 Open Access Article
Open Access ArticleA review on recent advances of cellulose acetate membranes for gas separation
Zunara Bashirab,
Serene Sow Mun Lock *ab,
Noor e Hiraab,
Suhaib Umer Ilyas
*ab,
Noor e Hiraab,
Suhaib Umer Ilyas c,
Lam Ghai Limd,
Irene Sow Mei Locke,
Chung Loong Yiin
c,
Lam Ghai Limd,
Irene Sow Mei Locke,
Chung Loong Yiin fg and
Mehtab Ali Darbanab
fg and
Mehtab Ali Darbanab
aDepartment of Chemical Engineering, Universiti Teknologi PETRONAS, 32610 Seri Iskandar, Perak Darul Ridzuan, Malaysia. E-mail: sowmun.lock@utp.edu.my
bCentre of Carbon Capture, Utilisation and Storage (CCCUS), Universiti Teknologi PETRONAS, 32610 Seri Iskandar, Perak Darul Ridzuan, Malaysia
cChemical Engineering Department, University of Jeddah, 23890 Jeddah, Kingdom of Saudi Arabia
dDepartment of Electrical and Robotics Engineering, School of Engineering, Monash University Malaysia, Jalan Lagoon Selatan, 47500, Bandar Sunway, Selangor, Malaysia
eGroup Technical Solutions, Project Delivery and Technology Division, PETRONAS, Kuala Lumpur 50088, Malaysia
fDepartment of Chemical Engineering and Energy Sustainability, Faculty of Engineering, Universiti Malaysia Sarawak (UNIMAS), 94300, Kota Samarahan, Sarawak, Malaysia
gInstitute of Sustainable and Renewable Energy (ISuRE), Universiti Malaysia Sarawak (UNIMAS), 94300, Kota Samarahan, Sarawak, Malaysia
First published on 18th June 2024
Abstract
This review thoroughly investigates the wide-ranging applications of cellulose-based materials, with a particular focus on their utility in gas separation processes. By focusing on cellulose acetate (CA), the review underscores its cost-effectiveness, robust mechanical attributes, and noteworthy CO2 solubility, positioning it as a frontrunner among polymeric gas separation membranes. The synthesis techniques for CA membranes are meticulously examined, and the discourse extends to polymeric blend membranes, underscoring their distinct advantages in gas separation applications. The exploration of advancements in CA-based mixed matrix membranes, particularly the incorporation of nanomaterials, sheds light on the significant versatility and potential improvements offered by composite materials. Fabrication techniques demonstrate exceptional gas separation performance, with selectivity values reaching up to 70.9 for CO2/CH4 and 84.1 for CO2/N2. CA/PEG (polyethylene glycol) and CA/MOF (metal–organic frameworks) demonstrated exceptional selectivity in composite membranes with favorable permeability, surpassing other composite CA membranes. Their selectivity with good permeability lies well above all the synthesised cellulose. As challenges in experimental scale separation emerge, the review seamlessly transitions to molecular simulations, emphasizing their crucial role in understanding molecular interactions and overcoming scalability issues. The significance of the review lies in addressing environmental concerns, optimizing membrane compositions, understanding molecular interactions, and bridging knowledge gaps, offering guidance for the sustainable evolution of CA-based materials in gas separation technologies.
1. Introduction
The escalating carbon dioxide (CO2) emissions in recent years pose a grave environmental threat, primarily contributing to global warming. The concentration of CO2 in the atmosphere is projected to surpass a critical threshold of 417 parts per million (ppm) by 2022, marking a staggering 50% increase from the 278 ppm recorded at the onset of the industrial era.1,2 This surge is predominantly attributed to the widespread utilization of fossil fuels for energy production, underscoring the urgent need for effective strategies to mitigate CO2 emissions.3,4 The primary motivation for CO2 separation from CH4 in natural gas purification is not directly related to environmental remediation, but rather to improve the calorific value and quality of the natural gas product. The presence of CO2 in natural gas can significantly reduce the calorific value and energy content of the fuel, making it less desirable for end-use applications. By selectively removing the CO2 from the natural gas stream, the purity and energy density of the methane-rich natural gas can be enhanced, ensuring it meets the necessary specifications for various applications, such as residential, commercial, and industrial use.5,6In contrast, the separation of CO2 from flue gas streams is more directly tied to environmental concerns and the need to mitigate greenhouse gas emissions. Flue gas, which is the exhaust gas from the combustion of fossil fuels in power plants and industrial facilities, is a major source of anthropogenic CO2 emissions. Capturing and separating the CO2 from these flue gas streams can enable its sequestration or utilization, thereby reducing the overall carbon footprint and contributing to environmental sustainability.7
Efficient separation techniques are essential in this context, driving the exploration of membrane-based CO2/CH4 separation technology. This approach has garnered significant attention due to its notable advantages, including high energy efficiency, minimal capital investment, operational reliability, and simplicity.8 Cellulose acetate (CA) is a compelling material for gas separation membranes, offering a combination of cost-effectiveness, robust mechanical properties, superior fouling resistance, ease of processing, and high CO2 solubility.9 The intrinsic characteristics of CA, such as sustainability and mechanical stability, position it as a prominent candidate for fabricating polymeric gas separation membranes, presenting an eco-friendly alternative to traditional petrochemical-based materials.10 The increasing need for gas separation membranes with enhanced performance is motivated by the prospect of substantially reducing energy consumption in chemical processing.8
Due to their notable chemical resistance and mechanical stability, CA membranes are extensively used in various gas separation applications, such as CO2 capture, hydrogen recovery, and nitrogen generation.11–13 CA possesses notable mechanical properties, including tensile strength and Young's modulus, which contribute to its reputation for mechanical strength compared to other polymers. Studies have reported that CA typically exhibits a tensile strength ranging from approximately 45 to 70 MPa, depending on factors such as the degree of acetylation and processing conditions. Moreover, Young's modulus of CA is usually in the range of 2 to 4 GPa. This range is comparable to polyimides (2–5 GPa) and polysulfones (2–3 GPa), which are known for their mechanical strength and rigidity. These mechanical characteristics position CA as a robust polymer, particularly in comparison to other polymers commonly used in similar applications.14–16
CA membranes for gas separation use various synthesis methodologies to enhance their performance and selectivity. Notably, the grafting of imidazole ionic liquid onto CA films demonstrated excellent CO2 permeation properties, showcasing a significant increase in selectivity compared to pure CA. Gamma irradiation of CA–polyethylene glycol composite membranes also proved effective, enhancing selectivity for CO2/CH4 gas separation. Additionally, the modification of CA films with fluorine treatment showcased a reduction in permeability, indicating potential applications in controlling gas transport. The use of room-temperature ionic liquid membranes, whether homogeneous or biphasic, revealed variations in permeability and selectivity, emphasizing the influence of casting methods on membrane performance.17–20
Different blends, such as CA/polysulfone(CA/PSF), CA/thiazole-based polyimine (PM-4), CA/polyimide (PI), and CA/Pebax, have notably enhanced gas separation performances. The incorporation of polymer has shown remarkable potential in enhancing the gas separation performance of membranes. CA/PI blends exhibited CO2/CH4 selectivity up to 50 compared to pure CA (∼30). CA/PSF blends doubled the CO2/N2 selectivity from 25 to 50. CA/Pebax blend membranes exhibit enhanced efficiency in CO2/N2 separation, with significant improvements in CO2 permeability and selectivity upon the addition of Pebax. CA/Pebax, there is an approximate 25% and 59% increase in CO2/N2 selectivity and CO2 permeability, respectively.21–23 Each blend presents a unique gas permeability sequence, emphasizing their application-specific advantages.
On the exploration of CA-based mixed matrix membranes (MMMs). Noteworthy examples include enhancing mechanical strength and permeability in cellulose acetate/nanoclay (CA/NC) composite membranes.24 The stability and CO2 adsorption performance improvement achieved by incorporating Cu-MOF-GO in CA.25 The studies extend to gas separation characteristics of MMMs based on metal–organic frameworks (MOF) and cellulose acetate/gamma-cyclodextrin MOF, showcasing advancements in CO2/CH4 separation with enhanced selectivity.26 Furthermore, the introduction of nanomaterials like multi-walled carbon nanotubes (MWCNTs) and ZIF-62 glass in cellulose acetate membranes demonstrates promising characteristics for liquid and gas separations.27,28
MMMs composed of CA/MOFs demonstrate enhanced selectivity and permeability for CO2/CH4 separation compared to other composite membranes illustrated in Fig. 7. The position of data for CA on the Robeson plot serves as a useful indicator of its potential as gas separation membranes. For instance, CA/MWCNT and CA/PI are positioned lower on the graph, indicating lower permeability, whereas CA/PEG exhibits greater permeability among the synthesized cellulose acetates. CA/IL and CA/silane show promising permeability potential, positioned between CA/MWCNT and CA/PEG on the Robeson plot. CA/MO and CA/MOF demonstrate exceptional selectivity in composite membranes with favorable permeability, surpassing other composite CA membranes.
As the research progresses, a distinct focus is placed on the challenges associated with experimental scale separation, emphasizing the need for cost-effective and time-efficient alternatives. This prompts a shift towards MD simulations, a powerful tool capable of elucidating physical properties and morphological alterations at the molecular level. The last section includes specific MD simulation studies exploring the performance of cellulose-based materials. For instance, the research a encompasses son CA dissolution behavior29,30 and the compatibility of cellulose acetobutyrate with plasticizers.31 The collective findings highlight the progress in enhancing gas separation efficiency and underscore the persistent challenges that necessitate a holistic approach, combining experimental insights and computational simulations.
This review navigates through cellulose-based materials; this research sets the stage for a comprehensive exploration of their potential applications, innovative compositions, and the critical role of MD simulations in advancing this field. This review comprehensively explores the properties and application of CA. Moreover, various synthesis methodologies employed for CA membranes are discussed, providing an in-depth examination of their application and advancements. The synthesis methods discussed encompass grafting techniques, showcasing their specific application advantages. The review further delves into the advancements of CA-based MMMs, emphasizing mechanical strength, permeability enhancement, stability, and CO2 adsorption performance. Additionally, the challenges linked to experimental scale separation, scalability issues, and the significance of MD simulations in addressing these challenges are discussed. Specific MD simulation studies on cellulose-based materials and their behavior are covered. The review concludes with a light on the difficulties associated with experimental scale separation. Furthermore, it outlines future research directions, emphasizing the need for systematic investigations into long-term stability and offering valuable guidance for advancing gas separation technology.
