Enhanced photocatalysis activity of ferroelectric KNbO3 nanofibers compared with antiferroelectric NaNbO3 nanofibers synthesized by electrospinning†
Yu Huana,
Xiaohui Wang*a,
Weichang Haob and
Longtu Lia
aState Key Laboratory of New Ceramics and Fine Processing, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, China. E-mail: wxh@mail.tsinghua.edu.cn
bDepartment of Physics, Key Laboratory of Micro-nano Measurement, Manipulation and Physics, Ministry of Education (MOE), Beihang University, Beijing 100191, China
First published on 10th August 2015
Abstract
Perovskite-type alkaline niobate nanofibers were prepared by electrospinning. KNbO3 nanofibers with lower BET exhibit higher photocatalytic efficiency than NaNbO3 with similar phase structure and morphology. The internal electric field, which does not exist in antiferroelectric NaNbO3, induced by spontaneous polarization in ferroelectric KNbO3 can promote the separation and accelerate the movement of photogenerated charge carriers.
Introduction
Since TiO2 was first reported by Honda et al.1 to display photocatalytic activity in hydrogen evolution, semiconductor photocatalysts have been found to have important applications in clean hydrogen energy generation and the treatment of organic pollutants. The study of novel semiconductor photocatalysts, such as Ti-, Nb-, and Ta-based photocatalysts, has been an important issue over the past few decades.2–4 Among these materials, perovskite-type oxides are attractive because of their high stability, good availability and low toxicity under light illumination. It is well known that the efficiency of photocatalysts is determined not only by their band gap but also by their ability to separate photogenerated charge carriers. Ferroelectric materials exhibit considerable photocatalytic performance because of the existence of an internal electric field created by spontaneous polarization. The internal electric field can effectively promote the separation of electrons and holes and accelerate the movement of electrons and holes in opposite directions.5 Thus, ferroelectric materials, such as PSZT6 and BiFeO3,7 have attracted a lot of attention in recent decades. However, these materials unfortunately contain large quantities of the toxic elements such as Pb and Bi. Environmentally friendly ANbO3 (A = K, Na) has attracted substantial interest among researchers and equipment designers because of its unique physical properties.8,9 Although a few studies have focused on the photocatalytic activity of ANbO3 powders,10–13 they do not shed any light on the correlation between the enhanced photocatalytic activity and the internal electric field in the ferroelectric material.In the light of scientific interest and potential commercial applications, the photocatalytic activities of previously reported ANbO3 micropowders are still not high enough because of their low Brunauer–Emmett–Teller (BET) surface areas.10–12 Recently, one-dimensional (1D) nanostructures are expected to result in considerably higher photocatalytic activities due to their large surface-volume ratio and high surface activity.14,15 Compared to hydrothermal methods16 and molten salt methods,17 electrospinning is the simplest and most versatile technique capable of generating nanofibers. Moreover, the electrospun nanofibers have a high aspect ratio, controllable fiber diameters and a precise stoichiometric chemical composition.18
In this study, ANbO3 nanofibers were prepared by a sol–gel-based electrospinning technique. We applied ANbO3 nanofibers in the degradation of rhodamine B (RhB), which is a preventative dye pollutant used in the determination of photocatalytic activity. To the best of our knowledge, this study is the first report on ANbO3 electrospun nanofibers in photocatalysis. Our experimental results indicate that higher photocatalytic activity could be obtained in ferroelectric KNbO3 nanofibers compared with antiferroelectric NaNbO3 nanofibers because of the internal polar field induced by spontaneous polarization. The synthesis and characterization of KNbO3 and NaNbO3 nanofibers are shown in the ESI.†
Results and discussion
KNbO3 and NaNbO3 binder-containing fibers after drying at 80 °C for 5 h in a vacuum oven are abbreviated as KN-NF and NN-NF, respectively. Fig. 1b displays the Fourier-Transform Infrared (FTIR) spectra of KNbO3 samples and raw materials. The characteristic band at ca. 1620 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of acetylacetone. 2-Methoxyethanol exhibits two C–O stretching modes, one at ca. 1124 cm−1, which is characteristic of an ether with a –CH2–O–CH2– group, and another at ca. 1066 cm−1, which is characteristic of a primary alcohol.19 It is difficult to detect these three peaks in the FTIR spectra of KN-NF and NN-NF, which illustrates that the solvent volatilizes completely. The double bands at ca. 1105 and 1066 cm−1 of niobium ethoxide do not appear in the spectra of KN-NF and NN-NF, which demonstrates that niobium ethoxide is decomposed to niobium hydroxide. The bands at ca. 1574 and 1425 cm−1 are assignable to stretching vibrations and antisymmetric stretching vibrations of carboxyl groups, respectively, which appear in the FTIR spectra of KN-NF and NN-NF and demonstrate that alkaline acetates exist in KN-NF and NN-NF.
