Open Access Article
Open Access ArticlePost-synthetic benzylation of the mRNA 5′ cap via enzymatic cascade reactions†
N. V.
Cornelissen‡
 a,
R.
Mineikaitė‡
a,
M.
Erguven
a,
R.
Mineikaitė‡
a,
M.
Erguven
 ab,
N.
Muthmann
a,
A.
Peters
a,
A.
Bartels
a and
A.
Rentmeister
ab,
N.
Muthmann
a,
A.
Peters
a,
A.
Bartels
a and
A.
Rentmeister
 *ab
*ab
aUniversity of Münster, Department of Chemistry, Institute of Biochemistry, Corrensstr. 36, 48149 Münster, Germany. E-mail: a.rentmeister@uni-muenster.de
bUniversity of Münster, Cells in Motion Interfaculty Centre, Waldeyerstr. 15, 48149, Münster, Germany
First published on 18th September 2023
Abstract
mRNAs are emerging modalities for vaccination and protein replacement therapy. Increasing the amount of protein produced by stabilizing the transcript or enhancing translation without eliciting a strong immune response are major steps towards overcoming the present limitations and improving their therapeutic potential. The 5′ cap is a hallmark of mRNAs and non-natural modifications can alter the properties of the entire transcript selectively. Here, we developed a versatile enzymatic cascade for regioselective benzylation of various biomolecules and applied it for post-synthetic modification of mRNA at the 5′ cap to demonstrate its potential. Starting from six synthetic methionine analogues bearing (hetero-)benzyl groups, S-adenosyl-L-methionine analogues are formed and utilized for N7G-cap modification of mRNAs. This post-synthetic enzymatic modification exclusively modifies mRNAs at the terminal N7G, producing mRNAs with functional 5′ caps. It avoids the wrong orientation of the 5′ cap—a problem in common co-transcriptional capping. In the case of the 4-chlorobenzyl group, protein production was increased to 139% during in vitro translation and to 128–150% in four different cell lines. This 5′ cap modification did not activate cytosolic pathogen recognition receptors TLR3, TLR7 or TLR8 significantly more than control mRNAs, underlining its potential to contribute to the development of future mRNA therapeutics.
Introduction
The past few years have witnessed a massive surge in the interest and use of mRNA for medical applications. In particular, the rapid development of mRNA-based vaccines against the SARS-CoV-2 coronavirus has not only boosted technology development and large-scale production, but also set the stage for approval of this therapeutic modality. In addition to its numerous applications as vaccine against infectious diseases1 or personalized cancer treatment,2 mRNA has potential for protein replacement therapy.3 However, this application will require repeated and much higher doses and the immune response to mRNA has to be restrained.4 A key challenge in the development of mRNA-based therapeutics is therefore to increase the amount of protein produced as well as the timespan of protein production without eliciting an overshooting immune response. To this end, a variety of modifications at the 5′ cap, the entire sequence and the poly(A) tail have been tested and shown to provide improvements.5The 5′ cap stabilizes eukaryotic mRNAs and is crucial for translation and reduced immune response compared to uncapped RNA.6 Within the >1000 nt long transcripts, it is a privileged structure, as even small modifications at the 5′ cap provide a powerful lever to modulate stability, translation, and immunogenicity.5b,7
To make mRNA with a 5′ cap, in vitro transcription (IVT) in the presence of a synthetic 5′ cap analogue which leads to a cap 0 (m7GpppG) or cap 1 (m7GpppAm) structure is most widely used. An anti-reverse cap analogue (7-methyl(3′-O-methyl)GpppG termed ARCA) is used to avoid wrong cap incorporation that would lead to translationally silent mRNA.8 Accordingly, to test modifications at the 5′ cap, the respective analogues can simply be introduced to the IVT.7c,d
However, despite the need to assess how altered 5′ caps change mRNA properties and function to get a comprehensive picture of the structure–activity relationship, modified 5′ cap analogues are not commercially available and their synthesis and purification requires technical expertise and equipment beyond routine.
