Assembly of an indium–porphyrin framework JLU-Liu7: a mesoporous metal–organic framework with high gas adsorption and separation of light hydrocarbons†
Jiahuan
Luo
,
Jing
Wang
,
Yu
Cao
,
Shuo
Yao
,
Lirong
Zhang
*,
Qisheng
Huo
and
Yunling
Liu
 *
*
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: zlr@jlu.edu.cn; yunling@jlu.edu.cn; Fax: +86-431-85168624; Tel: +86-431-85168614
First published on 25th November 2016
Abstract
By reacting with indium nitrate and tetrakis(4-carboxyphenyl)-porphyrin (H4TCPP), a mesoporous indium–porphyrin framework, named JLU-Liu7, which features a rare (4,4)-connected frl net based on 1D inorganic indium rod SBUs, has been constructed. It exhibits high performance for gas adsorption and light hydrocarbon separation.
Introduction
In the last three decades, assembly and functionalization of metal–organic frameworks (MOFs) have attracted significant attention. This is not only due to their intriguing varieties of architectural diversity, chemical multiformity and tailorability,1 but also because of their ground potential applications in storage, carbon dioxide capture, separation, catalysis, drug delivery, and sensing.2 Recently, metal–porphyrin frameworks (MPFs) as an important branch of MOFs have been widely investigated for their applications such as guest molecule adsorption and separation, catalysis, nano-thin films and light-harvesting.1e,3–6 To our knowledge, there are three effective synthetic strategies implemented to construct MPFs: (i) crystal engineering synthesis of MPFs by using porphyrin ligands;3,4 (ii) pillared-layer construction of MPFs with pyridyl–porphyrin as pillars;5 (iii) postsynthetic modification of MPFs. In addition, porphyrin molecules as guest molecules can also be encapsulated into the cavities of MOFs.6 The secondary building unit (SBU) strategy as an elementary and the most important synthetic method has been approved to be a triumphant strategy to assemble MOFs.7 M(OH)(COO)2 is a kind of common SBU which is comprised of bi/trivalent metal cations (such as Mg2+, Al3+, Sc3+, V3+, Cr3+, Mn2+, Fe3+ and In3+), a bridging OH− group and a ligand.2b,8 Plenty of MOFs based on M(OH)(COO)2 SBUs have been reported, such as the famous MIL-479a and MIL-53 series,9b In(OH)L (L = C17F6O4H8),9c JUC-77,9d In2(OH)2(TBAPy),9e Mg-CPF-1,9f InOF-1,9g Al-PMOF,9h NOTT-3009i and 437-MOF.9j However, examples of MPFs based on In(OH)(COO)2 SBU still remain scarce and need to be further developed.Light hydrocarbons are very essential rare chemicals and energy resources. Separation of propane (C3), ethene and ethane (C2s) from methane (C1) is a very significant industrial process. Recently, scientists have focused on separating light hydrocarbons by using porous MOF materials, which have tunable pore sizes, high surface areas and open metal coordination sites. The famous MOF-74/CPO-27 series reported by Long's group and Snurr's group were exploited to separate light hydrocarbons and demonstrated remarkable performance.10 In particular, MOF-74-Fe was successfully used in the separation of C3H8/C3H6 and CH4/C2H2/C2H4/C2H6.10b Moreover, some porous MOF materials are promising candidates for application in the separation of light hydrocarbons, such as UTSA-33, UTSA-34 and UTSA-35 reported by Chen's group,11 CID-5/CID-6 reported by Kitagawa's group,12 MCOF-1/ZnP-CTFs reported by Zhu's group,13 FIR-7 reported by Zhang's group,14 and some part of the JLU-Liu series by our group.15
Herein, we demonstrate a mesoporous MPF material [In2(OH)2(C48H26N4O8)]·(DMF)6(H2O)4 (named JLU-Liu7) based on In(OH)(COO)2 SBU, which exhibits a high gas uptake capacity (such as O2 and CO2 at low temperatures, and CO2, C2H4, C2H6 and C3H8 at room temperature) and separation ability (such as CO2/CH4, C2H4/CH4, C2H6/CH4 and especially C3H8/CH4).