O stretching vibration of acetylacetone. 2-Methoxyethanol exhibits two C–O stretching modes, one at ca. 1124 cm−1, which is characteristic of an ether with a –CH2–O–CH2– group, and another at ca. 1066 cm−1, which is characteristic of a primary alcohol.19 It is difficult to detect these three peaks in the FTIR spectra of KN-NF and NN-NF, which illustrates that the solvent volatilizes completely. The double bands at ca. 1105 and 1066 cm−1 of niobium ethoxide do not appear in the spectra of KN-NF and NN-NF, which demonstrates that niobium ethoxide is decomposed to niobium hydroxide. The bands at ca. 1574 and 1425 cm−1 are assignable to stretching vibrations and antisymmetric stretching vibrations of carboxyl groups, respectively, which appear in the FTIR spectra of KN-NF and NN-NF and demonstrate that alkaline acetates exist in KN-NF and NN-NF.
KN-NF was calcined at 500 °C, 600 °C, 700 °C, and 800 °C in air, which are abbreviated as KN-NF-500, KN-NF-600, KN-NF-700, and KN-NF-800, respectively. In the KN-NF thermogravimetric-differential scanning calorimetry (TG-DSC) curves, as shown in Fig. 1a, the endothermic peak at 130 °C in the DSC curve should be attributed to the decomposition of CH3COOK. Subsequently, about 30% weight loss is observed at 280 °C in the TG curve accompanied by a corresponding endothermic peak in the DSC curve, which results from the combustion of PVP. The weak endothermic peak at ca. 400 °C should be assigned to the elimination of water of crystallization in niobium hydroxide, because a part of the water of crystallization is difficult to be eliminated until 400 °C. The endothermic peak at ca. 580 °C should be ascribed to the crystallization of KNbO3. The bands at about 750 cm−1 and 850 cm−1 in FTIR spectrum of the KN-NF-500 are characteristic bands of the resident organism. All the characteristic peaks that correspond to organic functional groups in the FTIR spectrum have disappeared for KN-NF-600. The band around 500 cm−1 can be assigned to the edge-shared NbO6 octahedron and the broad, strong band centered at ca. 623 cm−1 represents O–Nb–O stretching vibrations in the corner-shared NbO6,12 which demonstrates that a perovskite structure forms when KN-NF is calcined above 600 °C, in agreement with the X-ray diffraction (XRD) patterns shown in Fig. 1c. KNbO3 could be well indexed to the orthorhombic phase with JCPDS no. 71-0946 without a second phase. The endothermic peak at ca. 670 °C should be ascribed to the grain growth of KNbO3, because no significant change appears in the FTIR spectra and XRD patterns. Because the crystallinity of KN-NF-600 is low, as seen from the Raman spectra (Fig. S1a†), the calcination temperature is designated to be 700 °C. The weak endothermic peak at ca. 860 °C should be attributed to the fracture of the nanofibers.
The TG-DSC curve of NN-NF shows similar features to that of KN-NF except for a few differences. First, the combustion of PVP occurs from ca. 200 to 380 °C accompanied by an endothermic peak in the DSC curve. Second, the decomposition of CH3COONa is assigned to the endothermic peak at ca. 390 °C in the DSC curve because CH3COONa decomposes at a higher temperature than CH3COOK. Third, the crystallization of NaNbO3 should occur at ca. 580 °C accompanied by a corresponding endothermic peak. To determine the calcination temperature, NN-NF was calcined at 400 °C, 500 °C, 600 °C, and 700 °C in air, which are abbreviated as NN-NF-400, NN-NF-500, NN-NF-600, and NN-NF-700, respectively. From the FTIR spectra shown in Fig. 1e, the bands of organic functional groups do not disappear until calcination at 500 °C. There are two different phases of NaNbO3, antiferroelectric (Pbma, JCPDS no. 89-8957) and ferroelectric (P21ma, JCPDS no. 82-0606).20 The XRD patterns of NN-NF calcined above 500 °C could be well indexed to the space group Pbma, as displayed in Fig. 1f, which demonstrates that NaNbO3 nanofibers exhibit antiferroelectric properties. The characteristic peaks of NbO6 octahedra distinctly appear when NN-NF is calcined at 600 °C, as seen from the Raman spectra (Fig. S1b†). Therefore, the best calcination temperature for the treatment of NN-NF is 600 °C.