2. Cellulose acetate properties and applications
Membrane-based CO2/CH4 separation technology has garnered significant interest because of its low start-up costs, excellent energy efficiency, dependability, and ease of use. Higher-performance gas separation membranes are becoming increasingly in demand because of their capability to minimize energy usage in chemical processing.8 The gas separation performance of CA membranes is typically evaluated in laboratory settings using various experimental techniques. One common method involves conducting permeation tests using a gas permeation setup. In this setup, the CA membrane is placed between two chambers, with one chamber containing a feed gas mixture and the other chamber acting as a permeate side or a vacuum. The gas mixture is then allowed to permeate through the membrane under controlled conditions such as pressure, temperature, and gas composition. The permeate gas is collected and analysed to determine the permeability and selectivity of the membrane for different gas pairs, such as CO2/CH4 or O2/N2.32–34 Fig. 1 illustrates the general experimental setup for conducting membrane permeation experiments, depicting the flow of gases and the critical components involved in the separation process.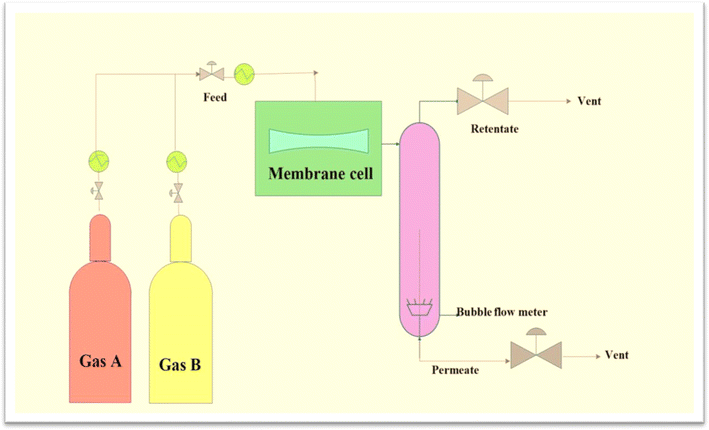 | ||
| Fig. 1 Flow diagram of membrane permeation experiment setup. Reproduced with permission from ref. 34, an open access article under the terms of the Creative Commons Attribution License. | ||
Natural gas processing membranes have dominated the gas separation industry since their initial commercialization for CO2 removal in the 1980s. Over time, these membranes have expanded their utility beyond CO2 removal and are now commercially employed in various phases of natural gas treatment. Notably, cellulose acetate membranes have been extensively utilized for CO2 removal from CH4 since the mid-1980s. Typically, these membranes combine cellulose forms including acetate, diacetate, and/or triacetate. Representing a significant 80% share in the natural gas processing membrane market, their widespread adoption underscores their efficacy and popularity across industries.35
This versatile material is characterized by good mechanical strength, making it suitable for various applications. Notably, CA is generally biodegradable, contributing to its eco-friendly profile.36 Its common uses span across industries, including film production and membrane manufacturing. With a balanced combination of physical properties, CA is a valuable material in diverse applications, aligning with functional and sustainable requirements. Table 1 shows the physical properties of CA.
| Physical properties | Description |
|---|---|
| Density | 1.3 g cm−3 |
| Molecular weight | ∼30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 000 g mol−1 |
| Melting point | 230 °C |
| Colour | White |
| Mechanical strength | Good |
| Biodegradability | Generally biodegradable |
| Common uses | Film production, membranes, etc. |
CA has been used commercially for almost 30 years and its pure gas permeability and selectivity values are quite competitive with other polymers for gas separation applications. CA is preferred over polymeric materials for gas separation applications due to several advantageous characteristics and properties. Its high CO2 solubility and permeability, favourable CO2/CH4 selectivity, and chemical and thermal properties. Its excellent film-forming ability yielding mechanically robust membranes, and cost-effectiveness compared to other polymers.
These advantageous properties, coupled with extensive research efforts on performance improvements through modifications and additives, position CA as a preferred choice, especially for natural gas purification processes involving CO2 separation.10,37,38 CA has a pure gas CO2 permeability of 4.8 Barrer, which is greater than the permeability value of TB BisA-PC, PSF, and PPO38 as illustrated in Fig. 2. CA emerges as the top polymer choice when compared to TB BisA-PC, PSF, and PPO, while Matrimid secures the leading position in the Robeson plot for CO2/CH4 separation.
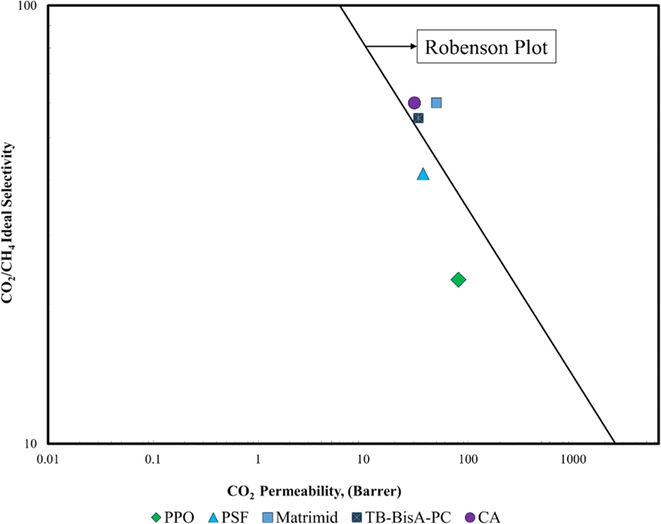 | ||
| Fig. 2 Robeson plot for different polymers.39 | ||
Analysing a 62 year publication trend as depicted in Fig. 3. The number of papers remained relatively constant between 1975 and 2009. However, a significant upturn in CA membrane-related publications occurred post-2009, highlighting CA's renewed interest and importance in designing and modifying separation membranes.
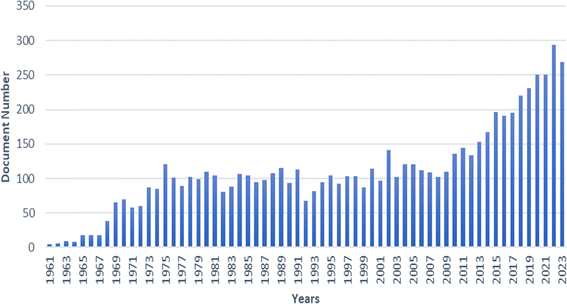 | ||
| Fig. 3 Publications on cellulose acetate membrane through the years (Source: Scopus: 01/02/2024, search in TITLE-ABS-KEY with keywords of cellulose acetate AND membrane). | ||
This surge in research activity may be attributed to the biodegradable nature of CA membranes and the potential for enhancements, particularly by incorporating nanomaterials, to augment membrane performance, coatings, and various other domains.30,40 Several studies have explored the biodegradability of CA through standardized tests and biological assessments, such as assessing the degradation of CA films in diverse environments such as soil, activated sludge, and seawater. It is observed that significant weight losses and structural changes in the CA films indicate biodegradation by microorganisms present in these environments. Furthermore, another study on biodegradability of CA using a combination of enzymatic and microbial tests. It is found that CA was effectively degraded by certain enzymes, such as cellulases and esterases, as well as by various microbial strains, including fungi and bacteria. Carboxymethyl cellulose degrades in a matter of days with the use of enzymes such as cellulase.10,41 The biodegradability of CA can be influenced by various factors, such as the degree of acetylation, crystallinity, and environmental conditions (e.g., temperature, pH, and microbial community).42
Selecting the suitable polymer is critical, considering its compatibility with inorganic fillers and its resistance to plasticization in harsh feed streams. Due to the increasing preference for environmentally friendly and biocompatible materials, cellulose and its derivatives have attracted considerable interest owing to their excellent chemical resistance.11 CA membranes are widely employed in gas separation, and energy generation applications due to their outstanding film-forming ability, high chemical and mechanical stability, elevated hydrophilicity, eco-friendly nature, and cost-effectiveness.38 CA membranes have been used for natural gas purification, flue gas treatment, acid gas removal, and hydrogen/methane separation.
CA is a cost-effective and easily accessible solution. CA improves the material's water binding, solubility, viscosity, film-forming, adhesiveness, swelling, and emulsifying capabilities, making it a versatile choice for various applications in industries such as pharmaceuticals, personal care, and advanced materials.10 However, one of the challenges associated with CA membranes is plasticization. Plasticization is a crucial issue that can significantly impact the performance of CA membranes in gas separation applications. Plasticization is a phenomenon that can occur in CA membranes particularly when exposed to high-pressure CO2 or CO2-rich gas mixtures. It refers to the swelling and disruption of the polymer matrix caused by the penetration and sorption of CO2 molecules, which act as a plasticizer. In CA membranes, the sorbed CO2 molecules interact with the polymer chains, increasing their mobility and altering the free volume distribution within the membrane structure. The sorbed CO2 increases polymer chain mobility and alters the free volume distribution, leading to increased permeability of all gas components and loss of selectivity. This plasticization effect can result in reduced separation performance, long-term stability issues, and operational limitations.9,22,27
3. Cellulose acetate-based membranes: emerging recent trends and advances
Owing to its higher chain mobility and ease of processing, CA is considered an efficient polymer for gas separation.43,44 CA also has a few other benefits, such as high CO2 solubility and production cost. In contrast to other commercial polyimides, CA has been widely utilized for CO2/CH4 separation.45 This review systematically covers CA properties, performances, and applications, offering insights into recent advances in the study of CA-based membranes. The focus is on elucidating the significant role of CA in addressing the methods to enhance CO2 removal and natural gas purification. As the research progresses, a distinct focus is placed on the challenges associated with experimental scale separation, emphasizing the need for cost-effective and time-efficient alternatives. This prompts a shift towards MD simulations.3.1 Synthesis methodology
The membrane-based gas separation method has garnered significant scientific attention in the last two decades due to its advantages over conventional separation technologies, including lower energy consumption and a smaller environmental imprint.46 Extensive research has been done using synthesized CA employing various synthesis methods. The synthesis of CA involves chemically modified cellulose to improve its characteristics and suitability for respective applications. Typically, the synthesis of CA starts with cellulose, a linear polysaccharide composed of glucose units linked by β-1,4-glycosidic bonds. The primary hydroxyl groups (–OH) on the cellulose chain were substituted with acetate groups (–OAc).47A homogeneous CA synthesis method without catalyst addition using the DBU/CO2 switchable solvent system is illustrated in Fig. 4. It was reported as an efficient and sustainable procedure for the homogeneous synthesis of CA with adjustable DS. Vinyl acetate is used in the DBU/CO2 switchable solvent system, and this process makes it simple to recycle the solvents used and results in better material qualities because there is less cellulose degradation.48
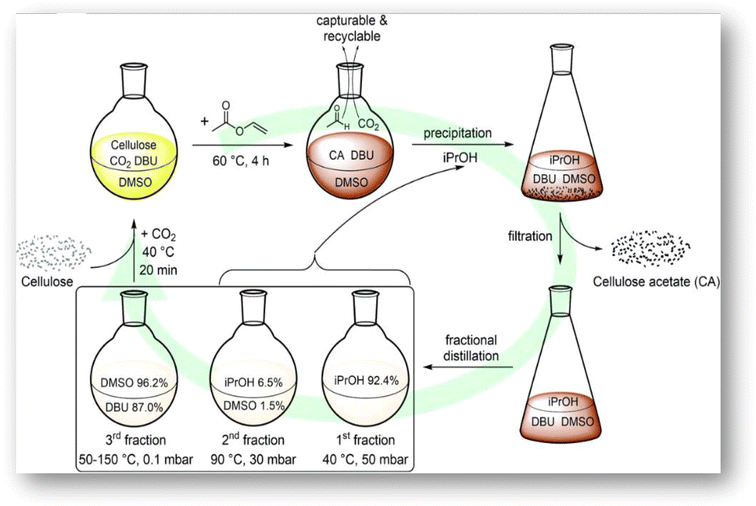 | ||
| Fig. 4 CA synthesis using DBU/CO2 switchable solvent system. Reprinted with permission from ref. 48, an open access article under the terms of the Creative Commons Attribution-Non Commercial 3.0 Unported License. | ||
The synthesis methods for CA can be broadly categorized into solution and heterogeneous processes. For the solution process, cellulose is dissolved in a solvent such as acetone or a mixture of solvents, allowing for a homogeneous reaction. On the other hand, the heterogeneous process involves the direct response from cellulose with acetylating agents in the presence of a catalyst, often under acidic conditions. In a recent study, the gas permeation capabilities of CA-HFM were analysed, unveiling selectivity values for the CO2/N2 and CO2/CH4 gas pairs falling within the range of 0.9 to 1.4, respectively.49
The synthesis and characterization of CA membranes for gas separation, using phase inversion and solution blending techniques, pure and MMMs were synthesized.50 ZIF-8-based membranes CO2/N2 gas separation revealed a small drop in gas selectivity and increased gas permeability.50 Table 2 presents research on CA membrane, covering synthesis methods and performance evaluation.