Experimental
Materials and methods
All the reagents were obtained from commercial sources and used without further purification. Powder X-ray diffraction (PXRD) data were collected on a Rigaku D/max-2550 diffractometer with CuKα radiation (λ = 1.5418 Å). Elemental analyses were performed on a Perkin-Elmer 2400 element analyzer. Thermogravimetric (TG) analyses were performed on a TGA Q500 thermogravimetric analyzer in the temperature range 35–800 °C under air flow with a heating rate of 5 °C min−1.Synthesis of JLU-Liu7
A mixture of H4TCPP (3 mg, 0.004 mmol), In(NO3)3·4H2O (12 mg, 0.025 mmol) and 0.2 mL HNO3 (0.35 M in DMF) in 1 mL DMF and 1 mL DMSO were sealed in a 20 mL vial, ultrasonically, then heated at 85 °C for 48 h to give purple rod crystals (85% yield based on H4TCPP). Elemental analysis: Found (wt%) C, 49.47; H, 4.88; N, 8.95. Calcd: C, 50.7; H, 5.0; N, 8.97.X-ray crystallography
Crystallographic data for JLU-Liu7 were collected on a Bruker X8 Prospector APEX II CCD diffractometer using Cu-Kα (λ = 1.54178 Å) radiation at 100 K. All non-hydrogen atoms were easily found from the difference Fourier map. The structure was solved by direct methods and refined by full-matrix least-squares on F2 using version 5.1.16 All non-hydrogen atoms were refined anisotropically. Since the highly disordered cations and guest molecules were trapped in the channels of JLU-Liu7 and could not be modeled properly, there are “Alert level A” about “Check Reported Molecular Weight” and “VERY LARGE Solvent Accessible VOID(S) in Structure” in the “checkCIF/PLATON report” files for JLU-Liu7. The final formula of JLU-Liu7 was derived from crystallographic data combined with elemental and thermogravimetric analysis data. Crystallographic data for JLU-Liu7 have been deposited with the Cambridge Crystallographic Data Centre. Topology information for the compound was calculated by using TOPOS 4.0.17Gas adsorption measurements
Gas sorption isotherm measurements were carried out on a Micromeritics ASAP 2420 and Micromeritics ASAP 2020 instrument. The as-synthesized samples were continuously extracted by Soxhlet extraction with acetone under reflux for 2 days to remove the guest molecules. The purity of the as-synthesized samples and guest-exchanged samples was evaluated using PXRD studies (Fig. S2†). About 110 mg of acetone-exchanged samples were activated at 80 °C for 10 h for adsorption test.Results and discussion
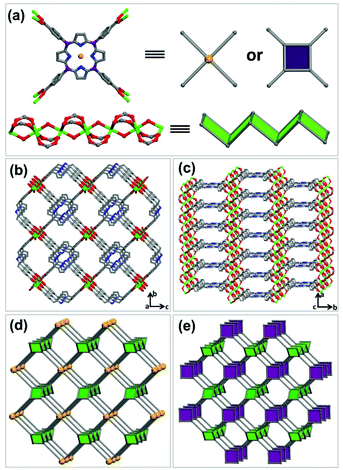
Solvothermal reaction of In(NO3)3·4H2O and tetrakis(4-carboxyphenyl)-porphyrin (H4TCPP) in DMF and DMSO at 85 °C for 48 h afforded red rod-shaped crystals of JLU-Liu7. Single crystal X-ray diffraction analysis reveals that JLU-Liu7 crystallizes in the Cmmm space group. There is one crystallographically independent indium atom in the structure, and the In atom is coordinated to six oxygen atoms, four of them at the equatorial sites are supplied by four different H4TCPP ligands, and the other two oxygen atoms are provided by two axial μ2trans hydroxide anions (Fig. 1a). The