In particular, the site-specific methylation of guanosine at the 5′ cap at a late stage of chemical synthesis can pose problems.9 Enzymatic methylation of 5′ caps or short synthetic RNAs with a 5′ cap at the late stage of chemical synthesis has repeatedly been used to solve this issue.10 We propose the enzymatic late-stage modification of commercially available 5′ caps or mRNAs with unmodified cap precursors (i.e. GpppG or GpppA) as an attractive alternative. The post-synthetic modification of mRNAs with a symmetric GpppG at the 5′ end solves the problem of statistical wrong cap incorporation encountered in co-transcriptional capping using modified 5′ caps.8a,11
Most natural modifications at the 5′ cap are methyl groups installed by methyltransferases (MTases) using the cosubstrate S-adenosyl-L-methionine (AdoMet or SAM). Previous work by several groups, including ours, demonstrated the use of various MTases in combination with AdoMet analogues to modify DNA, RNA, proteins and small molecules with various groups in a regio- and chemo-selective manner (Fig. 1).12 With respect to the 5′ cap, we reported on MTase-based modification of the N7G, N2G and N6A of m7GpppAm with the help of AdoMet analogues.7a,b,13
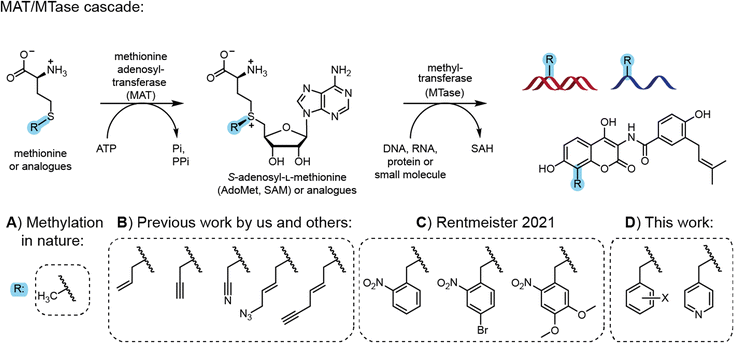 | ||
| Fig. 1 Cascade reactions for methylation in nature and application for site-specific alkylation and benzylation. (A) Methionine and ATP are reacted by methionine adenosyltransferase (MAT) to form the cofactor S-adenosyl-L-methionine (AdoMet or SAM). AdoMet can be used by methyltransferases (MTases) for the stereo-, regio- and chemo-selective methylation of DNA, RNA, proteins and small molecules. (B) Synthetic methionine analogues are used in MAT/MTase cascade reactions to transfer non-natural alkyl moieties to biomolecules.12 (C) Photocleavable groups derived from the 2-nitrobenzyl group can be transferred by an engineered MAT/MTase cascade to photocage DNA.14 (D) This work shows that MAT/MTase cascades are a general tool to transfer (hetero)-benzylic moieties and can be used to modify mRNA at the 5′ cap. | ||
However, these AdoMet analogues show limited half-lives in aqueous solution and their synthesis, although simple, is low yielding.15 Shipping the compounds can lead to partial degradation, limiting the exchange of materials and collaborations. Therefore, the enzymatic in situ generation of AdoMet analogues for direct use by MTases in cascade reactions presents an attractive alternative. Methionine adenosyltransferases (MATs) catalyse AdoMet formation from methionine and ATP.16 Starting from synthetic methionine analogues (Fig. 1B) a MAT/MTase cascade has been used in vitro for the late-stage alkylrandomization of natural products, such as rebeccamycin12b or rapamycin analogues.17 We reported MAT/MTase cascades for the modification of the 5′ cap with allyl and propargyl residues,18 but did not find activity with benzylic groups18a due to limited substrate promiscuity of the MAT. Thorson and coworkers systematically studied methionine analogues with several wild type MAT enzymes, but benzylation remained elusive due to the low promiscuity of most MATs.12b
Site-directed mutagenesis yielded MAT variants improved on methionine analogues with alkyl groups.12c,19 We then engineered a MAT variant from Methanocaldococcus jannaschii (MjMAT L147A/I351), termed PC-MjMAT, with high activity on 2-nitrobenzyl-containing methionine analogues (Fig. 1C), and solved the crystal structures in complex with an ortho-nitrobenzyl (ONB)-containing AdoMet analogue (AdoONB) and derivatives.14,20
The results from MAT engineering and crystal structure analysis led us to hypothesize that the binding pocket is not specific for AdoONB and PC-MjMAT might accommodate a variety of benzylic AdoMet analogues in a similar binding mode, involving π-stacking of the benzyl ring and the adenine moiety of such analogues (Fig. 1D). As MTases can be highly promiscuous, the enzymatic generation of benzylic AdoMet analogues could substantially broaden the scope of enzymatic alkyl randomization to benzyl randomization of biomolecules and 5′ cap modifications starting from simple amino acids.
Results and discussion
Enzymatic generation of AdoMet analogues with benzylic moieties
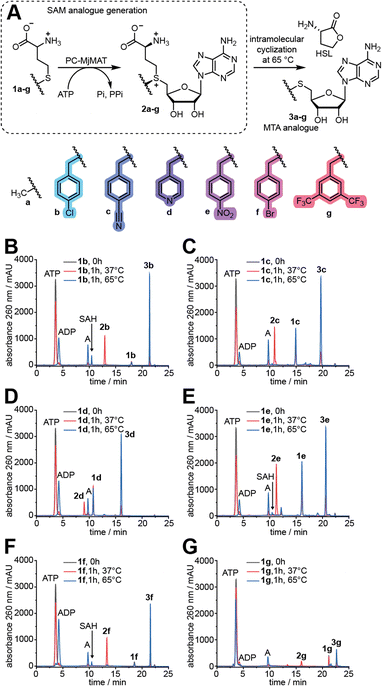
First, D,L-methionine analogues (1b–g, Fig. 2A) with benzylic side chains were synthesized in one-pot reactions from cheap D,L-homocysteine thiolactone (HCTL), purified by preparative HPLC (Fig. S1†) and analysed by LC-QTOF-MS (Fig. S2 and S3†) and 1H-,13C- or 19F-NMR (Fig. S4–S16†). The benzylic substituents all represent electron-poor aromatic systems, which we assumed to speed up the methyltransferase reaction based on the Hammett equation. Methionine analogues 1e and 1f have been previously synthesized by us and others and could not be converted by any MAT variant tested so far,12b,18a while the syntheses of 1b, 1c, 1d and 1g are reported for the first time to the best of our knowledge.We tested the activity of PC-MjMAT on methionine (1a) and these benzylic methionine analogues (1b–g). At 37 °C the AdoMet and AdoMet analogues (2a–g) can be detected in the reaction mixture. At 65 °C, however, they are degraded to stable methylthioadenosine (MTA) analogues (3a–g) (Fig. 2 and S17†). The reaction at 65 °C, leading to MTA formation, thus provides a fast and direct assay to measure MAT activity without product inhibition. To our delight, the HPLC analysis showed that PC-MjMAT is active on all methionine analogues 1b–g, as the respective AdoMet analogues (2b–g) and MTA analogues (3a–g) are formed (Fig. 2B–G and S17†). Formation of AdoMet analogues 2b–g was validated by LC-QTOF-MS (Fig. S18–S23†). The reactions without amino acid did not show a new peak and served as negative control (Fig. S17†). These data show that a broad range of benzylic methionine analogues are readily converted by PC-MjMAT at different temperatures.