| Aim | Synthesis method | Operating conditions | Application | Performance/result | References |
|---|---|---|---|---|---|
| CA film preparation via grafting with imidazole ionic liquid | Imidazole ionic liquid synthesis of CA grafted (CA–IL1, CA–IL2, and CA–IL3). At room temperature, pyridine was added to a flask containing CA. Subsequently, imidazole ionic liquid was dropwise injected, and the mixture was stirred at 70 °C | Temperature: 25–70 °C | CO2/CH4 separation | The CA–IL films exhibited CO2 permeation values of 65.5, 105.6, and 88.3 Barrer, with corresponding CO2/CH4 selectivity values of 15.6, 12.6, and 19.2 | Xu et al.17 |
| CA–polyethylene glycol 400 composite membranes subjected to gamma irradiation for gas separation | CA was dissolved using acetone and formamide. Polyethylene glycol or PEG was then added. Then, the mixture was stirred to ensure that the PEG and MBA were entirely dissolved | Temperature: 25–70 °C | CO2/CH4 separation | Irradiated membrane selectivity > non-irradiated membrane | Febriasari et al.18 |
| Evaluation and modeling of CA moisture absorption | A 20% w/w solution of CA in ethyl lactate with 98% purity was produced by progressively adding the powdered CA to the solvent | Temperature: 25–80 °C | — | Moisture absorption of dried CA membranes was observed. The diffusion coefficient (D), which accounts for polymer relaxation, remained constant throughout the process | Khoshtinatet at51 |
| Gas permeability of fluorine-treated CA films | CA films were cast onto a hydrophobized glass surface from a 3 wt% polymer solution in acetone; after separating from the surface, the film was vacuum-dried, forming properties for the specified molecular weight of CA polymer | Temperature: 25 °C | CO2/CH4, H2/CH4, He/CH4 | A decrease in permeability, corresponding to the penetration molecule's increased size, decreased from 3.5 to 0.67 Barrers for CO2 | Belov et al.20 |
| Ionic liquid membranes made of homogeneous and biphasic CA for gas separations | Characterization and preparation of the CA/[emim][SCN] solution, CA was dissolved in [emim][SCN] to a concentration of 12 wt% | Temperature: 50 °C, 60 °C | CO2/CH4 | The casting method membranes resulted in permeances of 22% for CO2 and 73% for CH4 | Khakpay et al.19 |
| Creation of a hollow fiber membrane made of CA for the separation of CO2 from N2 and CH4 | The CA powder was vacuum-dried. Then, the dope solution was prepared, followed by fiber spinning and solvent exchange | — | CO2/N2 CO2/CH4 | The gas pair selectivity of PDMS-coated CAHFM for CO2/CH4 and CO2/N2 was approximately 70.9% and 84.1%, respectively | Mubashir et al.75 |
| The effectiveness of cellulose-based poly-ionic membranes in CO2/N2 and CO2/CH4 gas separation | Synthesis of poly(diallyl dimethyl ammonium) bis(trifluoromethyl sulfonyl)imide (P[DADMA][Tf2N]). Synthesis of CA-based pyrrolidinium derivatized poly(ionic liquid)(P [CA][Tf2N]) | Temperature: 0, 25, 80 °C | CO2/N2 CO2/CH4 | In the investigated mixed gas conditions, introducing the ionic moieties to the polymer structure led to a threefold rise in absolute CO2 permeability values compared to pristine CA, accompanied by a slight decrease in selectivity | Nikolaevaet al52 |
| Impact of evaporation duration on gas separation using CA membrane | The critical polymer concentration in the dope solution was set at 23 wt%. The membranes were then prepared through the dry/wet phase inversion process | Temperature: ambient temperature, 56 °C | CO2/CH4 | As the solvent evaporation time increased, the permeability of both CO2 and CH4 through the CA asymmetric membrane decreased, resulting in an increased selectivity for CO2/CH4 gases | Jami'An et al.53 |
| Hollow-fiber membrane preparation from CA | Fabrication of asymmetric CA hollow-fiber membrane by spinning process | Temperature: 25, 50 °C | CO2/CH4 separation | The permeability of CO2 ranged from 5 to 62 GPU, while CH4 exhibited permeance between 0.1 and 84.5 GPU. Additionally, O2 and N2 displayed permeances in the 0.8–57 and 0.1–60 GPU range, respectively | Pak et al.49 |
| CA dense films modified with silane as materials for acid gas removal | CA powder dried at 100 °C under vacuum was dissolved in acetone to form a 20 wt% solution. A CA-dense film membrane was created | Temperature: 100, 150, 200 °C | Acid gas removal | The selectivity for H2S/CH4 and CO2/CH4 remains same with the modified material. On the other hand, pure CO2 and H2S have individual permeabilities of 139 and 165 Barrers, respectively | Achoundong et al.54 |
Synthesized CA has become essential to gas separation because of its mild chain structure, strong mechanical strength, and outstanding flexibility.55 Comparing the CA–IL films to pure CA (9.26), the CO2/CH4 selectivity of the films was 15.6, 12.6, and 19.2 increasing up to 1.7, 1.4, and 2.1 times, respectively. Studies on CA–polyethylene glycol 400 composite membrane subjected to gamma irradiation for gas separation performance test found that the irradiated membrane possesses a better selectivity than the non-irradiated membrane, as demonstrated by the perfect selectivity of CO2/CH4.18
The gas permeability of fluorine-treated CA films in perfluoro decalin was conducted, and it was found that the permeability of the films decreased when the duration of elemental fluorine treatment was extended.20 For gases, there is a drop in permeability as the size of the penetrant molecule increases (from 3.5 to 0.67 Barrers for CO2 and from 0.55 to 0.14 Barrers for oxygen). Another research on room temperature ionic liquid membranes made of homogeneous and biphasic cellulose acetate for gas separations found that comparing this casting approach to the homogeneous membrane casting method, the membranes produced had 22% and 73% lower CO2 and CH4 permeability, respectively.19 By comparison, the CO2/CH4 selectivity of the membranes made using the phase-inversion casting approach was three times higher than that of the homogeneous membranes. Overall, each research used a different synthesis method and yielded different permeability and selectivity values. The preparation of cellulose acetate membrane using ionic liquid results in good performance and high selectivity.
The results indicate promising performance in terms of CO2/CH4 separation, selectivity, and permeation values. However, the analysis reveals several gaps and areas for improvement. For instance, while CA–IL films exhibit high selectivity, their permeation values may not be optimal for certain applications. The impact of gamma irradiation on membrane selectivity suggests a need for further understanding of irradiation effects on membrane properties. Although CA-based membranes show potential for CO2/CH4 separation, there are challenges related to the trade-off between permeability and selectivity. The study underscores the importance of understanding moisture absorption behavior in CA membranes, as it directly affects their long-term performance and stability. The decrease in permeability observed in fluorine-treated CA films highlights the need for careful consideration of film preparation techniques to achieve desired gas separation properties.
While the introduction of ionic moieties enhances CO2 permeability, it also leads to a decrease in selectivity, indicating a trade-off that requires optimization. The impact of evaporation duration on gas separation suggests the need for precise control over membrane fabrication processes to achieve desired performance characteristics. The variability in permeability across different gases in hollow fiber membranes suggests the importance of tailoring membrane properties for specific gas separation applications. The study indicates the potential of CA-dense films modified with silane for acid gas removal, but further research is needed to optimize selectivity and permeability for practical applications. Thank you for your comment/suggestion.