H4TCPP ligand is unmetalized, which is confirmed by the single crystal X-ray diffraction analysis and UV/Vis solid-state absorption spectra, associated with eight indium atoms (Fig. 1a and S1, ESI†). As shown in Fig. 1b and c, JLU-Liu7 contains one-dimensional channels with dimensions of 5.2 × 10.8 Å along the [001] direction and there exists a pore with a diameter of 3.8 × 29.3 Å along the [100] direction. From one topological point of view, the 1D inorganic indium SBU can be viewed as a zigzag ladder with the points of extension (carboxylate C atoms), meanwhile, the ligand can be seen as a 4-connected node and link to the zigzag ladders to make up a (4,4)-connected network with frl topology (Fig. 1d). From another topological perspective, the ligand can be simplified as a 3-connected node and connect to the zigzag ladders to form a (3,4)-connected network with fry topology (Fig. 1e). The framework of JLU-Liu7 is similar to the Al-MOF based on the infinite Al(OH)(COO)2 rod SBU and H4TCPP ligand.18 Calculation performed using PLATON reveals that the pore volume of JLU-Liu7 is 0.69 cm3 g−1 and the total solvent-accessible volume is equal to 2398.2 Å3 per unit cell, which accounts for 60% of the cell volume, offering opportunities for gas adsorption.The TGA curve indicates that JLU-Liu7 is stable up to nearly 400 °C (Fig. S5†). The first weight loss of 4% before 100 °C corresponds to the release of four guest H2O molecules (calcd 4.6%). And the second weight loss of 28% from 100 °C to 250 °C corresponds to the release of six guest DMF molecules (calcd 28%). The framework of JLU-Liu7 starts to collapse with loss of the TCPP4− ligand from 400 to 800 °C (found 50%; calcd 50.4%). Moreover, the framework still holds its integrity when immersed in water for one week, which can be proved by the crystallinity in powder X-ray diffraction (PXRD) (Fig. S2†). The extraordinarily stable behaviour indicated that JLU-Liu7 may possess industrial applications in the future.
To investigate the porosity of JLU-Liu7, H2, N2 O2, Ar and CO2 adsorption experiments at low temperatures were executed (Fig. 2). The total N2 sorption of JLU-Liu7 is about 363 cm3 g−1 at 77 K and 1 atm (Fig. 2a), having BET and Langmuir surface areas of 879 and 1198 m2 g−1, respectively. The measured pore volume from the N2 sorption is up to 0.56 cm3 g−1. The total Ar sorption of JLU-Liu7 is about 421 cm3 g−1 at 1 atm (Fig. 3), having BET and Langmuir surface areas of 1001 m2 g−1 and 1315 m2 g−1, respectively.
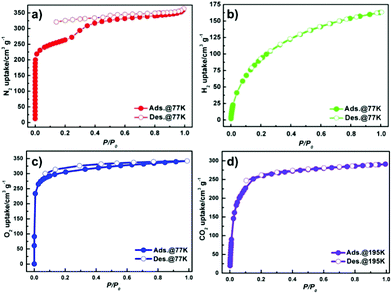 | ||
| Fig. 2 Gas isotherms for JLU-Liu7: N2 (a), H2 (b) and O2 (c) at 77 K and 1 atm; CO2 (d) at 195 K and 1 atm. | ||
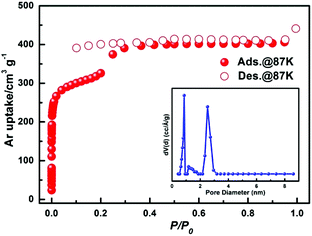 | ||
| Fig. 3 The argon isotherm of JLU-Liu7 at 87 K and 1 atm. Inset: The pore size distribution of JLU-Liu7 calculated by using the DFT method. | ||