MAT/MTase cascades for versatile site-specific benzylation of complex small molecules
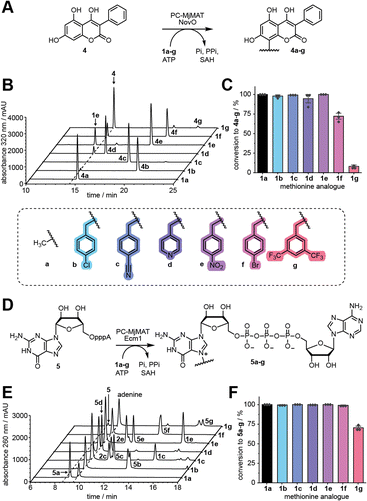
To test whether the generated AdoMet analogues can serve as MTase co-substrates in situ, we first used NovO, a coumarin MTase from Streptomyces niveus involved in the biosynthesis of the aminocoumarin antibiotic novobiocin (Fig. 3A).21 NovO selectively modifies the C8-position of a range of 7-hydroxycoumarine derivatives and was previously shown to accept bulky AdoMet analogues.22 We tested methionine (1a) and analogues 1b–g in the PC-MjMAT/NovO cascade using 3,4,7-trihydroxy-3-phenyl-coumarin (THPC, 4) as substrate and monitored the conversion via HPLC at 320 nm (Fig. 3B, S24 and S25†). To our delight, 1a–e resulted in almost quantitative conversions to 100% 4a, 98% 4b, 99% 4c, 95% 4d, and 100% 4e, respectively. Analogue 1f gave satisfactory conversion (72% 4f), whereas conversion of 1g was low (8% 4g) (Fig. 3C). Products 4a–g were confirmed by LC-QTOF-MS (Fig. S26–S32†).N7G-modified GpppA
Next, we turned our attention to the dinucleotide GpppA (5), which forms the 5′ cap of mRNA and can be used for the production of capped mRNA by in vitro transcription (IVT).23 We established a cascade of PC-MjMAT with Ecm1, the mRNA cap (guanine N7) methyltransferase from Encephalitozoon cuniculi,24 and monitored the N7G modification of 5 by HPLC (Fig. 3D and E, S33 and S34†). Using 1a–f, conversions were almost complete (>99% 5a–f). Using 1g, the conversion still reached 70%. Products 5a–g were confirmed by LC-QTOF-MS (Fig. S35–S41†). These results show that PC-MjMAT displays remarkable promiscuity for benzylic groups and compatibility with at least two different MTases.Evaluation of N7G-modified-mRNAs starting with GpppA
To test how modifications at the N7G of the 5′ cap affect protein production, we modified GpppA via PC-MjMAT/Ecm1 cascade reactions and purified 5b–d by HPLC to then use them for co-transcriptional capping of Gaussia luciferase (GLuc) mRNA. To ensure that exclusively capped mRNA is assessed in subsequent experiments, uncapped RNA was routinely digested by polyphosphatase and XRN1. Translation assays showed that 5b–d caps result in 40%, 22%, and 11% GLuc activity, respectively, compared to ARCA-GLuc in HeLa cells (Fig. S42A†). Due to this low amount of protein produced from 5b–d capped mRNAs in HeLa cells, we compared ARCA-, m7GpppA- and m7GpppG-capped mRNA in RRL and HeLa cells (Fig. S42B†). While m7GpppA- and m7GpppG-capped mRNAs performed similarly in vitro, protein production in HeLa cells was higher for m7GpppG-capped mRNA. We therefore switched to GpppG-based 5′ cap analogues from which higher amounts of protein are produced.N7-modified GpppG
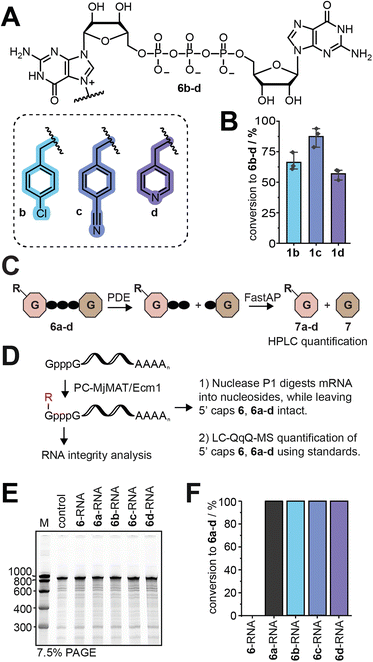
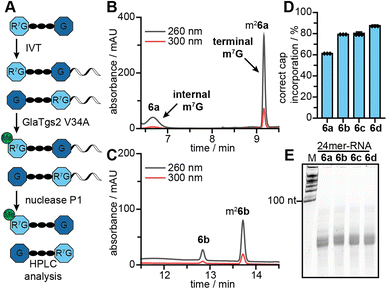
The PC-MjMAT/Ecm1 cascade with 1b–d was thus applied to GpppG (6). This can lead to N7G modification at one (6b–d) or both Gs. After optimization, we were able to convert GpppG (6) to 6b–d in good yields without formation of the double modified products (Fig. 4A, B and S43†). The products 6b–d were purified via semi-preparative HPLC and the identity was confirmed by LC-QTOF-MS (Fig. S44–S50†). Before using the isolated 5′ caps 6b–d as LC-QqQ-MS standards for quantification and analysis of the chemo-enzymatic