Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Concentrations and isomer profiles of hexabromocyclododecanes (HBCDDs) in floor, elevated surface, and outdoor dust samples from Basrah, Iraq†
Layla Salih
Al-Omran
 ab,
William A.
Stubbings
ab,
William A.
Stubbings
 *b and
Stuart
Harrad
*b and
Stuart
Harrad
 b
b
aDepartment of Chemistry, College of Science, University of Basrah, Basrah, Iraq
bSchool of Geography, Earth, and Environmental Sciences, University of Birmingham, Birmingham, B15 2TT, UK. E-mail: w.stubbings@bham.ac.uk
First published on 30th May 2022
Abstract
Concentrations of the α, β, and γ- diastereomers of hexabromocyclododecane (α-, β-, and γ-HBCDD) were measured in 60 dust samples from 20 homes across Basrah, Iraq. From each home, two indoor dust (ID) samples (specifically one collected from elevated surfaces (ESD) and one from the floor (FD)) were collected from the living room, with one outdoor dust (OD) sample collected from the front yard of the house. Concentrations of HBCDDs decreased in the following sequence ESD > FD > OD. For ID, ΣHBCDD concentrations varied from 5.3 ng g−1 in FD to 150 ng g−1 in ESD, with median levels of 60 and 40 ng g−1 in ESD and FD respectively. Concentrations of γ-HBCDD, and consequently of ΣHBCDDs in ESD, significantly (p < 0.05) exceeded those in FD. For adults, this implies that exposure assessments based on FD only may underestimate exposure, as adults are more likely to ingest ESD. Concentrations of ΣHBCDDs in OD ranged between 7.4 and 120 ng g−1 with a median of 35 ng g−1 and were significantly exceeded (p < 0.05) by those in ID samples. Concentrations of ΣHBCDDs in OD from houses with car parking areas exceeded (p < 0.05) those in OD from other homes, implying vehicles as potential emission sources of HBCDDs. Simultaneously, there was moderate correlation (R = 0.510–0.609, p < 0.05) between concentrations in ID and OD, implying that the indoor environment is an important source of OD contamination. The isomer pattern of HBCDDs in dust samples displayed a predominance of α-HBCDD, which represented 56%, 52% and 59% ΣHBCDD in ESD, FD and OD samples respectively. Derived from the concentrations reported in this study, the median and 95th percentile estimated daily intakes (EDI) for Iraqi adults and toddlers through house dust ingestion did not exceed the reference dose (RfD) value for HBCDD.
Environmental significanceBecause of its persistent and bioaccumulative properties, hexabromocyclododecane (HBCDD) has been listed in the Stockholm convention since 2013, including specific exemptions for the production and use in EPS and XPS foams. Ingestion of indoor dust constitutes an important pathway of exposure to HBCDDs. This study reveals that concentrations of HBCDD in dust collected from elevated surfaces were significantly higher than those in floor dust from the same rooms. This implies considerable uncertainty in human exposure assessment, particularly for adults, as they are likely more exposed to elevated surface dust. Our data represent a valuable baseline against which responses to actions designed to limit exposure to HBCDD may be evaluated in the future. |
1. Introduction
Hexabromocyclododecanes (HBCDDs) are cyclic aliphatic additive brominated flame retardants (BFRs). The main application of HBCDDs is in extruded (XPS) and expanded (EPS) polystyrene foams as thermal insulation in buildings, with another important application being in textiles used in upholstered furniture, curtains, wall coverings, and transportation seating. Other applications of HBCDDs include adhesives and paints, as well as in high-impact polystyrene (HIPS) and styrene–acrylonitrile resins employed in casings for electrical and electronic equipment.1–3 Due to bans and restrictions on another widely-used class of BFRs – polybrominated diphenyl ethers (PBDEs), HBCDDs were increasingly used as a flame retardant a substitute for penta- and octa -BDEs.4–7 The commercial mixtures of HBCDDs consist of three main stereoisomers that include γ-HBCDD (75–89%), α-HBCDD (10–13%) and β-HBCDD (1–12%). In addition, δ- and ε-HBCDD are present at minor concentrations in the commercial mixture.1,8,9 The HBCDD mixture is characterized by low vapour pressure (6 × 10−5 Pa at 21 °C), water solubility (3.4 μg L−1 at 20 °C), and high affinity to particulate matter and environmental lipids as exemplified by its log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW of 4.78.9,10
KOW of 4.78.9,10
Since the late 1960s, HBCDD has been on the world market, produced mainly in China, the United States of America, and the European Union. The global production of HBCDDs increased rapidly from 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 tonnes in 2001 to 22
700 tonnes in 2001 to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes in 2006, and 31
000 tonnes in 2006, and 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes in 2011.9,11,12 Because of its persistent and bio-accumulative properties, HBCDD has been listed in Annex A of the Stockholm convention since May 2013. However, the listing includes specific exemptions for the production and use of HBCDDs in EPS and XPS foams in building materials13, where use is still allowed until 2025.14
000 tonnes in 2011.9,11,12 Because of its persistent and bio-accumulative properties, HBCDD has been listed in Annex A of the Stockholm convention since May 2013. However, the listing includes specific exemptions for the production and use of HBCDDs in EPS and XPS foams in building materials13, where use is still allowed until 2025.14