The measured pore volume from the Ar sorption is up to 0.57 cm3 g−1. The two experimental pore volumes are similar, but a little less than the theoretical calculating value (0.69 cm3 g−1). The pore size distribution from about 0.66 to 3.79 nm is demonstrated by the fit of the adsorption data using the DFT method (Fig. 3). It shows that there exist two types of pores with diameters of 0.8 nm and 2.85 nm, which are homologous to the crystallographic data after considering the van der Waals radii. Both the N2 and Ar sorption isotherms are similar to the PCN-222 series4d and IRMOF-74 series,19 displaying typical type-IV sorption behaviours and exhibiting a steep increase at the point of P/P0 = 0.2, which is a characteristic of mesoporous materials. Both the PXRD data and single crystal X-ray diffraction analysis confirm that JLU-Liu7 is an example of mesoporous metal–organic frameworks (Meso-MOFs). The H2 adsorption isotherm at 77 K displays typical type-I sorption behaviours, showing that the hydrogen sorption volume JLU-Liu7 reaches 163 cm3 g−1 (1.46 wt%) at 77 K and 1 atm (Fig. 2b), which is comparable to those of the reported MOFs. In order to calculate the isosteric heat (Qst), an H2 adsorption isotherm at 87 K was also tested (Fig. S6a†). At low coverage, the Qst of JLU-Liu7 for H2 is 7.8 kJ mol−1 (Fig. S6b†), which is higher than that of MOF-5 (5.2 kJ mol−1), MIL-100 (6.3 kJ mol−1), HKUST-1 (6.6 kJ mol−1) or Soc-MOF (6.5 kJ mol−1), and comparable to that of SNU-4 and MOF-646.2e
The O2 sorption isotherm at 77 K shows typical type-I sorption behaviour (Fig. 2c), showing that the O2 sorption capacity of JLU-Liu7 is 342 cm3 g−1 (15.3 mmol g−1) at 77 K and 0.19 atm. The O2 capacity of JLU-Liu7 is pretty high around the reported MOFs, such as PCN-17 (210 cm3 g−1), Cu(BDT) (314 cm3 g−1), SNU-25 (233 cm3 g−1) and Zn(TCNQ-TCNQ)bpy (268 cm3 g−1).20
The CO2 adsorption isotherm at 195 K shows typical type-I sorption behaviour (Fig. 2d), showing that the CO2 sorption volume of JLU-Liu7 is 291 cm3 g−1 (13 mmol g−1) at 195 K and 1 atm. The CO2 adsorption isotherms at 273 and 298 K are obtained to investigate the isosteric heat of adsorption. It is found that the total CO2 adsorptions of JLU-Liu7 are 113 cm3 g−1 (22.2%, 273 K, 1 atm) and 60 cm3 g−1 (11.8%, 298 K, 1 atm), respectively (Fig. S7a†). The CO2 capacity of JLU-Liu7 is pretty high around the reported MOFs.2f The Qst for JLU-Liu7 was calculated to be 28.7 kJ mol−1 at zero-loading, indicating that there were strong interactions between CO2 molecules and the host framework of JLU-Liu7 (Fig. S7c†).
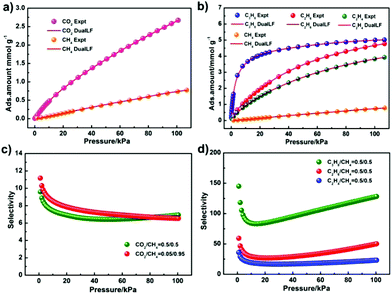
The pure component adsorption isotherms for CH4, C2H4, C2H6 and C3H8 of JLU-Liu7 were tested under ambient conditions to investigate its utilization in the adsorption and separation of small hydrocarbons (C1–C3). The different total gas uptake amounts are CH4 (34 and 17 cm3 g−1), C2H4 (114 and 88 cm3 g−1), C2H6 (140 and 107 cm3 g−1), and C3H8 (143 and 113 cm3 g−1) at 273 and 298 K under 1 atm, respectively (Fig. S8a–S11a†). At zero-coverage, the Qst values of CH4, C2H4, C2H6 and C3H8 are 20.9, 27.5, 34.8 and 28.5 kJ mol−1, respectively, which are estimated from the sorption isotherms at 273 and 298 K (Fig. S8c–S11c†).
