DOI:
10.1039/C5RA01281C
(Paper)
RSC Adv., 2015,
5, 40103-40110
In vivo evaluation of an anticancer drug delivery system based on heparinized mesoporous silica nanoparticles†
Received
22nd January 2015
, Accepted 13th April 2015
First published on 13th April 2015
Abstract
In this study, heparinized mesoporous silica nanoparticles (denoted as MSNs-HP) were used for loading an anticancer drug. MSNs-HP were found capable of penetrating into cancer cells and delaying the release of the anticancer drug doxorubicin (DOX) according to its in vitro and in vivo release profiles. More interestingly, in vivo evaluation with animal xenograft models showed that the tumor inhibitory rate of loaded MSNs-HP (58%) was much higher than that of DOX alone inside the nanoparticles (18%) and close to that of large doses of DOX (67%, 7-fold higher in dosage than DOX inside the nanoparticles), indicating that the use of MSNs-HP significantly increased the antitumor efficacy of DOX. The reason for the efficacy of this nanoparticle–drug system against tumor growth might be the synergy of these two components in inducing tumor cell apoptosis and tumor necrosis, and inhibiting tumor angiogenesis. Furthermore, this system was found safer than large doses of the drug. All the above might enable MSNs-HP to be a potential highly efficient and less toxic carrier for anticancer drug delivery.
Introduction
Heparin, a highly sulfated glycosaminoglycan, is a well-known anticoagulant. Except for its anticoagulant activity, heparin has also shown other activities such as regulation of the complement activity and inflammation, participation in the release of lipoprotein lipases, control of tumor growth by inhibiting tumor angiogenesis and metastasis, et al.1–6 These diverse activities show the potential of heparin for being exploited for therapeutic use to treat many different ailments and diseases. Recently, research has begun to combine the useful biological activities of heparin with the useful properties of nanomaterials. The combination of these two substances can provide synergistic improvements, enhancing already existing properties, and also create novel applications ranging from improving the anticoagulant activity for anticancer therapy to tissue engineering and biosensors.1 In particular, heparin is of interest for the use as a drug delivery system (DDS) in the treatment of cancer. Up to now, various heparin-based DDSs have been developed, in which the role of heparin is not only to improve the blood compatibility of nanomaterials but also to serve as an effective drug for cancer therapy.7–11
Mesoporous silica nanoparticles (MSNs) are a class of very promising candidates for anticancer drug delivery due to their biocompatibility and preferential accumulation in tumors.12–14 To further improve the performances of MSNs in cancer therapy, their surface is often functionalized with various bioactive molecules. For example, the modification with polyethylene glycol (PEG) derivatives improves the dispersion of the nanoparticles in saline, increases their circulatory half-life, reduces their uptake by the reticular endothelial system (RES) and thus enhances the enhanced permeability and retention (EPR) effect.15,16 The conjugation of specific ligands, such as folic acid and mannose, allows the targeting of cancer cells by the nanoparticles.14,17 Although these MSN-based DDSs along with their functional molecules are well-established or well-defined via in vitro assays, in vivo evaluations always exhibit unexpected results due to the complicated physiological environment. For example, camptothecin-loaded folic acid–MSN conjugates showed a higher cell killing in the in vitro study and a greater accumulation in tumors than the unloaded conjugates, but no significant difference was found in the tumor-suppressing effect when these two systems were transferred into animal xenograft models.14 This result may be correlated with the high dosage of drug injections that could suppress the tumors very quickly and then mask the enhanced effects of the carriers, and low expression of folate receptors on the tumor cells. Therefore, the most critical question regarding the use of functionalized MSNs in cancer therapy is its actual efficacy for suppressing tumor growth. In our previous study, a type of heparinized magnetic MSNs with good dispersity and blood compatibility has been developed for growth factor delivery.18 Herein, heparin was again covalently bound to the surfaces of bare MSNs. The resultant composite was denoted as MSNs-HP and used for loading the anticancer drug doxorubicin (DOX). Then, the drug release profile from the composite and the uptake of the composite itself by cancer cells were investigated. Finally, the antitumor effect and safeness of the DOX-loaded composite were evaluated using tumor-bearing mice models. The aim is to develop an effective carrier for anticancer drug delivery.
Materials and methods
Materials
Tetraethyl orthosilicate (TEOS), cetyltrimethlammonium bromide (CTAB), 3-aminopropyltriethoxysilane (APTES), polyvinyl pyrrolidone (PVP30, MW 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), heparin sodium (MW 12000) and DOX were purchased from Shanghai Chemical Corp. N-Hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), 4-morpholineethanesulfonic acid (MES), 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), fluorescein isothiocyanate (FITC), (2′-(4-ethoxyphenyl)-5-(4-methyl-1-piperazinyl)2,5′-bi-1H-benzimidazoletri hydrochloride) (Hoechst 33
000), heparin sodium (MW 12000) and DOX were purchased from Shanghai Chemical Corp. N-Hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), 4-morpholineethanesulfonic acid (MES), 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), fluorescein isothiocyanate (FITC), (2′-(4-ethoxyphenyl)-5-(4-methyl-1-piperazinyl)2,5′-bi-1H-benzimidazoletri hydrochloride) (Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342), and toluidine blue were obtained from Sigma-Aldrich (USA). Basic fibroblast growth factor (bFGF) was purchased from Pharmacia (Sweden). The antibodies used for western blotting were purchased from Santa Cruz Biotechnology (USA). All other chemicals are of analytical grade.
342), and toluidine blue were obtained from Sigma-Aldrich (USA). Basic fibroblast growth factor (bFGF) was purchased from Pharmacia (Sweden). The antibodies used for western blotting were purchased from Santa Cruz Biotechnology (USA). All other chemicals are of analytical grade.