As additive flame retardants and semi-volatile organic compounds (SVOCs), HBCDDs are not chemically bound to the polymeric products and emissions are likely during production, use, disposal and recycling processes.1,15 It has been reported that HBCDDs can leach from textiles even at room temperature.16 As a result, they are globally distributed in various environmental samples such as indoor and outdoor air,17–19 surface water,5,20,21 wastewater and sewage sludge,22,23 sediments,24,26 soil and road dust,6,27,28 indoor dust,19,29,30 food,31–33 and biota.34,35 In addition, several biomonitoring studies have detected HBCDDs in human tissues such as human milk,36–38 serum,39,40 and hair,41,42 indicating human exposure to HBCDDs. The main routes via which such exposure occurs are: inhalation,43 diet,31,44 dust ingestion,45–47 and dermal absorption.48,49 Based on animal test results, exposure to HBCDDs are reported to cause different toxicological effects and present potential human health concerns. The main targets for HBCDDs toxicity are: the liver, thyroid hormones, the immune and reproductive systems, as well as neurodevelopmental concerns.14,15,50–52
Indoor dust intake constitutes an important pathway of exposure to HBCDDs, particularly for young children due to the large time fraction spent indoors, small body mass and extensive hand-to-mouth behaviour.53–56 For example, in worst case scenarios, dust ingestion represents 95% of toddler intake for HBCDDs.57 The importance of this pathway is emphasised by the significant correlation between exposure to HBCDDs via dust ingestion and its concentration in serum.58 HBCDDs migrate from products into indoor dust via volatilisation, abrasion, and direct contact between dust and the surface of products.16,59,60 A large number of studies have reported various levels of HBCDD in indoor dust from different microenvironments such as homes,61–64 offices,65,66 schools67,68 and cars.69,70
Most studies of exposure via indoor dust measure HBCDDs based on floor dust only, however, for other SVOCs, significant differences between concentrations in elevated surface dust (i.e. dust from surfaces above floor level, such as shelves, tables, sofas etc.) and floor dust from the same microenvironment have been reported.71–78 In general, concentrations of PBDEs, NBFRs (“novel” brominated flame retardants) and OPEs (organophosphate esters) in ESD are significantly higher than those in FD.79 This implies considerable uncertainty in human exposure assessment, particularly for adults, as they are likely more exposed to ESD due to greater contact with elevated surfaces compared to floors.75 Nevertheless, no available data have compared HBCDD concentrations between ESD and FD specifically from homes. To our knowledge, only one study has examined HBCDDs in both ESD and FD collected from various US microenvironments (a bus, scientific laboratory, computer laboratory, gymnasium, and two each of domestic apartments, classrooms, and offices). However, despite the small sample size (n = 20 in total comprising paired ESD and FD samples from 10 microenvironments), this study revealed median concentrations of α-HBCDD and γ-HBCDD in ESD exceeded those in FD by factors of 4 and 1.6 respectively.80 In addition, many studies focusing on human exposure to flame retardants via dust ingestion have indicated that exposure to outdoor dust, such as road dust, is not negligible.81,82 In other words, it can be concluded that both indoor and outdoor dust will be valuable for human risk assessments. Considerable levels of HBCDDs were found in road and street dust from different areas, such as agricultural, residential, industrial27 and e-waste recycling areas,28 suggesting that indoor environments and industrial activities are important sources of HBCDDs to outdoor environments. Nonetheless, to the best of our knowledge, so far, no investigation has attempted to measure concentrations of HBCDDs in paired indoor/outdoor dust samples from the same microenvironment to assess to what extent indoor environments may contribute to outdoor contamination. From our previous study of the most widespread OPEs, significant correlations were found in their concentrations between indoor and outdoor locations from the same house.83 Furthermore, despite a large number of studies that measured human exposure to HBCDDs via indoor dust ingestion, only a limited number have been performed in the Middle East.84,85 This study is the first to report HBCDD concentrations in dust samples from Iraq. Basrah (or Basra) province was selected for dust sampling because it is one of the important cities in the Middle East, a major port, a main economic city, particularly in the oil industry and the second-largest city by population in Iraq.
Given this background, the aims of this work are to: (1) determine the concentrations of three HBCDD diastereomers (α-HBCDD, β-HBCDD, and γ-HBCDD) in dust samples from Basrah, Iraq to provide an indication of the use of these chemicals in Iraq; (2) examine within-room spatial variability of HBCDDs in dust samples taken from elevated surfaces and floor dust from the same microenvironment, to facilitate assessment of the impact of any such variation on human exposure assessment; (3) determine the relationship between concentrations of HBCDDs in house dust and dust from outdoor (house entrance) surfaces adjoining the sampled houses, to assess to what extent that HBCDD outdoor contamination is influenced by indoors; and (4) provide the first evaluation of the exposure of the Iraqi population to HBCDDs via ingestion of indoor and outdoor settled dust.
