Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solvent-free temperature gradient melt formation of efficient visible-to-UV photon upconversion organic films with subsolar threshold and over 100 h photostability in air†
Riku
Enomoto
 ab and
Yoichi
Murakami
ab and
Yoichi
Murakami
 *ab
*ab
aLaboratory for Zero-Carbon Energy, Institute of Innovative Research, Tokyo Institute of Technology, 2-12-1 Ookayama, Meguro-ku, Tokyo 152-8550, Japan. E-mail: murakami.y.af@m.titech.ac.jp
bDepartment of Mechanical Engineering, Tokyo Institute of Technology, 2-12-1 Ookayama, Meguro-ku, Tokyo 152-8552, Japan
First published on 20th December 2022
Abstract
Completely solvent-free “green” formation of bicomponent organic polycrystalline films for efficient visible-to-ultraviolet photon upconversion (UV–UC) were achieved by temperature-gradient solidification from the melt. The UV–UC film formed under optimal conditions exhibited an ultralow excitation threshold (0.3 suns, AM1.5 spectrum with a 413 nm long-pass filter) and a record-long photostability (>100 h) in air.
Ultraviolet (UV) light (wavelength < 400 nm) consisting of high-energy photons has broad utility; including photocatalytic generation of green hydrogen1 and hydrocarbons,2 photo-polymerization,3 and sanitization.4 However, only ca. 4% of photons in terrestrial sunlight5 constitute UV light, which has hindered efficient and effective uses of sunlight.
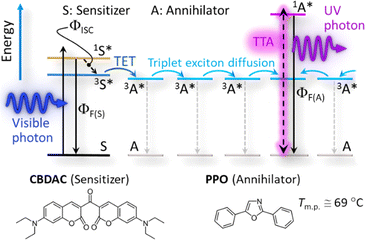
Photon upconversion (UC) based on triplet–triplet annihilation (TTA) (TTA-UC, Scheme 1) is an active area of research because of its potential of converting low-intensity light.6,7 Since a visible-to-UV UC (UV–UC) was reported8 in deaerated benzene by using biacetyl and 2,5-diphenyloxazole (PPO) as a metal-free sensitizer and annihilator, respectively, many UV–UCs in organic solvents have been reported;8–12 although such systems generally exhibit photodegradation.9 Recently, a photostability of 1 h was reported for UV–UCs in deaerated solvents with a UC quantum yield (ΦUC) of ca. 10% or a normalized UC emission efficiency (ηUC ≡ 2ΦUC) of ca. 20%.10,11 However, use of volatile, flammable, and bio-toxic solvents should be avoided for applications that usually demand safety, stability, and greenness. To date, after some early works that embedded chromophores into polymers,13,14 solid-state UC has been intensively investigated.15–18 However, no UV–UC solids with an excitation threshold comparable with or lower than terrestrial solar intensity have been reported.14,18
Recently, we developed van der Waals (vdW) solid–solution crystals of a hydrocarbon annihilator in which only 0.002 mol% of the porphyrin sensitizer was stably dissolved.17 Although such slowly grown vdW crystals have afforded substantially high performance in air17 and resolved the previous issue of low sample crystallinity,‡ the corresponding preparation used organic solvents during the recrystallization for 1–3 days.17 Therefore, a solvent-free method to controllably and reproducibly generate high-performance UV–UC solids, preferably in the form of a film that is compatible with broad applications, is highly desired. Such a target would substantially impact our manner of using visible light and solve the problem of the low number of UV photons in sunlight.
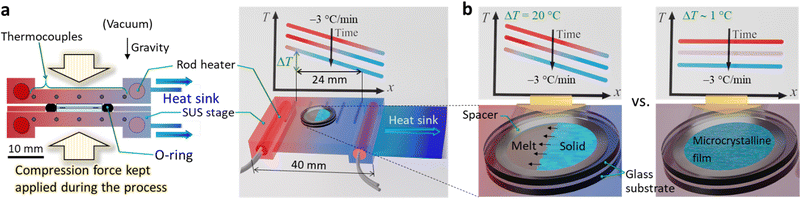
Herein, we report completely solvent-free “green” formation of UV–UC solid thin films with outstanding high performance by using a temperature gradient controlled over a surface (Fig. 1a). This strategy, for the first time applied towards preparation of UC solids, enables formation of a bicomponent polycrystalline organic film from a melt of a sensitizer and an annihilator blend. Our investigations of this concept reveal an essential importance of optimizing the temperature gradient or cooling rate (Fig. 1b). Consequently, the UC film formed under optimal conditions exhibited a high ΦUC = 4.3% (ηUC = 8.6%), substantially low excitation threshold (ca. 0.3× that of natural sunlight intensity), and record-long (>100 h) photostability ever reported for TTA–UC, all of which were achieved in air, as follows.