Synthesis of FITC–APTES
A solution of FITC (8.6 mg) in anhydrous ethanol (2 mL) was mixed with APTES (0.197 mL) and then the mixture was stirred at room temperature for 24 h. The prepared FITC–APTES stock solution was kept at 4 °C.
Synthesis of amino-modified MSNs (denoted as MSNs-NH2)
The synthesis of MSNs-NH2 was similar to that of magnetic MSNs-NH2.18 Typically, 0.3 g CTAB and 0.1 g PVP30 were added to a solution containing 90 mL of deionized water and 60 mL of methanol. The solution was adjusted to pH 10.2 by an ammonia solution, and then 600 μL TEOS and 50 μL APTES were added. After stirring for 2.5 h at room temperature, the resultant particles were separated and washed with ethanol and water. The extraction of organic templates for the as-synthesized particles was performed by dispersing these particles into 50 mL of a mixed solution of ethanol and NH4NO3 (0.3 g) at 60 °C for 1 h. The synthesis of FITC-labeled MSNs-NH2 is similar to that of the unlabeled ones, except that FITC-APTES (50 μL) and APTES were added together during the condensation process.
Synthesis of MSNs-HP and FITC-labeled MSNs-HP
The immobilization of heparin onto MSNs-NH2 and FITC-labeled MSNs-NH2 was performed according to our previous report.18 Typically, 100 mg of MSNs-NH2 or FITC-labeled MSNs-NH2 was suspended in 30 mL of 0.1 M MES buffer (pH 5.5) and hydrated for 2 h, after which EDC (287.4 mg) and NHS (172.8 mg) were added, and then 20 mg of heparin was slowly added. The reaction mixture was incubated at 4 °C by rotating overnight. Samples were collected by centrifugation and then dialyzed against deionized water to get rid of the residual EDC, NHS and heparin. The obtained products were lyophilized and kept at 4 °C. The content of heparin incorporated into MSNs-NH2 was examined using the toluidine blue assay.19
Loading/release of DOX into/from MSNs-HP
MSNs-HP (100 mg) were dispersed in 20 mL of a DOX solution (0.5 mg mL−1). After shaking for 24 h in the dark, the solid particles were centrifuged and washed with PBS solution (pH 7.4). To evaluate the loading efficiency of the drug, the supernatant solutions were collected and the residual drug was measured by UV-vis spectroscopy at 480 nm. The loading efficiency of the drug was expressed as percentage of the drug in MSN-HP with respect to the initial amount of the drug.
The in vitro release test was performed by immersing the drug-loaded sample (10 mg) in 10 mL PBS solution. The suspension was gently shaken at 37 °C in a water bath. At a given time, the medium was replaced with a fresh medium. The amount of the released DOX from MSNs-HP was determined by UV-vis spectrophotometry. To determine the amount of DOX left in the nanoparticles at the end of the release test, the nanoparticles were collected and dissolved using 2 M NaOH. Then, the undissolved DOX was separated and again dissolved using 1 M HCl. Finally, the remaining drug was quantified by UV-vis spectrophotometry.
Concentration profile of DOX in rat plasma
MSNs-HP containing 4% of DOX were intravenously administered via a sublingual vein of a male Sprague-Dawley rat (200 ± 20 g) as a single dose of 8 mg kg−1 (in which the content of DOX was 0.32 mg). Blood was sampled from the tail vain at each time interval, and the blood samples (1 mL) were collected using heparinized tubes, and then centrifuged at 2500 g for 15 min at 4 °C to separate the plasma, and stored at −20 °C until analysis. Subsequently, the plasma (300 μL) was mixed with 20 μL of daunorubicin as the internal standard (20 μg mL−1) and 50 μL of hydrochloric acid solution (1 mol L−1), and then the suspension was extracted with 1 mL of ethyl acetate for 2 min. After standing for stratification, the upper organic layer was transferred to another tube and evaporated to dryness at 40 °C under a stream of nitrogen (about 40 min). The residue was dissolved in 100 μL of the HPLC mobile phase (65% of H2O, 25% of acetonitrile and 10% of trifluoroacetic acid) by vortexing and 20 μL of the solution was used for the measurement of the DOX concentration with a HPLC system (HP Agilent 1100). The flow rate of the mobile phase was 0.8 mL min−1. The detection wavelength was 210 nm.
Cell cultures
Hela cells, HepG2 cells and H22 mouse sarcoma cells were originally obtained from the Shanghai Institute of Biochemistry and Cell Biology (Chinese Academy of Sciences). The cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, GIBCO) supplemented with 10% fetal calf serum, 2% L-glutamine, 1% penicillin, and 1% streptomycin stock solutions, and then seeded in a 96-well plate at a density of 104 cells per well and cultured in 5% CO2 at 37 °C for 24 h.
Uptake of FITC-labeled MSNs-HP by the Hela cells
The cellular uptake of FITC-labeled MSNs-HP was investigated by high content screening (Thermo Scientific ArrayScan VTI 600). In brief, 1.0 × 105 Hela cells were incubated with the nanoparticles on an 8-well plate for 30 min and then washed with DMEM and PBS to remove the nanoparticles that did not enter the cells. The cells were then stained with Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 and DiI before measurement.
342 and DiI before measurement.
Establishment of the H22 xenograft model
50 Male Kunming mice (8–10 weeks old, Code number SCXK2010-0007, weighing 18–22 g) were obtained from the Medical Experimental Animal Center, Henan Province, China. The H22 model was established by subcutaneous injection as previously described,20 in which 0.2 mL of H22 cell suspensions (1.0 × 106 cells per mL) were subcutaneously inoculated into the right armpit region of the mice. The experimental procedures described in this section and the previous section to investigate the concentration profile of DOX in plasma using male Sprague-Dawley rats were performed in accordance with the Guidelines of Animal Experiments from the Committee of Medical Ethics, National Health Department of China (1998), and followed the standards and policies of the Henan University of Science and Technology’s Animal Care and Use Committee.