2. Materials and methods
2.1. Sampling
Details of how samples of indoor and outdoor dust were collected during September–November of 2019 are given in our previous study.83 In brief, from 20 urban houses in Basrah, Iraq, 60 dust samples were collected using a vacuum cleaner with a nylon sock (25 μm pore size) inserted into the nozzle of the vacuum. From each house, three different types of dust were collected: two indoor dust (ID) samples comprising one floor dust (FD) and one elevated surface dust (ESD) sample, along with one outdoor dust (OD) sample. FD samples were collected from the most frequently used floor areas in the main living room, while ESD was collected from available elevated surfaces such as tables, shelves, and chairs in the same living room for 2–4 min.75,86 OD samples were vacuumed from available areas next to the wall and surfaces above the ground in the main house entrance area which represents the front yard of the house located between the house and the outer boundary of the property. After sampling, socks were closed, sealed in a plastic bag and stored at −20 °C. Characteristics of the participating houses such as floor material, furniture, electronic equipment, ventilation system and the presence of car parking area contiguous with the entrance of the house were recorded in the ESI (Table S1†).2.2. Sample preparation, extraction, and clean-up
Dust samples were analysed at the University of Birmingham, UK. The sample extraction and clean-up methods were described in detail elsewhere with slight modification.87 Briefly, dust samples were passed through a stainless steel 125 μm mesh size sieve, weighed accurately (50–75 mg) and spiked with 50 ng of 13C-α-HBCDD, 13C-β-HBCDD and 13C-γ-HBCDD as internal standards. Two mL of extraction solvent (n-hexane: acetone, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v ratio) were added to each sample, vortexed for 2 min, sonicated for 5 min, centrifuged for 5 min at 3500 rev per min and the supernatant transferred to a separate tube. The extraction process was repeated three times with all extracts combined, evaporated to incipient dryness, and resolubilised in 1 mL of hexane. The concentrated extract was fractionated into two fractions (F1 and F2) using Florisil cartridges. F1 (containing some HBCDDs) was eluted with 8 mL n-hexane, with F2 (containing the remainder of the HBCDDs, as well as OPEs (data reported elsewhere83)) eluted with 10 mL ethyl acetate. F1 was evaporated to 1 mL and transferred onto 2 g acidified silica 44% cartridges, eluted with 10 mL of Hex/DCM (1
1, v/v ratio) were added to each sample, vortexed for 2 min, sonicated for 5 min, centrifuged for 5 min at 3500 rev per min and the supernatant transferred to a separate tube. The extraction process was repeated three times with all extracts combined, evaporated to incipient dryness, and resolubilised in 1 mL of hexane. The concentrated extract was fractionated into two fractions (F1 and F2) using Florisil cartridges. F1 (containing some HBCDDs) was eluted with 8 mL n-hexane, with F2 (containing the remainder of the HBCDDs, as well as OPEs (data reported elsewhere83)) eluted with 10 mL ethyl acetate. F1 was evaporated to 1 mL and transferred onto 2 g acidified silica 44% cartridges, eluted with 10 mL of Hex/DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), evaporated until dryness and then reconstituted in n-hexane. Following analysis of F2 for OPEs,83 F1 and F2 were combined and evaporated to incipient dryness before reconstitution in 100 μL methanol containing 50 ng d18-γ-HBCDD as recovery determination standard.
1, v/v), evaporated until dryness and then reconstituted in n-hexane. Following analysis of F2 for OPEs,83 F1 and F2 were combined and evaporated to incipient dryness before reconstitution in 100 μL methanol containing 50 ng d18-γ-HBCDD as recovery determination standard.
2.3. Instrumental analysis
α-, β- and γ- HBCDDs were separated and analysed using LC-MS/MS. The equipment used was a dual pump Shimadzu LC-20AB Prominence liquid chromatograph (Shimadzu, Kyoto, Japan) equipped with a SIL-20A autosampler, a DGU-20A3 vacuum degasser, and a Varian Pursuit XRS3 C18 reversed phase analytical column (150 mm × 4.6 mm i.d., 3 μm particle size). A mobile phase program based upon (mobile phase A) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol/water and (mobile phase B) methanol at a flow rate of 0.18 mL min−1. The elution program was started at 50% (B) then increased linearly to 100% (B) over 4 min, held for 9 min followed by a linear decrease to 88% (B) over 10 min, held for 0.1 min and ending with 50% (A) for 14 min. The three HBCDD stereoisomers were baseline separated with retention times of 10.8, 11.4 and 11.9 min for α-, β- and γ-HBCDD, respectively.
1 methanol/water and (mobile phase B) methanol at a flow rate of 0.18 mL min−1. The elution program was started at 50% (B) then increased linearly to 100% (B) over 4 min, held for 9 min followed by a linear decrease to 88% (B) over 10 min, held for 0.1 min and ending with 50% (A) for 14 min. The three HBCDD stereoisomers were baseline separated with retention times of 10.8, 11.4 and 11.9 min for α-, β- and γ-HBCDD, respectively.
Mass spectrometric analysis was performed using a Sciex API 2000 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA) equipped with an ESI ion source operated in negative ionisation mode. MS/MS detection operated in the multiple reaction monitoring (MRM) mode was used for the quantitative determination of the target compounds based on m/z 640.6 → 78.9, m/z 652.4 → 79.0 and m/z 657.6 → 78.9 for the native, 13C-labelled and d18-labelled HBCDD diastereomers, respectively.