In order to examine the separation ability of JLU-Liu7, the gas selectivities of CO2/CH4, C2H4/CH4, C2H6/CH4 and C3H8/CH4 in a binary mixture were calculated by using ideal solution adsorbed theory (IAST). The selectivity of CO2/CH4 (50% and 50%, 5% and 95%) is 6.9 and 6.5. And the selectivities of C2H4/CH4, C2H6/CH4 and C3H8/CH4 are 23.3, 50.4 and 128.5, respectively (Fig. 4). It is obvious that the selectivity of C3H8 over CH4 is higher than many reported MOFs, such as FIR-7,14 JLU-Liu515a and JLU-Liu18,15b but lower than JLU-Liu2215c and JLU-Liu15.15d
Conclusions
In summary, we have successfully synthesized a stable Meso-MPF JLU-Liu7 based on an In(OH)(COO)2 SBU and porphyrin-containing ligand. The novel framework is constructed by using a 1D inorganic indium chain to form a rare (4,4)-connected frl net. JLU-Liu7 exhibits high adsorption performance for small gases and selectivity for CO2, C2H4, C2H6 and C3H8 over CH4. Furthermore, this material may be used for gas storage and purification in the future.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21373095, 21371067 and 21621001).Notes and references
- (a) H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS; (b) O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS PubMed; (c) J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213–1214 RSC; (d) H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed; (e) T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734–777 CrossRef CAS PubMed; (f) M. Li, D. Li, M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2014, 114, 1343–1370 CrossRef CAS PubMed.
- (a) R. B. Getman, Y.-S. Bae, C. E. Wilmer and R. Q. Snurr, Chem. Rev., 2011, 112, 703–723 CrossRef PubMed; (b) P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2011, 112, 1232–1268 CrossRef PubMed; (c) L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2011, 112, 1105–1125 CrossRef PubMed; (d) J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2011, 112, 869–932 CrossRef PubMed; (e) M. P. Suh, H. J. Park, T. K. Prasad and D.-W. Lim, Chem. Rev., 2011, 112, 782–835 CrossRef PubMed; (f) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2011, 112, 724–781 CrossRef PubMed; (g) M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2011, 112, 1196–1231 CrossRef PubMed; (h) H. Wu, Q. Gong, D. H. Olson and J. Li, Chem. Rev., 2012, 112, 836–868 CrossRef CAS PubMed; (i) H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 974 CrossRef CAS PubMed; (j) Q. Yang, D. Liu, C. Zhong and J.-R. Li, Chem. Rev., 2013, 113, 8261–8323 CrossRef CAS PubMed.
- (a) M. E. Kosal, J.-H. Chou, S. R. Wilson and K. S. Suslick, Nat. Mater., 2002, 1, 118–121 CrossRef CAS PubMed; (b) K. S. Suslick, P. Bhyrappa, J. H. Chou, M. E. Kosal, S. Nakagaki, D. W. Smithenry and S. R. Wilson, Acc. Chem. Res., 2005, 38, 283–291 CrossRef CAS PubMed; (c) W.-Y. Gao, M. Chrzanowski and S. Ma, Chem. Soc. Rev., 2014, 43, 5841–5866 RSC; (d) L. D. DeVries and W. Choe, J. Chem. Crystallogr., 2008, 39, 229–240 CrossRef; (e) F. Cao, M. Zhao, Y. Yu, B. Chen, Y. Huang, J. Yang, X. Cao, Q. Lu, X. Zhang, Z. Zhang, C. Tan and H. Zhang, J. Am. Chem. Soc., 2016, 138, 6924–6927 CrossRef CAS PubMed; (f) Q. Lin, X. Bu, A. Kong, C. Mao, X. Zhao, F. Bu and P. Feng, J. Am. Chem. Soc., 2015, 137, 2235–2238 CrossRef CAS PubMed; (g) K. Wang, X.-L. Lv, D. Feng, J. Li, S. Chen, J. Sun, L. Song, Y. Xie, J.-R. Li and H.-C. Zhou, J. Am. Chem. Soc., 2016, 138, 914–919 CrossRef CAS PubMed.