Animal groups and treatments
Twenty-four hours after establishing the H22 models, the transplanted mice were allocated into five groups (10 mice in each group) and treated as follows: normal saline (control group), MSNs-HP nanoparticles (8 mg kg−1), DOX-loaded MSNs-HP nanoparticles (8 mg kg−1), low doses of DOX (0.3 mg kg−1) and high doses of DOX (2.0 mg kg−1). These agents were denoted as control, unloaded MSNs-HP, loaded MSNs-HP, DOX0.3 and DOX2.0, respectively. All the agents were injected once per day via the lateral tail vein, and the body weight was daily checked. After 10 days, the antitumor activity was evaluated by weighing the tumor tissues. The tumor inhibitory rate (%) was calculated as: (1–(average tumor weight of each group)/(average tumor weight of control group)) × 100%. Herein, it should be noted that the amount of loaded MSNs-HP administered was approximately equal to the sum of unloaded MSNs-HP and DOX0.3.
Histomorphometric determination
The tumor tissue was fixed in 10% formalin, and then dehydrated, embedded in paraffin blocks, cut into 5 μm sections, and finally mounted on APTES-coated slices. Next, the sections were deparaffinized, rehydrated, washed with H2O, stained with hematoxylin–eosin (HE) and finally observed under a light microscope at 100× and 400× magnifications.
Western blot analysis
Tumor tissues were homogenized in lysis buffer containing proteinase inhibitors, and then western blotting was performed as previously described.21,22 Briefly, equal amounts of protein (60 μg) as quantified by the Bradford method were separated by a 12% SDS-PAGE and then transferred onto PVDF membranes at 100 V for 60 min. Blocking solution (5% skim milk) was loaded over the membranes for 1 h at room temperature. The membranes were incubated with Bcl-2, Bax, the VEGF polyclonal antibody and β-actin antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution), respectively, overnight at 4 °C, and then incubated with the corresponding horseradish peroxidase-labeled secondary antibodies at room temperature for 1 h. Finally, the immunoreactivity was detected using the enhanced chemiluminescence method. Quantization of the protein band density was performed using Gel-Pro Analyzer 4.0. The data are reported as normalized protein band densities.
1000 dilution), respectively, overnight at 4 °C, and then incubated with the corresponding horseradish peroxidase-labeled secondary antibodies at room temperature for 1 h. Finally, the immunoreactivity was detected using the enhanced chemiluminescence method. Quantization of the protein band density was performed using Gel-Pro Analyzer 4.0. The data are reported as normalized protein band densities.
Blood biochemistry and immune function analysis
At the end of the tumor growth assays, blood was collected from the eye-orbit of the transplanted mice and left at room temperature for 30 min. Then, the blood samples were centrifuged at 4 °C for 10 min. The separated serum was stored at −70 °C for biochemical analysis. Finally, biochemical criteria such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine, white blood cell count, thymus index and spleen index, were measured.
MTT assays
To study the interaction between DOX and MSNs-HP, the HepG2 cells were treated with various dilutions of DOX in the presence or absence of MSNs-HP, the cell viability was determined after 48 h and IC50 values were determined by plotting the percentage of cell survival as a function of DOX or MSNs-HP concentration. The interactions between DOX and MSNs-HP were evaluated by the combination index (CI) (synergism, additive effect, and antagonism) according to the Chou and Talalay equation.23 For 50 percent toxicity, the combination index (CI) values were calculated based on the equation stated below: Combination Index (CI) = (D)1/(Dx)1 + (D)2/(Dx)2, where (Dx)1 is the dose of DOX to produce 50 percent cell kill alone, (D)1 is the dose of DOX to produce 50 percent cell kill in combination with MSNs-HP, (Dx)2 is the dose of MSNs-HP to produce 50 percent cell kill alone, and (D)2 is the dose of MSNs-HP to produce 50 percent cell kill in combination with DOX.
Chicken chorioallantoic membrane assay (CAM)
To investigate the effect of MSNs-HP on the growth factor-induced angiogenesis, in vitro CAM assays were performed according to a previous report.24 Briefly, the embryos were allocated to four groups (8 eggs in each group) and treated as follows: normal saline (control), bFGF (100 ng), bFGF (100 ng) + heparin (10 μg), and bFGF (100 ng) + MSNs-HP (100 μg). After incubation at 37 °C for 10 days in a humidified atmosphere, the fertilized eggs were opened and the shell membranes were taken off in order to expose the chorioallantoic membranes. Then, the above agents in PBS were applied to the top of the chorioallantoic membranes, the windows were recovered with glass-adhesive plaster and the fertilized eggs were incubated continuously for another 96 h. Finally, the chorioallantoic membranes were taken off and fixed with formaldehyde. The tube formation of CAM was monitored by the BI-2000 photograph system.
Statistical analysis
Data were expressed as mean ± S. D. Statistical analysis was performed by Student’s t test and one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test when appropriate. Differences were considered statistically significant at P < 0.05.
Characterization
Transmission electron microscopy (TEM) analyses were conducted on a JEM-2100F electron microscope operating at 200 kV. UV-vis spectra were recorded on a UV-3101PC Shimadzu UV-vis spectroscope. Nitrogen adsorption–desorption isotherms at 77 K were measured on a Micrometitics Tristar 3000 system. Zeta potentials of the nanoparticles were measured by a Malvern Zetasizer Nano ZS90. Fluorescence images were obtained by Leica DMI4000B fluorescence microscopy.