2.4. Quality assurance/quality control
All the test tubes were ultrasonicated and Pasteur Pipettes rinsed with n-hexane and baked at 450 °C overnight before use. To assess any possible contamination during sample preparation and analysis, one laboratory blank was conducted in with every set of 6 dust samples. In total 12 blanks were analysed. All target HBCDD isomers in the blanks were below the limit of detection (LOD), and hence data are not corrected for blank concentrations. Similar results were obtained for field blanks (n = 3). LOD was estimated based on a signal to noise ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with limits of quantification (LOQ) estimated based on signal to noise ratio of 10
1, with limits of quantification (LOQ) estimated based on signal to noise ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (Table S2,† ESI). For on-going evaluation of the accuracy and precision of the analytical method, an aliquot of SRM-2585 organic contaminants in house dust was analysed with each batch of 10 dust samples. The results show good agreement with previously reported indicative concentrations of HBCDDs (Keller et al., 2007)88 (Table S3†). Recoveries (average ± standard deviation) of the 13C-labelled internal standards added to dust samples were: α-HBCDD = 76 ± 14%, β-HBCDD = 83 ± 12%, and γ-HBCDD = 86 ± 15%.
1, (Table S2,† ESI). For on-going evaluation of the accuracy and precision of the analytical method, an aliquot of SRM-2585 organic contaminants in house dust was analysed with each batch of 10 dust samples. The results show good agreement with previously reported indicative concentrations of HBCDDs (Keller et al., 2007)88 (Table S3†). Recoveries (average ± standard deviation) of the 13C-labelled internal standards added to dust samples were: α-HBCDD = 76 ± 14%, β-HBCDD = 83 ± 12%, and γ-HBCDD = 86 ± 15%.
2.5. Statistical analysis
Statistical analysis of the data was conducted using Excel (Microsoft Office 2019) and IBM SPSS statistics software (V. 20). Due to the skewness of the data, all concentration data were first log-transformed to achieve normal distributions. For the purposes of statistical evaluation, all concentrations below LOQ were assigned a value of 0.5 LOQ. ΣHBCDD indicates summation of α-, β- and γ-HBCDD. Potential correlations between various parameters were investigated using Pearson's correlation. Results were considered significant where P values were less than 0.05.3. Results and discussion
3.1. Levels and distribution profiles of HBCDDs in dust samples
The three HBCDD stereoisomers were detected in at least 70–80% of samples for α- and β- HBCDDs, while γ-HBCDD was detected in all indoor and outdoor dust samples. The detection frequency, mean, median, minimum, maximum, 5th percentile and 95th percentile concentrations of α-, β-, γ-HBCDD and ΣHBCDDs in ESD, FD, and OD samples are shown in Table 1. The concentrations of the three stereoisomers and ΣHBCDDs in each analysed sample can be found in Tables S4–S6.† Overall, the highest concentrations of ΣHBCDDs were observed in ESD samples ranging from 17 to 150 ng g−1 with an average of 65 ng g−1 followed by FD and OD with averages of 46 and 38 ng g−1, respectively. Compared with our previous studies of indoor dust in Basrah, Iraq,75,83 concentrations of HBCDDs were substantially lower than those of PBDEs and OPEs, indicating the limited application of HBCDD in Iraqi homes. Based on the recorded information, only three of the participating houses were insulated using polystyrene foam boards as thermal insulation. Thus, it is likely that the main sources of HBCDDs in Iraqi houses are textiles, as well as electrical and electronic equipment.| α-HBCDD | β-HBCDD | γ-HBCDD | ΣHBCDDs | |
|---|---|---|---|---|
| Elevated surface dust (ESD) | ||||
| Average | 36 | 6.6 | 22 | 65 |
| Median | 35 | 7.0 | 20 | 60 |
| Min | 0.66 | 0.66 | 9.2 | 17 |
| Max | 100 | 17 | 39 | 150 |
| SD | 26 | 4.9 | 10 | 35 |
| DF% | 85 | 70 | 100 | 100 |
| 5% | 0.66 | 0.66 | 10 | 22 |
| 95% | 77 | 13 | 38 | 120 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Floor dust (FD) | ||||
| Average | 24 | 6.3 | 16 | 46 |
| Median | 23 | 6.3 | 12 | 40 |
