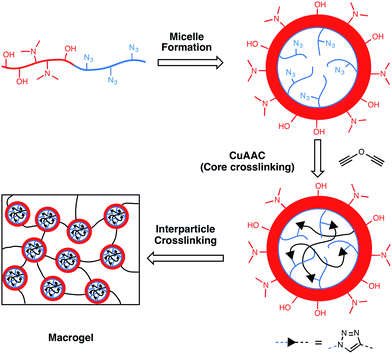
Controlled nanogel and macrogel structures from self-assembly of a stimuli-responsive amphiphilic block copolymer†
JianCheng Liu a,
Christina Uhlira,
Parag K. Shaha,
Fang Sunbc and
Jeffrey W. Stansbury*ad
a,
Christina Uhlira,
Parag K. Shaha,
Fang Sunbc and
Jeffrey W. Stansbury*ad
aDepartment of Chemical and Biological Engineering, University of Colorado, Boulder, Colorado 80309, USA
bState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, PR China
cCollege of Science, Beijing University of Chemical Technology, Beijing 100029, PR China
dDepartment of Craniofacial Biology, School of Dental Medicine, University of Colorado, 12800 19th Ave, RC1-N Mail Stop 8310, Aurora, Colorado 80045, USA. E-mail: jeffrey.stansbury@ucdenver.edu
First published on 4th July 2016
Abstract
RAFT polymerization was utilized to prepare an amphiphilic block copolymer containing both hydrophilic and hydrophobic segments. The self-assembly behavior of the block copolymer into nano-scale particulate structures was studied in both water and polar organic solvents. Uniform micelle assemblies were stabilized by reaction within the hydrophobic core, which contained pendant azide groups, through copper-catalyzed azide-alkyne cycloaddition (CuAAC) click chemistry with a dialkyne crosslinker. The reaction preceded efficiently with negligible residual azide functionality and resulted in core–shell nanogel structures that were analyzed by a variety of techniques including light scattering, electron microscopy and the ability to take up hydrophobic molecules. Both thermo- and pH-responsive character of the nanogels and the linear polymers from which they were made were studied through cloud point testing at different pH levels. It was found that these nanogel dispersions in water exhibited the highest cloud point temperatures indicating a highly stable nanogel structure. The solvent-dispersed nanogels were used as building blocks to form extended polymer networks through the inter- as well as intra-particle reaction between hydroxyl groups within the hydrophilic domain of the nanogel shell by crosslinking with a diisocyanate. It was found that as little as 10 wt% nanogel dispersions in solvent reached the percolation threshold to yield highly porous macroscopic networks; while 50 wt% concentrations achieved densely packed and interdigitated nanogels to afford relatively homogeneous structures.
Introduction
Despite the rapid growth of chemistry and methods in polymer synthesis, it is still a challenge to easily and efficiently realize precise control over highly branched polymers. Much effort has been directed toward dendrimer synthesis, which can yield uniform structures and desired chemical composition at the molecular level.1 However, it generally requires many steps to synthesize dendrimers and the de Genne's dense packing effect2 limits the final material generation or molecular weight. In order to simplify synthesis procedures, one-step reactions have been used to form hyper-branched polymers with either single monomer or multi-monomer methods mostly by a variety of step-growth chemical reactions.3 Chain-growth reactions have also been applied to form polymers with multi-chain structures. Random structured, highly branched polymers were synthesized through solution polymerization of a dimethacrylate and monomethacrylate with a chain transfer agent.4–6 Primary chain length and branching density of these polymers can be tuned by varying the concentration of the dimethacrylate crosslinker and chain transfer agent. This type of randomly highly branched or internally crosslinked polymeric particles have been referred to as nanogels when the polymeric structures are less than 100 nm in dimension.7–9 Secondary reactions, such as cyclization and polymer–polymer coupling, are inevitable in single-stage reactions. So the formed nanogels typically offer limited control over polymer structure, molecular weight and polydispersity.10,11 Amphiphilic block copolymer self-assembly associated with hydrophilic and hydrophobic prepolymer segments can form many complex nano- or micro-domain structures12 including spheres, vesicles and cylinders under appropriate conditions. Many studies have demonstrated that polymer micelles with core–shell structures can be generated in aqueous solutions when polymer concentration exceeds critical micelle concentration (CMC).13 The micelle structures can be altered by varying monomers, polymer repeating units and the ratio of polymer blocks. However, the micelle structures can be destabilized and disrupted with the change of polymer concentration as well as environment including polarity, pH and temperature. Crosslinking reactions have been used