- (a) B. J. Burnett, P. M. Barron, C. Hu and W. Choe, J. Am. Chem. Soc., 2011, 133, 9984–9987 CrossRef CAS PubMed; (b) C. Y. Lee, O. K. Farha, B. J. Hong, A. A. Sarjeant, S. T. Nguyen and J. T. Hupp, J. Am. Chem. Soc., 2011, 133, 15858–15861 CrossRef CAS PubMed; (c) X.-S. Wang, L. Meng, Q. Cheng, C. Kim, L. Wojtas, M. Chrzanowski, Y.-S. Chen, X. P. Zhang and S. Ma, J. Am. Chem. Soc., 2011, 133, 16322–16325 CrossRef CAS PubMed; (d) D. Feng, Z.-Y. Gu, J.-R. Li, H.-L. Jiang, Z. Wei and H.-C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 10307–10310 CrossRef CAS PubMed; (e) W. Morris, B. Volosskiy, S. Demir, F. Gándara, P. L. McGrier, H. Furukawa, D. Cascio, J. F. Stoddart and O. M. Yaghi, Inorg. Chem., 2012, 51, 6443–6445 CrossRef CAS PubMed; (f) H.-L. Jiang, D. Feng, K. Wang, Z.-Y. Gu, Z. Wei, Y.-P. Chen and H.-C. Zhou, J. Am. Chem. Soc., 2013, 135, 13934–13938 CrossRef CAS PubMed; (g) Q. Lin, X. Bu, A. Kong, C. Mao, X. Zhao, F. Bu and P. Feng, J. Am. Chem. Soc., 2015, 137, 2235–2238 CrossRef CAS PubMed; (h) K. Wang, D. Feng, T.-F. Liu, J. Su, S. I. Yuan, Y.-P. Chen, M. Bosch, X. Zou and H.-C. Zhou, J. Am. Chem. Soc., 2014, 136, 13983–13986 CrossRef CAS PubMed; (i) T.-F. Liu, D. Feng, Y.-P. Chen, L. Zou, M. Bosch, S. Yuan, Z. Wei, S. Fordham, K. Wang and H.-C. Zhou, J. Am. Chem. Soc., 2015, 137, 413–419 CrossRef CAS PubMed.
- (a) H.-J. Son, S. Jin, S. Patwardhan, S. J. Wezenberg, N. C. Jeong, M. So, C. E. Wilmer, A. A. Sarjeant, G. C. Schatz, R. Q. Snurr, O. K. Farha, G. P. Wiederrecht and J. T. Hupp, J. Am. Chem. Soc., 2012, 135, 862–869 CrossRef PubMed; (b) S. Jin, H.-J. Son, O. K. Farha, G. P. Wiederrecht and J. T. Hupp, J. Am. Chem. Soc., 2013, 135, 955–958 CrossRef CAS PubMed.
- (a) M. H. Alkordi, Y. Liu, R. W. Larsen, J. F. Eubank and M. Eddaoudi, J. Am. Chem. Soc., 2008, 130, 12639–12641 CrossRef CAS PubMed; (b) R. W. Larsen, L. Wojtas, J. Perman, R. L. Musselman, M. J. Zaworotko and C. M. Vetromile, J. Am. Chem. Soc., 2011, 133, 10356–10359 CrossRef CAS PubMed; (c) Z. Zhang, L. Zhang, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, J. Am. Chem. Soc., 2011, 134, 928–933 CrossRef PubMed; (d) Z. Zhang, L. Zhang, L. Wojtas, P. Nugent, M. Eddaoudi and M. J. Zaworotko, J. Am. Chem. Soc., 2011, 134, 924–927 CrossRef PubMed; (e) Z. Zhang, W.-Y. Gao, L. Wojtas, S. Ma, M. Eddaoudi and M. J. Zaworotko, Angew. Chem., Int. Ed., 2012, 51, 9330–9334 CrossRef CAS PubMed; (f) Z. Zhang, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, J. Am. Chem. Soc., 2013, 135, 5982–5985 CrossRef CAS PubMed.