modification of mRNAs, we needed to determine their exact concentrations. To achieve this, we digested 6b–d with snake venom phosphodiesterase (PDE) and quantified the resulting guanosine (7) peak by HPLC (Fig. 4C and S51A†). This enzymatic approach provides rapid access to synthetic standards of modified 5′ cap analogues, which are required for quantification but not commercially available and tedious to synthesize by chemical means alone.Post-synthetic modification of mRNA and analysis
With LC-MS-based methodology to quantify modified 5′ caps from mRNA at hand, we converted GpppG-capped GLuc-mRNA via the PC-MjMAT/Ecm1 cascade (Fig. 4D). The approach relies on addition of symmetric GpppG during IVT and benefits from the site-specific post-transcriptional modification at the N7 position of the 5′ terminal G. It elegantly circumvents wrong cap incorporation (where the N7-modified G is not the terminal nucleotide) as either orientation of GpppG yields identically capped mRNA, and once in mRNA only the terminal G is recognized by the enzyme Ecm1.To ensure the quality of mRNA, we routinely removed uncapped RNA and tested the integrity of mRNA before biological assays (Fig. 4E). After optimization, we achieved quantitative modification of GpppG-capped-mRNA using 1a–d without affecting RNA integrity. The conversion of mRNA modification at the 5′ cap was determined for each mRNA sample, using the LC-QqQ method and synthetic standards (Fig. 4F). To this end, we digested mRNA with nuclease P1 into nucleosides and the intact cap. LC-QqQ-MS was then used to quantify modified and non-modified caps via external calibration, using optimized multiple reaction monitoring (MRM) settings for each fragment (Fig. S51B and C†).
Evaluation of the effect of N7G-modified-mRNAs starting with GpppG on the amount of protein produced
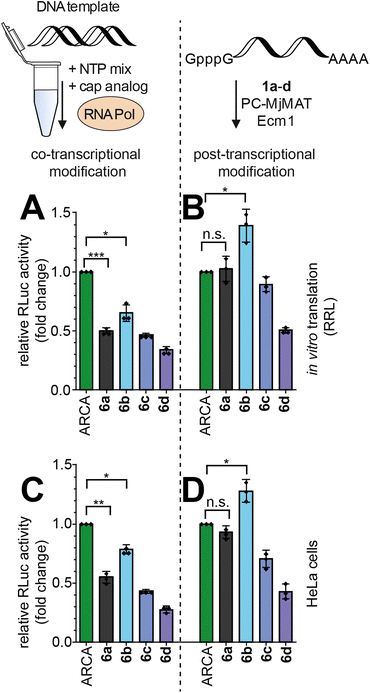
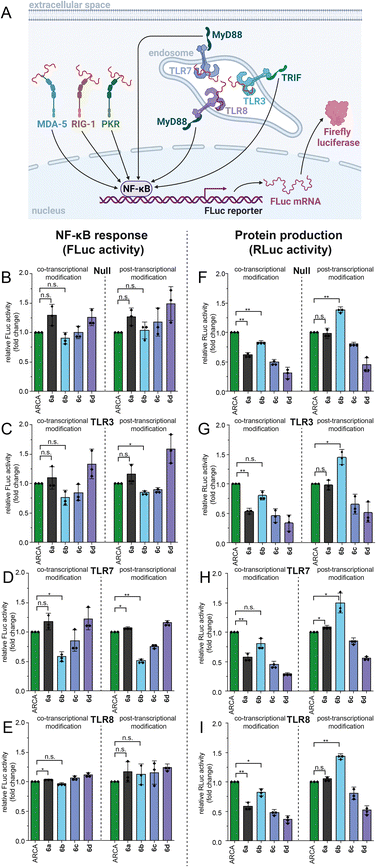
With these high-quality mRNA samples, we sought to investigate the effect of non-natural 5′ cap modifications on protein production. We prepared Renilla luciferase (RLuc) reporter mRNAs with different 5′ cap modifications. To ensure that we are looking at the effect of 5′ cap modifications and rule out effects by the mRNA preparation, we prepared the mRNAs by two different procedures (Fig. 5): in the co-transcriptional approach, the N7-modified GpppG caps are added to the IVT mix (Fig. 5A and C) whereas in the post-transcriptional approach, the cascade reaction is performed on complete GpppG-capped mRNAs (Fig. 5B and D).Both approaches yielded good amounts of intact mRNA (Fig. 4E and S52†). The amount of protein produced from differently prepared and cap-modified mRNAs was first tested in rabbit reticulocyte lysate (RRL), a eukaryotic cell-free expression system (Fig. 5A and B). The signals of luminescence were measured after 90 minutes of in vitro translation, and normalized to RLuc-mRNA capped with ARCA. N7G-modified caps used for co-transcriptional modification in IVT can be incorporated in different orientations and resulted in ∼50% luciferase activity for m7GpppG (6a) compared to ARCA (Fig. 5A), in line with previous reports.8a,b,25 RLuc-mRNA with benzylic modifications, i.e. 5′ caps 6b–d, resulted in 65%, 46% and 34% luciferase activity respectively (Fig. 5A).