extensively to yield nanoparticles or nanogels with permanent shapes by selective fixation of one block of the micelles (core or shell). Thermoresponsive units have been incorporated into nanogel structures to realize particle solubility difference related to temperature change. Poly(N-isopropyl acrylamide) (PNIPAM) is by far the most studied thermoresponsive polymer since its lower critical solution temperature (LCST) is close to physiological conditions. PNIPAM, as one segment of block copolymer, has been selected to form core structures of polymer micelles at elevated temperature when the PNIPAM block solubility in water is limited.14 Poly(2-dimethylaminoethyl methacrylate) (PDMAEMA), among other types of thermoresponsive polymers, has also been studies due to its additional responsiveness to pH. The cloud points of PDMAEMA strongly depend on the solution pH since the tertiary amine group can be protonated and thus promote hydrophilic character at lower pH. Cloud point temperatures as low as 25 °C were found for these PDMAEMA polymers in solution at pH = 10 while under neutral pH conditions, they remained stable until 80 °C.15In the current study, an amphiphilic block copolymer containing DMAEMA was synthesized and further assembled into micelle structures with styrenic monomers as the hydrophobic block. Nanogel particles were formed after crosslinking the core region. The thermoresponsive character was studied for the copolymer, block copolymer and nanogel at different pH values. The ability to form macroscopic networks was explored by additional reaction of nanogel solutions at concentration above the percolation threshold.
Experimental
Materials
Poly(ethylene glycol) methacrylate (Mn = 360, PEGMA), 4-vinylbenzyl chloride (90%), sodium azide (99.5%, NaN3), 2-cyano-2-propyl benzodithioate (97%, CPBD), 2-(dimethylamino)ethyl methacrylate (98%, DMAEMA), styrene (99%, St), propargyl ether (98%, dipropargyl ether), azobisisobutyronitrile (98%, AIBN), tin(II) ethyl hexanoate (95%), hexamethylene diisocyanate (99%, HMDI) copper(II) chloride (97%, CuCl2), N,N,N′,N′,N′′-pentamethyldiethylenetriamine (99%, PMDETA) and ascorbic acid (reagent grade) were purchased from Sigma Aldrich. PEGMA, DMAEMA, and St were purified with activated alumina (basic, Brockman I); AIBN was recrystallized twice from methanol. All other reagents were used as received.4-Azidomethyl styrene (AzMSt) synthesis
4-Vinylbenzyl chloride (5.00 g, 32.76 mmol) was dissolved in 40 ml dimethylformamide (DMF). Sodium azide (6.38 g, 98.28 mmol) was then added in the solution and the reaction was conducted at room temperature for 24 h. Afterwards the reaction mixture was combined with deionized water (DI H2O; 100 ml) and subsequently extracted with diethyl ether (3 × 100 ml). The collected organic phase was further washed with DI H2O (3 × 100 ml). It was then dried over Na2SO4 for 1 h. The AzMSt was obtained as a yellow oil after solvent removal under vacuum (yield: 5.17 g, 97.5%). 1H NMR (400 Hz, CDCl3): δ (ppm) 7.47–7.43 (m, 2H), 7.33–7.29 (m, 2H), 6.79–6.70 (m, 1H), 5.80 (dd, J = 17.6, 1.2 Hz, 1H), 5.31 (dd, J = 11.2, 0.8 Hz, 1H), 4.35 (s, 2H).Copolymer synthesis
PEGMA (6.02 g, 16.7 mmol) and DMAEMA (10.52 g, 66.9 mmol) were dissolved in methyl ethyl ketone (MEK, 50 ml). AIBN (0.07 g, 0.42 mmol) and CPBD (0.28 g, 1.25 mmol) were added in the solution (Scheme 1). The reaction was carried out at 75 °C for 12 h in a nitrogen-purged environment. The polymer was precipitated by the dropwise addition of the reaction mixture into a 10-fold excess of ethyl ether. A pink-colored powdery solid polymer was collected through centrifugation and residual solvent removal under high vacuum (yield: 14.63 g, 87%).Block copolymer synthesis
The copolymer PPEGMA-co-PDMAEMA (7.30 g), was dissolved into MEK (40 ml) and then mixed with St (5.88 g, 56.5 mmol) and AzMSt (3.00 g, 18.9 mmol) (Scheme 2). AIBN was added as the initiator (0.03 g, 0.19 mmol) and the reaction was carried out for 24 h at 75 °C in a nitrogen environment. After reaction, the solution was dialyzed in MEK for 2 days to remove residual monomers in a dialysis tube (molecular weight cut-off of MWCO: 1000 Da) and followed by vacuum to obtain an orange polymer (yield: 8.75 g, 55%). | ||
| Scheme 2 Synthesis of block copolymer with PPEGMA-co-PDMAEMA with styrene (St) and azidomethyl styrene (AzMSt) in MEK. | ||
Block copolymer assembly and nanogel formation (Scheme S1†)