Results and discussion
Characterization of MSNs-HP and their drug-release behavior
The preparation of MSNs-HP and the drug-loaded sample were performed partially according to our previous report18 and the details are described as follows: (i) synthesis of MSNs-NH2 using sol–gel methods incorporated by APTES, (ii) conjugation of heparin with MSNs-NH2 via carbodiimide chemistry, and (iii) loading of DOX into heparinized MSNs by physical adsorption. As shown in SEM and TEM images (Fig. 1a and b), the MSNs-NH2 substrate exhibits a very uniform particle size distribution around 120–160 nm, a regular spherical morphology, a disordered porous structure, and a perfect monodispersity. The immobilization of heparin onto MSNs-NH2 has no effect on the morphology (data not shown), but the BET surface area, pore volume, pore size and the zeta potential of MSNs-NH2 were reduced from 552 cm2 g−1, 0.74 cm3 g−1, 2.58 nm and 34 mV to 329 cm2 g−1, 0.40 cm3 g−1, 2.07 nm, and −43 mV, respectively (Fig. 2a and b, and S1a and b†), and the immobilization efficiency was approximately 2% (w/w) as determined by the toluidine blue assay. Furthermore, a 4% (w/w) DOX loading capacity onto MSNs-HP was confirmed by UV-vis measurements at a wavelength of 480 nm. The in vitro release profile of DOX from the nanoparticles is shown in Fig. 3a. It can be seen that the burst release of the drug occurred within the initial 8 h. After 70 h, the release curve reached a plateau and only around 70% of the drug were released. No further drug release was found at the end of the study. According to the dissolution experiment of the material (please see the experimental section), the other 30% of the drug were still inside the nanoparticles. When loaded MSNs-HP were intravenously administered, it can be seen from Fig. 3b that the peak concentration state of DOX from the nanoparticles in the plasma could be maintained for up to 6 h, its whole concentration profile of a single dose of administration was sustained for up to 72 h, and the calculated half-life was approximately 20.5 h, whereas the concentration profile of the DOX administration alone was reported to be less than 10 min.7 These results suggested that MSNs-HP were able to extend circulation of the drug and thus could be helpful for improving its therapeutic efficacy. Herein, the high dosage of drug incorporated into the materials and the controlled drug release might be attributed to porous and electrostatic interactions between the negatively charged heparin and positively charged DOX.
 |
| | Fig. 1 SEM (a) and TEM (b) images of MSNs-NH2. | |
 |
| | Fig. 2 Nitrogen adsorption–desorption curves of MSNs-NH2 (a) and MSNs-HP (b). The insets show the corresponding pore size of these nanoparticles. | |
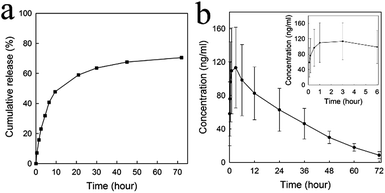 |
| | Fig. 3 (a) Release profile of DOX from MSNs-HP in PBS solution (pH 7.4). (b) Plasma concentration–time curve of DOX following the administration (i.v.) of a single dose of loaded MSNs-HP (8 mg kg−1) to a normal Sprague-Dawley rat. The inset shows the profile of the initial peak concentration of the drug in the plasma. | |
Cellular uptake analysis
Cellular uptake was considered as a prerequisite for those nanoparticles to be developed as drug delivery carriers administrated by intravenous injection. Then, we labeled MSNs-HP with FITC dye and investigated their interaction with Hela cells using High Content Screening. As shown in Fig. 4a, FITC-labeled MSNs-HP alone exhibited strong green fluorescence emission. Fig. 4b shows the fluorescence images of Hela cells in the presence of the Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 and DiI dyes. It can be seen that the cell nuclei stained with Hoechst 33
342 and DiI dyes. It can be seen that the cell nuclei stained with Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 exhibited blue fluorescence, the cell membranes stained with DiI exhibited red fluorescence, and the cytoplasm also exhibited red fluorescence due to the diffusion of DiI from the cell membranes to the cytoplasm. Fig. 4c shows the merged images of Hela cells in the presence of FITC-labeled MSNs-HP and the other two dyes. It can be seen that the cell nuclei still exhibited blue fluorescence, but the fluorescence of the cell membranes and cytoplasm changed from red to yellow-green due to the overlapping of red fluorescent spots and green fluorescent spots, indicating that MSNs-HP could penetrate into the living cells. Herein, it should be mentioned that MSNs-HP is negatively charged which does not benefit its cellular uptake. The great cellular uptake of the nanoparticles may be related to the heparin targeting effect because a lot of natural products such as bleomycin also have tumor targeting properties.25
342 exhibited blue fluorescence, the cell membranes stained with DiI exhibited red fluorescence, and the cytoplasm also exhibited red fluorescence due to the diffusion of DiI from the cell membranes to the cytoplasm. Fig. 4c shows the merged images of Hela cells in the presence of FITC-labeled MSNs-HP and the other two dyes. It can be seen that the cell nuclei still exhibited blue fluorescence, but the fluorescence of the cell membranes and cytoplasm changed from red to yellow-green due to the overlapping of red fluorescent spots and green fluorescent spots, indicating that MSNs-HP could penetrate into the living cells. Herein, it should be mentioned that MSNs-HP is negatively charged which does not benefit its cellular uptake. The great cellular uptake of the nanoparticles may be related to the heparin targeting effect because a lot of natural products such as bleomycin also have tumor targeting properties.25
 |
| | Fig. 4 Fluorescence image of FITC-labeled MSNs-HP (a), high content screening images of Hela cells simultaneously stained with Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 (blue fluorescence) and DiI (red fluorescence) (b), high content screening images of Hela cells in the presence of Hoechst 33 342 (blue fluorescence) and DiI (red fluorescence) (b), high content screening images of Hela cells in the presence of Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342, DiI and FITC-labeled MSNs-HP (c). 342, DiI and FITC-labeled MSNs-HP (c). | |
Tumor suppression analysis