During the synthesis of CA, several challenges can arise, including the need for precise control over reaction conditions, such as temperature, pressure, and reaction time, to achieve the desired degree of substitution (DS) and molecular weight (MW) of the resulting polymer. Additionally, the purification of CA to remove impurities and by-products generated during synthesis can be challenging, as it requires efficient separation techniques to ensure high product purity.45
The techniques explained in the literature are often preferred over others due to several reasons. For instance, methods such as solution polymerization offer advantages in terms of scalability, reproducibility, and control over the DS and MW of CA. In contrast, techniques like heterogeneous acetylation may involve more complex reaction conditions and purification steps, leading to lower yields and higher production costs. Moreover, solution-based methods allow for the incorporation of additives or modifiers, facilitating the synthesis of CA-based composite materials with tailored properties for specific applications.50
It is necessary to compare the technical difficulties between different methods of CA preparation to identify the most efficient and cost-effective approach. This comparison involves evaluating factors such as reaction efficiency, product yield, purity, scalability, and environmental impact. By assessing these aspects, researchers can determine the most suitable synthesis method based on the desired CA properties and intended applications. Overall solution casting might produce less homogeneous membranes with greater defect rates, but it gives control over thickness while phase inversion provides a regulated pore structure, although it needs to be optimized to have the right gas separation characteristics. On the other hand, in situ polymerization yields accurate control over the DS, but extra processes for membrane production and optimization might be needed.45,50,56
3.2 Polymeric blend
Gas separation using polymer blend membranes has emerged as a dynamic and promising field in membrane technology. Several researchers have been actively exploring enhancing separation performances by integrating various polymers. Various polymeric additions were used to improve the CA matrix performance. Different blends, such as CA/PSP, thiazole-based polyamine CA, CA/Polyimide (PI), CA with acetyl concentration (CA-39/CA-56), and CA/Pebax, have been examined for their potential in gas separation applications present in Table 3. Incorporating PSF into pure CA membranes enhanced gas separation performance, yielding a membrane with a CO2 permeability of 80.51 Barrer and a CO2/H2 selectivity.21 The structure representation of cellulose acetate (CA) and polysulfone (PSF) is shown in Fig. 5.| Polymer blend | Research domain | Application | Operating conditions | Separation performance | References |
|---|---|---|---|---|---|
| CA/polyimide (PI) | Blend membrane of CA and PI | CO2/CH4 | Pressure: 1.5 bar, temperature: 25 °C | CO2 permeability: 19.71 Barrer selectivity: 9.42 | Nayak et al.57 |
| CA/PSF | The addition of PSF improved the separation performance of the pure CA membrane | CO2/H2 separation | Temperature: 25 °C, pressure: 2.5 bar | Permeability of CO2 (P = 80.51 Barrer) selectivity of CO2/H2 = 1.83 of CA/PSF 2 wt% | Douna et al.21 |
| Thiazole-based polyimine CA CA/PM-4 | Thiazole-based polyimine with a thioether linkage was synthesized with glassy CA | CO2/CH4 CO2/N2 | Pressure: 3 bar, temperature: 35 °C | The selectivity ratios for CO2/N2 and CO2/CH4 are 59 and 33.7, respectively, while the gas permeability sequence of blend membranes is CO2 > CH4 > N2. Remarkable permeation (P = 3000 Barrer) of CA/PM-4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3% w/w) 3% w/w) |
Akbarzadeh et al.22 |
| CA/PI | To change the structure and improve the gas separation performance from a polymer to a carbon-based material, CA is added to PI | H2/N2 | Temperature: 600 °C | H2 permeability 5300 Barrer | Li et al.23 |
| CA with 39% acetyl concentration (CA-39) and 56% acetyl content (CA-56) | Membranes were fabricated by incorporating CA-39 and CA-56. The gas separation performance for the CA-39/CA-56 blend membranes was observed | CO2/N2 | Pressure: 0.5–3.5 bar | CO2 permeance 99.26 ± 3.08 GPU N2 permeance of 87.12 ± 0.81 GPU CO2/N2 selectivity of 1.139 ± 0.037 | Jin et al.58 |
| CA/Pebax | CA/Pebax blend membranes for CO2/N2 separation and their effectiveness in separating CO2 from N2 was investigated | CO2/N2 separation | Pressure: 2–10 bar, temperature: 25 °C | The addition of Pebax at up to 8 wt% is added to improve the efficiency in gas separation while the remaining 92 wt% is CA. CA/Pebax: (8wt%) permeability (Barrer) CO2: 2.71 N2: 0.093 with CA/Pebax (8wt%), there is an approximate 25% and 59% increase in CO2/N2 selectivity and CO2 permeability, respectively | Sanaeepur et al.59 |
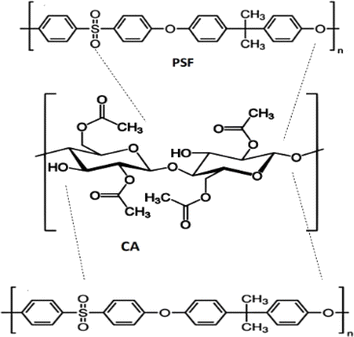 | ||
| Fig. 5 Structure representation of cellulose acetate (CA) and polysulfone (PSF). Reproduced from ref. 21 with permission from Springer Nature, copyright 2022. | ||
Fabricating membranes using thiazole-based polyamine (PM-4) with a thioether linkage combined with glassy CA. Results showed selectivity ratios of CO2/N2 = 59 and CO2/CH4 = 33.7, with gas permeability following the sequence CO2 > CH4 > N2.22 The incorporation of CA into PI to enhance membrane structure led to remarkable H2 permeability of 5300 Barrer in high-temperature H2/N2 separation experiments.23
Membranes are fabricated by incorporating CA with different acetyl concentrations, CA-39 and CA-56.58 The results demonstrate an improvement in performance as compared to pure CA. Studies are conducted on CA/Pebax blend membranes for CO2/N2 separation. The effectiveness of these membranes in separating CO2 from N2 was explored under varying pressures (2–10 bar) and at a temperature of 25 °C. The CO2/N2 selectivity and CO2 permeability for the CA/Pebax (8 wt%) blend increased by approximately 25% and 59%, respectively, According to gas permeation, the addition of PSF to CA shows potential for improved separation performance for CO2/H2 separation, indicating applicability in various gas separation processes. However, there might be a need for further investigation into the optimization of operating conditions and blend ratios to maximize selectivity. The thiazole-based polyimine CA blend membranes exhibit promising selectivity ratios for CO2/N2 and CO2/CH4, suggesting potential for efficient gas separation. However, there is a need for optimization to achieve a more desirable gas permeability sequence for practical applications. The incorporation of CA into PI for structure modification leads to significant improvements in H2 permeability, indicating potential for applications in hydrogen separation processes.
Further research could focus on optimizing the blend composition to enhance both permeability and selectivity. Moreover, the blend of CA with different acetyl concentrations shows reasonable CO2 permeance and selectivity, but adjustments in blend ratios or fabrication techniques may be required to enhance membrane properties.CA/Pebax blend membranes demonstrate notable increases in CO2/N2 selectivity and CO2 permeability, indicating progress in gas separation technology. However, additional research is needed to optimize operational conditions and blend compositions for optimal performance in various gas separation processes.
Overall, the findings present promising advancements in polymer blend membranes for gas separation, further research and optimization are necessary to address challenges such as selectivity improvement, permeability enhancement, and optimization of operational conditions to realize their full potential in practical applications.
By comparing all the studies, it is observed that for CO2 permeability, the thiazole-based polyimine CA blend exhibits the highest, followed by the CA/PSF blend. The CA/PI blend stands out with an exceptional H2 permeability of 5300 Barrer for H2/N2 separation. The thiazole-based polyimine CA blend demonstrates a specific gas permeability sequence of CO2 > CH4 > N2. Selectivity values vary across the blends, with the highest CO2/N2 selectivity observed in the CA/Pebax blend. These comparisons highlight different polymer blends diverse gas separation performances under various operating conditions and applications.
3.3 Mixed matrix membrane
The notable advancements in membrane technology, particularly in the development of MMMs, have unveiled a promising performance in the separation of gases.60 Gas separation membranes play a pivotal role in addressing the increasing demand for efficient and selective separation of gases in various industrial applications, such as natural gas processing, carbon capture, and hydrogen purification. Pursuing enhanced membrane performance has led to exploring innovative polymer blends and nanomaterial incorporations to achieve improved permeability, selectivity, and mechanical properties. In this section, the research works focusing on gas separation through mixed matrix membranes from 2019 to 2024 are highlighted and analyzed.The research on mixed matrix membranes (MMMs) containing CA explores various strategies and materials aimed at improving the efficiency of gas separation. Incorporating MgO nanorods into CA improved gas permeation for H2/CH4, CO2/CH4, and H2/CH4 mixtures at different pressures and temperatures, showcasing the versatility of MMMs.61 Another investigation involved the addition of zeolitic tetrazolate-imidazolate frameworks (ZTIFs) to CA, emphasizing enhanced CO2 separation.62 UiO-66-NH2 nanoparticles in CA MMMs also demonstrated improved CO2 separation in biogas mixtures.63 These studies highlight the potential of MMMs with diverse fillers for specific gas separation applications are shown in Table 4.CA/NC membranes are designed to enhance mechanical strength and permeability for gas separation, with a notable improvement observed in the mechanical strength of the 20 wt% CA/0.5 wt% nanoclay (NC) hollow fiber compared to the 20 wt% CA hollow Fiber without NC.24
| Main polymer | Class | Research domain | Operating conditions | Application | Separation performance | References |
|---|---|---|---|---|---|---|
| CA metal/MgO nanorods | Metal oxide | To fabricate MgO/CA MMMs to enhance the separation of H2/CH4, CO2/CH4, and H2/CO2 | Temperature: 25 °C, pressure: 1–3 bar | H2/CH4 CO2/CH4 H2/CH4 | MgO-5% wt./CA MMMs show CO2/CH4 selectivity of 24.30 and the highest CO2 permeability of 62.90 Barrer. Furthermore, at 77.80 Barrer, MgO-15/CA MMMs showed the greatest H2 permeability | Rajpure et al.61 |
| CA/zeolitic tetrazolate-imidazolate (ZTIFs) | MOFs | Enhancing CO2 separation by incorporating ZTIFs framework additive in CA MMMs | Temperature: 35 °C | CO2/CH4 CO2/N2 CO2/O2 | Permeability (Barrer) CO2: 15.854, CH4: 1.426 N2: 0.870, O2: 2.490 selectivity CO2/CH4: 19.698 CO2/N2: 21.842 CO2/O2: 6.914 | Li et al.62 |
| CA/ZIF-8 | MOFs | Enhanced separation of CO2 through incorporation of inorganic fillers (ZIF-8) in MMMs | Pressure: 1.5–2 bar | CH4/CO2 | CO2 permeability 9.65 Barrer CO2/CH4 selectivity 10.37 | Tanvidkar et al.65 |
| CA/ZIF-62 glass | Composite materials. This type of material is formed by embedding metal–organic framework (MOF) nanoparticles | The creation of composite gas separation membranes using ZIF-62 MOF nano-glass is demonstrated to enhance permeation performance and provide resistance to CO2 plasticization | Temperature: 25 °C | CO2/CH4 | Compared to the original CA membrane, the membrane incorporating 8 wt% ZIF-62 glass demonstrated superior CO2 permeability and CO2/CH4 ideal selectivity, with values of 84.8 Barrer and 35.3, respectively, marking increases of 436.7% and 189.3%, respectively | Mubashir et al.28 |
| CA/UiO-66-NH2 | MOFs | This research aims to enhance CO2 separation in biogas mixtures by developing and evaluating UiO-66-NH2/CA MMMs | Pressure: 1.5–2 bar | CH4/CO2 | CO2 permeability: 11 Barrer CO2/CH4 selectivity: 10 | Tanvidkar et al.63 |