Performing the cascade on GpppG-capped RLuc circumvents the problem of wrong cap incorporation, as Ecm1 selectively modifies the terminal guanosine.26 Post-transcriptional modification of GpppG-mRNA via the cascade starting from 1a–d resulted in relative luciferase activities of 102%, 139%, 89% and 50%, respectively, in vitro (Fig. 5B). The commonly used ARCA cap was outperformed by the N7-(4-chlorobenzyl) modification installed in the cascade reaction (Fig. 5B).
To independently assess the effect of 5′ cap modifications in cells, we transfected HeLa cells with the differently modified RLuc-mRNAs and assayed 24 h after transfection (Fig. 5C and D). Again, both the co- and post-transcriptional methods were used for preparation. Compared to ARCA, co-transcriptionally modified mRNAs prepared by IVT gave luciferase activity of 55%, 79%, 43% and 27% for 6a–d (Fig. 5C). Again, the post-transcriptionally modified mRNAs showed higher luciferase activity (Fig. 5D). With the cascade starting from 1a–d, 93%, 128%, 70% and 43% luciferase activity relative to ARCA-capped mRNA were determined (Fig. 5D).
The direct comparison shows that the relative performance of all differently capped mRNAs is essentially the same in vitro and in cells (Fig. 5Avs.5C and 5Bvs.5D). It is thus unlikely that other factors, such as different mRNA levels in cells, are the reason for the effects observed.
The comparison of co-transcriptionally and post-transcriptionally modified mRNAs (Fig. 5A to B and C to D) shows that mRNAs with 5′ caps 6a–d generated via the enzymatic cascade produce 148–200% protein, compared to ARCA-mRNAs, which cannot be incorporated in the wrong orientation and are not affected.
Analysis of 5′ cap orientation and eIF4E binding
The amount of protein produced from different 5′ cap modified mRNAs is the most relevant readout. It results, however, from several contributing factors, which can have convoluted and even contradicting effects. We aimed to further dissect the positive effect of post-transcriptional modification by determining the contribution of correct cap incorporation. To this end, we produced short mRNAs (24-mers) with different 5′ caps by IVT in the presence of 6a–d. The integrity of the 24-mer mRNAs was confirmed by gel electrophoresis (Fig. 6E).To analyse the orientation of the 5′ cap, i.e. whether the N7-modified G is terminal or at the TSN, we capitalized on the site-specificity of the 5′ cap methyltransferase GlaTgs2, which can only methylate terminal m7G but not terminal G or internal m7G.13a,24,27 We incubated the mRNAs with GlaTgs2 and SAM. This leads to additional complete N2 methylation only in the case of correct 5′ cap orientation (Fig. 6A), even when the N7G is modified with non-natural groups.18b After digestion into 5′ cap and nucleosides, the fraction of correct orientation can then be determined from the ratio of methylated and unmethylated product by HPLC analysis (Fig. 6B, C and S53†). For mRNAs with 5′ caps 6a–d, we found 61%, 79%, 80% and 88% of them in the correct orientation, respectively (Fig. 6D). These data indicate that bulkier N7-modifications improve correct 5′ cap incorporation during IVT. The effect can be rationalized by the bulkiness of the modifications and a stronger effect on the G that serves as TSN than the terminal G, which is per se already an extension. The result suggests that 5′ caps with bulky N7G modifications also alleviate the problem of wrong cap incorporation in a co-transcriptional approach. However, our data for wrong cap incorporation of 6b–d cannot explain the trend for protein production observed in RRL and in cells.
Another factor contributing to improved translational output of mRNAs could be higher affinity to the translation initiation factor eIF4E.7c,28 To test the effect of benzylic 5′ cap modifications on binding to eIF4E, we performed binding assays of 6a–d with fluorescently labelled eIF4E, using microscale thermophoresis (MST). ARCA and 6a showed the highest affinities with Kd values in the low μM range. All N7-benzylated 5′ caps bound less tightly (Fig. S54†). Based on this data, the affinity to eIF4E is not responsible for the observed increase in protein production.
Evaluation of the effect of N7G-modified-mRNAs starting with GpppG on the immune response in HEK-NF-κB cell lines
Next, we sought to evaluate the effects of the non-natural modifications on the immune response. To study if 5′ cap modifications affect the activation of pathogen recognition receptors (PRRs), we used four HEK-NF-κB cell lines (Null, TLR3, TLR7, TLR8). All these cell lines express the firefly luciferase gene driven by the NF-κB response element after activation of PRRs such as RIG-I, MDA5, and PKR (Fig. 7A). Additionally, HEK-NF-κB-TLR3, HEK-NF-κB-TLR7 and HEK-NF-κB-TLR8 overexpress the respective Toll-like receptor (TLR). TLRs recognize foreign RNAs and initiate another signalling pathway triggering expression of genes driven by the NF-κB response element (Fig. 7A). Transfection of different HEK-NF-κB cell lines with GpppG-RLuc-mRNA that was modified either co- or post-transcriptionally via PC-MjMAT/Ecm1 revealed that 5′ cap modifications can, in some cases, alter the immunogenicity of RNA compared to control RNA (m7GpppG-RLuc) that was used as a reference (Fig. 7B–E). The overall effect on NF-κB activation from co- and post-transcriptionally modified mRNAs was similar for all tested HEK-NF-κB cell lines. While ARCA-mRNA and 6b-mRNA led to similar levels of activation of the NF-κB pathway in HEK-NF-κB-TLR Null, TLR3 and TLR8 cells, we found significantly lower activation by 6b-mRNA in TLR 7 cells.In parallel, we measured protein production via a luminescence assay in HEK-NF-κB cell lines (Fig. 7F–I). The protein production for different mRNA 5′ cap modifications showed the same trend as the data in HeLa cells (Fig. 5C and D). Of note, we observed for 6b-mRNAs made by the post-synthetic approach that even more protein is produced compared to ARCA-mRNAs, specifically, HEK-NF-κB for the null (139%), TLR3 (146%), TLR7 (150%), and TLR8 (143%) cell lines, respectively (Fig. 7F–I). As higher activation of the immune response has a negative impact on translation, our data suggest that lower activation of TLR7 might play a role in the observed effects of 6b-mRNA on protein output.