After our initial search for a suitable combination of the sensitizer and annihilator (some examples shown in Table S1, ESI†), we selected 3,3′-carbonylbis(7-diethylaminocoumarin) (CBDAC)11,19,20 as the sensitizer and PPO as the annihilator (Scheme 1). CBDAC has a high intersystem-crossing quantum yield (ΦISC ≅ 92%, in benzene),19 whereas PPO has a low melting point (Tm.p. ≅ 69 °C, Fig. S6a, ESI†) and a high fluorescence quantum yield (ΦF(A) ≅ 79%, cf.Scheme 1) in the solid state as measured with an absolute photoluminescence quantum yield spectrometer (Quantaurus-QY, Hamamatsu). Herein, a blended powder of CBDAC and PPO was heated above the melting point of PPO on a heating stage and then the melt was cooled at a controlled rate with a lateral temperature gradient, which resulted in filmwise crystals of PPO doped with CBDAC (Fig. 1 and Section 1 of the ESI†). We identified the optimal mole ratio of CBDAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPO to be 1
PPO to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, which minimized the excitation threshold intensity (Ith) (Fig. S7, ESI†), used hereafter unless otherwise specified. Thus, only ca. 0.0033 mol% of CBDAC was dissolved in the melt liquid of PPO during the process described as follows.
000, which minimized the excitation threshold intensity (Ith) (Fig. S7, ESI†), used hereafter unless otherwise specified. Thus, only ca. 0.0033 mol% of CBDAC was dissolved in the melt liquid of PPO during the process described as follows.
We custom-designed and fabricated the processing apparatus (Fig. 1a and Fig. S1 of the ESI†). The main part of the apparatus consisted of a pair of stainless-steel (SUS) stages over which the same temperature gradients were formed. Each stage has two rod heaters embedded in the both ends, five thermocouples embedded between the heaters, and a heatsink attached to the cold end of the stage. We placed this setup in a vacuum chamber (Fig. S1, ESI†); the details regarding the apparatus are in Section 1.4 of the ESI.† Briefly, we sandwiched a blend of CBDAC and PPO (ca. 32 mg), placed inside a spacer ring (SUS, thickness: 200 μm, inner diameter: 8 mm), with two round glass substrates (EAGLE XG, Corning; diameter: 12 mm, thickness: 0.7 mm); we deposited the contacting surface to the SUS stage with a thin aluminum layer that served as a light reflector (Section 1.3 of the ESI†). During the process carried out under vacuum, we sealed the periphery of the stack with a perfluoroelastomer O-ring to prevent loss of the material, and we applied a compression force to the stack to ensure that the (i) O-ring was effective, (ii) resulting UC film had the same thickness as that of the spacer (Fig. 1a and Fig. S1, ESI†), and (iii) stage and substrate were in good thermal contact. We define ΔT as the difference between the temperatures measured with the two thermocouples at both ends separated by 24 mm (Fig. 1a and Fig. S2 of the ESI†). The minimum possible ΔT was 0.9 ± 0.4 °C because of the heatsink, which we denote “ΔT ∼ 1 °C.” We digitally controlled the temperature profile over the SUS stages such that it decreased at −3 °C min−1 (see Fig. S2 in the ESI† for the recorded changes of the temperature profiles). After the stack was taken out of the apparatus, the thin film was adhered to only one of the substrates because of the weaker adhesion of the film to the substrate than the integrity of the film. Thus, we performed all of the experiments in air; i.e., with one surface of the film exposed to air. Before measurements, the film was annealed in dry nitrogen at 66 °C for 30 min for improving the crystallinity; previously, we found that annealing is important for crystalline UC solids.17