Block copolymer (2 g) was dissolved in acetone (37 ml). Methanol (13 ml) was charged into the solution to dilute the polymer concentration to 40 mg ml−1 at a rate of 5 ml h−1 controlled by a syringe pump. Propargyl ether (0.054 g, 0.57 mmol) was added to the solution in a nitrogen environment together with catalytic amounts of CuCl2/PMDETA and ascorbic acid. The copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction was allowed to proceed for 24 h at room temperature followed by dialysis in H2O to remove residual monomers and catalysts (dialysis tube MWCO: 1000). The solution of the resulting nanogel product was further freeze-dried to yield a light yellow solid with a lyophilizer (VirTis, SP Scientific).Sol–gel transition
The nanogel (0.2 g) was dispersed in toluene at 5–50 wt% concentrations. Hexamethylene diisocyanate (1.74 mg, 0.01 mmol) was introduced along with a trace amount of tin(II) ethyl hexanoate as the catalyst (Scheme S2†). The molar ratio of –NCO with –OH group was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The reaction was allowed to proceed for overnight and formation of a macroscopic polymer network was verified when the material maintained its shape without flow or deformation (Fig. 1).
1. The reaction was allowed to proceed for overnight and formation of a macroscopic polymer network was verified when the material maintained its shape without flow or deformation (Fig. 1).
Characterization
Results and discussion
From the 1H NMR spectrum of the hydrophilic copolymer (Fig. 2), the peak around 2.6 ppm is associated with the methylene group (–CH2–) adjacent to the amine functionality in DMAEMA, while the resonance signal at 3.7 ppm belongs to the methylene (–CH2–) adjacent to the ether or hydroxyl groups (except for the –CH2 next to the ester group) in PEGMA. By comparing the relative integrals of these two peaks, the molar ratio of DMAEMA to PEGMA in the final copolymer was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The initial monomer feed ratio was 7
1. The initial monomer feed ratio was 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 M for DMAEMA and PEGMA indicating PEGMA has a modestly lower reactivity than DMAEMA but the copolymer structure is assumed to be essentially random. The use of the CPBD/AIBN initiating system has previously been demonstrated to promote RAFT polymerization behaviour with the ability to produce block copolymer structures.8 After the reaction with styrene and AzMSt, the amphiphilic block copolymer was formed confirmed by the appearance of peaks around 6.3–7.3 ppm (aromatic protons) in the NMR (Fig. 2). The methylene peaks around 4.0–4.5 ppm included CH2 (adjacent to azide) from AzMSt and also CH2 (adjacent to ester group) in PEGMA and DMAEMA. By comparing the relative intensity of this peak to the 2.6 ppm peak (that solely belongs to –CH2– adjacent to amine in DMAEMA) in the block copolymer, the AzMSt concentration can be obtained. Furthermore, the styrene to AzMSt ratio was determined by separation of the aromatic proton signals of these two monomers at 6.3–7.3 ppm given the known AzMSt contribution. The molar ratio of St and AzMSt is 5
3 M for DMAEMA and PEGMA indicating PEGMA has a modestly lower reactivity than DMAEMA but the copolymer structure is assumed to be essentially random. The use of the CPBD/AIBN initiating system has previously been demonstrated to promote RAFT polymerization behaviour with the ability to produce block copolymer structures.8 After the reaction with styrene and AzMSt, the amphiphilic block copolymer was formed confirmed by the appearance of peaks around 6.3–7.3 ppm (aromatic protons) in the NMR (Fig. 2). The methylene peaks around 4.0–4.5 ppm included CH2 (adjacent to azide) from AzMSt and also CH2 (adjacent to ester group) in PEGMA and DMAEMA. By comparing the relative intensity of this peak to the 2.6 ppm peak (that solely belongs to –CH2– adjacent to amine in DMAEMA) in the block copolymer, the AzMSt concentration can be obtained. Furthermore, the styrene to AzMSt ratio was determined by separation of the aromatic proton signals of these two monomers at 6.3–7.3 ppm given the known AzMSt contribution. The molar ratio of St and AzMSt is 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, which is close to the 3
2, which is close to the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 monomer feed ratio used in the preparation of this block, while the overall molar ratio of the hydrophobic units to hydrophilic units is 2
1 monomer feed ratio used in the preparation of this block, while the overall molar ratio of the hydrophobic units to hydrophilic units is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The molecular weight (Mw) of the hydrophilic copolymer was estimated about 48.8 kDa while the block copolymer had a Mw of 66.6 kDa based on the comparison of repeat monomer units with RAFT end group. The NMR end-group analysis method was adopted after attempts with GPC analysis failed due to strong interactions between the copolymers and GPC columns.
3. The molecular weight (Mw) of the hydrophilic copolymer was estimated about 48.8 kDa while the block copolymer had a Mw of 66.6 kDa based on the comparison of repeat monomer units with RAFT end group. The NMR end-group analysis method was adopted after attempts with GPC analysis failed due to strong interactions between the copolymers and GPC columns.
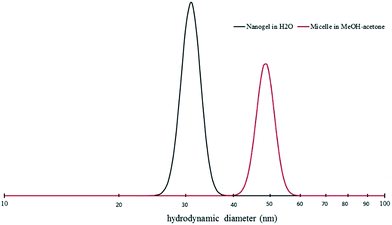
The block copolymer was further assembled in organic solvent. Most of the amphiphilic block copolymer self-assembly studies were carried out in water due to the strong repulsion of the hydrophobic segments with water. There have been some reports using organic solvents for block copolymer self-assembly primarily for reverse micelle formation or non-water dispersible polymers.16,17 The choice of solvents can affect final assembled polymer structure significantly including micelle morphology and dimension.18 In this study, it was demonstrated that water could disperse the block copolymer by forming core–shell micelle structures at dilute concentration (i.e. 1 mg ml−1). However, a higher concentration of micelles is desirable in order to generate large amounts of nanogel particles without conducting time-consuming multi-batch reactions. However, a cloudy solution was formed when the block copolymer concentration was increased to higher content (e.g. 20 mg ml−1) due to the aggregation of the micelle structures in the absence of any surfactant additive. From light scattering (not shown here), there were two peaks in size distribution in the solution at 37 nm and 170 nm. The 37 nm particle dimension was likely associated with the self-assembled micelle structures while the 170 nm average size structures represented aggregated particles that appeared at the higher polymer concentrations. Methanol, a poor solvent for the hydrophobic block, was added to the polymer solution in acetone slowly to yield an optically clear solution at 40 mg ml−1 concentration with methanol to acetone volume ratio of 0.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Under these solvent conditions, the average particle size was 47 nm with narrow distribution indicating the formation of uniform micelles (Fig. 3). With the addition of propargyl ether in the presence of Cu(I) catalyst, an ambient CuAAC reaction was carried out to crosslink the hydrophobic block. CuAAC click chemistry has been demonstrated to provide an efficient coupling between alkyne and the azide groups in the core structure of block copolymer micelles.19,20 The styrene-based azide group of the block copolymer in the solution was 1.2 mmol through calculation based on the relative concentration of the AzMSt to the overall polymer (based on NMR data). By the reaction of azide with the stoichiometric ratio of alkyne groups, 95% of the azide groups in the block copolymer were consumed based on the signal reduction of azide IR absorption peak (2000–2200 cm−1, asymmetric stretching) (Fig. 4). As propargyl ether is hydrophobic, it is expected that the alkyne groups are concentrated in the hydrophobic core region of the block copolymer micelles. The high conversion of azide groups indicates the crosslinking reaction between azide and alkyne groups was highly efficient, similar to what has been reported previously.16 The proton in newly-formed triazole connecting group appeared in the 1H NMR spectrum at 8.1 ppm (Fig. 2). Since the mobility of the core structure was restricted compared to the hydrophilic shell, especially in a hydrophilic solvent – d-DMSO, broadened peaks for styrene and trizole were observed with low intensity. The resulting nanogel was also able to disperse in water as achieved through solvent exchange with dialysis. The nanogel size was reduced to 30 nm when dispersed in water rather than the methanol/acetone co-solvent (Fig. 3). Nanogels shrank in a more hydrophilic environment not only due to collapse of the hydrophobic core but probably also because the relatively hydrophilic shell layer of the copolymer from PEGMA and DMAEMA were found to have better solubility in the co-solvent than in neutral water. The collapsed nanogel particles freeze-dried in water had an average diameter of 15.4 ± 4.8 nm as characterized by SEM (Fig. S1;† analysis with ImageJ, n = 919). The relatively uniform globular particles confirmed the low polydispersity of nanogels after block copolymer self-assembly.