- (a) J. J. Perry Iv, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400–1417 RSC; (b) D. J. Tranchemontagne, J. L. Mendoza-Cortes, M. O'Keeffe and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1257–1283 RSC; (c) S. Wang, T. Zhao, G. Li, L. Wojtas, Q. Huo, M. Eddaoudi and Y. Liu, J. Am. Chem. Soc., 2010, 132, 18038–18041 CrossRef CAS PubMed; (d) S.-T. Zheng, J. T. Bu, Y. Li, T. Wu, F. Zuo, P. Feng and X. Bu, J. Am. Chem. Soc., 2010, 132, 17062–17064 CrossRef CAS PubMed; (e) J. F. Eubank, L. Wojtas, M. R. Hight, T. Bousquet, V. C. Kravtsov and M. Eddaoudi, J. Am. Chem. Soc., 2011, 133, 17532–17535 CrossRef CAS PubMed.
- (a) S. Bourrelly, P. L. Llewellyn, C. Serre, F. Millange, T. Loiseau and G. Férey, J. Am. Chem. Soc., 2005, 127, 13519–13521 CrossRef CAS PubMed; (b) G. Ferey and C. Serre, Chem. Soc. Rev., 2009, 38, 1380–1399 RSC; (c) L. Chen, J. P. S. Mowat, D. Fairen-Jimenez, C. A. Morrison, S. P. Thompson, P. A. Wright and T. Düren, J. Am. Chem. Soc., 2013, 135, 15763–15773 CrossRef CAS PubMed.
- (a) K. Barthelet, J. Marrot, D. Riou and G. Férey, Angew. Chem., Int. Ed., 2002, 41, 281–284 CrossRef CAS; (b) C. Serre, F. Millange, C. Thouvenot, M. Noguès, G. Marsolier, D. Louër and G. Férey, J. Am. Chem. Soc., 2002, 124, 13519–13526 CrossRef CAS PubMed; (c) F. Gándara, B. Gomez-Lor, E. Gutiérrez-Puebla, M. Iglesias, M. A. Monge, D. M. Proserpio and N. Snejko, Chem. Mater., 2007, 20, 72–76 CrossRef; (d) Z. Jin, H.-Y. Zhao, X.-J. Zhao, Q.-R. Fang, J. R. Long and G.-S. Zhu, Chem. Commun., 2010, 46, 8612–8614 RSC; (e) K. C. Stylianou, R. Heck, S. Y. Chong, J. Bacsa, J. T. A. Jones, Y. Z. Khimyak, D. Bradshaw and M. J. Rosseinsky, J. Am. Chem. Soc., 2010, 132, 4119–4130 CrossRef CAS PubMed; (f) Q. Lin, T. Wu, S.-T. Zheng, X. Bu and P. Feng, Chem. Commun., 2011, 47, 11852–11854 RSC; (g) J. Qian, F. Jiang, D. Yuan, M. Wu, S. Zhang, L. Zhang and M. Hong, Chem. Commun., 2012, 48, 9696–9698 RSC; (h) A. Fateeva, P. A. Chater, C. P. Ireland, A. A. Tahir, Y. Z. Khimyak, P. V. Wiper, J. R. Darwent and M. J. Rosseinsky, Angew. Chem., Int. Ed., 2012, 51, 7440–7444 CrossRef CAS PubMed; (i) S. Yang, J. Sun, A. J. Ramirez-Cuesta, S. K. Callear, I. F. DavidWilliam, D. P. Anderson, R. Newby, A. J. Blake, J. E. Parker, C. C. Tang and M. Schröder, Nat. Chem., 2012, 4, 887–894 CrossRef CAS PubMed; (j) M. Du, M. Chen, X.-G. Yang, J. Wen, X. Wang, S.-M. Fang and C.-S. Liu, J. Mater. Chem. A, 2014, 2, 9828–9834 RSC.
- (a) Y.-S. Bae, C. Y. Lee, K. C. Kim, O. K. Farha, P. Nickias, J. T. Hupp, S. T. Nguyen and R. Q. Snurr, Angew. Chem., Int. Ed., 2012, 51, 1857–1860 CrossRef CAS PubMed; (b) E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown and J. R. Long, Science, 2012, 335, 1606–1610 CrossRef CAS PubMed.