With ΔT ∼ 1 °C, microcrystalline films with strong light scattering were generated (Fig. 2a, left), ascribed to a sudden solidification over the entire area. Notably, with ΔT = 20 °C, the film was comprised mostly of single-crystalline stripes grown along the temperature gradient (Fig. 2a, middle); the width of the stripes ranged mainly between 30 and 80 μm. We found some macroscopic cracks in the film, ascribed to the difference in the thermal expansion coefficients between the film and substrate. This result, which is highly reproducible, indicates a strong influence of the temperature gradient on the nature of the obtained film. The literature reports similar directional crystallization along the temperature gradient in films formed on a substrate from the melt of an organic p-type and n-type semiconductor blend.21
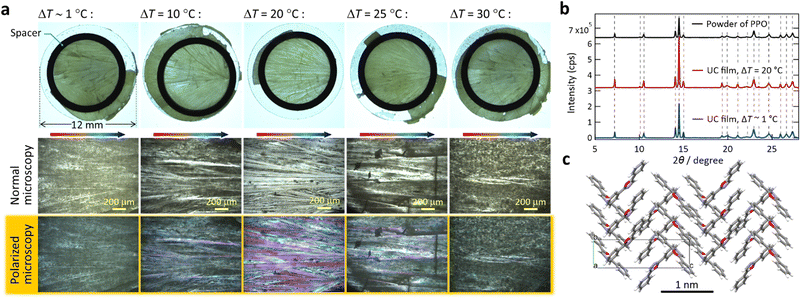 | ||
Fig. 2 Morphology and microscopic structure of the UC films prepared at CBDAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPO = 1 PPO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (in mol). (a) Digital photographs acquired with a stereo microscope (top) and magnified optical microscope images (bottom) of the samples prepared with various ΔT, all of which we acquired with transmitted illumination. In the polarized microscope images, crystalline domains were evident as purple-colored areas. (b) PXRD patterns acquired from the samples and as-received PPO powder, indicating the absence of polymorphism. (c) The crystal structure determined after Pawley and Rietveld refinements. See Section 6 of the ESI† for the results of the crystallographic analysis. 000 (in mol). (a) Digital photographs acquired with a stereo microscope (top) and magnified optical microscope images (bottom) of the samples prepared with various ΔT, all of which we acquired with transmitted illumination. In the polarized microscope images, crystalline domains were evident as purple-colored areas. (b) PXRD patterns acquired from the samples and as-received PPO powder, indicating the absence of polymorphism. (c) The crystal structure determined after Pawley and Rietveld refinements. See Section 6 of the ESI† for the results of the crystallographic analysis. | ||
Interestingly, when we increased ΔT to 25–30 °C, the films were again microcrystalline and highly opaque (Fig. 2a, right). This indicates that ΔT = 20 °C afforded an optimal moving speed of the solidification front line in a manner that facilitated growth of crystalline stripes. Aforementioned previous work also reported an optimal cooling rate, in which both too-slow and too-fast cooling rates perturbed the directional growth.21 Although the report did not provide an explanation,21 we confirmed this aspect by conducting a supporting experiment; use of a lower cooling rate of −1 °C min−1 with ΔT = 20 °C yielded similarly microcrystalline films (Fig. S8, ESI†). The UC films exhibited the same powder X-ray diffraction (PXRD) pattern as that of as-supplied PPO (Fig. 2b), indicating an absence of polymorphs. The Pawley and Rietveld refinements22 indicate herringbone packing of PPO (Fig. 2c and Section 6 in the ESI†).