1. Under these solvent conditions, the average particle size was 47 nm with narrow distribution indicating the formation of uniform micelles (Fig. 3). With the addition of propargyl ether in the presence of Cu(I) catalyst, an ambient CuAAC reaction was carried out to crosslink the hydrophobic block. CuAAC click chemistry has been demonstrated to provide an efficient coupling between alkyne and the azide groups in the core structure of block copolymer micelles.19,20 The styrene-based azide group of the block copolymer in the solution was 1.2 mmol through calculation based on the relative concentration of the AzMSt to the overall polymer (based on NMR data). By the reaction of azide with the stoichiometric ratio of alkyne groups, 95% of the azide groups in the block copolymer were consumed based on the signal reduction of azide IR absorption peak (2000–2200 cm−1, asymmetric stretching) (Fig. 4). As propargyl ether is hydrophobic, it is expected that the alkyne groups are concentrated in the hydrophobic core region of the block copolymer micelles. The high conversion of azide groups indicates the crosslinking reaction between azide and alkyne groups was highly efficient, similar to what has been reported previously.16 The proton in newly-formed triazole connecting group appeared in the 1H NMR spectrum at 8.1 ppm (Fig. 2). Since the mobility of the core structure was restricted compared to the hydrophilic shell, especially in a hydrophilic solvent – d-DMSO, broadened peaks for styrene and trizole were observed with low intensity. The resulting nanogel was also able to disperse in water as achieved through solvent exchange with dialysis. The nanogel size was reduced to 30 nm when dispersed in water rather than the methanol/acetone co-solvent (Fig. 3). Nanogels shrank in a more hydrophilic environment not only due to collapse of the hydrophobic core but probably also because the relatively hydrophilic shell layer of the copolymer from PEGMA and DMAEMA were found to have better solubility in the co-solvent than in neutral water. The collapsed nanogel particles freeze-dried in water had an average diameter of 15.4 ± 4.8 nm as characterized by SEM (Fig. S1;† analysis with ImageJ, n = 919). The relatively uniform globular particles confirmed the low polydispersity of nanogels after block copolymer self-assembly.
 | ||
| Fig. 4 IR spectra for the block copolymer (black line) and formed nanogels after CuAAC reaction (red line). The absorbance at 2000–2200 cm−1 belongs to azide groups. | ||
The LCST of DMAEMA polymer can be significantly affected by the addition of other monomer units. It has been shown that the LCST increased with PEGMA content by copolymerizing the two monomers together.21 This is due to PEG being more hydrophilic with greater affinity for water compared with the neutral tertiary amine. For the copolymer with 25 mol% PEGMA units, the cloud point was 69 °C in a neutral pH environment (Table 1). The pKa of DMAEMA is around 7.5 (ref. 22) and protonation of DMAEMA in pH 7 solution would be expected to enhance polymer solubility in water. In water at pH 4, no phase separation or cloud point was detected at any temperature since the higher degree of protonation increased polymer–solvent interaction even at high temperatures with reduced hydrogen bonding effect. Meanwhile, a lower cloud point (47 °C) was found due to deprotonation of amine groups in a basic environment (pH = 10). It has been reported previously that LCST is decreased by the incorporation of hydrophobic monomer units.23 The hydrophobic units preferably interact with each other in aqueous systems so that the interaction between water and the macromolecules can be minimized. However, at a certain temperature, polymer aggregation or phase separation can take place once the internal thermodynamic stabilization within a polymer coil (or a polymeric particle) no longer presents an energy minimum. With the incorporation of styrene and AzMSt, the greater hydrophobicity of the block copolymer was introduced with a corresponding decrease to a cloud point of 4–5 °C was found for pH 7 and 10 buffer solutions. This is not surprising since a significant decrease in the cloud point temperature was expected with the incorporation of 2/5 molar content of these quite hydrophobic monomers.23 Different from randomly copolymerizing hydrophobic monomers with hydrophilic monomers, a block copolymer can form relatively stable micelles in water solution with the hydrophobic block segregated in the core structure to avoid significant interaction with water. Thus, the shell comprised mainly of the hydrophilic monomer-based segments was able to interact with water in a similar way as did the simple hydrophilic copolymer. With an increase of temperature, the chain mobility is increased in the micelles so that the core structure could become unstable through enhanced interaction with water. By chemical crosslinking of the core, stable core–shell nanogels were formed and no cloud point was detected for the nanogel solution in pH 4 and 7 buffers (Fig. S2†). With the increase of temperature, the interaction between hydrophilic blocks was promoted within single nanogel particles when the hydrogen bonding with solvent was reduced. Notably, the nanogel solution remained stable and transparent at any given temperature in a neutral pH buffer indicating no large particles formed through multi-nanogel aggregation. The cloud point was 58 °C for nanogel solution in a pH 10 buffer, which is still considerably higher than either of the other two precursor components. Nanogel did precipitate out of the solution when the temperature was increased to 100 °C. The phase separation process was irreversible in that the nanogel-based precipitant remained insoluble when the sample was cooled back to room temperature. This is different than the behavior of the copolymer and block copolymer samples where the cloudy solutions rapidly transitioned back to transparent solutions when the temperature was dropped below the corresponding cloud point. This is probably because at high temperatures, once the isolated nanogels undergo aggregation and precipitation, the multiple chain entanglements and affinity between particles was strong enough to prevent redispersal without other more aggressive intervention.