From the scanning electron microscopy (SEM) images, shown in Fig. 2a1 and b1, it can be inferred that KN-NF and NN-NF have relatively uniform diameters of ca. 500 nm. After calcining, the surface of the nanofibers is not quite smooth because of grain growth at high temperature, as shown in Fig. 2a2 and b2. These nanofibers compactly stack together with small nanoparticles, as shown in the transmission electron microscopy (TEM) bright-field images presented in Fig. 2a3 and b3. The nanofibers are polycrystalline as inferred from the selected-area electron diffraction (SAED) patterns with several sets of spot patterns obtained from partial nanofibers, as shown in the insets of Fig. 2a3 and b3. Moreover, the regular atomic arrangement, as depicted in high-resolution transmission electron microscopy (HRTEM) images in Fig. 2a4 and b4, indicates that the synthesized nanofibers have good crystallinity. The crystal lattice spacings of 0.406 nm and 0.285 nm correspond to the (011) and (200) crystal planes of orthorhombic KNbO3, respectively, which agree well with the results obtained from the SAED pattern shown in the inset. The angle between adjacent spots labeled in the SAED pattern is 90°, which is identical to the theoretical value of the angle between the (011) and (200) planes of orthorhombic KNbO3. Analogously, the crystal lattice spacings of 0.391 nm and 0.389 nm in NN-NF-600 are well ascribed to the (101) and (040) crystal planes of orthorhombic NaNbO3, respectively. To visualize the grain size of nanofibers, dark-field TEM (DFTEM) images are illustrated in Fig. 2. The DFTEM images in Fig. 2a5 and a6 were obtained using the A and B reflections shown in the inset of Fig. 2a3, respectively. Typically, the nanofibers are stacked one by one with small nanoparticles together with a grain size of about 200 nm. Similar conclusions apply to NN-NF-600. Furthermore, energy-dispersive X-ray spectroscopy (EDS) mapping indicates that all the chemical elements are distributed homogeneously, as shown in Fig. S2.†
The photocatalytic activity of the as-prepared samples was evaluated using the degradation of an RhB aqueous solution under UV-vis light illumination. The change in the relative concentration of the RhB aqueous solution as a function of the irradiation time (t) is shown in Fig. 3a. For the blank experiment, the degradation of the RhB aqueous solution under UV-vis light illumination is negligible. The concentration of the RhB aqueous solution decreases with an increase in reaction time, which demonstrates that the RhB molecule is decomposed. The decomposition rate declines because the concentration of the RhB aqueous solution decreases. To evaluate the photoreactivity quantitatively, the reaction rate constants were calculated and are depicted in Fig. 3b. The photodegradation of RhB in the presence of KNbO3 and NaNbO3 can be considered as a pseudo-first-order reaction13 and its kinetics can be expressed as follows:
| C = C0e−kt |
| Sample | BET surface area (m2 g−1) | Band gap (eV) |
|---|---|---|
| KN-NF-700 | 10.098 | 3.626 |
| NN-NF-600 | 15.668 | 3.804 |
 | ||
| Fig. 4 (a) Top view of an individual KNbO3 nanofiber, (b) displacement–voltage curve (black line) and d33-voltage curve (red line). | ||
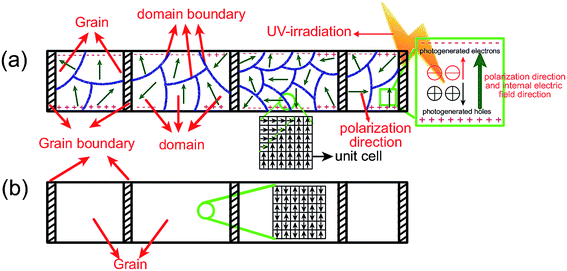 | ||
| Fig. 5 Schematic diagram of the domain structure and spontaneous polarization direction in a unit cell in (a) KN-NN-700 and (b) NN-NF-600. | ||
Conclusion
KNbO3 and NaNbO3 nanofibers with uniform diameters of ca. 500 nm were prepared by sol–gel-based electrospinning. Perovskite-type KNbO3 and NaNbO3 nanofibers were well crystallized after being calcined at 700 and 600 °C, respectively. The nanofibers are composed of small nanoparticles that are compactly stacked one by one, as revealed by DFTEM analysis. Ferroelectric KNbO3 nanofibers, whose ferroelectric properties were confirmed by modified AFM, with lower BET surface areas exhibit higher photocatalytic activity than antiferroelectric NaNbO3 nanofibers with a similar phase structure and micromorphology. It was found that spontaneous polarization in the ferroelectric domain can induce an internal polar electric field, which can promote the separation of photogenerated electrons and holes and accelerate the movement of electrons and holes in opposite directions. In addition, KNbO3 and NaNbO3 nanofibers with good availability and low toxicity display high stability under irradiation. Therefore, there is a potential to develop nanoscale multifunctional devices because KNbO3 has applications in actuators, sensors, transducers, and capacitors.Acknowledgements
The study was supported by the Ministry of Sciences and Technology of China through the National Basic Research Program of China (973 Program 2015CB654604), the National Natural Science Foundation of China for Creative Research Groups (Grant No. 51221291), and the National Natural Science Foundation of China (Grant No. 51272123, 51332002), and also supported by CBMI Construction Co., Ltd W. Hao thanks the National Natural Science Foundation of China for partial support to this study (Grant Nos. 51272015, 51472016).References
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- M. R. Hoffmann, S. T. Martin, W. Y. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- Q. J. Xiang, J. G. Yu and M. Jaroniec, Chem. Soc. Rev., 2012, 41, 782–796 RSC.
- Y. Inoue, Energy Environ. Sci., 2009, 2, 364–386 CAS.
- J. Jiang, K. Zhao, X. Xiao and L. Zhang, J. Am. Chem. Soc., 2012, 134, 4473–4476 CrossRef CAS PubMed.
- Y. Inoue, K. Sato and K. Sato, J. Chem. Soc., Faraday Trans. 1, 1989, 85, 1765–1774 RSC.
- F. Gao, Y. Yuan, K. Wang, X. Chen, F. Chen, J.-M. Liu and Z. Ren, Appl. Phys. Lett., 2006, 89, 102506 CrossRef PubMed.
- P. Günter, Phys. Rep., 1982, 93, 199–299 CrossRef.
- J. Richter, A. Steinbrück, M. Zilk, A. Sergeyev, T. Pertsch, A. Tünnermann and R. Grange, Nanoscale, 2014, 6, 5200–5207 RSC.
- G. Q. Li, T. Kako, D. F. Wang, Z. G. Zou and J. H. Ye, J. Phys. Chem. Solids, 2008, 69, 2487–2491 CrossRef CAS PubMed.
- L. S. Yan, J. Zhang, X. M. Zhou, X. X. Wu, J. Y. Lan, Y. S. Wang, G. Liu, J. G. Yu and L. J. Zhi, Int. J. Hydrogen Energy, 2013, 38, 3554–3561 CrossRef CAS PubMed.
- T. T. Zhang, K. Zhao, J. G. Yu, J. Jin, Y. Qi, H. Q. Li, X. J. Hou and G. Liu, Nanoscale, 2013, 5, 8375–8383 RSC.
- J. Y. Lan, X. Zhou, G. Liu, J. G. Yu, J. C. Zhang, L. J. Zhi and G. J. Nie, Nanoscale, 2011, 3, 5161–5167 RSC.
- Y. Wang, X. Zhan, F. Wang, Q. Wang, M. Safdar and J. He, J. Mater. Chem. A, 2014, 2, 18413–18419 CAS.
- N. C. Hildebrandt, J. Soldat and R. Marschall, Small, 2015, 11, 2051–2057 CrossRef CAS PubMed.
- Y. Xu, Q. Yu and J. F. Li, J. Mater. Chem., 2012, 22, 23221–23226 RSC.
- L. Li, J. Deng, J. Chen, X. Sun, R. Yu, G. Liu and X. Xing, Chem. Mater., 2009, 21, 1207–1213 CrossRef CAS.
- X. F. Lu, C. Wang and Y. Wei, Small, 2009, 5, 2349–2370 CrossRef CAS PubMed.
- C. Guha, J. M. Chakraborty, S. Karanjai and B. Das, J. Phys. Chem. B, 2003, 107, 12814–12819 CrossRef CAS.
- T. Y. Ke, H. A. Chen, H. S. Sheu, J. W. Yeh, H. N. Lin, C. Y. Lee and H. T. Chiu, J. Phys. Chem. C, 2008, 112, 8827–8831 CAS.
- G. Bastard, E. Mendez, L. Chang and L. Esaki, Phys. Rev. B: Condens. Matter Mater. Phys., 1983, 28, 3241 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra13680f |
| This journal is © The Royal Society of Chemistry 2015 |