| CA/Cu-MOF-GO | Class of hybrid materials, combining MOFs | The objective is to develop MMMs by adding 1–5 wt% Cu-MOF-GO to CA to achieve improved stability and enhanced CO2 adsorption performance compared to the pure polymer | Temperature: 45 °C, pressure: 15 bars | CO2 separation | CO2 adsorption capacity 4.1 wt% 2 wt% loadings of Cu MOF-GO reveal the highest CO2 uptake was 1.79 mmol g−1 compared to the CO2 adsorption capacity of the CA membrane, which was 0.92 mmol g−1 | Rehman et al.25 |
| CA/gamma-cyclodextrin MOF | MOFs | The is to examine the CO2/CH4 separation permeation characteristics of MMMs based on metal–organic frameworks (MOF) and CA/γ-CD | Pressure 1–5 bar | CO2/CH4 | The CA/γ-CD-MOF MMM with a 0.4 wt% loading attained the highest selectivity for CO2/CH4, reaching 38.49. The CA/γ-CD-MOF MMM with a 0.2 wt% loading achieved the highest CO2 permeability, reaching 18 Barrer under a pressure of 5 bar | Mehmood et al.26 |
| CA/NH2-MIL-53(Al) | MOFs | The investigation focused on process parameters, including CO2 feed composition, feed pressure, and temperature, using the design of experiment (DoE) approach to study CO2 separation from a binary gas mixture of CO2/CH4 in HFMMM | Temperature: 50 °C, pressure: 3 bar | CO2/CH4 separation | The maximum CO2 permeance of 3.82 GPU significantly impacts feed pressure on separation | Mubashir et al.66 |
| CA/NH2-MIL-53(Al) | MOFs | To enhance the CO2 separation by fabricating CA/NH2-MIL-53(Al) hollow fiber MMMs | Temperature: 50 °C, pressure: 3 bar | CO2/CH4 CO2/N2 | CO2/CH4 ideal selectivity of 16.0 and CO2/N2 ideal selectivity of 12.0 permeance (GPU) for pure gas CO2: 6.7 CH4: 0.4 N2: 0.6 | Mubashir et al.67 |
| CA-bentonite clay (Bt) | — | For this study, CO2/CH4 and CO2/N2 separation, the phase-inversion technique was used to create MMMs made CA with varying loadings of (Bt) clay | CO2/CH4 pressure: 2 bar; CO2/N2 pressure: 4 bar | CO2/CH4 CO2/N2 separation | 1% Bt loading achieves 79% greater ideal selectivity for CO2/CH4, and 1 wt% Bt loading at 4 bars exhibits a 123% higher ideal selectivity for CO2/N2 | Jamil et al.34 |
| CA/NC | To enhance mechanical characteristics and increased permeability in CA/NC composite membranes for gas separation | Temperature: 200–300 °C | Gas separation | Superior mechanical strength observed with 20 wt% CA and 0.5 wt% NC. 20 wt% CA/0.5 wt% NC permeances is 6.50 × 10−10 | Juntadech et al.24 | |
| (Multi-walled carbon nanotubes) MWCNTs-based CA | Carbon-based nanofillers | The primary goal is to create CA MMM based on nanocarbons that may be utilized in liquid and gas separation processes | He/N2 pressure: 1.3 bar, temperature: 28 °C; CO2/CH4 pressure 5 bar, temperature: 28 °C | CO2/CH4 He/N2 | The membranes display promising attributes for He/N2 and CO2/CH4 separations, achieving the highest mixed gas separation coefficients recorded at 55.4 and 87, respectively | Esser et al.27 |
| CA butyrate/functionalised multi-walled carbon nanotubes | Carbon-based nanofillers | The functionalized multi-walled carbon nanotubes (MWCNTs-F) were added to the CAB blend polymer matrix; more precisely, the blend MMMs have demonstrated promising performance in separating CO2 and nitrogen N2 | Pressure: 1–3 × 105 Pa | CO2/N2 separation | Solubility coefficient of 7.58 × 1012 cm3 (STP)/cm4 cm Hg. Selectivity of 7.85 ± 1.48 CO2 permeance of 341.15 ± 1.19 GPU | Lee et al.60 |
| CA/palladium acetate | Metal salts | The fabrication of CA/palladium blend membranes. And their contribution to the separation process | — | H2/CO2, H2/CH4, CO2/CH4 | The ideal membrane has selectivity values of 2.02, 68.5, and 34 for H2/CO2, H2/CH4, and CO2/CH4 separation, respectively, and permeability in H2 > CO2 > CH4 | Sajjan et al.64 |
Most of the nanofiller materials mentioned in polymeric membranes are simple nanofillers, such as metal–organic frameworks (MOFs), zeolitic imidazolate frameworks (ZIFs), metal oxides (MgO), clays (Bentonite), and carbon-based nanofillers (MWCNTs). However, the material “CA/Cu-MOF-GO” is a hybrid nanofiller, as it combines a copper-based MOF with graphene oxide (GO). This hybrid material aims to leverage the synergistic effects of the high surface area and porosity of the MOF for gas adsorption and the excellent gas barrier properties and mechanical strength of graphene oxide.25 The incorporation of 1–5 wt% Cu-MOF-GO in CA aimed to enhance stability and CO2 adsorption performance, with 2 wt% loading of Cu-MOF-GO showing the highest CO2 uptake compared to the pristine CA membrane.25 Schematic MMMs of Cu-MOF-GO loading in CA-matrix are shown in Fig. 6.
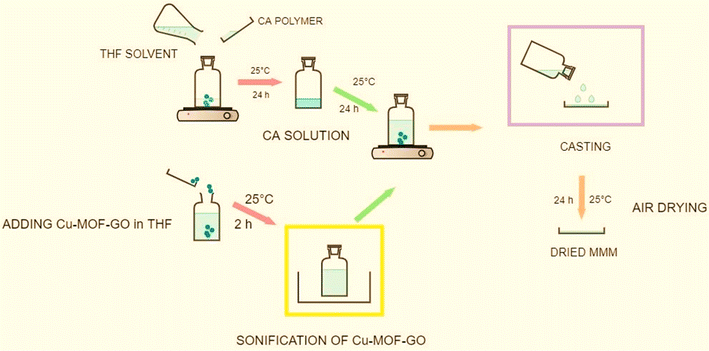 | ||
| Fig. 6 Schematic of MMMs of Cu-MOF-GO loading in CA-matrix. Reproduced with permission from ref. 25, an open access article distributed under the terms of the Creative Commons Attribution License. | ||
Moreover, Optimal compositions, such as 2 wt% Cu-MOF-GO and 0.4 wt% CA/γ-CD-MOF, have demonstrated promising outcomes for CO2 uptake and selectivity, highlighting the effectiveness of synergistic combinations.26 The integration of GO and MOFs has proven particularly successful in surpassing the performance of pristine CA membranes. Furthermore, utilizing MWCNTs has showcased enhanced conductivity and superior gas separation capabilities in specific scenarios. These membranes show promising characteristics for He/N2 and CO2/CH4 separations, with the highest mixed gas separation coefficients achieved.27
The fabrication of composite gas separation membranes utilizing ZIF-62 MOF nano-glass in CA has been illustrated. The composite membrane loaded with 8 wt% ZIF-62 glass exhibits the highest CO2 permeability and CO2/CH4 ideal selectivity, significantly higher than the pristine CA membrane.28 The study of CA/palladium acetate-based blend membranes has focused on H2/CO2, H2/CH4 and CO2/CH4 separations. The membranes exhibit a permeability order of H2 > CO2 > CH4, with the selectivity of 2.02, 68.5, and 34, respectively, for ideal separation.64
The review acknowledges the various types of nanoparticle fillers investigated in CA membranes, including MOFs, carbon-based nanofillers such as graphene and carbon nanotubes, as well as metal oxides. The review emphasizes recent advancements in the field, incorporating findings from studies published in 2022 and 2023 that investigate the incorporation of these nanoparticle fillers in CA membranes for gas separation applications. By evaluating various types of nanoparticles, MOFs emerge as the predominant and most effective choice among the various nanoparticles utilized for gas separation applications.
Bentonite (Bt) clay in CA membranes for CO2/CH4 and CO2/N2 separation has been explored. The 1% Bt loading achieves a 79% greater ideal selectivity for CO2/CH4, while the 1 wt% Bt loading at 4 bars exhibits a 123% higher ideal selectivity for CO2/N2.34 MMMs incorporating TiO2 at concentrations of 0, 5, 10, and 20 wt%, and NiFe2O4 nanoparticles at concentrations of 0, 0.5, 1, 1.5, and 2 wt%, were prepared to examine their CH4/CO2 selectivity and permeability.68 These studies highlight diverse approaches with distinct membrane compositions, offering a range of performances tailored for specific separation applications. The choice of membrane composition depends on the desired separation objectives and operating conditions. This collection of studies shows the diverse applications of MMMs for gas separation, each addressing specific objectives and operating conditions.
While comparing, it has been seen that the CA/NC system enhances mechanical characteristics at elevated temperatures, demonstrating superior strength. Cu-MOF-GO/CA focuses on stability and CO2 adsorption, promising performance under specific temperature and pressure conditions. CA/gamma-cyclodextrin MOF excels in CO2/CH4 separation with remarkable selectivity and permeability. MWCNTs-based cellulose acetate showcases versatility in liquid and gas separation. ZIF-62 glass/CA offers improved permeation and CO2 plasticization resistance. Cellulose acetate/palladium acetate targets H2/CO2, H2/CH4, and CO2/CH4 separations.NH2-MIL-53(Al)/cellulose acetate investigates the impact of CO2 separation on binary mixtures. CA butyrate/functionalized MWCNTs excel in CO2/N2 separation. CA-bentonite achieves superior selectivity in CO2/CH4 and CO2/N2 separations. CA/PEG explores NiFe2O4 and TiO2 nanoparticles impact on permeation.
The studies highlighted significant advancements in gas separation performance, several research gaps and avenues for improvement remain. Most of the research focuses on enhancing selectivity and permeability for specific gas pairs like CO2/CH4 or H2/CH4, neglecting other important gas mixtures relevant to industrial applications. Exploring a wider range of gas combinations and operating conditions could provide insights into the broader applicability of the developed membranes. The scalability and cost-effectiveness of the fabrication methods need consideration. Many studies utilize complex synthesis techniques or expensive additives, which may limit large-scale production and commercial viability. Developing simpler and more cost-effective fabrication methods while maintaining or improving gas separation performance is crucial for real-world applications. Additionally, the long-term stability and durability of the membranes under various operating conditions, including pressure and temperature fluctuations, remain largely unexplored.
Investigating the robustness and reliability of the developed membranes over extended periods is essential to assess their practical feasibility and durability in industrial settings. There is a need for comprehensive techno-economic analyses to evaluate the overall feasibility and competitiveness of the developed membranes compared to existing separation technologies. Assessing factors such as energy consumption, maintenance costs, and lifecycle assessments could provide valuable insights into the economic viability and sustainability of the developed membranes. In short, addressing these research gaps and challenges would pave the way for the development of more practical, efficient, and commercially viable membrane-based gas separation technologies.
CA-based polymers synthesized using both MMMs and polymeric membrane techniques exhibit distinct effectiveness for specific gas pairs, each with its unique advantages. MMMs, incorporating CA, have been shown to enhance gas separation performance, particularly for gas pairs like CO2/CH4 and CO2/N2. The addition of nanomaterials, such as MOFs and carbon nanotubes (CNTs), significantly enhances selectivity and permeability.66 The porous structure of MOFs provides a high surface area for gas adsorption, improving selectivity, while CNTs offer molecular-sized channels that facilitate gas transport, enhancing permeability. These synergistic effects with the CA matrix improve gas separation properties in MMMs. On the other hand, CA-based polymeric membranes also demonstrate effectiveness in gas separation, particularly for acid gas removal and CO2 capture. These membranes exhibit high selectivity for acidic gases like CO2 while maintaining adequate permeability. The inherent properties of CA, such as its high CO2 solubility and chemical stability, contribute to the effectiveness of polymeric membranes.7
The flexibility of the polymer allows for the fabrication of thin, dense membranes, which are favorable for gas separation applications. While MMMs excel in achieving higher selectivity and permeability through the incorporation of nanomaterials, polymeric membranes offer advantages in terms of simplicity of fabrication and suitability for specific gas separation applications like acid gas removal. The choice between MMMs and polymeric membranes depends on the desired gas separation performance. A comparison of the CA composite membrane with the Robenson plot is done as shown in Fig. 7.