- (a) Y. He, Z. Zhang, S. Xiang, F. R. Fronczek, R. Krishna and B. Chen, Chem. Commun., 2012, 48, 6493–6495 RSC; (b) Y. He, Z. Zhang, S. Xiang, F. R. Fronczek, R. Krishna and B. Chen, Chem. – Eur. J., 2012, 18, 613–619 CrossRef CAS PubMed; (c) Y. He, Z. Zhang, S. Xiang, H. Wu, F. R. Fronczek, W. Zhou, R. Krishna, M. O'Keeffe and B. Chen, Chem. – Eur. J., 2012, 18, 1901–1904 CrossRef CAS PubMed.
- S. Horike, Y. Inubushi, T. Hori, T. Fukushima and S. Kitagawa, Chem. Sci., 2012, 3, 116–120 RSC.
- (a) H. Ma, H. Ren, S. Meng, Z. Yan, H. Zhao, F. Sun and G. Zhu, Chem. Commun., 2013, 49, 9773–9775 RSC; (b) H. Ma, H. Ren, S. Meng, F. Sun and G. Zhu, Sci. Rep., 2013, 3, 2611 Search PubMed.
- Y.-P. He, Y.-X. Tan and J. Zhang, Chem. Commun., 2013, 49, 11323–11325 RSC.
- (a) D. Wang, T. Zhao, Y. Cao, S. Yao, G. Li, Q. Huo and Y. Liu, Chem. Commun., 2014, 50, 8648–8650 RSC; (b) D. Wang, B. Liu, S. Yao, T. Wang, G. Li, Q. Huo and Y. Liu, Chem. Commun., 2015, 51, 15287–15289 RSC; (c) S. Yao, D. Wang, Y. Cao, G. Li, Q. Huo and Y. Liu, J. Mater. Chem. A, 2015, 3, 16627–16632 RSC; (d) X. Luo, L. Sun, J. Zhao, D.-S. Li, D. Wang, G. Li, Q. Huo and Y. Liu, Cryst. Growth Des., 2015, 15, 4901–4907 CrossRef CAS; (e) S. Yao, X. Sun, B. Liu, R. Krishna, G. Li, Q. Huo and Y. Liu, J. Mater. Chem. A, 2016, 4, 15081–15087 RSC.
- G. M. Sheldrick, SHELXTL-NT, version 5.1, Bruker AXS Inc., Madison, WI, 1997 Search PubMed.
- V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576–3683 CAS.
- A. Fateeva, P. A. Chater, C. P. Ireland, A. A. Tahir, Y. Z. Khimyak, P. V. Wiper, J. R. Darwent and M. J. Rosseinsky, Angew. Chem., Int. Ed., 2012, 51, 7440–7444 CrossRef CAS PubMed.
- H. Deng, S. Grunder, K. E. Cordova, C. Valente, H. Furukawa, M. Hmadeh, F. Gándara, A. C. Whalley, Z. Liu, S. Asahina, H. Kazumori, M. O'Keeffe, O. Terasaki, J. F. Stoddart and O. M. Yaghi, Science, 2012, 336, 1018–1023 CrossRef CAS PubMed.
- (a) S. Q. Ma, X. S. Wang, D. Q. Yuan and H.-C. Zhou, Angew. Chem., Int. Ed., 2008, 47, 4130–4133 CrossRef CAS PubMed; (b) M. Dincă, A. F. Yu and J. R. Long, J. Am. Chem. Soc., 2006, 128, 8904–8913 CrossRef PubMed; (c) Y. E. Cheon, J. Park and M. P. Suh, Chem. Commun., 2009, 36, 5436–5438 RSC; (d) S. Shimomura, M. Higuchi, R. Matsuda, K. Yoneda, Y. Hijikata, Y. Kubota, Y. Mita, J. Kim, M. Takata and S. Kitagawa, Nat. Chem., 2010, 2, 633–637 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystal data and structure refinement, structure information, XRD, TGA, gas adsorption and adsorptive selectivity. CCDC 1443466. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qi00440g |
| This journal is © the Partner Organisations 2017 |