Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mechanochemical polymerization – controlling a polycondensation reaction between a diamine and a dialdehyde in a ball mill†
S.
Grätz
and
L.
Borchardt
*
Department of Inorganic Chemistry, Technische Univeristät Dresden, Bergstraße 66, 01062 Dresden, Germany. E-mail: lars.borchardt@chemie.tu-dresden.de; Tel: +49-35146334960
First published on 1st July 2016
Abstract
The mechanochemical polycondensation between a diamine and a dialdehyde constitutes a sustainable alternative to classical solvent-based polymerization reactions. This process not only allows for a higher conversion and a shorter reaction time as compared to standard solvent-based syntheses of this conjugated polymer, but the reaction can also be adjusted by the energy introduced via the ball mill.
Lately, mechanochemical reactions have proven to be some of the most successful solvent-free synthetic approaches.1 Initiated and/or sustained by mechanical energy these reactions offer a sustainable alternative to many wet-chemical methods. Innovative uses have made their way into the fields of pharmaceuticals,2–4 material synthesis4–9 and foremost to organic chemistry,10–14 where the vast potential of this rediscovered field has been demonstrated. Common reactions can be undergone with high yields, short reaction time and in the absence of solvents. While publications in the field of polymerization are scarce, some groups have indeed used the ball mill for oxidative and GILCH polymerizations,15–17 but up to now no attempt has been made to perform a polycondensation reaction mechanochemically. To investigate whether such an approach is possible and to further understand the method, we focused on a model reaction – namely the formation of a Schiff base by the reaction between a primary amine and an aldehyde.18 We choose the formation of poly(1,4-phenylene-methylidynenitrilo-1,4-phenylenenitrilo-methylidyne) (PPI) from p-benzoldicarbaldehyde and p-phenylenediamine (Fig. 1). This system is prone to be investigated, not only is the reaction fast but the polymerisation by solution methods suffers from the bad solubility of the forming polymer – an issue not relevant for a solid state reaction.19 To overcome this obstacle either high temperatures, multi-step reactions or toxic solvents such as hexamethylphosphoramide have been employed in the past.20 The resulting poly(azomethine)s (PA) are conjugated polymers that show a high thermal stability and photoluminescence.21 Therefore they are especially interesting for a potential application as transparent conductors,22 liquid crystals,23 aerospace coatings24 and light emitting diodes.25,26 Here we present the first solvent-free, mechanochemical synthesis of PPI in a planetary ball mill. Advantages over traditional method arise from the short reaction time, low temperatures, higher molecular weights and the sustainable nature of the process.
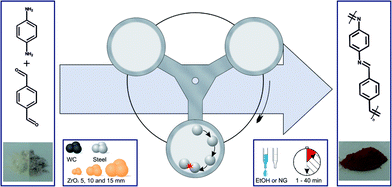
In a surprisingly simple manner equal molar amounts of both monomers are mixed together in a zirconium oxide milling cup (V = 45 mL) with 22 zirconium oxide milling balls (d = 10 mm). Further details can be found in the ESI.† After 45 minutes of grinding at 800 rpm the resulting polymer was investigated via IR and UV/vis spectroscopy, dynamic scanning calorimetry, thermogravimetric analysis and SEM imaging. Due to the insolubility of the polymer in common solvents none of the textbook polymer characterization methods could be applied and we had to resort to other means. At first, we determined the general success of the reaction by IR spectroscopy (Fig. 2). The appearance of a vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group at 1609 cm−1 as well as the fading of the vibrations of the carbonyl and amine groups at 1686 cm−1 and 1514 cm−1 respectively, proof the formation of an imine group.27 The solid state UV/Vis (SFig. 1†) showed coherent results. After 1 minute of grinding the formation of the product could already be confirmed by the appearance of the band around 400 nm (π-system) but monomer absorption bands at 259 nm and 305 nm from p-benzoldicarbaldehyde still remained in the spectrum. Interestingly, only 5 minutes into the reaction the monomer peaks had almost disappeared and longer reaction times only lead to a bathochromic shift of λMAX to 427 nm. This is accompanied by a decrease of the optical band gap to 2.18 eV (STable 1, SFig. 2 and 3†) – far superior to literature values of 2.50 eV.27 This change can be attributed to the longer chains and therefore bigger conjugated π-system achieved by the mechanochemical approach.
N group at 1609 cm−1 as well as the fading of the vibrations of the carbonyl and amine groups at 1686 cm−1 and 1514 cm−1 respectively, proof the formation of an imine group.27 The solid state UV/Vis (SFig. 1†) showed coherent results. After 1 minute of grinding the formation of the product could already be confirmed by the appearance of the band around 400 nm (π-system) but monomer absorption bands at 259 nm and 305 nm from p-benzoldicarbaldehyde still remained in the spectrum. Interestingly, only 5 minutes into the reaction the monomer peaks had almost disappeared and longer reaction times only lead to a bathochromic shift of λMAX to 427 nm. This is accompanied by a decrease of the optical band gap to 2.18 eV (STable 1, SFig. 2 and 3†) – far superior to literature values of 2.50 eV.27 This change can be attributed to the longer chains and therefore bigger conjugated π-system achieved by the mechanochemical approach.
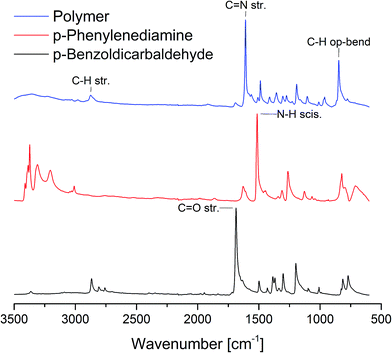 | ||
| Fig. 2 IR spectra of the monomers and the polymer yielded by ball milling after 40 min with 10 mm ZrO2 milling balls. | ||
A glimpse onto the SEM images showed agglomerated micrometer sized particles with a smooth surface. This is in contrast to the reference material, synthesized in a classical solution polymerization, which formed flaky particles (SFig. 4 and 5†). Differences between the two methods could also be observed in the thermochemical behaviour of the materials (SFig. 6–8†). While the reference shows a relatively strong melting peak at 375 °C with a melt enthalpy of −15.27 J g−1, the melting peaks for the products in the ball mill were much broader with enthalpies only half as big and peak temperatures between 350 °C and 360 °C. Both effects are expected for PA with growing chain length and diminishing crystallite size.28 The thermal resistance of the prepared materials is with an onset of decomposition around 490 °C quite high for polymers (SFig. 9†).
For an in depth investigation of the mechanochemical polymerization we focused on the influence of the milling material and the ball size in the second part of the study. In addition to the analysis methods presented above we also took a look at the kinetics of the reaction to compare said parameters better. Due to the poor solubility of the polymer, textbook methods could not be applied to our system. We resorted to the intensity of the carboxyl group in the infrared spectrum in order to follow the progress of the polymerization. To compare the integrals with one another they have to be normed to the CH vibration at 2850 cm−1. Since the intensity of a vibration in the IR range is linear proportional to the concentration of the specific group, the peak integrals presents an easy way to monitor the reaction. Looking at the conversion curves, one can see that the reaction is proceeding swiftly and reaches a plateau after around 20 minutes (Fig. 3A). In addition we plotted the rate laws and determined that the polymerization follows a second order law. Furthermore, one can see the dependence of the conversion on the reaction parameters.
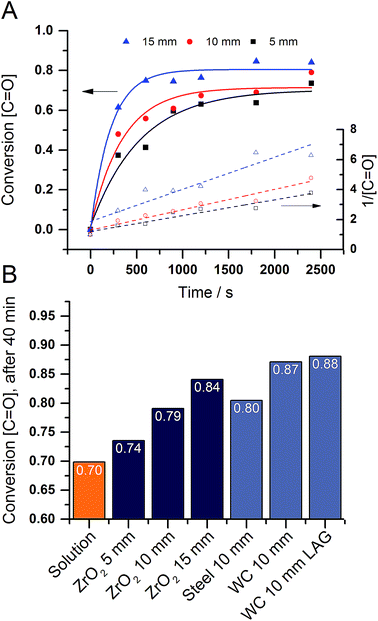 | ||
| Fig. 3 (A) Conversion (full lines) and 2nd order rate law (dashed lines) in function of the zirconium oxide ball size. (B) Conversion after 40 minutes in function of different parameters. For detailed spectra see SFig. 10–15.† | ||
Consequently, the ball diameter was varied from 5 mm over 10 mm to 15 mm. As the milling balls became bigger the conversion of carbonyl groups became quicker and higher (Fig. 3A). This effect can be attributed to the higher collision energies of the bigger balls. To further proof this assumption we went on to investigate different milling materials. A wide range of material densities from 14.89 g cm−3 for tungsten carbide, over 7.9 g cm−3 for steel to 5.7 g cm−3 for zirconium dioxide has been explored. As expected the higher density materials led to a higher conversion (Fig. 3B). In an attempt to increase the conversion further we investigated the influence of small amounts of solvents (liquid assisted grinding, LAG) on the reaction. As described by Kaitner and co-workers ethanol has a positive influence on the formation of azomethines.28 For the polymerization a similar trend can be observed. The samples created by LAG show a higher and faster conversion than the ones prepared by neat grinding (SFig. 16†). Moreover, the ethanol is also promoting a crosslinking reaction in the polymer backbone (eqn (1)).29,30
Possible cross-linking process (adopted from ref. 27).
 | (1) |
Since this is breaking the conjugated π-system and thereby increasing the optical band gap, the crosslinking can be seen in the solid state UV/vis spectra (SFig. 17†). In addition, λMAX is shifted to 456 nm indicating a strong change in the conjugated π-system. All utilized analysis methods point towards longer chains created by the mechanochemical approach but the absolute chain length is hard to determine for an insoluble polymer. However, with a volume mean diameter (determined by dynamic light scattering of the soluble part of the polymer (SFig. 18†)) of 140 nm the ball milled sample is way bigger than the reference sample with 5.5 nm. In addition the MADLI-TOF analysis of two samples (SFig. 19†) proves that the reference shows a short oligomeric character (Mn = 1700 g mol−1; Đ = 1.14) and the milled sample a nearly twice as high molecular weight (Mn = 3010 g mol−1; Đ = 1.36).
Conclusion
Summing up, we demonstrated for the first time that a polycondensation reaction can be carried out in a solvent- and catalyst-free manner in a ball mill. The model system of a conjugated PA achieved high conversion in a short time. The polymers showed high thermal stability and low optical bandgaps. Furthermore, the influence of the milling ball size as well as the milling material was investigated. Tungsten carbide milling material lead to the highest conversion but all mechanochemical syntheses yielded higher conversions than the classical solution polymerisation. The energy input seems to be the key parameter which can easily be influenced by the milling material. In combination with different monomers these findings can lead to a scalable and sustainable production of PA. Via polycondensation reactions a vast variety of polymers are accessible and the ball mill may overcome solubility problems faced by a multitude of systems.Acknowledgements
We gratefully acknowledge the Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF) for support of the Mechanocarb project (award number 03SF0498). We acknowledge Valerya Tkachova for the MALDI-TOF measurements, Dr Ilka Kunert for conducting the thermal analysis, Sebastian Ehrling for taking the SEM images and Sven Roediger for help in the preparative work.Notes and references
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- F.-J. Wang, H. Xu, M. Xin and Z. Zhang, Mol. Diversity, 2016, 3, 659–666 CrossRef PubMed.
- K. Lien Nguyen, T. Friščić, G. M. Day, L. F. Gladden and W. Jones, Nat. Mater., 2007, 6, 206–209 CrossRef PubMed.
- C. Mottillo and T. Friščić, Chem. Commun., 2015, 51, 8924–8927 RSC.
- K. Užarević, T. C. Wang, S.-Y. Moon, A. M. Fidelli, J. T. Hupp, O. K. Farha and T. Friščić, Chem. Commun., 2016, 52, 2133–2136 RSC.
- A. L. Garay, A. Pichon and S. L. James, Chem. Soc. Rev., 2007, 36, 846–855 RSC.
- V. Š. Epelák, A. Düvel, M. Wilkening, K.-D. Becker and P. Heitjans, Chem. Soc. Rev., 2013, 42, 7507–7520 RSC.
- S.-E. Zhu, F. Li and G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7535 RSC.
- P. Baláž, M. Baláž and Z. Bujňáková, Chem. Eng. Technol., 2014, 37, 747–756 CrossRef.
- G. Kaupp, J. Schmeyers and J. Boy, Chemosphere, 2001, 43, 55–61 CrossRef CAS PubMed.
- A. Stolle, T. Szuppa, S. E. S. Leonhardt and B. Ondruschka, Chem. Soc. Rev., 2011, 40, 2317–2329 RSC.
- G. N. Hermann, P. Becker and C. Bolm, Angewandte Chemie, 2015, 127, 7522–7525 CrossRef.
- A. Stolle, R. Schmidt and K. Jacob, Faraday Discuss., 2014, 170, 267–286 RSC.
- G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668 RSC.
- S. Palaniappan and P. Manisankar, Electrochim. Acta, 2011, 56, 6123–6130 CrossRef CAS.
- J. B. Ravnsbæk and T. M. Swager, ACS Macro Lett., 2014, 3, 305–309 CrossRef.
- M. Pascu, A. Ruggi, R. Scopelliti and K. Severin, Chem. Commun., 2013, 49, 45–47 RSC.
- J. Schmeyers, F. Toda, J. Boy and G. Kaupp, J. Chem. Soc., Perkin Trans. 2, 1998, 4, 989–994 RSC.
- M. Grigoras and C. O. Catanescu, J. Macromol. Sci., Part C: Polym. Rev., 2004, 44, 131–173 CrossRef.
- T. Miyaji, C. Azuma, E. Asaoka and S. Nakamura, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 1064–1072 CrossRef CAS.
- A. Iwan and D. Sek, Prog. Polym. Sci., 2008, 33, 289–345 CrossRef CAS.
- C.-L. Liu, F.-C. Tsai, C.-C. Chang, K.-H. Hsieh, J.-L. Lin and W.-C. Chen, Polymer, 2005, 46, 4950–4957 CrossRef CAS.
- J. M. Adell, M. P. Alonso, J. Barberá, L. Oriol, M. Piñol and J. L. Serrano, Polymer, 2003, 44, 7829–7841 CrossRef CAS.
- N. Chantarasiri, C. Chulamanee, T. Mananunsap and N. Muangsin, Polym. Degrad. Stab., 2004, 86, 505–513 CrossRef CAS.
- M. Bruma, E. Hamciuc, B. Schulz, T. Köpnick, Y. Kaminorz and J. Robison, Macromol. Symp., 2003, 199, 511–521 CrossRef CAS.
- A. Kimoto, J.-S. Cho, M. Higuchi and K. Yamamoto, Macromolecules, 2004, 37, 5531–5537 CrossRef CAS.
- C. Yang and S. A. Jenekhe, Chem. Mater., 1991, 3, 878–887 CrossRef CAS.
- D. Cinčić, I. Brekalo and B. Kaitner, Chem. Commun., 2012, 48, 11683–11685 RSC.
- K. Suematsu, K. Nakamura and J. Takeda, Polym. J., 1983, 15, 71–79 CrossRef CAS.
- A. Padwa, W. Bergmark and D. Pashayan, J. Am. Chem. Soc., 1969, 91, 2653–2660 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra15677k |
| This journal is © The Royal Society of Chemistry 2016 |