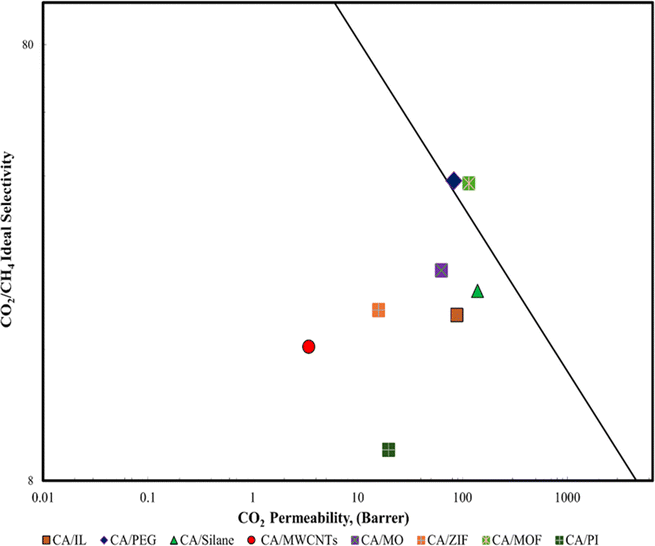 | ||
| Fig. 7 Robeson plot of the different CA composite membranes.39 | ||
In the Robeson plot, the position of the data for CA provides a useful indicator of its potential performance as gas separation membranes. A CA/MWCNT and CA/PI are at a lower position in the graph and demonstrate a lower permeability value while CA/PEG showed greater permeability among all the synthesized cellulose acetates. The CA/IL and CA/Silane also showed good permeability potential as both lie in between CA/MWCNT and CA/PEG. CA/PEG (polyethylene glycol) and CA/MOF demonstrated exceptional selectivity with good permeability as they lie well above all the synthesized cellulose.
3.4 Molecular simulation
Conducting experimental-scale separations can be cost-intensive and time-consuming and often requires specific parameters in challenging conditions, including high pressure and temperature.69 The constructed molecular structures can be adapted to elucidate physical properties and in situ morphological alteration (e.g., energy, free volume, and cavity size distribution) associated with varying membrane operating factors and parameters that are impossible to obtain from experimental work considering the limitation in instrument capacity. Process simulation modulation can provide a valid digital representation of the process.70 Simulation software creates a dynamic environment for the analysis of the process. MD simulations offer practical methods to model structures and simulate absolute gas separation at a molecular level. This is achieved by applying suitable computational algorithms and established empirical functions to explore physical characteristics such as density, cavity sizes, and glass transition temperature.71Examining CA through MD simulation and free-energy calculations to examine cellulose acetate's amorphous state with varying acetyl substitution degrees revealed that within the DS range of 2 to 2.5, CA demonstrates a preference order for solutes as H2O > CO2 > CH4, and emphasizes the dependence of dissolution free energy on DS, especially for H2O.29 Using MD simulation, the impact of substitution positions on the dissolution of CA in different solvents, finding that increased substituents lead to decreased cohesive energy and polarity, enhancing CA solubility.30
The study investigates the impact of adding pristine carbon nanotubes (CNT) and CNT functionalized with –NH2 and –COOH groups on CAB structure for gas separation. It reveals that incorporating these materials significantly increases the free volume fraction (FFV) of the polymer, leading to enhanced adsorption of CO2, CH4, and N2 gases compared to pristine CAB. The simulation results demonstrate stronger interactions between gas molecules and composites, particularly with CO2. The composites exhibit high selectivity for gas separation, especially in CO2/CH4 and CO2/N2 mixtures. While the presence of water vapor minimally affects gas separation, the adsorption of water molecules increases with pressure due to the nature of CAB. Overall, the findings suggest that CNT and F-CNT additives hold promise for developing high-performance composites for CO2 separation, albeit caution is advised when dealing with gas mixtures containing water vapor (Table 5).72
| Polymer | Research domain | Application | Operating conditions | Main findings | References |
|---|---|---|---|---|---|
| CA | MD simulation examines amorphous state with varied acetyl substitutions for CO2, CH4, and H2O | CO2, CH4, H2O | Temperature: 25 to 225 °C | Acetyl substitution (DS) from 2 to 2.5, cellulose acetate served as a more favorable medium for solutes, with a preference order of H2O > CO2 > CH4 | Matsuba et al.29 |
| CA | Examining effects of substitution positions on dissolution in different solvents for CO2, CH4, and H2O MD simulation | CO2, CH4, H2O | — | As the number of substituents increased, the cohesive energy and polarity of CAs decreased, enhancing solubility in solvents. Solubility order: Acetone > acetic acid > water | Shi et al.30 |
| CA butyrate (CAB) | Assessing separation efficiency for CO2/CH4 and CO2/N2 through Grand Canonical Monte Carlo (GCMC) simulations | CO2/CH4 CO2/N2 | Temperature: 27 °C, pressure: 5 bar | Adsorption isotherms, selectivity, and isosteric heats were computed. Thermodynamic parameters and Henry's constant (KH). Were determined | Barzegar et al.72 |
| Poly(ether-block-amide)–CA (Pebax–CA) | Investigating transport properties and morphological structures of CO2, CH4, N2, and H2 gases in hybrid blend MMMs using the material studio software | CO2, CH4, H2 and N2 | Temperature: 25 °C, pressure: 10 bars | Solubility for Pebax: 19.917 (J cm−3)0.5 CA: 18.174 (J/cm3)0.5 and Pebax/CA blend was 18.174 (J cm−3)0.5. Solubility values and selectivity for CO2/CH4, CO2/N2, and CO2/H2 increased with an increasing weight of 10 to 20% NH2-MIL-53 in the membranes. Selectivity CO2/CH4: 132.06 at PCNM 20% CO2/N2: 90.64 at PCM10%, CO2/H2: 15.35 PCNM20% | Salahshoori et al.73 |
| Cellulose acetatobutyrate (CAB) | Assessing compatibilities between CAB and seven plasticizers through MD simulations | — | Initial temperature 298 K, mid-cycle temperature 500 K | The tensile strengths (σ) and Young's moduli (E) of CAB/plasticizer systems follow the order of CAB/A3 > CAB/Bu-NENA > CAB/GAPA, suggesting that CAB/A3 exhibits the highest mechanical strength and deformation resistance among them. Specifically, CAB/A3 demonstrates a tensile strength of 0.497 MPa and a Young's modulus of 0.273 MPa | Wang et al.31 |
| CA | Developing amorphous CA with varying degrees of substitution (DS) to assess changes in chain conformation | — | Temperature 300 K, pressure 1 bar | Hydrogen bonds among hydroxyl groups contributed to cellulose stabilization, while dipolar interactions between acetyl groups played a stabilizing role in cellulose triacetate (CTA). Hydrogen bonds formed between hydroxyl and acetate groups further stabilized partially substituted cellulose acetates | Bacahut et al.74 |
The mechanical properties of CAB with different plasticizers are investigated, seven plasticizers, including bis(2,2-dinitro propyl) formal/acetal (BDNPF/A) or A3, azide-terminated glycidyl azide (GAPA), n-butyl-N-(2-nitroxy-ethyl) nitramine (Bu-NENA), ethylene glycol bis(azidoacetate) (EGBAA), diethylene glycol bis(azidoacetate) (DEGBAA), trimethylol nitromethane tris(azidoacetate) (TMNTA) and pentaerythritol tetrakis (azidoacetate) [PETKAA], were studied for the plasticization of CAB. Identifying CAB/A3 as the system with the highest mechanical strength. The tensile strengths (σ) and Young's moduli (E) of CAB/plasticizer systems follow the order of CAB/A3 > CAB/Bu-NENA > CAB/GAPA, suggesting that CAB/A3 exhibits the highest mechanical strength and deformation resistance among them. Specifically, CAB/A3 demonstrates a tensile strength of 0.497 MPa and a Young's modulus of 0.273 MPa. This information is crucial for designing CAB formulations with enhanced mechanical properties for diverse applications.31 The geometric structures of CAB/plasticizer amorphous models are optimized by geometry optimization to establish the final molecular models as shown in Fig. 8.
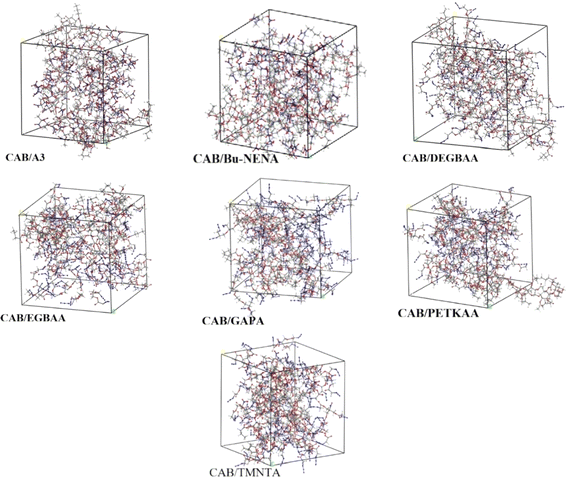 | ||
| Fig. 8 Molecular structures of CAB/plasticizer systems. CAB cellulose acetate butyrate. Reprinted with permission from ref. 31, an open access article under the terms of the Creative Commons Attribution Licence. | ||
In the exploration of hybrid membranes (Pebax–CA) using Material Studio software, the study reveals gas solubility and selectivity values, uncovering a rise in CO2/CH4, CO2/N2, and CO2/H2 selectivity with increasing NH2-MIL-53 loading from 10 to 20%. These insights contribute to understanding gas solubility and selectivity behavior in MMMs.73 Additionally, the study explores changes in chain conformation and interactions in amorphous CA with varying degrees of substitution (DS). The findings highlight the stabilizing roles of hydrogen bonds and dipolar interactions, contributing to a deeper understanding of CA structural dynamics and stability, with potential implications for material design and synthesis.74
All the studies collectively emphasize MD simulation's crucial role in unraveling CA-based materials' diverse properties and applications. MD simulations serve as a powerful tool for gaining insights into the behavior of these materials at the molecular level, offering a virtual platform to explore their interactions under different conditions. In short, these cellulose-based materials cater to distinct applications, showcasing diverse strengths, from enhanced adsorption capacities to efficient gas separation and mechanical robustness. Their collective contributions underscore the broad utility and potential advancements in cellulose-derived materials for various industrial and environmental challenges.