| Min | 0.66 | 0.66 | 4.1 | 5.3 |
| Max | 64 | 17 | 41 | 120 |
| SD | 18 | 4.5 | 11 | 32 |
| DF% | 80 | 80 | 100 | 100 |
| 5% | 0.66 | 0.66 | 4.5 | 9.2 |
| 95% | 55 | 12 | 40 | 100 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Outdoor dust (OD) | ||||
| Average | 22 | 4.8 | 11 | 38 |
| Median | 19 | 4.4 | 8.4 | 35 |
| Min | 0.66 | 0.66 | 4.2 | 7.4 |
| Max | 75 | 13 | 32 | 120 |
| SD | 20 | 3.9 | 6.9 | 28 |
| DF% | 80 | 70 | 100 | 100 |
| 5% | 0.66 | 0.66 | 4.3 | 10 |
| 95% | 60 | 11 | 22 | 86 |
The concentrations of HBCDDs in house dust samples detected in this study were comparable with those in indoor dust from Vietnamese houses (median ΣHBCDDs = 29 ng g−1),89 but higher than those in indoor dust from Egypt (median 6.2 ng g−1)84 and residential homes surrounding e-waste recycling workshops from China (median = 19 ng g−1).28 By comparison with other regions, median concentrations of ΣHBCDDs in Iraqi houses were lower than those reported from Europe and North America, and substantially lower than in house dust from France,70 Canada,53 and Ireland19 by factors of 34, 16, and 12 respectively (Table 2 and Fig. S1†). Most recently, the highest median concentration of ΣHBCDDs reported in indoor dust (4 homes, 2 offices and 3 schools) was 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 ng g−1 in Spain,68 while the highest dust concentration recorded globally was 570
400 ng g−1 in Spain,68 while the highest dust concentration recorded globally was 570![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng g−1 from a house in Birmingham, UK.64
000 ng g−1 from a house in Birmingham, UK.64
| Country | Sampling year | n | α-HBCDD | β-HBCDD | γ-HBCDD | ΣHBCDDs |
|---|---|---|---|---|---|---|
| a GM, n.a. = not available. | ||||||
| Iraq, this study | 2019 | 20 | 23 | 6.3 | 12 | 41 |
| Turkey85 | 2012 | 10 | n.a. | n.a. | n.a. | 250 |
| Egypt84 | 2013 | 17 | n.a. | n.a. | n.a. | 6.0 |
| Kazakhstan70 | 2009 | 10 | 78 | 20 | 190 | 280 |
| Japan46 | 2009–2010 | 10 | n.a. | n.a. | n.a. | 70 |
| Korea63 | 2011 | 42 | 140 | 12 | 60 | 280 |
| Vietnam89 | 2008 | 33 | 10 | 3.0 | 9.0 | 29 |
| China28 | 2016–2017 | 25 | 13 | 2.0 | 4.0 | 19 |
| China25 | 2014 | 30 | 64 | 21 | 64 | 150 |
| Nigeria70 | 2014 | 10 | 99 | 81 | 130 | 400 |
| UK64 | 2019 | 14 | 130 | 66 | 72 | 280 |
| France70 | 2008 | 9.0 | 600 | 140 | 420 | 1400 |
| Sweden103 | 2010 | 27 | 56 | 18 | 37 | 330 |
| New Zealand61 | 2009–2010 | 50 | 99 | 12 | 96 | 210 |
| Norway105 | 2014 | 61 | 94 | 23 | 43 | 190 |
| Germany90 | NR | 20 | 180 | 35 | 110 | 330 |
| Ireland19 | 2016–2017 | 32 | 200 | 100 | 200 | 490 |
| Portugal104 | 2010–2011 | 28 | 91 | 16 | 35 | 150 |
| Czech Republic69 | 2008 | 25 | 26 | 7.0 | 61 | 94 |
| Romania106 | 2010 | 47 | 200 | 40 | 30 | 270 |
| Belgium58 | 2007–2008 | 16 | 69 | 14 | 31 | 110 |
| USA52 | 2012 | 30 | 210a | 28a | 70a | 340a |
| Canada53 | 2006 | 8.0 | 300 | 72 | 230 | 640 |
| Antarctica107 | 2006 | 11 | 150 | 35 | 43 | 230 |
The isomer pattern of HBCDDs in the two indoor (ID) dust (ESD and FD) and OD samples collected from each house, displayed a predominance of α-HBCDD. On average, samples consisted of 56%, 52% and 59% α-HBCDD in ESD, FD and OD samples respectively (Fig. 1). Similar composition profiles were also found in indoor dust collected in the UK,64 France,70 Germany,90 the USA,62 China28 and Korea,63 where α-HBCDD represented 46%, 44%, 55%, 63%, 68%, and 52% of ΣHBCDDs respectively. A higher proportion of α-HBCDD (63–67%) in OD was observed in China.27,28,91 The proportion of γ-HBCDD in our dust samples was lower than in commercial HBCDD formulations (which are reported to consist of 75–89% γ-HBCDD).1,5,11 It has been reported that during the production or processing of materials containing HBCDDs, thermal rearrangement at elevated temperatures (>160 °C), will result in a substantially altered profile of HBCDDs (78%, 13%, and 9% for α-, β-, and γ-HBCDD respectively).1,92 In addition, experimental data on light-exposed dust samples show a significant shift from γ-HBCDD to α-HBCDD.29 This may explain the slightly higher ratio of α-HBCDDs in outdoor samples of the current study (59%), compared to that in ID (52–56%).