Hereafter, the photophysical properties are shown (Fig. 3; see Section 1 of the ESI† for experimental details). All Ith values were determined by fitting experimental data with eqn (35) or (36) of ref. 23. By irradiating the film with a laser light at a wavelength (λ) of 440 nm (diameter: 3 mm, beam profile: top hat, Fig. S3 in the ESI†), we observed an UC emission peaked at 390–393 nm with maximum fluorescence from CBDAC at 480–490 nm (Fig. 3a). The UC fluorescence was substantially redshifted compared with the fluorescence of PPO in solution (see Fig. S10, ESI† for absorption and emission spectra in dilute solutions). The UC emission from the sample prepared with ΔT = 20 °C was more intense than that prepared with ΔT ∼ 1 °C, ascribed to the higher crystallinity of the former (Fig. 2a). This explanation is supported by the ca. 2 × longer decay time-constant of the UC emission intensity for the former than the latter (Fig. S11, ESI†). The emission intensity distribution along the temperature-gradient direction was highly uniform (Fig. S13, ESI†), indicating that the crystals in this report were solid solutions similar to the crystals in our previous report.17
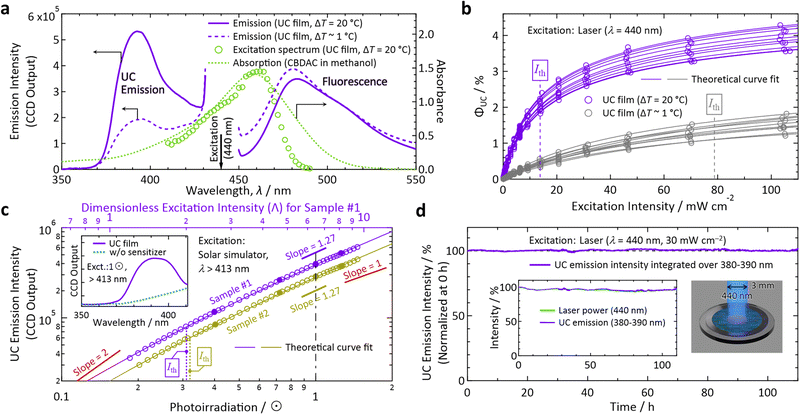 | ||
| Fig. 3 Photoemission properties measured in air. (a) Spectra of emission from the films prepared with ΔT = 20 °C (solid curves) and ∼ 1 °C (dashed curves) acquired with an incident laser light at λ = 440 nm. Also shown are an excitation spectrum (monitored at 380–390 nm) acquired with a wavelength-tunable laser from the film made with ΔT = 20 °C (circles) and an absorption spectrum of a methanol solution of CBDAC (dotted curve, 2 × 10−4 M, optical path length = 1 mm). (b) Dependence of ΦUC on the intensity of excitation at 440 nm measured for samples prepared with ΔT = 20 °C (purple) and ∼ 1 °C (gray), each of which we measured for 10 independently prepared samples. (c) Dependence of the UC emission intensity on the irradiance (in unit of sun, ⊙) of simulated sunlight, which passed through a λ > 413 nm long-pass filter placed above the sample (see Section 1.11 in the ESI†). Here, we measured two different samples #1 and #2, prepared with ΔT = 20 °C, to check the reproducibility. The inset shows the emission spectrum from the sample under 1 ⊙ irradiance (solid curve) and that from the reference prepared without the sensitizer (dotted curve). In panels (b) and (c), we first acquired the data represented by open marks by increasing the excitation light power, and then acquired the data represented by filled marks to confirm the reproducibility and sample stability. The theoretical fit curves used in panels (b) and (c) are eqn (35) and (36) of ref. 23, respectively. See eqn (28) and (29) of ref. 23 for details of the dimensionless excitation intensity Λ; Λ = 2 corresponds to Ith. (d) Test of photostability of the UC film prepared with ΔT = 20 °C by continuous irradiation of 440-nm laser light at an intensity of 30 mW cm−2 (see Fig. S3 in the ESI† for the setup). The vertical axis represents the UV–UC emission intensity corrected by the temporal fluctuation of the laser power shown in the inset. | ||
Because we determined the ΦF(S) (cf.Scheme 1) of the UC film to be 5.1 ± 0.23% (0.23%: standard deviation for measurements on five different samples) with an absolute photoluminescence quantum yield spectrometer, ΦUC could be determined by referencing to the fluorescence intensity; see Section 1.10 of the ESI† for details of the determination of ΦUC. Herein, we define photoemission ranging in λ ≤ 425 nm as UC emission. With this definition, 60.1% of the UC photons from the sample prepared with ΔT = 20 °C were UV photons with λ < 400 nm. The samples made with ΔT = 20 °C exhibited much higher efficiency (up to ΦUC = 4.3% and ηUC = 8.6%) and a lower Ith than those made with ΔT ∼ 1 °C (Fig. 3b).