Conclusions
This work presents a convenient and robust method for the regioselective modification of small molecules and mRNAs, which can likely be readily extended to other methyltransferase targets. Stable D,L-methionine analogues can be accessed in one pot in up to half-gram scale starting from cheap homocysteine thiolactone (HCTL). The engineered MAT (PC-MjMAT) efficiently generates the corresponding AdoMet analogues that are substrates for promiscuous methyltransferases, as shown here for NovO and Ecm1. The post-transcriptional modification via the PC-MjMAT/Ecm1 cascade ensures specificity for the terminal G when applied to full-length GpppG-capped mRNAs. When N7-modified 5′ caps are added co-transcriptionally to the IVT, however, they can be incorporated in different orientations.We found in several different systems consistently that mRNAs post-transcriptionally modified with an N7-(4-chlorobenzyl) group (6b-mRNAs) led to more protein output than the widely used ARCA-mRNAs. This was true in vitro (139%), in HeLa cells (128%) and in four different HEK cell lines (139–150%).
The effect of this 5′ cap modification could not be pinpointed to a single contributing factor; however, a number of factors, such as differences in transfection, better incorporation in IVT or binding to eIF4E could be ruled out. Our data suggest a role in lower activation of TLR7, however studies with primary cells will be required for validation. The low activation of cytosolic PRRs, in particular of TLR7, by 6b-mRNAs suggests that benzylic 5′ cap modifications can be a useful approach contributing to future mRNA therapy.
Data availability
All data are available in the ESI.†Author contributions
N. V. C. and A. R. conceived the project. N. V. C., R. M., M. E., N. M., A. P. and A. B. designed and performed experiments. All authors discussed the results. N. V. C., R. M. and A. R. wrote the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. R. thanks the DFG (CRC 1459 Project ID 433682494, IRTG2678) for financial support. R. M. was supported by a fellowship from the CRC 1459. We thank Ann-Marie Lawrence-Dörner and Dr Wolfgang Dörner for excellent technical assistance, Sabine Hüwel for culturing HeLa cells, Dr Petr Špaček for assistance with LC-QqQ-MS measurements, and Melissa van Dülmen for discussions. The mass spectrometry and NMR facilities of the organic chemistry department are gratefully acknowledged for analytical services. We thank TRON (Translational Oncology at the University Medical Center of the Johannes Gutenberg University Mainz) for providing us with the HEK-NF-κB cell lines.References
- L. A. Jackson, E. J. Anderson, N. G. Rouphael, P. C. Roberts, M. Makhene, R. N. Coler, M. P. McCullough, J. D. Chappell, M. R. Denison, L. J. Stevens, A. J. Pruijssers, A. McDermott, B. Flach, N. A. Doria-Rose, K. S. Corbett, K. M. Morabito, S. O'Dell, S. D. Schmidt, P. A. Swanson 2nd, M. Padilla, J. R. Mascola, K. M. Neuzil, H. Bennett, W. Sun, E. Peters, M. Makowski, J. Albert, K. Cross, W. Buchanan, R. Pikaart-Tautges, J. E. Ledgerwood, B. S. Graham and J. H. Beigel, N. Engl. J. Med., 2020, 383, 1920–1931 CrossRef CAS PubMed
.
- C. L. Lorentzen, J. B. Haanen, Ö. Met and I. M. Svane, Lancet Oncol., 2022, 23, e450–e458 CrossRef CAS PubMed
.
-
(a) S. Ramaswamy, N. Tonnu, K. Tachikawa, P. Limphong, J. B. Vega, P. P. Karmali, P. Chivukula and I. M. Verma, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E1941–e1950 CrossRef CAS PubMed
; (b) C. G. Perez-Garcia, R. Diaz-Trelles, J. B. Vega, Y. Bao, M. Sablad, P. Limphong, S. Chikamatsu, H. Yu, W. Taylor, P. P. Karmali, K. Tachikawa and P. Chivukula, Mol. Ther.--Nucleic Acids, 2022, 28, 87–98 CrossRef CAS PubMed
.
- E. Rohner, R. Yang, K. S. Foo, A. Goedel and K. R. Chien, Nat. Biotechnol., 2022, 40, 1586–1600 CrossRef CAS PubMed
.
