Development of a novel nitrite electrochemical sensor by stepwise in situ formation of palladium and reduced graphene oxide nanocomposites†
Abstract
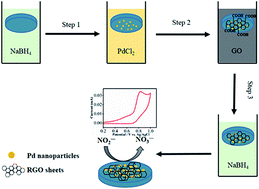
In this paper, a novel and sensitive electrochemical nitrite sensor was prepared by in situ electroless deposition of Pd nanoparticles and reduced graphene oxide (RGO) stepwisely on a glassy carbon electrode. The deposition process was very fast and the amount of Pd/RGO deposited on the electrode was well controlled by the number of the deposition cycle. The fabrication process was monitored by UV-vis spectroscopy, and the as prepared composites were characterized by FTIR, Raman spectroscopy, XRD and SEM. The results confirmed the successful formation of Pd and the chemical reduction of graphene oxides. Moreover, the incorporation of Pd in between RGO sheets effectively prevented the agglomeration of RGO. The Pd/RGO modified electrode exhibited significant enhancement to the oxidation of nitrite with increased current response and reduced over-potential which was a result of the synergistic catalytic effect of RGO and Pd nanoparticles. The influence of various experimental parameters on the detection of nitrite was studied in detail. Under optimum conditions, the developed nitrite sensor had a linear response in the concentration range of 1–1000 μM and a detection limit of 0.23 μM. Additionally, the fabricated nitrite sensor showed excellent selectivity, reproducibility and stability.


 Please wait while we load your content...
Please wait while we load your content...