| pH | Copolymer (°C) | Block copolymer (°C) | Nanogel (°C) |
|---|---|---|---|
| 4 | N/A | N/A | N/A |
| 7 | 69 | 65 | N/A |
| 10 | 47 | 42 | 58 |
Since these nanogel particles are composed of a hydrophobic core, it could be used to selectively absorb hydrophobic molecules that display little affinity for water. Here we used a hydrophobic dye, Nile Red, to test if selective absorption was available with the nanogel. From the images (Fig. 5), essentially no coloration was found by the addition of Nile Red to water due to its negligible solubility. The nanogel used here has a yellow color due to the presence of RAFT and AzMSt groups. However, due to the low concentration of nanogel (5 mg ml−1), the resulting dispersion did not have any noticeable color. However, with nanogel dispersed in the water, an obvious red color was displayed by adding Nile Red, which confirms that the self-assembled nanogel could be employed as a water-compatible absorbent or carrier for hydrophobic molecules. Under UV irradiation, once, again the dye only sample was colorless due to very limited aqueous solubility; however, a bluish color was observed for the nanogel dispersion (Fig. 5) due to the light scattering effect of nanogel particles. UV light with shorter wavelength is scattered by smaller particles than is visible light. For the nanogel and dye sample under UV light, Nile Red fluoresces resulting in a purple color as shown in the dye-loaded nanogel dispersion.
 | ||
| Fig. 5 Sample images of water solutions with just Nile Red or nanogel or both: (left) under visible light; and (right) under UV light. | ||
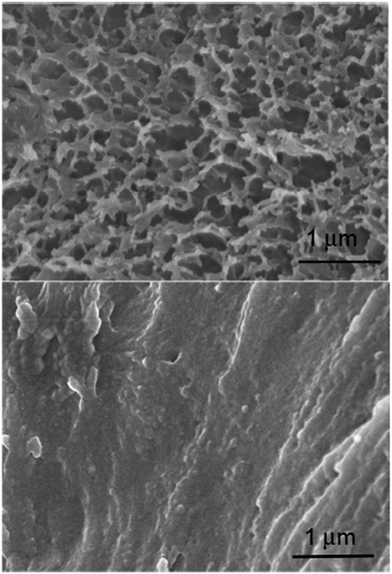
The pendent –OH groups in PEGMA units were able to crosslink nanogels together to form macrogel networks. This nanogel is dispersible in hydrophilic solvents like DMSO, DMF and acetonitrile as well as more hydrophobic solvent like toluene. The reaction between hydroxyl and isocyanate groups yields urethane functionality, which is less hydrophilic than –OH groups. Thus, in hydrophilic solvents, phase separation was observed during the crosslinking reaction. By dispersing nanogel in toluene from 5–66.7 wt%, initially homogeneous, transparent dispersions were formed with a linear relationship between the logarithm of viscosity and nanogel concentration (wt%) (Fig. 6). This indicates that the viscosity scales with the nanogel concentration at a single exponent despite the nanogel inter-particle distance. This loading range was selected to span isolated/discontinuous nanogel dispersions at the lower limit and overlapping, confluent solvent-swollen particles at the upper limit. Prior studies have found the slope of viscosity increasing when the polymer concentration is increased from a semi-dilute to an entangled state due to the additional contribution from significant polymer–polymer interaction.24 The possible reason that a constant slope was observed including the relatively dilute state (5 wt%) is that the nanogel–nanogel interaction is not negligible even at low concentrations since the hydrophilic nanogel shell preferentially interact with other nanogels due to the hydrophobicity of the solvent. At the lower nanogel concentration limit (5 wt%), the HMDI crosslinking reaction only led to an increase in solution viscosity instead of macrogelation. Under these conditions, intra-particle and limited inter-particle coalescence occurred with aggregate formation not large enough to induce observable visible light scattering. From loadings of 10 wt% and up, macrogel structures were formed in the crosslinking process. In a polar solvent like DMF, 10 wt% is also the lowest concentration to achieve macrogelation similar to the toluene solution. Opaque polymer samples were formed in DMF due to phase separation. The hydrophobic core has better swelling in toluene than in polar solvents, but the hydrophilic shell would likely be collapsed on the surface. These macrogelation experiments in various dispersion media and nanogel loading levels can be used to examine nanogel interspacing. At the 10 wt% loading level, a loosely crosslinked material was formed with significant interconnected porous structure as shown by imaging the fracture surface of the gel (Fig. 7). The polymers only occupied a small percentage of the volume, which further supported that the 10 wt% loading was just beyond the nanogel percolation threshold. With the increase of the nanogel content to 50 wt%, the crosslinked polymer presents a more uniform, continuous surface. The densely packed polymer consisted of a matrix with secondary nodular features that appeared in the dimension of less than 100 nm. The small features could be individual nanogels and nanogel aggregates were also formed what appeared as larger spherical morphologies. The interparticle spacing at 50 wt% loading is negligible so nanogels were able to interpenetrate significantly given the low Tg of the nanogel.
Conclusion
An amphiphilic block copolymer was synthesized through RAFT polymerization. Uniform micelles were formed by dispersing the block copolymer in a hydrophilic environment. A CuAAC click reaction was applied for the core crosslinking of the hydrophobic block with nearly complete consumption of the appended azide functionality. Nanogel structures were achieved with an average dimension of 30 nm in water. Due to the stability of the nanogel structures, the cloud point temperatures were higher for the nanogels compared with the component copolymer and block copolymer at pH 7 and 10. It was further demonstrated that the nanogel hydrophobic core could be applied to absorb and stabilize hydrophobic molecules in water. Extended polymeric networks were able to be formed when the nanogel concentration exceeded the critical percolation concentration (∼10 wt%) during secondary crosslinking between hydrophilic blocks that make up the nanogel shell. A transition from highly porous macrogel structure to densely packed morphology was observed when the nanogel concentration increased from 10 wt% to 50 wt%.Acknowledgements
This study was supported by NIH/NIDCR R01DE022348 and U01DE023774. The assistance provided by Dr Tao Liu during preparation of this manuscript is gratefully acknowledged.References
- D. A. Tomalia, Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry, Prog. Polym. Sci., 2005, 30, 294–324 CrossRef CAS.
- P. G. Degennes and H. Hervet, Statistics of starburst polymers, J. Phys. Lett., 1983, 44, L351–L360 CrossRef.
- B. Voit, Hyperbranched polymers – all problems solved after 15 years of research?, J Polym Sci Pol Chem, 2005, 43, 2679–2699 CrossRef CAS.
- N. O'brien, A. McKee, D. C. Sherrington, A. T. Slark and A. Titterton, Facile, versatile and cost effective route to branched vinyl polymers, Polymer, 2000, 41, 6027–6031 CrossRef.
- C. Chen, J. Liu, F. Sun and J. W. Stansbury, Control of microstructure and gradient property of polymer network by photopolymerizable silicone-containing nanogel, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2830–2840 CrossRef CAS.
- C. Chen, J. Liu, F. Sun and J. W. Stansbury, Tuning the surface microstructure and gradient properties of polymers with photopolymerizable polysiloxane-modified nanogels, RSC Adv., 2014, 4, 28928–28936 RSC.
- R. R. Moraes, J. W. Garcia, M. D. Barros, S. H. Lewis, C. S. Pfeifer, J. C. Liu and J. W. Stansbury, Control of polymerization shrinkage and stress in nanogel-modified monomer and composite materials, Dent. Mater., 2011, 27, 509–519 CrossRef CAS PubMed.
- J. Liu and J. W. Stansbury, Raft-mediated control of nanogel structure and reactivity: chemical, physical and mechanical properties of monomer-dispersed nanogel compositions, Dent. Mater., 2014, 30, 1252–1262 CrossRef CAS PubMed.
- J. C. Liu, I. Y. Rad, F. Sun and J. W. Stansbury, Photo-reactive nanogels as a means to tune properties during polymer network formation, Polym. Chem., 2014, 5, 227–233 RSC.
- A. T. Slark, D. C. Sherrington, A. Titterton and I. K. Martin, Branched methacrylate copolymers from multifunctional comonomers: the effect of multifunctional monomer functionality on polymer architecture and properties, J. Mater. Chem., 2003, 13, 2711–2720 RSC.
- J. C. Liu, G. D. Howard, S. H. Lewis, M. D. Barros and J. W. Stansbury, A study of shrinkage stress reduction and mechanical properties of nanogel-modified resin systems, Eur. Polym. J., 2012, 48, 1819–1828 CrossRef CAS PubMed.
- C. Park, J. Yoon and E. L. Thomas, Enabling nanotechnology with self assembled block copolymer patterns, Polymer, 2003, 44, 6725–6760 CrossRef CAS.
- J. Rodriguez-Hernandez, F. Checot, Y. Gnanou and S. Lecommandoux, Toward ‘smart’ nano-objects by self-assembly of block copolymers in solution, Prog. Polym. Sci., 2005, 30, 691–724 CrossRef CAS.
- S. Pascual and M. J. Monteiro, Shell-crosslinked nanoparticles through self-assembly of thermoresponsive block copolymers by raft polymerization, Eur. Polym. J., 2009, 45, 2513–2519 CrossRef CAS.
- F. A. Plamper, M. Ruppel, A. Schmalz, O. Borisov, M. Ballauff and A. H. E. Muller, Tuning the thermoresponsive properties of weak polyelectrolytes: aqueous solutions of star-shaped and linear poly(n,n-dimethylaminoethyl methacrylate), Macromolecules, 2007, 40, 8361–8366 CrossRef CAS.
- J. F. Ding and G. J. Liu, Hairy, semi-shaved, and fully shaved hollow nanospheres from polyisoprene block poly(2-cinnamoylethyl methacrylate), Chem. Mater., 1998, 10, 537–542 CrossRef CAS.
- H. S. Peng, D. Y. Chen and M. Jiang, Self-assembly of formic acid/polystyrene-block-poly(4-vinylpyridine) complexes into vesicles in a low-polar organic solvent chloroform, Langmuir, 2003, 19, 10989–10992 CrossRef CAS.
- Y. S. Yu and A. Eisenberg, Control of morphology through polymer–solvent interactions in crew-cut aggregates of amphiphilic block copolymers, J. Am. Chem. Soc., 1997, 119, 8383–8384 CrossRef CAS.
- R. K. O'reilly, M. J. Joralemon, K. L. Wooley and C. J. Hawker, Functionalization of micelles and shell cross-linked nanoparticles using click chemistry, Chem. Mater., 2005, 17, 5976–5988 CrossRef.
- J. Y. Zhang, Y. M. Zhou, Z. Y. Zhu, Z. S. Ge and S. Y. Liu, Polyion complex micelles possessing thermoresponsive coronas and their covalent core stabilization via “click” chemistry, Macromolecules, 2008, 41, 1444–1454 CrossRef CAS.
- D. Fournier, R. Hoogenboom, H. M. L. Thijs, R. M. Paulus and U. S. Schubert, Tunable pH- and temperature-sensitive copolymer libraries by reversible addition–fragmentation chain transfer copolymerizations of methacrylates, Macromolecules, 2007, 40, 915–920 CrossRef CAS.
- P. van de Wetering, E. E. Moret, N. M. E. Schuurmans-Nieuwenbroek, M. J. van Steenbergen and W. E. Hennink, Structure–activity relationships of water-soluble cationic methacrylate/methacrylamide polymers for nonviral gene delivery, Bioconjugate Chem., 1999, 10, 589–597 CrossRef CAS PubMed.
- H. Feil, Y. H. Bae, J. Feijen and S. W. Kim, Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of n-isopropylacrylamide copolymers, Macromolecules, 1993, 26, 2496–2500 CrossRef CAS.
- P. Gupta, C. Elkins, T. E. Long and G. L. Wilkes, Electrospinning of linear homopolymers of poly(methyl methacrylate): exploring relationships between fiber formation, viscosity, molecular weight and concentration in a good solvent, Polymer, 2005, 46, 4799–4810 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra03933b |
| This journal is © The Royal Society of Chemistry 2016 |