-
(a) D. Strzelecka, M. Smietanski, P. J. Sikorski, M. Warminski, J. Kowalska and J. Jemielity, RNA, 2020, 26, 1815–1837 CrossRef CAS PubMed
; (b) B. A. Wojtczak, P. J. Sikorski, K. Fac-Dabrowska, A. Nowicka, M. Warminski, D. Kubacka, E. Nowak, M. Nowotny, J. Kowalska and J. Jemielity, J. Am. Chem. Soc., 2018, 140, 5987–5999 CrossRef CAS PubMed
; (c) K. Drazkowska, R. Tomecki, M. Warminski, N. Baran, D. Cysewski, A. Depaix, R. Kasprzyk, J. Kowalska, J. Jemielity and P. J. Sikorski, Nucleic Acids Res., 2022, 50, 9051–9071 CrossRef CAS PubMed
.
- N. Sonenberg and A. C. Gingras, Curr. Opin. Cell Biol., 1998, 10, 268–275 CrossRef CAS PubMed
.
-
(a) J. M. Holstein, L. Anhauser and A. Rentmeister, Angew. Chem., Int. Ed., 2016, 55, 10899–10903 CrossRef CAS PubMed
; (b) M. van Dülmen, N. Muthmann and A. Rentmeister, Angew. Chem., Int. Ed., 2021, 60, 13280–13286 CrossRef PubMed
; (c) N. Klöcker, F. P. Weissenboeck, M. van Dülmen, P. Spacek, S. Hüwel and A. Rentmeister, Nat. Chem., 2022, 14, 905–913 CrossRef PubMed
; (d) P. J. Sikorski, M. Warminski, D. Kubacka, T. Ratajczak, D. Nowis, J. Kowalska and J. Jemielity, Nucleic Acids Res., 2020, 48, 1607–1626 CrossRef CAS PubMed
; (e) M. Warminski, J. Kowalska, E. Nowak, D. Kubacka, R. Tibble, R. Kasprzyk, P. J. Sikorski, J. D. Gross, M. Nowotny and J. Jemielity, ACS Chem. Biol., 2021, 16, 334–343 CrossRef CAS PubMed
.
-
(a) J. Stepinski, C. Waddell, R. Stolarski, E. Darzynkiewicz and R. E. Rhoads, Rna, 2001, 7, 1486–1495 CAS
; (b) J. Jemielity, T. Fowler, J. Zuberek, J. Stepinski, M. Lewdorowicz, A. Niedzwiecka, R. Stolarski, E. Darzynkiewicz and R. E. Rhoads, Rna, 2003, 9, 1108–1122 CrossRef CAS PubMed
; (c) F. Muttach, N. Muthmann and A. Rentmeister, Org. Biomol. Chem., 2017, 15, 278–284 RSC
.
- J. Jemielity, R. Stolarski and E. Darzynkiewicz, Nucleosides, Nucleotides Nucleic Acids, 2007, 26, 1315–1319 CrossRef CAS PubMed
.
-
(a) N. Muthmann, T. Guez, J. J. Vasseur, S. R. Jaffrey, F. Debart and A. Rentmeister, ChemBioChem, 2019, 20, 1693–1700 CrossRef CAS PubMed
; (b) J. Leiter, D. Reichert, A. Rentmeister and R. Micura, ChemBioChem, 2020, 21, 265–271 CrossRef CAS PubMed
; (c) A. Shannon, B. Sama, P. Gauffre, T. Guez, F. Debart, J. J. Vasseur, E. Decroly, B. Canard and F. Ferron, Nucleic Acids Res., 2022, 50, 11186–11198 CrossRef CAS PubMed
.
- A. E. Pasquinelli, J. E. Dahlberg and E. Lund, RNA, 1995, 1, 957–967 CAS
.
-
(a) F. Muttach and A. Rentmeister, Methods, 2016, 107, 3–9 CrossRef CAS PubMed
; (b) S. Singh, J. Zhang, T. D. Huber, M. Sunkara, K. Hurley, R. D. Goff, G. Wang, W. Zhang, C. Liu, J. Rohr, S. G. Van Lanen, A. J. Morris and J. S. Thorson, Angew. Chem., Int. Ed., 2014, 53, 3965–3969 CrossRef CAS PubMed
; (c) R. Wang, K. Islam, Y. Liu, W. Zheng, H. Tang, N. Lailler, G. Blum, H. Deng and M. Luo, J. Am. Chem. Soc., 2013, 135, 1048–1056 CrossRef CAS PubMed
; (d) K. Islam, I. Bothwell, Y. Chen, C. Sengelaub, R. Wang, H. Deng and M. Luo, J. Am. Chem. Soc., 2012, 134, 5909–5915 CrossRef CAS PubMed
.
-
(a) D. Schulz, J. M. Holstein and A. Rentmeister, Angew. Chem., Int. Ed., 2013, 52, 7874–7878 CrossRef CAS PubMed
; (b) J. M. Holstein, D. Schulz and A. Rentmeister, Chem. Commun., 2014, 50, 4478–4481 RSC
; (c) J. M. Holstein, D. Stummer and A. Rentmeister, Chem. Sci., 2015, 6, 1362–1369 RSC
; (d) A. Ovcharenko, F. P. Weissenboeck and A. Rentmeister, Angew. Chem., Int. Ed., 2020, 60, 4098 CrossRef PubMed
; (e) N. Klöcker, L. Anhäuser and A. Rentmeister, ChemBioChem, 2022, e202200522 Search PubMed
; (f) A. Bollu, A. Peters and A. Rentmeister, Acc. Chem. Res., 2022, 55, 1249–1261 CrossRef CAS PubMed
.
