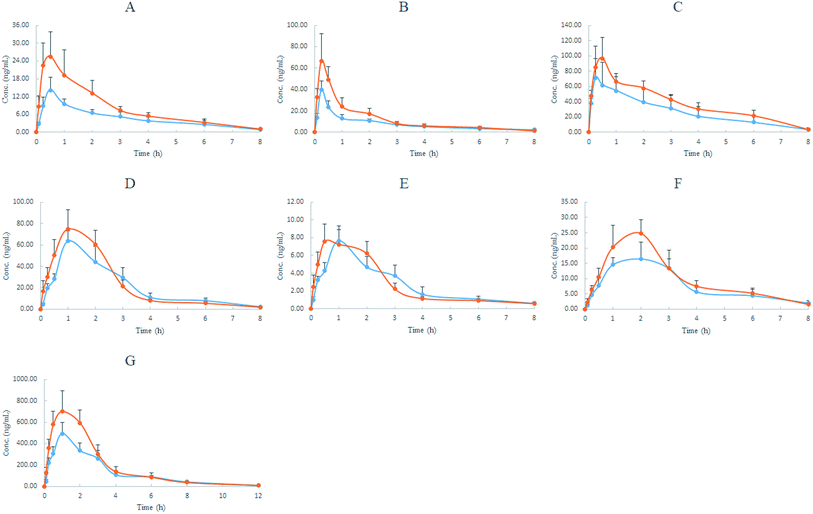
The results showed that after the oral administration of YCZFD and YCZFD VO, these volatile components had similar pharmacokinetic characteristics, such as rapid absorption (tmax ≤ 2 h), especially camphene, β-phellandrene, and eucalyptol (tmax < 0.5 h), as well as rapid elimination (almost T1/2 ≤ 4 h). However, their exposure levels varied widely. The AUC0–t of atractylon was the highest, the AUC0–t of eucalyptol was approximately 13% that of atractylon, and the AUC0–t of other components was less than 10% that of atractylon (Table 8). In the present study, atractylon and eucalyptol from YCZFD//YCZFD VO possessed a shorter T1/2 and a higher dose-normalized Cmax and AUC0–t than that of atractylon from Atractylodis extract21 and that of the eucalyptol monomer,23 respectively. These results indicate that some coexisting components in YCZFD may promote absorption of both components, but speed up their elimination. This mechanism needs to be studied further. After the administration of YCZFD, the exposure (AUC0–t) of most analytes was increased compared with that after YCZFD VO administration. In fact, the increase in exposure of camphene (p < 0.01), β-phellandrene, and atractylon (p < 0.05) was significant. In addition, compared with the YCZFD VO group, the YCZFD group showed short Tmax of curcumene; increased Cmax of camphene, β-phellandrene, copaene, and atractylon; and decreased t1/2 of β-phellandrene and copaene. The synergistic effect of traditional Chinese medicine formulae results from the interaction of multiple components in the formulae. The findings of this study showed that the WE of YCZFD increased the exposure of some volatile components, which may be beneficial to the overall effect of YCZFD.