The Ith values for the cases of ΔT = 20 °C and ΔT ∼ 1 °C, from measurements of 10 different samples each, ranged over 13.8–17.0 and 78.8–93.5 mW cm−2, respectively. In Fig. 3b, the minimum values are indicated. The aluminum light reflector deposited on the backside of the substrate decreased Ith by ca. 40%. Owing to the reflector, despite the low concentration of CBDAC (ca. 0.0033 mol% ≅ 1.92 × 10−4 M) in the PPO crystals, the absorptance by CBDAC in the film at 440 nm was ca. 61%, from which the excitation rate (kex in ref. 23) and excitation density (ex in ref. 24) of CBDAC at Ith are found to be ca. 7.7 × 10−4 M s−1 and ca. 2 × 1016 s−1 cm−2, respectively (see Section 11 of the ESI† for these derivations). The emission intensities were maximal when the polarization direction of the incident laser light matched the direction of the crystal growth, whereas the intensity ratio of the UC fluorescence to CBDAC fluorescence was independent of the incident light polarization (Section 10, ESI†).
Notably, the value of Ith for sunlight irradiation cannot be determined from the value of Ith obtained by monochromatic excitation.17,24 Therefore, we used broadband light generated with a solar simulator, which consisted only of the wavelength range of λ > 413 nm obtained with a long-pass filter (see Section 1.11 in the ESI† for details), representing the intensity in unit of suns (⊙). As shown in Fig. 3c for two samples prepared with ΔT = 20 °C (samples #1 and #2), the values of Ith were ca. 0.3 ⊙, indicating that the developed materials can be used for sunlight without concentration optics. These Ith values are reliable because the slope changed from ca. 1.6 to ca. 1.2, which is sufficient to perform theoretical curve fitting, and the vertical-axis values of Fig. 3c were highly reproducible (see the figure caption). We note that aforementioned annealing has improved ΦUC by ca. 38% and decreased Ith by ca. 34% for samples prepared with ΔT = 20 °C (Fig. S15, ESI†) whereas the appearance of the films did not change.
To test the photostability, we irradiated the sample with laser light at λ = 440 nm at an intensity of 30 mW cm−2, which was well above Ith (cf.Fig. 3b). Despite the fact that we carried out this test in air, the sample exhibited an outstanding photostability of >100 h (Fig. 3d), which is the longest record of photostability ever reported for TTA–UC to the best of our knowledge.
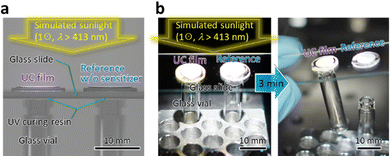
Finally, we exemplify a utility of this material. As graphically indicated in Fig. 4a, we applied a small quantity of UV-curing resin (BD-SKCJ, Bondic) to the mouth of two empty glass vials (outer diameter: 8 mm, height: 35 mm) and placed a glass slide onto them. Then, on the position of each vial's mouth, we placed the substrate on which the film was made with ΔT = 20 °C with and without including the sensitizer, termed “UC film” and “reference,” respectively. After 3 min irradiation of 1 ⊙ light from the solar simulator with a λ > 413 nm long-pass filter, only the vial placed under the UC film was bonded to the glass slide, whereas that placed under the reference was not (Fig. 4b); we have confirmed reproducibility of this result. This demonstration conducted in air indicates a practical utility of the UV–UC films developed in this work.
Conclusions
For the first time, we created polycrystalline UV–UC films exhibiting a record-long photostability (>100 h), subsolar excitation threshold (ca. 0.3 ⊙), and high efficiency (up to ΦUC = 4.3% and ηUC = 8.6%) in air by a solvent-free method that used a controlled temperature gradient. We revealed the impact of ΔT on the film properties and elucidated an essential importance of optimizing the temperature profile as well as the cooling speed. This novel class of UV-light-generating materials will substantially broaden the utility of ubiquitous terrestrial sunlight towards the extensive domain of UV-light-necessitating areas.Author contributions
Y. M. conceived the idea and led this project. R. E. and Y. M. designed and developed the apparatus. R. E. conducted all of the experiments. Both authors wrote the manuscript and agreed with this submission.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by JSPS KAKENHI Grant Numbers JP17H03183 and JP20H02082 (Y. M.).Notes and references
- T. Takata, J. Jiang, Y. Sakata, M. Nakabayashi, N. Shibata, V. Nandal, K. Seki, T. Hisatomi and K. Domen, Nature, 2020, 581, 411 CrossRef CAS PubMed; H. Nishiyama, T. Yamada, M. Nakabayashi, Y. Maehara, M. Yamaguchi, Y. Kuromiya, Y. Nagatsuma, H. Tokudome, S. Akiyama, T. Watanabe, R. Narushima, S. Okunaka, N. Shibata, T. Takata, T. Hisatomi and K. Domen, Nature, 2021, 598, 304 CrossRef PubMed; Y. Fang, Y. Hou, X. Fu and X. Wang, Chem. Rev., 2022, 122, 4204 CrossRef PubMed; H. Song, S. Luo, H. Huang, B. Deng and J. Ye, ACS Energy Lett., 2022, 7, 1043 CrossRef.