-
(a) F. Michailidou, N. Klöcker, N. V. Cornelissen, R. K. Singh, A. Peters, A. Ovcharenko, D. Kümmel and A. Rentmeister, Angew. Chem., Int. Ed., 2021, 60, 480–485 CrossRef CAS PubMed
; (b) A. Peters, E. Herrmann, N. V. Cornelissen, N. Klöcker, D. Kümmel and A. Rentmeister, ChemBioChem, 2022, 23, e202100437 CrossRef CAS PubMed
.
- G. Lukinavicius, V. Lapiene, Z. Stasevskij, C. Dalhoff, E. Weinhold and S. Klimasauskas, J. Am. Chem. Soc., 2007, 129, 2758–2759 CrossRef CAS PubMed
.
-
(a) J. L. Hoffman, Biochemistry, 1986, 25, 4444–4449 CrossRef CAS PubMed
; (b) G. de la Haba, G. A. Jamieson, S. H. Mudd and H. H. Richards, J. Am. Chem. Soc., 1959, 81, 3975–3980 CrossRef CAS
; (c) Z. J. Lu and G. D. Markham, J. Biol. Chem., 2002, 277, 16624–16631 CrossRef CAS PubMed
; (d) R. S. Firestone and V. L. Schramm, J. Am. Chem. Soc., 2017, 139, 13754–13760 CrossRef CAS PubMed
.
- B. J. C. Law, A. W. Struck, M. R. Bennett, B. Wilkinson and J. Micklefield, Chem. Sci., 2015, 6, 2885–2892 RSC
.
-
(a) F. Muttach and A. Rentmeister, Angew. Chem., Int. Ed., 2016, 55, 1917–1920 CrossRef CAS PubMed
; (b) J. M. Holstein, F. Muttach, S. H. H. Schiefelbein and A. Rentmeister, Chemistry, 2017, 23, 6165–6173 CrossRef CAS PubMed
.
- M. Dippe, W. Brandt, H. Rost, A. Porzel, J. Schmidt and L. A. Wessjohann, Chem. Commun., 2015, 51, 3637–3640 RSC
.
- M. Erguven, N. V. Cornelissen, A. Peters, E. Karaca and A. Rentmeister, ChemBioChem, 2022, 23, e202200511 CrossRef CAS PubMed
.
- M. Pacholec, J. Tao and C. T. Walsh, Biochemistry, 2005, 44, 14969–14976 CrossRef CAS PubMed
.
-
(a) H. Stecher, M. Tengg, B. J. Ueberbacher, P. Remler, H. Schwab, H. Griengl and M. Gruber-Khadjawi, Angew. Chem., Int. Ed., 2009, 48, 9546–9548 CrossRef CAS PubMed
; (b) J. C. Sadler, C.-w. H. Chung, J. E. Mosley, G. A. Burley and L. D. Humphreys, ACS Chem. Biol., 2017, 12, 374–379 CrossRef CAS PubMed
; (c) I. J. W. McKean, J. C. Sadler, A. Cuetos, A. Frese, L. D. Humphreys, G. Grogan, P. A. Hoskisson and G. A. Burley, Angew. Chem., Int. Ed., 2019, 58, 17583–17588 CrossRef CAS PubMed
.
- T. M. Coleman, G. Wang and F. Huang, Nucleic Acids Res., 2004, 32, e14 CrossRef PubMed
.
- S. Hausmann, S. Zheng, C. Fabrega, S. W. Schneller, C. D. Lima and S. Shuman, J. Biol. Chem., 2005, 280, 20404–20412 CrossRef CAS PubMed
.
- I. Kocmik, K. Piecyk, M. Rudzinska, A. Niedzwiecka, E. Darzynkiewicz, R. Grzela and M. Jankowska-Anyszka, Cell Cycle, 2018, 17, 1624–1636 CrossRef CAS PubMed
.
- N. Muthmann, P. Spacek, D. Reichert, M. van Dülmen and A. Rentmeister, Methods, 2021, 203, 196–206 CrossRef PubMed
.
- S. Hausmann, C. P. Vivares and S. Shuman, J. Biol. Chem., 2002, 277, 96–103 CrossRef CAS PubMed
.
-
(a) A. R. Kore, Z. Xiao and M. Li, Bioorg. Med. Chem., 2013, 21, 4570–4574 CrossRef CAS PubMed
; (b) R. Wojcik, M. R. Baranowski, L. Markiewicz, D. Kubacka, M. Bednarczyk, N. Baran, A. Wojtczak, P. J. Sikorski, J. Zuberek, J. Kowalska and J. Jemielity, Pharmaceutics, 2021, 13 Search PubMed
; (c) A. Niedzwiecka, J. Marcotrigiano, J. Stepinski, M. Jankowska-Anyszka, A. Wyslouch-Cieszynska, M. Dadlez, A.-C. Gingras, P. Mak, E. Darzynkiewicz, N. Sonenberg, S. K. Burley and R. Stolarski, J. Mol. Biol., 2002, 319, 615–635 CrossRef CAS PubMed
; (d) D. Barnes-Seeman, S. L. Cohen, J. L. Diener, C. Gampe, J. Roache, A. White, S. L. Williams, J. Yuan and F. Zecri, in 3′ end caps, 5′ end caps and combinations thereof for therapeutic RNA, Novartis AG, 2020 Search PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc03822j |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |