Production of 2-hydroxyacetylfuran from lignocellulosics treated with ionic liquid–water mixtures
Abstract
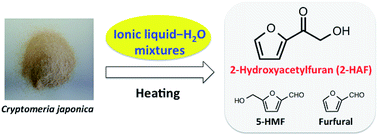
Japanese cedar (Cryptomeria japonica) was treated with 12 ionic liquid (IL)–water mixtures at 120 °C for 1 h. Production of 5-hydroxymethylfurfural, furfural and 2-hydroxyacetylfuran (2-HAF) was observed by HPLC and GC-MS. This is the first report to identify 2-HAF from lignocellulosics using ILs. The optimal IL–water mixture was found to be a 90% pyridinium chloride and 10% water w/w solution, although any IL–water mixture that contained pyridinium or imidazolium salts produced all three compounds in varying yields.

 Please wait while we load your content...
Please wait while we load your content...