Despite the valuable contributions of these studies, several gaps and opportunities for improvement can be identified. There is a need for further experimental validation of the computational findings to ensure their applicability in real-world scenarios. While the studies provide insights into the solvation behavior and separation efficiency of CA-based materials, there is a lack of investigation into the scalability and practical implementation of these findings. The further exploration of alternative additives or modifications to enhance the performance of CA-based membranes could be a promising avenue for future research. Considering the environmental impact of polymer processing and material disposal, future studies could focus on the sustainability aspects of CA-based materials, such as biodegradability and recyclability. Overall, by addressing these gaps and focusing on practical implementation and sustainability, future research can further advance the understanding and application of CA-based polymers in various fields.
4. Challenges and future prospective
The comprehensive research on cellulose-based materials for gas separation revealed several challenges warranting future advancements. Key challenges include scalability issues, high costs associated with experimental scale separation, and the need for parameters under extreme conditions. These challenges identified through various studies,27,75 highlight the limitations of experimental work in addressing industrial-scale demands.The comprehensive exploration of various polymeric additions to enhance CA membranes reveals promising advancements in gas separation applications. However, amidst these developments, a notable research gap exists in understanding these modified membranes' long-term stability, scalability, and real-world applicability. While the reported studies demonstrate enhanced gas permeability and selectivity under specific operating conditions, there is a need for systematic investigations that address the durability of these membranes over extended periods of use and their performance in diverse industrial environments. Additionally, the scalability of the synthesis methods and the economic feasibility of large-scale production remain areas requiring further attention. Bridging these research gaps will contribute to the practical implementation of modified CA membranes in commercial gas separation processes, paving the way for more sustainable and efficient applications in this field.
Challenges in optimizing mixed matrix membrane (MMM) compositions, ensuring stability, and enhancing performance under diverse operating conditions were identified.25,60 Integrating nanomaterials, such as Cu-MOF-GO and ZIF-62 glass, into cellulose acetate membranes introduces complexities in achieving optimal loading for improved properties.26 Moreover, challenges faced in MMM development, including issues related to materials, scalability, stability, selectivity/permeability trade-offs, interfacial adhesion, economic viability, and long-term durability. Addressing these challenges is crucial for successfully implementing MMMs in practical gas separation scenarios. Moreover, there is a need for comprehensive assessments of MMMs under large-scale industrial conditions to address real-world challenges. The commercially available polymeric membranes, such as CA, exhibit several difficulties, particularly when employed for high-pressure natural gas sweetening applications. The major disadvantages associated with these membranes are their relatively low separation performance, specifically low CO2/CH4 selectivity, and low CO2 permeances. These limitations can be attributed to membrane compaction and plasticization phenomena. The low separation performance and permeances of these membranes necessitate the use of a larger membrane area, leading to increased costs. Furthermore, the issues of compaction and plasticization can result in a shorter membrane lifetime, further contributing to the overall operational expenses.27 Bridging these gaps is essential for developing reliable, scalable, and sustainable MMMs that can meet the demands of practical gas separation applications.
These challenges require a delicate balance between material properties, operating conditions, and application-specific demands. Furthermore, MD simulations can bridge the gap between simulation and experimental outcomes for an accurate representation of the behavior of cellulose-based materials. However, Future research avenues could optimize MMM compositions, explore novel nanomaterials, and refine fabrication techniques to address specific gas separation needs. Advancements in MD simulations offer a promising avenue for understanding intricate molecular interactions, paving the way for the design of tailored cellulose-based materials with enhanced properties. Collaboration between experimental and computational approaches can refine the material design, providing a holistic understanding of cellulose-based materials behavior under varying conditions.
In the context of gas separation applications, the use of CA membranes offers both advantages and limitations. One significant advantage of CA membranes lies in their inherent biocompatibility and eco-friendliness, stemming from their renewable cellulose-based origin. This characteristic makes CA membranes appealing for environmentally conscious applications, aligning with the growing demand for sustainable separation technologies. Additionally, CA membranes exhibit good film-forming properties and can be easily fabricated into thin, uniform films, facilitating cost-effective manufacturing processes.
However, alongside these benefits, several limitations of CA membranes must be considered. One key concern is membrane stability, particularly in harsh operating conditions. CA membranes may suffer from degradation over time when exposed to factors such as high temperatures, aggressive chemical environments, or prolonged exposure to UV radiation. Such degradation can compromise membrane integrity and lead to reduced separation efficiency, highlighting the need for robust membrane materials or protective coatings to enhance stability and prolong membrane lifespan.
The performance of CA membranes in gas separation applications can be influenced by various environmental factors. Changes in temperature, humidity, and gas composition can affect membrane permeability and selectivity, leading to fluctuations in separation efficiency. Additionally, fouling from contaminants in the feed gas stream or deposition of impurities on the membrane surface can further impair membrane performance over time. Understanding these environmental influences and implementing strategies to mitigate their effects are crucial for optimizing CA membrane performance and ensuring long-term operational stability. By systematically evaluating membrane performance under varying temperature, pressure, and gas composition conditions, researchers can identify potential challenges and develop targeted solutions to enhance membrane stability and overall separation efficiency.
Using CA in gas separation membranes presents challenges and opportunities, with plasticization being notable. Challenges arise in optimizing CA membranes for enhanced gas separation performance while mitigating the effects of plasticization. Plasticization occurs when certain gases, typically those with smaller kinetic diameters, permeate the polymer matrix, causing swelling and reduced membrane selectivity. This phenomenon poses a challenge in maintaining membrane integrity and performance over extended periods of use. Studies are actively exploring strategies to overcome plasticization effects, to address the challenges posed by plasticization, future studies should focus on membrane modifications with plasticization-resistant materials or crosslinking agents, exploring advanced membrane architectures like mixed matrix membranes (MMMs) or thin-film composites (TFCs), conducting molecular simulations for a better understanding of CO2-polymer interactions, developing operational strategies like feed pretreatment or pressure cycling, and investigating alternative cellulose-based or bio-derived polymers with improved plasticization resistance.76,77 Conducting computational simulations and molecular dynamics studies can provide insights into the interactions between CO2 molecules and the CA polymer chains, enabling the development of more plasticization-resistant CA membranes. Addressing plasticization is crucial for realizing the full potential of CA membranes in gas separation applications, particularly CO2 capture and natural gas purification, ensuring both efficiency and durability in diverse industrial settings.9,22,27,76,77
5. Conclusion
In conclusion, the review of CA studies helps understand gas separation performance. It illuminates the extraordinary adaptability and potential applications of cellulose-based materials for gas separation. The research showcases notable strides in MMMs, utilizing inventive blends and integrating nanomaterials to improve gas separation efficiency, permeability, and mechanical strength. Moreover, the exploration of polymeric blend membranes, specifically those incorporating cellulose acetate (CA), has showcased promising advancements in gas separation technology. Different blends, such as CA/polysulfone, thiazole-based polyimine CA, CA/polyimide (PI), and CA/Pebax, have notably enhanced gas separation performances. Furthermore, the synthesis of membranes for gas separation has been the subject of extensive research, utilizing various methodologies to improve their performance and selectivity. CA/PEG and CA/MOF composite membranes exhibited remarkable selectivity for CO2/CH4 separation, exceeding other composite CA membranes. These membranes demonstrated exceptional selectivity while maintaining favorable permeability, outperforming the selectivity and permeability trade-off observed in other synthesized cellulose-based membranes.The hurdles associated with experimental scale separation are effectively tackled through MD simulations, presenting a cost-effective and time-efficient alternative for comprehending molecular interactions. Through cost-effective and efficient virtual explorations at the molecular level, simulations contribute valuable insights that aid in optimizing and designing cellulose-based materials for specific industrial and environmental challenges. Overall, the collective findings underscore the broad utility and potential advancements in cellulose-derived materials, emphasizing their role in addressing various scientific and technological needs. While demonstrating advancements in gas separation, the research also underscores persistent challenges, underscoring the pivotal role of MD simulations in advancing our comprehension and application of cellulose-based materials in varied industrial contexts.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We want to acknowledge the financial support provided by the Ministry of Higher Education, Malaysia, under Fundamental Research Grant Scheme (FRGS) (Ref. No. FRGS/1/2022/TK09/UTP/03/3, Cost Centre: 015MA0-153), The Murata Science Foundation (Grant No. 23MP07, Cost Centre: 015ME0-351), Centre of Carbon Capture, Utilisation and Storage (CCCUS), Institute of Sustainable Energy, Universiti Teknologi PETRONAS (Cost Centre: 015NEC-001) and YUTP Fundamental Research Grant (Cost Centre: 015LC0-498).References
- A. Iulianelli, F. Russo, F. Galiano, M. Manisco and A. Figoli, Int. J. Greenhouse Gas Control, 2022, 117, 103657 CrossRef CAS.
- H. Ali, A. S. Maulud, H. Zabiri, M. Nawaz, H. Suleman and S. A. A. Taqvi, ACS Omega, 2022, 7, 9496–9512 CrossRef CAS PubMed.
- U. Naveed, N. E. Mohammad Rozali and S. Mahadzir, Energies, 2022, 15, 8605 CrossRef CAS.
- H. Ali, Z. Zhang and F. Gao, Process Saf. Environ. Prot., 2023, 180, 1053–1075 CrossRef CAS.
- T. E. Rufford, S. Smart, G. C. Watson, B. Graham, J. Boxall, J. D. Da Costa and E. May, J. Pet. Sci. Eng., 2012, 94, 123–154 CrossRef.
- J. Zhang, J. Zhang, H. Bai, Q. Tan, H. Wang, B. He, Y. Xiang and S. Lu, J. Membr. Sci., 2019, 572, 496–503 CrossRef CAS.
- X. Li, Y. Yu, Q. Liu and Y. Meng, J. Membr. Sci., 2013, 436, 202–212 CrossRef CAS.
- W.-g. Kim, J. S. Lee, D. G. Bucknall, W. J. Koros and S. Nair, J. Membr. Sci., 2013, 441, 129–136 CrossRef CAS.
- M. Mubashir, Y. Fong, C. Leng and L. Keong, Int. J. Automot. Mech. Eng., 2018, 15, 4978–4986 CrossRef CAS.
- B. Y. Gul, E. Pekgenc, V. Vatanpour and I. Koyuncu, Carbohydr. Polym., 2023, 121296 Search PubMed.
- D. Aoki, Y. Teramoto and Y. Nishio, Biomacromolecules, 2007, 8, 3749–3757 CrossRef CAS PubMed.
- H. Ali, A. S. Maulud, H. Zabiri, M. Nawaz and L. Ismail, AIP Conf. Proc., 2022, 2472, 040002 CrossRef.
- H. Ali and F. Gao, IFAC-PapersOnLine, 2023, 56, 11675–11680 CrossRef.
- J. M. Polfus, W. Xing, G. Pećanac, A. Fossdal, S. M. Hanetho, Y. Larring, J. Malzbender, M.-L. Fontaine and R. Bredesen, J. Membr. Sci., 2016, 499, 172–178 CrossRef CAS.
- M. Mariano, Applications of cellulose nanocrystals: thermal, rheological and mechanical properties of new materials, Université Grenoble Alpes, 2016 Search PubMed.
- R. M. Sheltami, H. Kargarzadeh, I. Abdullah and I. Ahmad, Handbook of Nanocellulose and Cellulose Nanocomposites, 2017, vol. 2, pp. 523–552 Search PubMed.