- Y. Xu, S. Wang, J. Yang, B. Han, R. Nie, J. Wang, J. Wang and H. Jing, Nano Energy, 2018, 51, 442 CrossRef CAS; J. Low, B. Dai, T. Tong, C. Jiang and J. Yu, Adv. Mater., 2019, 31, 1802981 CrossRef; A. Behera, A. K. Kar and R. Srivastava, Mater. Horiz., 2022, 9, 607 RSC.
- G. Barroso, Q. Li, R. K. Bordia and G. Motz, J. Mater. Chem. A, 2019, 7, 1936 RSC; H. Samadian, H. Maleki, Z. Allahyari and M. Jaymand, Coord. Chem. Rev., 2020, 420, 213432 CrossRef CAS.
- M. Raeiszadeh and B. Adeli, ACS Photonics, 2020, 7, 2941 CrossRef CAS.
- National Renewable Energy Laboratory, Reference Air Mass 1.5 Spectra, ASTM G–173–03, https://www.nrel.gov/grid/solar-resource/spectra-am1.5.html, accessed on October 22, 2022.
- S. Baluschev, T. Miteva, V. Yakutkin, G. Nelles, A. Yasuda and G. Wegner, Phys. Rev. Lett., 2006, 97, 143903 CrossRef CAS; T. N. Singh-Rachford and F. N. Castellano, Coord. Chem. Rev., 2010, 254, 2560 CrossRef; V. Gray, D. Dzebo, M. Abrahamsson, B. Albinsson and K. Moth-Poulsen, Phys. Chem. Chem. Phys., 2014, 16, 10345 RSC; T. F. Schulze and T. W. Schmidt, Energy Environ. Sci., 2015, 8, 103 RSC; P. Bharmoria, H. Bildirir and K. Moth-Poulsen, Chem. Soc. Rev., 2020, 49, 6529 RSC; A. Vasilev, R. Dimitrova, M. Kandinska, K. Landfester and S. Baluschev, J. Mater. Chem. C, 2021, 9, 7119 RSC; J. K. Gallaher, K. M. Wright, L. Frazer, R. W. MacQueen, M. J. Crossley, F. N. Castellano and T. W. Schmidt, Energy Environ. Sci., 2021, 14, 5541 RSC; F. Edhborg, A. Olesund and B. Albinsson, Photochem. Photobiol. Sci., 2022, 21, 1143 CrossRef PubMed.
- A. Monguzzi, R. Tubino, S. Hoseinkhani, M. Campione and F. Meinardi, Phys. Chem. Chem. Phys., 2012, 14, 4322 RSC.
- T. N. Singh-Rachford and F. N. Castellano, J. Phys. Chem. A, 2009, 113, 5912 CrossRef CAS.
- M. Majek, U. Faltermeier, B. Dick, R. Pérez-Ruiz and A. J. von Wangelin, Chem. – Eur. J., 2015, 21, 15496 CrossRef CAS PubMed; H. L. Lee, M. S. Lee, H. Park, W. S. Han and J. H. Kim, Korean J. Chem. Eng., 2019, 36, 1791 CrossRef; Y. Murakami, A. Motooka, R. Enomoto, K. Niimi, A. Kaiho and N. Kiyoyanagi, Phys. Chem. Chem. Phys., 2020, 22, 27134 RSC; A. Olesund, J. Johnsson, F. Edhborg, S. Ghasemi, K. Moth-Poulsen and B. Albinsson, J. Am. Chem. Soc., 2022, 144, 3706 CrossRef PubMed; T. J. B. Zähringer, M. S. Bertrams and C. Kerzig, J. Mater. Chem. C, 2022, 10, 4568 RSC; Y. Wei, K. Pan, X. Cao, Y. Li, X. Zhou and C. Yang, CCS Chem., 2022, 4, 3852 CrossRef.
- N. Harada, Y. Sasaki, M. Hosoyamada, N. Kimizuka and N. Yanai, Angew. Chem., Int. Ed., 2021, 60, 142 CrossRef CAS PubMed.
- M. Uji, N. Harada, N. Kimizuka, M. Saigo, K. Miyata, K. Onda and N. Yanai, J. Mater. Chem. C, 2022, 10, 4558 RSC.
- P. Duan, N. Yanai and N. Kimizuka, Chem. Commun., 2014, 50, 13111 RSC; N. Yanai, M. Kozue, S. Amemori, R. Kabe, C. Adachi and N. Kimizuka, J. Mater. Chem. C, 2016, 4, 6447 RSC; V. Gray, P. Xia, Z. Huang, E. Moses, A. Fast, D. A. Fishman, V. I. Vullev, M. Abrahamsson, K. Moth-Poulsen and M. L. Tang, Chem. Sci., 2017, 8, 5488 RSC; M. Barawi, F. Fresno, R. Pérez-Ruiz and V. A. de la Pena O’Shea, ACS Appl. Energy Mater., 2019, 2, 207 CrossRef CAS; S. He, X. Luo, X. Liu, Y. Li and K. Wu, J. Phys. Chem. Lett., 2019, 10, 5036 CrossRef PubMed; B. Pfund, D. M. Steffen, M. R. Schreier, M. S. Bertrams, C. Ye, K. Börjesson, O. S. Wenger and C. Kerzig, J. Am. Chem. Soc., 2020, 142, 10468 CrossRef; L. Hou, A. Olesund, S. Thurakkal, X. Zhang and B. Albinsson, Adv. Funct. Mater., 2021, 31, 2106198 CrossRef; X. Lin, Z. Chen, Y. Han, C. Nie, P. Xia, S. He, J. Li and K. Wu, ACS Energy Lett., 2022, 7, 914 CrossRef.
- P. E. Keivanidis, S. Baluschev, T. Miteva, G. Nelles, U. Scherf, A. Yasuda and G. Wegner, Adv. Mater., 2003, 15, 2095 CrossRef CAS; S. Baluschev, P. E. Keivanidis, G. Wegner, J. Jacob, A. C. Grimsdale and K. Müllen, Appl. Phys. Lett., 2005, 86, 061904 CrossRef; R. R. Islangulov, J. Lott, C. Weder and F. N. Castellano, J. Am. Chem. Soc., 2007, 129, 12652 CrossRef; A. Monguzzi, R. Tubino and F. Meinardi, J. Phys. Chem. A, 2009, 113, 1171 CrossRef; T. N. Singh-Rachford, J. Lott, C. Weder and F. N. Castellano, J. Am. Chem. Soc., 2009, 131, 12007 CrossRef PubMed; Y. C. Simon and C. Weder, J. Mater. Chem., 2012, 22, 20817 RSC.
- P. B. Merkel and J. P. Dinnocenzo, J. Lumin., 2009, 129, 303 CrossRef CAS.
- M. Wu, D. N. Congreve, M. W. B. Wilson, J. Jean, N. Geva, M. Welborn, T. V. Voorhis, V. Bulović, M. G. Bawendi and M. A. Baldo, Nat. Photonics, 2016, 10, 31 CrossRef CAS; V. Gray, K. Moth-Poulsen, B. Albinsson and M. Abrahamsson, Coord. Chem. Rev., 2018, 362, 54 CrossRef; B. Joarder, N. Yanai and N. Kimizuka, J. Phys. Chem. Lett., 2018, 9, 4613 CrossRef; K. M. Felter, M. C. Fravventura, E. Koster, R. D. Abellon, T. J. Savenije and F. C. Grozema, ACS Energy Lett., 2020, 5, 124 CrossRef PubMed; S. Izawa and M. Hiramoto, Nat. Photonics, 2021, 15, 895 CrossRef; J. Alves, J. Feng, L. Nienhaus and T. W. Schmidt, J. Mater. Chem. C, 2022, 10, 7783 RSC; S. E. Seo, H. S. Choe, H. Cho, H. Kim, J. H. Kim and O. S. Kwon, J. Mater. Chem. C, 2022, 10, 4483 RSC; L. Wei, C. Fan, M. Rao, F. Gao, C. He, Y. Sun, S. Zhu, Q. He, C. Yang and W. Wu, Mater. Horiz., 2022, 9, 3048 RSC.