Having verified the uptake of MSNs-HP by cancer cells, solid H22 tumors were then established in mice to determine whether DOX-loaded MSNs-HP can suppress tumor growth in vivo. As shown in Fig. 5, the administration of unloaded/loaded MSNs-HP, DOX0.3 and DOX2.0 all exhibited the inhibition of tumor growth relative to the saline control. Interestingly, although the DOX dosage in loaded MSNs-HP was approximately 7-fold lower than that of DOX2.0, its inhibitory efficiency (58%) was only a little lower than that of the latter (67%) and much higher than those of DOX0.3 (18%, in which the DOX dosage was equal to that of the loaded nanoparticles) and unloaded MSNs-HP (20%, see Table 1). Bcl-2 is a protein that can protect cells from external insults and afford anti-apoptotic properties. Bax is a protein that can oppose the anti-apoptotic activity of Bcl-2. The Bcl-2/Bax ratio as an apoptosis index is believed to determine whether tumor cells undergo apoptosis.26 Fig. 6 shows the expression of the Bcl-2 and Bax proteins in the tumor tissues of mice treated with different agents. It can be seen that all the agents except the saline control caused a decrease in Bcl-2/Bax ratio, indicating their apoptosis-inducing activities. It is likely that similar tendencies as those observed in the tumor growth assays also appeared, in which the ratio induced by loaded MSNs-HP was close to that induced by DOX2.0 and less than those induced by unloaded MSNs-HP and DOX0.3. Furthermore, histological examination of the solid tumors showed the presence of large size areas of tumor necrosis in the loaded MSNs-HP-treated and DOX2.0-treated mice, whereas tumor necrosis induced by unloaded MSNs-HP and DOX0.3 was clearly lower than those induced by the above two agents (Fig. 7). The similar performances of loaded MSNs-HP in the above investigations as those of DOX2.0 rather than those of DOX0.3, as well as the positive performance of MSNs-HP itself suggested that MSNs-HP play a synergistic role in the antitumor activity using DOX and greatly improved its efficacy. The synergy of MSNs-HP and DOX was further verified by cell viability assays, in which the combination index (CI) of these two components against the proliferation of HepG2 cells was clearly less than 1 at medium dose levels (IC50) for MSNs-HP/DOX (Table S1 and Fig. S2†). All of these make the resultant DDS highly effective in suppressing tumor growth.
 |
| | Fig. 5 The effects of the different agents on the tumor growth in the transplanted H22 mice: control (a), unloaded MSNs-HP (b), loaded MSNs-HP (c), DOX0.3 (d), DOX2.0 (e). | |
Table 1 The effects of the different agents on the tumor growth in the transplanted H22 mice (mean ± S. D., n = 10)
| Groups |
Weight (g) |
Tumor weight (g) |
Inhibitory rate (%) |
| Pre-treatment |
Post-treatment |
| P < 0.05, compared to control group. P < 0.05, compared to DOX2.0 group. |
| Control |
19.5 ± 2.5 |
24.7 ± 2.9 |
1.6 ± 0.2 |
— |
| Unloaded MSNs-HP |
20.7 ± 2.0 |
27.2 ± 3.5 |
1.3 ± 0.2a |
20.5b |
| Loaded MSNs-HP |
23.0 ± 2.4 |
28.9 ± 3.1 |
0.7 ± 0.3a,b |
58.8 |
| DOX0.3 |
22.3 ± 1.2 |
29.3 ± 2.5 |
1.3 ± 0.3a,b |
18.0b |
| DOX2.0 |
21.2 ± 3.6 |
22.6 ± 5.1 |
0.5 ± 0.2a,b |
67.5 |
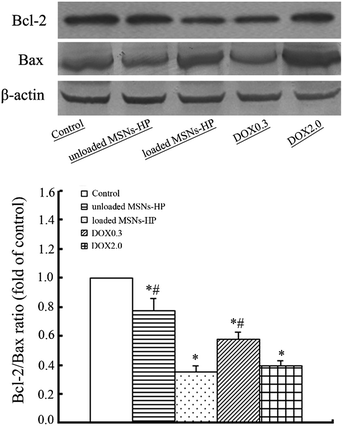 |
| | Fig. 6 The expression of the Bcl-2 and Bax proteins in the tumor tissue of the transplanted H22 mice treated with the different agents (top), and analysis of the Bcl-2/Bax ratio (bottom). (*P < 0.05 vs. control group, and #P < 0.05 vs. unloaded MSNs-HP group). | |
 |
| | Fig. 7 Histological examination of the solid tumors in the H22 transplanted mice treated with the different agents (100× magnification). Cell nuclei were stained with blue and necrotic areas without cell nuclei were stained with light red. | |
Tumor angiogenesis analysis
Growth factors such as the vascular endothelial growth factor (VEGF) and bFGF play a crucial role in angiogenesis,27 a key event during tumor formation. DOX and heparin were reported to be able to inhibit angiogenesis by decreasing the expression of these pro-angiogenetic factors in tumor tissues, or by disrupting their interaction with endothelial cell-surface receptors.6,28 Fig. 8 shows the expression of VEGF in xenograft tumors treated with the different agents. It can be seen that all the agents except the saline control were able to decrease protein expression and loaded MSNs-HP exerted the strongest inhibitory effect. Furthermore, it has been reported that several heparinized nanomaterials were capable of disturbing growth factor-induced angiogenesis.29–31 We then examined the effect of MSNs-HP itself on the pro-angiogenetic activity of the growth factors by in vitro CAM assays. Fig. 9 shows the formation of new blood vessels of the CAM models in the presence of the different agents. It can be seen that the growth factor bFGF induced a pronounced angiogenetic response in the treated model compared to that of the control (Fig. 9a and b), whereas the presence of heparin or MSNs-HP seriously disturbed such a response (Fig. 9c and d). Therefore, loaded MSNs-HP could have multiple antitumor actions, by firstly inducing tumor cell apoptosis and tumor necrosis, and secondly inhibiting tumor angiogenesis by reducing the expression of pro-angiogenetic factors in the tumor tissues and disrupting their pro-angiogenetic activities. Beside these contributions, other factors might also be responsible for the strong tumor cytotoxicity of this nanoparticle–drug system, including the preferential accumulation of MSNs in tumor sites as described in previous studies:13,14 the MSN-mediated endocytosis and the sustained intracellular release of the DOX drug as indicated by the in vitro/in vivo release profiles in Fig. 3. Recently, a global gene expression analysis technology was used by Shi et al. for exploring the underlying pathways and mechanisms of cancer cell death induced by MSN-mediated drug delivery.32 They found that by virtue of a certain kind of synergetic biological effect between the nanoparticles and drug, DOX@MSN DDS was capable of increasing the intracellular levels of reactive oxygen species by triggering the mitochondria-related autophagic lysosome pathway, thus activating a specific pathway of necrosis different from those activated by the free drug and the carrier molecules. Since the strong antitumor effect of loaded MSNs-HP also involved a synergetic effect between the nanoparticles and drug, the possibility of a unique antitumor mechanism generated by this system could not be ruled out. Further investigations at the molecular level are needed in the future. Herein, it should be noteworthy that the above two types of synergetic effects between mesoporous silica nanoparticles (MSNs) and the anticancer drug were observed from different levels. One was from the cell level and another was from the animal level.
 |
| | Fig. 8 The expression of VEGF in the tumor tissue of the transplanted H22 mice treated with the different agents. (*P < 0.05 vs. control group, and #P < 0.05 vs. DOX2.0 group). | |
 |
| | Fig. 9 The effects of different agents on the bFGF-induced tube formation of CAM. | |
Safety evaluation
As loaded MSNs-HP might induce damage in the organs and tissues of the transplanted mice except for those in the tumor areas, its safeness was examined at the end of the experiments. As shown in Table 1, the weight of the mice treated with loaded MSNs-HP was not statistically different from those of the mice treated with other agents. Furthermore, slightly decreased creatinine/BUN (representative for renal function) and significantly elevated ALT/AST ratios (representative for liver function) were detected in the mice treated with DOX2.0 compared to those of the control mice, whereas the levels of these biomarkers from mice treated with the other agents were lower than those of the control mice (Table 2). Finally, the unmodified white blood cell count and thymus and spleen indexes were observed in all mice except for the DOX2.0-treated mice (Table 3). According to these data, it can be seen that among these agents, only DOX2.0 caused unexpected side effects and the others did not show any effects on the growth normal tissues of the transplanted H22 mice, which means that loaded MSNs-HP were safer than large doses of the DOX drug, and thus the use of MSNs-HP not only could improve the efficacy of DOX but also reduce its side effects. As for the low toxicity of this system, we speculated that it might be related to the preferential accumulation of MSNs in tumor sites and their low dose of administration. Those details await further investigation for the biodistribution and in vivo clearance profiles in the future.
Table 2 Blood biochemistry in the transplanted H22 mice treated with the different agents (mean ± S. D., n = 10)
| Groups |
Creatinine (mg dL−1) |
BUN (mg dL−1) |
ALT (U L−1) |
AST (U L−1) |
| P < 0.05, compared to control group. P < 0.05, compared to DOX2.0 group. |
| Control |
65.2 ± 4.6 |
8.7 ± 0.9 |
52.8 ± 7.8 |
872.5 ± 231.5 |
| Unloaded MSNs-HP |
66.5 ± 7.6 |
8.8 ± 1.1 |
43.5 ± 6.2a,b |
674.4 ± 180.2a,b |
| Loaded MSNs-HP |
65.1 ± 9.0 |
8.7 ± 2.7 |
38.4 ± 5.1a,b |
558.1 ± 175.1a,b |
| DOX0.3 |
61.3 ± 7.7 |
7.3 ± 1.2 |
39.5 ± 9.5a,b |
562.0 ± 175.4a,b |
| DOX2.0 |
60.5 ± 6.9 |
7.0 ± 1.2 |
57.3 ± 15.6 |
1181.2 ± 325.5a |
Table 3 Influence of the different agents on the immune function of the transplanted H22 mice (mean ± S. D., n = 10)
| Groups |
White blood cell count (× 109 L−1) |
Thymus index (× 10−3) |
Spleen index (× 10−3) |
| P < 0.05, compared to control group. P < 0.05, compared to DOX2.0 group. |
| Control |
6.1 ± 1.2 |
2.2 ± 0.4 |
8.9 ± 0.1 |
| Unloaded MSNs-HP |
7.6 ± 1.3 |
2.5 ± 0.1b |
8.5 ± 0.2b |
| Loaded MSNs-HP |
7.8 ± 1.1 |
2.2 ± 0.8b |
8.5 ± 0.3b |
| DOX0.3 |
7.2 ± 0.7 |
2.0 ± 0.4b |
9.6 ± 0.2b |
| DOX2.0 |
7.4 ± 1.4 |
1.0 ± 0.6a |
3.9 ± 1.9a |
Conclusions
In this study, the synthesis and characterization of heparinized mesoporous silica nanoparticles, MSNs-HP, were firstly described. Then, MSNs-HP were found capable of penetrating into cancer cells and delaying the release of the anticancer drug DOX in vitro and in vivo. More interestingly, in vivo investigations using animal xenograft models showed that, by only carrying a low amount of the drug, the loaded MSNs-HP could achieve a similar antitumor efficacy to that of large doses of the drug alone. Such a strong tumor cytotoxicity of this nanoparticle–drug system could be correlated to the synergistic effect of these two components in inducing tumor cell apoptosis and tumor necrosis and inhibiting tumor angiogenesis. Furthermore, this system was found safer than large doses of the drug. All the above might enable MSNs-HP to be a potential highly efficient and less toxic carrier for anticancer drug delivery. However, considering the possible anticoagulant side effects, the in vivo evaluation was performed only at a low dose range of these heparinized nanoparticles. To increase the applied dosage, we intend to modify the surfaces of MSNs with non-anticoagulant and anti-angiogenetic heparin derivatives in the next stage.33–35
Acknowledgements
The authors should be grateful to the financial support of the National Natural Science Foundation of China (grant no. 21471046), the Important National Science & Technology Specific Projects (grant no. 2012ZX09301003-001-009) and the State Key Laboratory of Antitoxic Drugs and Toxicology.
Notes and references
- M. M. Kemp and R. J. Linhardt, WIREs Nanomed. Nanobiotechnol., 2010, 2, 77 CrossRef CAS PubMed.
- M. D. Sharath, Z. M. Merchant, Y. S. Kim, K. G. Rice, R. J. Linhardt and J. M. Weiler, Immunopharmacology, 1985, 9, 73 CrossRef CAS.
- J. M. Weiler, R. E. Edens, R. J. Linhardt and D. P. Kapelanski, J. Immunol., 1992, 148, 3210 CAS.
- G. Liu, M. Hultin, P. Oestergaard and T. Olivecrona, Biochem. J., 1992, 285, 731 CAS.
- R. Crum, S. Szabo and J. Folkman, Science, 1985, 230, 1375 CAS.
- J. Folkman, R. Langer, R. J. Linhardt, C. Haudenschild and S. Taylor, Science, 1983, 221, 719 CAS.
- K. Park, G. Y. Lee, Y. S. Kim, M. Yu, R. W. Park, I. S. Kim, S. Y. Kim and Y. Byun, J. Controlled Release, 2006, 114, 300 CrossRef CAS PubMed.
- C. Passirani, G. Barratt, J. P. Devissaguet and D. Labarre, Pharm. Res., 1998, 15, 1046 CrossRef CAS.
- M. H. Dufresne and J. C. Leroux, Pharm. Res., 2004, 21, 160 CrossRef CAS.
- K. Na, K. Park, S. W. Kim and Y. H. Bae, J. Controlled Release, 2000, 69, 225 CrossRef CAS.
- S. Kwon, J. H. Park, H. Chung, I. C. Kwon and S. Y. Jeong, Langmuir, 2003, 19, 10188 CrossRef CAS.
- H. Meng, M. Xue, T. Xia, Z. Ji, D. Y. Tarn, J. I. Zink and A. E. Nel, ACS Nano, 2011, 5, 4131 CrossRef CAS PubMed.
- V. Mamaeva, J. M. Rosenholm, L. T. Bate-Eya, L. Bergman, E. Peuhu, A. Duchanoy, L. E. Fortelius, S. Landor, D. M. Toivola, M. Linden and C. Sahlgren, Mol. Ther., 2011, 19, 1538 CrossRef CAS PubMed.
- J. Lu, M. Liong, Z. Li, J. I. Zink and F. Tamanoi, Small, 2010, 6, 1794 CrossRef CAS PubMed.
- H. Meng, M. Xue, T. Xia, Z. X. Ji, D. Y. Tarn, J. I. Zink and A. E. Nel, ACS Nano, 2011, 5, 4131 CrossRef CAS PubMed.
- Q. J. He, J. M. Zhang, J. L. Shi, Z. Y. Zhu, L. X. Zhang, W. B. Bu, L. M. Guo and Y. Chen, Biomaterials, 2010, 31, 1085 CrossRef CAS PubMed.
- M. Gary-Bobo, Y. Mir, C. Rouxel, D. Brevet, I. Basile, M. Maynadier, O. Vaillant, O. Mongin, M. Blanchard-Desce, A. Morere, M. Garcia, J.-O. Durand and L. Raehm, Angew. Chem., 2011, 123, 11627 CrossRef PubMed.
- Q. Wu, C. Liu, L. Fan, J. Shi, Z. Liu, R. Li and L. Sun, Nanotechnology, 2012, 23, 485703 CrossRef PubMed.
- H. J. Chung, H. K. Kim, J. J. Yoon and T. G. Park, Pharm. Res., 2006, 23, 1835 CrossRef CAS PubMed.
- L. P. Zhu, J. Xing, Q. X. Wang, L. Kou, C. Li, B. Hu, Z. W. Wu, J. J. Wang and G. X. Xu, Eur. J. Pharmacol., 2009, 617, 23 CrossRef CAS PubMed.
- K. Le, R. Li, S. Xu, X. Wu, H. Huang, Y. Bao, Y. Cai, T. Lan, J. Moss, C. Li, J. Zou, X. Shen and P. Liu, Arch. Biochem. Biophys., 2012, 518(1), 71 CrossRef CAS PubMed.
- R. Li, W. Zheng, R. Pi, J. Gao, H. Zhang, P. Wang, K. Le and P. Liu, FEBS Lett., 2007, 581, 3311 CrossRef CAS PubMed.
- A. Saleem, M. Husheem, P. Harkonen and K. Pihlaja, J. Ethnopharmacol., 2002, 81, 327 CrossRef CAS.
- H. Chen, L. You and S. Li, Cancer Lett., 2004, 211, 163 CrossRef CAS PubMed.
- Z. Q. Yu, R. M. Schmaltz, T. C. Bozeman, R. Paul, M. J. Rishel, K. S. Tsosie and S. M. Hecht, J. Am. Chem. Soc., 2013, 135, 2883 CrossRef CAS PubMed.
- T. Sato, M. Hanada, S. Bodrug, S. Irie, N. Iwama, L. H. Boise, C. B. Thompson, E. Golemis, L. Fong and H. G. Wang, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 9238 CrossRef CAS.
- M. J. Cross and L. Claesson-Welsh, Trends Pharmacol. Sci., 2001, 22, 201 CrossRef CAS.
- N. Tarasenko, S. M. Cutts, D. R. Phillips, A. Inbal, A. Nudelman, G. Kessler-Icekson and A. Rephaeli, PLoS One, 2012, 7, e31393 CAS.
- M. M. Kemp, A. Kumar, S. Mousa, E. Dyskin, M. Yalcin, P. Ajayan, R. J. Linhardt and S. A. Mousa, Nanotechnology, 2009, 20, 455104 CrossRef PubMed.
- Y. Matsumura and H. Maeda, Cancer Res., 1986, 46, 6387 CAS.
- H. Maeda, J. Wu, T. Sawa, Y. Matsumura and K. Hori, J. Controlled Release, 2000, 65, 271 CrossRef CAS.
- X. Li, Q. He and J. Shi, ACS Nano, 2014, 8, 1309 CrossRef CAS PubMed.
- L. Lundin, H. Larsson, J. Kreuger, S. Kanda, U. Lindahl, M. Salmivirta and L. Claesson-Welsh, J. Biol. Chem., 2000, 275, 24653 CrossRef CAS PubMed.
- Y. Yoshitomi, H. Nakanishi, Y. Kusano, S. Munesue, K. Oguri, M. Tatematsu, I. Yamashina and M. Okayama, Cancer Lett., 2004, 207, 165 CrossRef CAS PubMed.
- K. Ono, M. Ishihara, K. Ishikawa, Y. Ozeki, H. Deguchi, M. Sato, H. Hashimoto, Y. Saito, H. Yura, A. Kurita and T. Maehara, Br. J. Cancer, 2002, 86, 1803 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Zeta potential of nanoparticles. See DOI: 10.1039/c5ra01281c |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), heparin sodium (MW 12000) and DOX were purchased from Shanghai Chemical Corp. N-Hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), 4-morpholineethanesulfonic acid (MES), 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), fluorescein isothiocyanate (FITC), (2′-(4-ethoxyphenyl)-5-(4-methyl-1-piperazinyl)2,5′-bi-1H-benzimidazoletri hydrochloride) (Hoechst 33
000), heparin sodium (MW 12000) and DOX were purchased from Shanghai Chemical Corp. N-Hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), 4-morpholineethanesulfonic acid (MES), 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), fluorescein isothiocyanate (FITC), (2′-(4-ethoxyphenyl)-5-(4-methyl-1-piperazinyl)2,5′-bi-1H-benzimidazoletri hydrochloride) (Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342), and toluidine blue were obtained from Sigma-Aldrich (USA). Basic fibroblast growth factor (bFGF) was purchased from Pharmacia (Sweden). The antibodies used for western blotting were purchased from Santa Cruz Biotechnology (USA). All other chemicals are of analytical grade.
342), and toluidine blue were obtained from Sigma-Aldrich (USA). Basic fibroblast growth factor (bFGF) was purchased from Pharmacia (Sweden). The antibodies used for western blotting were purchased from Santa Cruz Biotechnology (USA). All other chemicals are of analytical grade.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 and DiI before measurement.
342 and DiI before measurement.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution), respectively, overnight at 4 °C, and then incubated with the corresponding horseradish peroxidase-labeled secondary antibodies at room temperature for 1 h. Finally, the immunoreactivity was detected using the enhanced chemiluminescence method. Quantization of the protein band density was performed using Gel-Pro Analyzer 4.0. The data are reported as normalized protein band densities.
1000 dilution), respectively, overnight at 4 °C, and then incubated with the corresponding horseradish peroxidase-labeled secondary antibodies at room temperature for 1 h. Finally, the immunoreactivity was detected using the enhanced chemiluminescence method. Quantization of the protein band density was performed using Gel-Pro Analyzer 4.0. The data are reported as normalized protein band densities.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 and DiI dyes. It can be seen that the cell nuclei stained with Hoechst 33
342 and DiI dyes. It can be seen that the cell nuclei stained with Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 exhibited blue fluorescence, the cell membranes stained with DiI exhibited red fluorescence, and the cytoplasm also exhibited red fluorescence due to the diffusion of DiI from the cell membranes to the cytoplasm. Fig. 4c shows the merged images of Hela cells in the presence of FITC-labeled MSNs-HP and the other two dyes. It can be seen that the cell nuclei still exhibited blue fluorescence, but the fluorescence of the cell membranes and cytoplasm changed from red to yellow-green due to the overlapping of red fluorescent spots and green fluorescent spots, indicating that MSNs-HP could penetrate into the living cells. Herein, it should be mentioned that MSNs-HP is negatively charged which does not benefit its cellular uptake. The great cellular uptake of the nanoparticles may be related to the heparin targeting effect because a lot of natural products such as bleomycin also have tumor targeting properties.25
342 exhibited blue fluorescence, the cell membranes stained with DiI exhibited red fluorescence, and the cytoplasm also exhibited red fluorescence due to the diffusion of DiI from the cell membranes to the cytoplasm. Fig. 4c shows the merged images of Hela cells in the presence of FITC-labeled MSNs-HP and the other two dyes. It can be seen that the cell nuclei still exhibited blue fluorescence, but the fluorescence of the cell membranes and cytoplasm changed from red to yellow-green due to the overlapping of red fluorescent spots and green fluorescent spots, indicating that MSNs-HP could penetrate into the living cells. Herein, it should be mentioned that MSNs-HP is negatively charged which does not benefit its cellular uptake. The great cellular uptake of the nanoparticles may be related to the heparin targeting effect because a lot of natural products such as bleomycin also have tumor targeting properties.25