| Components | Group | Tmax (h) | Cmaxd (ng mL−1) | Cmax/dosee (μg mL−1) | T1/2 (h) | AUC0–td (ng h mL−1) | AUC0–t/dosee (μg h mL−1) |
|---|---|---|---|---|---|---|---|
| a Data are expressed as the mean ± SD, n = 6.b p < 0.05 vs. VO group.c p < 0.01 vs. VO group.d The observed value.e Dose-normalized value. | |||||||
| Camphene | VO | 0.50 ± 0.00 | 14.4 ± 4.1 | 303 ± 86 | 2.20 ± 0.35 | 38.5 ± 9.0 | 811 ± 189 |
| YCZFD | 0.42 ± 0.13 | 26.0 ± 8.0c | 535 ± 165c | 1.78 ± 0.21 | 66.5 ± 17.9c | 1370 ± 377c | |
| β-Phellandrene | VO | 0.25 ± 0.00 | 40.2 ± 4.1 | 332 ± 34 | 3.59 ± 1.48 | 63.7 ± 10.4 | 526 ± 86 |
| YCZFD | 0.29 ± 0.10 | 68.0 ± 23.9b | 581 ± 204b | 1.77 ± 0.19b | 99.2 ± 22.5b | 848 ± 192b | |
| Eucalyptol | VO | 0.42 ± 0.30 | 81.7 ± 19.9 | 3620 ± 881 | 1.84 ± 0.30 | 216 ± 98 | 9560 ± 4340 |
| YCZFD | 0.46 ± 0.10 | 101 ± 27 | 4390 ± 1170 | 1.69 ± 0.23 | 305 ± 43 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 ± 1870 300 ± 1870 |
|
| Copaene | VO | 2.00 ± 0.63 | 17.7 ± 4.2 | 1510 ± 359 | 2.39 ± 0.65 | 64.0 ± 19 | 5470 ± 1620 |
| YCZFD | 1.83 ± 0.41 | 25.1 ± 4.5b | 2110 ± 378b | 1.72 ± 0.17b | 82.2 ± 16.2 | 6910 ± 1360 | |
| Zingiberene | VO | 1.00 ± 0.00 | 63.8 ± 9.6 | 716 ± 108 | 1.76 ± 0.81 | 171 ± 38 | 1920 ± 426 |
| YCZFD | 1.00 ± 0.00 | 75.0 ± 18.3 | 823 ± 203 | 1.59 ± 0.28 | 191 ± 41 | 2120 ± 455 | |
| Curcumene | VO | 1.00 ± 0.00 | 7.66 ± 1.26 | 323 ± 53 | 2.57 ± 0.57 | 21.8 ± 4.2 | 920 ± 177 |
| YCZFD | 0.58 ± 0.20c | 7.94 ± 1.66 | 321 ± 67 | 4.14 ± 0.89 | 22.3 ± 4.7 | 903 ± 190 | |
| Atractylon | VO | 0.92 ± 0.20 | 495 ± 105 | 1070 ± 227 | 1.86 ± 0.34 | 1610 ± 330 | 3480 ± 713 |
| YCZFD | 1.08 ± 0.49 | 721 ± 153c | 1570 ± 334c | 1.84 ± 0.20 | 2230 ± 460b | 4870 ± 1000b | |
In present study, the pharmacokinetic behaviors of multiple volatile components of YCZFD and the characteristics of the influence of water extract on the pharmacokinetics of volatile components were firstly clarified, which can provide valuable information for understanding the potential effect of YCZFD components. However, to date, to the best of our knowledge, there have been no studies on the CYP enzymes and transporters involved in these volatile compounds. The mechanism of the pharmacokinetic interactions between the components of the WE and volatile components of YCZFD is still unclear and requires further research.
4. Conclusions
In the present study, 85 volatile compounds were identified in the VO of YCZFD, and 11 highly abundant volatile components in YCZFD and YCZFD VO were quantified using an established GC-MS/MS method. An HS-SPDE-GC-MS/MS method for the simultaneous quantification of camphene, β-phellandrene, eucalyptol, copaene, borneol, zingiberene, curcumene, and atractylon in rat plasma was developed, which provides a methodological basis for the pharmacokinetic study of volatile components in YCZFD and other related Chinese materia medica. The pharmacokinetic laws of camphene, β-phellandrene, eucalyptol, copaene, zingiberene, curcumene, and atractylon from YCZFD, as well as the characteristics of exposure of volatile components increased by WE of YCZFD, were established. These results elucidate the potentially effective components of YCZFD that can be used to promote progression in the development of YCZFD.Author contributions
Bin Zan performed the experiments and completed this manuscript. Yuanyuan Li guided the experiment. Xiaoshu Sun and Tianming Wang assisted the experiments. Rong Shi guided the experiment and revised the article. Yueming Ma designed the experiment, guided and revised the article.Conflicts of interest
The authors declare no conflict of interests, financial or otherwise.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (81773871).References
- Q. Wang, R. Shi, Y. M. Ma, P. Jiang, J. Zhong, H. Y. Cui, P. Liu and C. H. Liu, Content determination of the major constituents of Yinchenzhufu decoction via ultra high-performance liquid chromatography coupled with electrospray ionisation tandem mass spectrometry, J. Pharm. Biomed. Anal., 2013, 77, 88–93 CrossRef CAS PubMed.
- D. W. Mao, N. Tang, Y. Q. Chen, L. H. Liu, N. Wang and S. Wang, A clinical study on the efficacy of modified Yinchenzhufu decoction in chronic liver failure, Chin. J. Integr. Tradit. West. Med. Liver Dis., 2015, 25, 74–76 Search PubMed.
- C. J. Qu, A. Z. Wu, W. L. Wang, W. Qin and J. Qu, Experimental study of Yinchenzhufu decoction on jaundice animal model with Yin yellow syndrome, J. Liaoning Univ. Tradit. Chin. Med., 2006, 8, 89–90 Search PubMed.
- G. F. Wang, Y. Y. Li, R. Shi, T. M. Wang, Y. F. Li, W. K. Li, M. Zheng, F. B. Fan, J. Zou, B. Zan, J. S. Wu and Y. M. Ma, Yinchenzhufu decoction protects against alpha-naphthylisothiocyanate-induced acute cholestatic liver injury in mice by ameliorating disordered bile acid homeostasis and inhibiting inflammatory responses, J. Ethnopharmacol., 2020, 254, 112672 CrossRef CAS PubMed.
- Q. Wang, P. Jiang, F. Y. Ye, R. Shi, Y. M. Ma, J. Zhong, J. S. Wu, P. Liu, C. H. Liu and Y. Q. Jia, Identification and pharmacokinetics of multiple constituents in rat plasma after oral administration of Yinchenzhufu decoction, J. Ethnopharmacol., 2014, 153, 714–724 CrossRef CAS PubMed.
- J. D. Cha, S. E. Moon, H. Y. Kim, J. C. Lee and K. Y. Lee, The essential oil isolated from Artemisia capillaris prevents LPS-induced production of NO and PGE2 by inhibiting MAPK-mediated pathways in RAW 264.7 macrophages, Immunol. Invest., 2009, 38, 483–497 CrossRef CAS PubMed.
- J. D. Cha, M. R. Jeong, S. I. Jeong, S. E. Moon, J. Y. Kim, B. S. Kil and Y. H. Song, Chemical composition and antimicrobial activity of the essential oils of Artemisia scoparia and A. capillaris, Planta Med., 2005, 71, 186–190 CrossRef CAS PubMed.
- C. Yang, D. H. Hu and Y. Feng, Essential oil of Artemisia vestita exhibits potent in vitro and in vivo antibacterial activity: Investigation of the effect of oil on biofilm formation, leakage of potassium ions and survival curve measurement, Mol. Med. Rep., 2015, 11, 2852–2860 CrossRef CAS PubMed.
- B. Sabulal, M. Dan, A. J. J, R. Kurup, N. S. Pradeep, R. K. Valsamma and V. George, Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity, Phytochemistry, 2006, 67, 2469–2473 CrossRef CAS PubMed.
- G. Singh, I. P. Kapoor, P. Singh, C. S. de Heluani, M. P. de Lampasona and C. A. Catalan, Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale, Food Chem. Toxicol., 2008, 46, 3295–3302 CrossRef CAS PubMed.
- A. J. Akinyemi and P. A. Adeniyi, Effect of essential oils from ginger (Zingiber officinale) and turmeric (Curcuma longa) rhizomes on some inflammatory biomarkers in cadmium induced neurotoxicity in rats, J. Toxicol., 2018, 2018, 4109491 Search PubMed.
- S. Gu, L. Li, H. Huang, B. Wang and T. Zhang, Antitumor, Antiviral, and Anti-Inflammatory Efficacy of Essential Oils from Atractylodes macrocephala Koidz. Produced with Different Processing Methods, Molecules, 2019, 24(16), 2956 CrossRef CAS PubMed.
- K. Vaillancourt, G. LeBel, L. Yi and D. Grenier, Arch. In vitro antibacterial activity of plant essential oils against Staphylococcus hyicus and Staphylococcus aureus, the causative agents of exudative epidermitis in pigs, Microbiol, 2018, 200, 1001–1007 CAS.
- Y. El Atki, I. Aouam, F. El Kamari, A. Taroq, K. Nayme, M. Timinouni, B. Lyoussi and A. Abdellaoui, Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics, J. Adv. Pharm. Technol. Res., 2019, 10, 63–67 CrossRef CAS PubMed.
- C. Pannee, I. Chandhanee and L. Wacharee, Antiinflammatory effects of essential oil from the leaves of Cinnamomum cassia and cinnamaldehyde on lipopolysaccharide-stimulated J774A.1 cells, J. Adv. Pharm. Technol. Res., 2014, 5, 164–170 CrossRef PubMed.
- L. Sun, S. B. Zong, J. C. Li, Y. Z. Lv, L. N. Liu, Z. Z. Wang, J. Zhou, L. Cao, J. P. Kou and W. Xiao, The essential oil from the twigs of Cinnamomum cassia Presl alleviates pain and inflammation in mice, J. Ethnopharmacol., 2016, 194, 904–912 CrossRef CAS PubMed.
- C. J. Qu, A. Z. Wu, W. L. Wang, W. Qin and J. Qu, Study the effect of Yinchenzhufu Decoction against Yin jaundice syndrome in an animal model, Journal of Liaoning College of Traditional Chinese Medicine, 2006, 8, 89–90 Search PubMed.
- K. Heberger, M. Gorgenyi and T. Kowalska, Temperature dependence of Kováts indices in gas chromatography revisited, J. Chromatogr. A, 2002, 973, 135–142 CrossRef CAS PubMed.
- D. Lenz, L. Kroner and M. A. Rothschild, Determination of gamma-hydroxybutyric acid in serum and urine by headspace solid-phase dynamic extraction combined with gas chromatography-positive chemical ionization mass spectrometry, J. Chromatogr. A, 2009, 1216, 4090–4096 CrossRef CAS PubMed.
- M. Z. Hou, L. L. Chen, C. Chang, J. F. Zan and S. M. Du, Pharmacokinetic and tissue distribution study of eight volatile constituents in rats orally administrated with the essential oil of Artemisiae argyi Folium by GC-MS/MS, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2021, 1181, 122904 CrossRef CAS PubMed.
- H. Yan, Y. Sun, Y. Ma, B. Ji, X. Hou, Z. Yu and Y. Zhao, Determination of atractylon in rat plasma by a GC-MS method and its application to a pharmacokinetic study, J. Pharm. Anal., 2015, 5, 327–331 CrossRef CAS PubMed.
- T. T. Zhao, L. L. Zhu, M. Chen and Q. Zhou, Is it appropriate regarding patient preference to take Myrtol standardized enteric-coated soft capsules after a meal rather than at fasted state? A food-drug pharmacokinetic interaction study in healthy Chinese volunteers, Patient Prefer. Adherence, 2016, 10, 2031–2037 CrossRef PubMed.
- C. Sa, J. Liu, Y. Dong, L. Jiang, G. Gentana and A. Wurita, Quantification of eucalyptol(1,8-cineole) in rat serum by gas chromatography-mass/mass spectrometry and its application to a rat pharmacokinetic study, Biomed. Chromatogr., 2021, 35, e5080 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra08584k |
| This journal is © The Royal Society of Chemistry 2022 |