- S. H. Lee, J. R. Lott, Y. C. Simon and C. Weder, J. Mater. Chem. C, 2013, 1, 5142 RSC; R. Vadrucci, C. Weder and Y. C. Simon, J. Mater. Chem. C, 2014, 2, 2837 RSC; R. Karpicz, S. Puzinas, V. Gulbinas, A. Vakhnin, A. Kadashchuk and B. P. Rand, Chem. Phys., 2014, 429, 57 CrossRef CAS; H. Goudarzi and P. E. Keivanidis, J. Phys. Chem. C, 2014, 118, 14256 CrossRef; M. Hosoyamada, N. Yanai, T. Ogawa and N. Kimizuka, Chem. – Eur. J., 2016, 22, 2060 CrossRef; S. Raišys, K. Kazlauskas, S. Juršėnas and Y. C. Simon, ACS Appl. Mater. Interfaces, 2016, 8, 15732 CrossRef; K. Kamada, Y. Sakagami, T. Mizokuro, Y. Fujiwara, K. Kobayashi, K. Narushima, S. Hirata and M. Vacha, Mater. Horiz., 2017, 4, 83 RSC; K. Kamada, Y. Sakagami, T. Mizokuro, Y. Fujiwara, K. Kobayashi, K. Narushima, S. Hirata and M. Vacha, Mater. Horiz., 2018, 5, 1219 RSC (correction); H. Goudarzi and P. E. Keivanidis, ACS Appl. Mater. Interfaces, 2017, 9, 845 CrossRef; T. Ogawa, M. Hosoyamada, B. Yurash, T. Q. Nguyen, N. Yanai and N. Kimizuka, J. Am. Chem. Soc., 2018, 140, 8788 CrossRef PubMed; T. Ogawa, N. Yanai, H. Kouno and N. Kimizuka, J. Photonics Energy, 2018, 8, 022003 Search PubMed; A. Abulikemu, Y. Sakagami, C. Heck, K. Kamada, H. Sotome, H. Miyasaka, D. Kuzuhara and H. Yamada, ACS Appl. Mater. Interfaces, 2019, 11, 20812 CrossRef PubMed; E. M. Rigsby, T. Miyashita, D. A. Fishman, S. T. Roberts and M. L. Tang, RSC Adv., 2021, 11, 31042 RSC; S. Raišys, O. Adomėnienė, P. Adomėnas, A. Rudnick, A. Köhler and K. Kazlauskas, J. Phys. Chem. C, 2021, 125, 3764 CrossRef; N. Tripathi, M. Ando, T. Akai and K. Kamada, J. Mater. Chem. C, 2022, 10, 4563 RSC.
- R. Enomoto, M. Hoshi, H. Oyama, H. Agata, S. Kurokawa, H. Kuma, H. Uekusa and Y. Murakami, Mater. Horiz., 2021, 8, 3449 RSC.
- X. Jiang, X. Guo, J. Peng, D. Zhao and Y. Ma, ACS Appl. Mater. Interfaces, 2016, 8, 11441 CrossRef CAS.
- D. P. Specht, P. A. Martic and S. Farid, Tetrahedron, 1982, 38, 1203 CrossRef CAS.
- D. Huang, J. Sun, L. Ma, C. Zhang and J. Zhao, Photochem. Photobiol. Sci., 2013, 12, 872 CrossRef CAS.
- G. Liu, J. Liu, A. S. Dunn, P. Nadazdy, P. Siffalovic, R. Resel, M. Abbas, G. Wantz and Y. H. Geerts, Cryst. Growth Des., 2021, 21, 5231 CrossRef CAS.
- H. M. Rietveld, Acta Crystallogr., 1967, 22, 151 CrossRef CAS; G. S. Pawley, J. Appl. Crystallogr., 1981, 14, 357 CrossRef.
- Y. Murakami and K. Kamada, Phys. Chem. Chem. Phys., 2021, 23, 18268 RSC.
- Y. Zhou, F. N. Castellano, T. W. Schmidt and K. Hanson, ACS Energy Lett., 2020, 5, 2322 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tc04578h |
| ‡ Low crystallinity of organic UC solids was often resulted from the use of rapid solidification (or kinetically controlled) approaches, which were used to mitigate aggregation of the sensitizer species during formation of bicomponent organic solids.7,16 See our previous paper17 for detailed discussion on this point. |
| This journal is © The Royal Society of Chemistry 2023 |