- S. Xu, H. Zhou, H. Jia, J. Xu, L. Ma, Y. Zang, P. Jiang, W. Ma, Y. Zhang and W. Zhao, ACS Omega, 2021, 6, 12500–12506 CrossRef CAS PubMed.
- A. Febriasari, M. Suhartini, A. L. Yunus, R. Rahmawati, S. Sudirman, B. Hotimah, R. F. Hermana, S. Kartohardjono, A. Fahira and I. P. Permatasari, Int. J. Technol., 2021, 12, 1198–1206 CrossRef.
- A. Khakpay, P. Scovazzo and S. Nouranian, J. Membr. Sci., 2019, 589, 117228 CrossRef CAS.
- N. Belov, I. Blinov, A. Suvorov, R. Y. Nikiforov, S. Chirkov, A. Y. Alentiev, M. Kambur, Y. V. Kostina, I. Levin and A. Shapagin, Membr. Membr. Technol., 2021, 3, 114–123 CrossRef CAS.
- I. Douna, S. Farrukh, A. Hussain, Z. Salahuddin, T. Noor, E. Pervaiz, M. Younas and X. F. Fan, Korean J. Chem. Eng., 2022, 39, 189–197 CrossRef CAS.
- E. Akbarzadeh, A. Shockravi and V. Vatanpour, Carbohydr. Polym., 2021, 252, 117215 CrossRef CAS PubMed.
- H. Li, S. Xu, B. Zhao, Y. Yu and Y. Liu, Membranes, 2021, 11, 618 CrossRef CAS PubMed.
- N. C. Juntadech, T. Juntadech, S. Niamlang and B. Jongsomjit, J. Appl. Polym. Sci., 2023, e54032 CrossRef CAS.
- A. Rehman, Z. Jahan, F. Sher, T. Noor, M. B. K. Niazi, M. A. Akram and E. K. Sher, Chemosphere, 2022, 307, 135736 CrossRef CAS PubMed.
- O. Mehmood, S. Farrukh, A. Hussain, M. Younas, Z. Salahuddin, E. Pervaiz and M. Ayoub, Greenhouse Gases: Sci. Technol., 2021, 11, 313–330 CrossRef CAS.
- T. Esser, T. Wolf, T. Schubert, J. Benra, S. Forero, G. Maistros, S. Barbe, G. V. Theodorakopoulos, D. S. Karousos and A. A. Sapalidis, Nanomaterials, 2021, 11, 280 CrossRef CAS PubMed.
- M. Mubashir, L. F. Dumée, Y. Y. Fong, N. Jusoh, J. Lukose, W. S. Chai and P. L. Show, J. Hazard. Mater., 2021, 415, 125639 CrossRef CAS PubMed.
- R. Matsuba, H. Kubota and N. Matubayasi, Cellulose, 2022, 29, 5463–5478 CrossRef CAS.
- Y.-X. Shi, S.-H. Li and Z.-P. Zhao, Carbohydr. Polym., 2022, 291, 119610 CrossRef CAS PubMed.
- W. Wang, L. Li, S. Jin, Y. Wang, G. Lan and Y. Chen, Polymers, 2020, 12, 1272 CrossRef CAS PubMed.
- Q. Gan, D. Rooney, M. Xue, G. Thompson and Y. Zou, J. Membr. Sci., 2006, 280, 948–956 CrossRef CAS.
- S. S. Dhingra and E. Marand, J. Membr. Sci., 1998, 141, 45–63 CrossRef CAS.
- A. Jamil, M. Zulfiqar, U. Arshad, S. Mahmood, T. Iqbal, S. Rafiq and M. Z. Iqbal, Adv. Polym. Technol., 2020, 2020, 1–12 CrossRef.
- C. A. Scholes, G. W. Stevens and S. E. Kentish, Fuel, 2012, 96, 15–28 CrossRef CAS.
- A. Alexander, J. Macromol. Sci., Part A: Pure Appl. Chem., 1993, 3, 733–740 Search PubMed.
- M. D. Islam, F. J. Uddin, T. U. Rashid and M. Shahruzzaman, Mater. Adv., 2023, 4, 4054–4102 RSC.
- V. Vatanpour, M. E. Pasaoglu, H. Barzegar, O. O. Teber, R. Kaya, M. Bastug, A. Khataee and I. Koyuncu, Chemosphere, 2022, 295, 133914 CrossRef CAS PubMed.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- K. Golzar, S. Amjad-Iranagh, M. Amani and H. Modarress, J. Membr. Sci., 2014, 451, 117–134 CrossRef CAS.
- W. Hu, S. Chen, J. Yang, Z. Li and H. Wang, Carbohydr. Polym., 2014, 101, 1043–1060 CrossRef CAS PubMed.
- N. Yadav and M. Hakkarainen, Chemosphere, 2021, 265, 128731 CrossRef CAS PubMed.
- A. Zulhairun, Z. Fachrurrazi, M. N. Izwanne and A. Ismail, Sep. Purif. Technol., 2015, 146, 85–93 CrossRef CAS.
- A. E. Amooghin, M. Omidkhah and A. Kargari, J. Membr. Sci., 2015, 490, 364–379 CrossRef.
- A. Moghadassi, Z. Rajabi, S. Hosseini and M. Mohammadi, J. Ind. Eng. Chem., 2014, 20, 1050–1060 CrossRef CAS.
- F. Moghadam, E. Kamio, T. Yoshioka and H. Matsuyama, J. Membr. Sci., 2017, 530, 166–175 CrossRef CAS.
- B. J. Sundell, D. J. Harrigan, S. C. Hayden, J. T. Vaughn, K. A. Guzan, J. A. Lawrence III and M. L. Ostraat, J. Membr. Sci., 2019, 573, 448–454 CrossRef CAS.
- J. Wolfs and M. A. Meier, Green Chem., 2021, 23, 4410–4420 RSC.
- S.-H. Pak, Y.-W. Jeon, M.-S. Shin and H. C. Koh, Environ. Eng. Sci., 2016, 33, 17–24 CrossRef CAS.
- P. D. Sutrisna, E. Savitri, M. A. Gunawan, I. H. F. Putri and S. G. de Rozari, Polym.-Plast. Technol. Mater., 2020, 59, 1300–1307 CAS.
- S. Khoshtinat, V. Carvelli and C. Marano, Cellulose, 2021, 28, 9039–9050 CrossRef CAS.
- D. Nikolaeva, I. Azcune, M. Tanczyk, K. Warmuzinski, M. Jaschik, M. Sandru, P. I. Dahl, A. Genua, S. Lois and E. Sheridan, J. Membr. Sci., 2018, 564, 552–561 CrossRef CAS.
- W. Jami’An, H. Hasbullah, F. Mohamed, N. Yusof, N. Ibrahim and R. Ali, Effect of evaporation time on cellulose acetate membrane for gas separation, in IOP Conference Series: Earth and Environmental Science, IOP Publishing, 2016, vol. 36, p. 012008 Search PubMed.
- C. S. Achoundong, N. Bhuwania, S. K. Burgess, O. Karvan, J. R. Johnson and W. J. Koros, Macromolecules, 2013, 46, 5584–5594 CrossRef CAS.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed.
- T. Araújo, G. Bernardo and A. Mendes, Molecules, 2020, 25, 3532 CrossRef PubMed.
- P. Tanvidkar, B. Nayak and B. V. R. Kuncharam, J. Polym. Environ., 2023, 31(8), 3404–3417 CrossRef CAS.
- R. P. W. X. Jin, Z. A. Jawad, P. C. Tan, B. L. F. Chin, T. L. Chew and A. Saptoro, J. Phys. Sci., 2020, 31(2), 15–31 CrossRef CAS.
- H. Sanaeepur, R. Ahmadi, M. Sinaei and A. Kargari, J. Membr. Sci. Res., 2019, 5, 25–32 CAS.
- R. Lee, Z. Jawad, H. Chua and A. Ahmad, J. Environ. Chem. Eng., 2020, 8, 104212 CrossRef CAS.
- M. M. Rajpure, R. B. Mujmule, U. Kim and H. Kim, Int. J. Hydrogen Energy, 2024, 50, 615–628 CrossRef CAS.
- C. Li, Y. Ding, W. Xu, M. Li and W. Li, React. Funct. Polym., 2023, 182, 105463 CrossRef CAS.
- P. Tanvidkar, A. Jonnalagedda and B. V. R. Kuncharam, J. Appl. Polym. Sci., 2023, 140, e53264 CrossRef CAS.
- P. Sajjan, V. Nayak, M. Padaki, V. Y. Zadorozhnyy, S. N. Klyamkin and P. Konik, Energy Fuels, 2020, 34, 11699–11707 CrossRef CAS.
- P. Tanvidkar, A. Jonnalagedda and B. V. R. Kuncharam, Environ. Technol., 2023, 1–12 Search PubMed.
- M. Mubashir, C. T. Leng, L. K. Keong and N. Jusoh, Polym. Test., 2020, 81, 106223 CrossRef CAS.
- M. Mubashir, Y. F. Yeong, T. L. Chew and K. K. Lau, Sep. Purif. Technol., 2019, 215, 32–43 CrossRef CAS.
- I. Shakeel, A. Hussain and S. Farrukh, J. Polym. Environ., 2019, 27, 1449–1464 CrossRef CAS.
- N. e. Hira, S. S. M. Lock, N. F. Shoparwe, I. S. M. Lock, L. G. Lim, C. L. Yiin, Y. H. Chan and M. Hassam, Sustainability, 2023, 15, 1510 CrossRef CAS.
- N. E. Hira, S. S. M. Lock, U. Arshad, K. Asif, F. Ullah, A. S. Farooqi, C. L. Yiin, B. L. F. Chin and Z. e. Huma, ACS Omega, 2023, 8, 48130–48144 CrossRef CAS PubMed.
- K. Asif, S. S. M. Lock, S. A. A. Taqvi, N. Jusoh, C. L. Yiin and B. L. F. Chin, Chemosphere, 2023, 311, 136936 CrossRef CAS PubMed.
- B. Barzegar and F. Feyzi, J. Mol. Liq., 2022, 368, 120788 CrossRef CAS.
- I. Salahshoori, I. Cacciotti, A. Seyfaee and A. Babapoor, J. Polym. Res., 2021, 28, 223 CrossRef CAS.
- A. Bocahut, J.-Y. Delannoy, C. Vergelati and K. Mazeau, Cellulose, 2014, 21, 3897–3912 CrossRef CAS.
- M. Mubashir, Y. F. Yeong, K. K. Lau and T. L. Chew, Polym. Test., 2019, 73, 1–11 CrossRef CAS.
- M. Aroon, A. Ismail, T. Matsuura and M. Montazer-Rahmati, Sep. Purif. Technol., 2010, 75, 229–242 CrossRef CAS.
- E. Favvas, Innovations in Nanomaterials, ed. A. B. Imran, and J. Shapter, 2015, pp. 105–153 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |