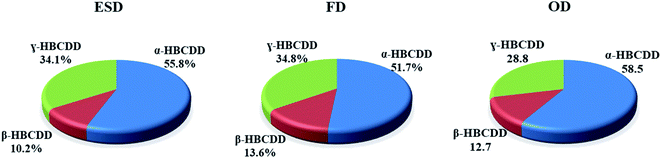 | ||
| Fig. 1 Average contribution profiles of α-, β- and γ-HBCDD to HBCDDs in elevated surface dust (ESD), floor dust (FD) and outdoor dust (OD) samples from Iraqi homes. | ||
3.2. Within-room spatial variability of HBCDDs in dust samples
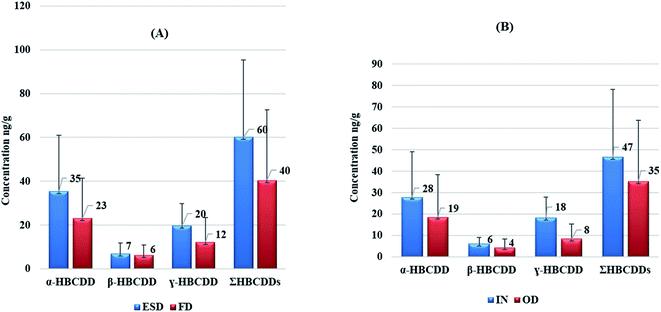
Statistically significant differences were observed between concentrations of HBCDDs in ESD and FD. A paired t-test revealed that concentrations of γ-HBCDD, and consequently ΣHBCDDs in ESD exceeded those in FD with p values of 0.001, and 0.002 respectively. In addition, median ESD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FD ratios of HBCDD stereoisomers were found to be 1.5, 1.1, 1.6, and 1.5 for α-HBCDD, β-HBCDD, γ-HBCDD, and ΣHBCDDs respectively. Fig. 2A illustrates within-room spatial variability in HBCDDs between ESD and FD. This is consistent with HBCDD concentrations from various microenvironments within a university campus in the USA, where the median ESD
FD ratios of HBCDD stereoisomers were found to be 1.5, 1.1, 1.6, and 1.5 for α-HBCDD, β-HBCDD, γ-HBCDD, and ΣHBCDDs respectively. Fig. 2A illustrates within-room spatial variability in HBCDDs between ESD and FD. This is consistent with HBCDD concentrations from various microenvironments within a university campus in the USA, where the median ESD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FD ratios exceeded 1.2.80 However, no significant differences were apparent between ESD and FD levels in that study, this is likely due to the small sample size that was collected from 10 different microenvironments. For other flame retardants such as PBDEs, NBFRs, and OPEs, significant differences in concentrations of such chemicals were apparent between ESD and FD in Japan,72 Norway,71 Iraq,75,83 UK,76 and China.93 This implies that HBCDDs are likely to have similar distribution patterns between ESD and FD from the same microenvironment. As SVOCs and additive flame retardants, HBCDDs can be released from treated products into the environment via volatilisation and subsequent sorption to dust particles depending on their vapour pressures and octanol–air partition coefficient (KOA).94 However, for low volatility compounds like HBCDDs, abrasion of fibres or particles from treated products and transfer via direct source-dust contact were identified as the dominant mechanisms of emission into the indoor environment.59,95 This may explain the higher concentrations of HBCDDs in ESD since most such samples were collected directly from sofas, tables, and shelves on which electronic devices were present. Pearson correlation analysis revealed concentrations of both α-HBCDD and γ-HBCDD in our ESD samples were significantly (p < 0.05) correlated with those in FD, with respective correlation coefficient values of 0.652, 0.682, and 0.718 for α-, γ-, and ΣHBCDDs, respectively. This implies a similar source of HBCDD to both ESD and FD samples.
FD ratios exceeded 1.2.80 However, no significant differences were apparent between ESD and FD levels in that study, this is likely due to the small sample size that was collected from 10 different microenvironments. For other flame retardants such as PBDEs, NBFRs, and OPEs, significant differences in concentrations of such chemicals were apparent between ESD and FD in Japan,72 Norway,71 Iraq,75,83 UK,76 and China.93 This implies that HBCDDs are likely to have similar distribution patterns between ESD and FD from the same microenvironment. As SVOCs and additive flame retardants, HBCDDs can be released from treated products into the environment via volatilisation and subsequent sorption to dust particles depending on their vapour pressures and octanol–air partition coefficient (KOA).94 However, for low volatility compounds like HBCDDs, abrasion of fibres or particles from treated products and transfer via direct source-dust contact were identified as the dominant mechanisms of emission into the indoor environment.59,95 This may explain the higher concentrations of HBCDDs in ESD since most such samples were collected directly from sofas, tables, and shelves on which electronic devices were present. Pearson correlation analysis revealed concentrations of both α-HBCDD and γ-HBCDD in our ESD samples were significantly (p < 0.05) correlated with those in FD, with respective correlation coefficient values of 0.652, 0.682, and 0.718 for α-, γ-, and ΣHBCDDs, respectively. This implies a similar source of HBCDD to both ESD and FD samples.
3.3. Within-home (between indoor and outdoor) spatial variability of HBCDDs in dust samples
A one-way repeated measures ANOVA test was performed to evaluate to what extent HBCDD concentrations in both ESD and FD exceeded those in OD samples. Our results revealed that γ-HBCDD and subsequently ΣHBCDDs in ESD were significantly higher than those in OD with p values of <0.001 and 0.02 respectively. A paired t-test also revealed that concentrations of β-HBCDD, γ-HBCDD, and ΣHBCDDs in ID (i.e. the average concentration in each house of ESD and FD) were significantly higher than those in OD samples with a p value of 0.008, <0.001, and 0.006 respectively. Furthermore, the median ratio of ID![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
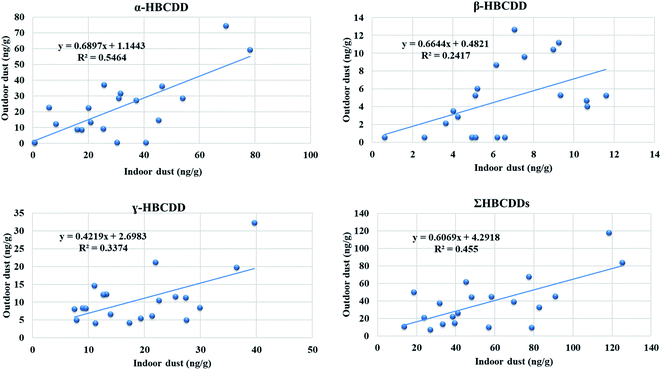
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OD concentrations for the three stereoisomers and ΣHBCDDs ranged between 1.1 for β-HBCDD and 1.6 for γ-HBCDD. Concentrations of our target HBCDDs in ID were moderately correlated with those in OD, with correlation coefficient values of 0.609, 0.551, and 0.509 for α-, β-HBCDD and ΣHBCDDs, respectively. This indicates that indoor sources may contribute significantly to concentrations of HBCDDs outdoors via windows, doors, mechanical ventilation systems, and shoes. It has been reported that HBCDD concentrations in soil decline on moving away from housing which was attributed to the decreasing influence of emissions from HBCDD-treated building insulation.96Fig. 2B demonstrates the observed within-home spatial variability between ID and OD and Fig. 3 illustrates correlations between concentrations of HBCDDs in IN and OD dust samples.
OD concentrations for the three stereoisomers and ΣHBCDDs ranged between 1.1 for β-HBCDD and 1.6 for γ-HBCDD. Concentrations of our target HBCDDs in ID were moderately correlated with those in OD, with correlation coefficient values of 0.609, 0.551, and 0.509 for α-, β-HBCDD and ΣHBCDDs, respectively. This indicates that indoor sources may contribute significantly to concentrations of HBCDDs outdoors via windows, doors, mechanical ventilation systems, and shoes. It has been reported that HBCDD concentrations in soil decline on moving away from housing which was attributed to the decreasing influence of emissions from HBCDD-treated building insulation.96Fig. 2B demonstrates the observed within-home spatial variability between ID and OD and Fig. 3 illustrates correlations between concentrations of HBCDDs in IN and OD dust samples.
It is worth noting that the outdoor locations sampled in this study are not on roads or streets, but the front yards of the investigated homes which are commonly used for parking vehicles. A paired t-test revealed that concentrations of ΣHBCDDs in OD from houses with front yards used as parking areas exceeded those in OD from other homes with a p value of 0.014 (Fig. S2†). This may indicate vehicles as potential emission sources of HBCDDs in parking areas. Several studies have reported high concentrations of HBCDDs in car dust samples45,70,97,98 with a higher relative abundance of γ-HBCDD. Further research is needed to investigate the influence of vehicles parked in front yards on the concentrations of HBCDDs and related chemicals in OD and ID environments.
3.4. Estimation of HBCDDs daily intake via dust ingestion
Measured concentrations of HBCDD in house dust were used to estimate exposure for the Iraqi population via oral ingestion. Two scenarios were considered, based on ingestion of median and 95th percentile concentrations at mean and high rates of 20 and 60 mg day−1 for adults, and 50 and 100 mg day−1 for toddlers, respectively.99 Estimated daily intakes via house dust ingestion (EDIingestion; ng kg−1 bw per day) were calculated as shown in the equation below and described elsewhere83,85,100| EDI = (Cdust × IRdust × AFgastro × FTH)/BW |
Table 3 summarises EDIs of HBCDDs via dust ingestion under various exposure scenarios. For adults, EDIs of ΣHBCDDs assuming median concentrations in dust and mean dust ingestion rates were between 0.00041 (assuming ingestion of OD only) and 0.0096 ng kg−1 bw per day (assuming ingestion of ESD only); while for toddlers, they were between 0.11 and 0.13 ng kg−1 bw per day assuming ingestion of OD and FD respectively. Assuming ingestion at the high intake rate of dust contaminated at the 95th percentile concentration (i.e. high-end exposure scenario); EDIs of ΣHBCDDs for adults were 0.058, 0.059, and 0.041 ng kg−1 bw per day, and for toddlers were 0.76, 0.64 and 0.53 ng kg−1 bw per day assuming ingestion of ESD, FD and OD respectively. This implies that compared with adults, EDIs of ΣHBCDDs for toddlers are substantially higher (by factors of 13–20) due to their lower body mass and greater assumed dust ingestion rate. However, these EDIs are uncertain as adults are more likely to ingest ESD, while toddlers are more likely to ingest FD. Taking this into account and assuming adults and toddlers ingest ESD and FD respectively, the EDIs of ΣHBCDDs for toddlers will exceed those of adults by approximately 8–13 times. Even under the high-end exposure scenario, our EDIs were orders of magnitude lower than the reference dose (RfD) value for HBCDD of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng kg−1 bw/day promulgated by the United States National Academy of Sciences (NAS).101 On this basis, the current study suggest HBCDD exposure of the Iraqi population via dust ingestion is not a health concern. As a cautionary note however, the NAS has expressed low confidence in this RfD for HBCDD, and in 2016, the UK's Committee on Toxicity reported that “high levels (of HBCDD) in household dust continues to be a cause for concern” and cited a toxicological reference point of 3000 ng kg−1 bw per day.102
000 ng kg−1 bw/day promulgated by the United States National Academy of Sciences (NAS).101 On this basis, the current study suggest HBCDD exposure of the Iraqi population via dust ingestion is not a health concern. As a cautionary note however, the NAS has expressed low confidence in this RfD for HBCDD, and in 2016, the UK's Committee on Toxicity reported that “high levels (of HBCDD) in household dust continues to be a cause for concern” and cited a toxicological reference point of 3000 ng kg−1 bw per day.102
| HBCDDs | ESD | FD | OD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adults | Toddlers | Adults | Toddlers | Adults | Toddlers | |||||||
| Median | 95th %ile | Median | 95th %ile | Median | 95th %ile | Median | 95th %ile | Median | 95th %ile | Median | 95th %ile | |
| Mean dust ingestion scenario | ||||||||||||
| α-HBCDD-ESD | 0.0056 | 0.012 | 0.11 | 0.24 | 0.0037 | 0.0087 | 0.072 | 0.17 | 0.00022 | 0.00071 | 0.058 | 0.19 |
| β-HBCDD-ESD | 0.0011 | 0.0021 | 0.022 | 0.041 | 0.0010 | 0.0019 | 0.020 | 0.038 | 0.00005 | 0.00013 | 0.014 | 0.035 |
| γ-HBCDD-ESD | 0.0032 | 0.0061 | 0.062 | 0.12 | 0.0020 | 0.0064 | 0.038 | 0.13 | 0.00010 | 0.00026 | 0.026 | 0.068 |
| ΣHBCDDs-ESD | 0.0096 | 0.019 | 0.19 | 0.38 | 0.0065 | 0.016 | 0.13 | 0.32 | 0.00041 | 0.0010 | 0.11 | 0.27 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
| High dust ingestion scenario | ||||||||||||
| α-HBCDD-ESD | 0.017 | 0.037 | 0.22 | 0.48 | 0.011 | 0.026 | 0.14 | 0.34 | 0.0089 | 0.029 | 0.12 | 0.38 |
| β-HBCDD-ESD | 0.0033 | 0.0063 | 0.043 | 0.082 | 0.0030 | 0.0058 | 0.040 | 0.075 | 0.0021 | 0.0054 | 0.027 | 0.070 |
| γ-HBCDD-ESD | 0.0095 | 0.018 | 0.12 | 0.24 | 0.0059 | 0.019 | 0.077 | 0.25 | 0.0040 | 0.010 | 0.053 | 0.14 |
| ΣHBCDDs-ESD | 0.029 | 0.058 | 0.38 | 0.76 | 0.019 | 0.049 | 0.25 | 0.64 | 0.017 | 0.041 | 0.22 | 0.53 |
4. Conclusion
This is the first study to report concentrations of HBCDDs in dust samples from Iraq, in either indoor or outdoor house dust. In general, the concentrations of HBCDDs were much lower than those in Europe and North America. This likely demonstrates lower application in Iraq of EPS and XPS foams as insulation material (the primary use of HBCDDs). Thus, we propose that the low levels of HBCDDs in this study can be attributed to emissions from electronic and electrical equipment, and textiles in the homes studied. Concentrations of HBCDDs were significantly higher in elevated surface dust compared to those in floor dust from the same rooms. This implies that human exposure assessments based on FD only are underestimates, particularly for adults as they interact more with ESD. The presence of non-negligible concentrations of HBCDDs in outdoor dust from front yards indicates the importance of this area when assessing human exposure. Despite the valuable contribution to the body of knowledge on human exposure to HBCDDs via indoor and outdoor dust ingestion, the limitation of this study was in sampling design. Monitoring dust from the interior of cars parked in the front yards as well as dust from the same yards would further assist evaluation of the significance of cars as a source of HBCDDs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge gratefully the assistance of all donors of the dust samples in this study. Layla Salih Al-Omran acknowledges the Iraqi Ministry of Higher Education and Scientific Research for the research leave based on the ministerial directive no. 29685 on 11/11/2019, University of Basrah for the research scholarship based on the university directive no. 7/17/28946 on 8/12/2019 and the School of Geography, Earth & Environmental Sciences, University of Birmingham, UK for the academic visitor invitation.References
- A. Covaci, et al., Hexabromocyclododecanes (HBCDs) in the Environment and Humans: A Review, Environ. Sci. Technol., 2006, 40(12), 3679–3688 CrossRef CAS PubMed.
- W. A. Stubbings and S. Harrad, Extent and mechanisms of brominated flame retardant emissions from waste soft furnishings and fabrics: A critical review, Environ. Int., 2014, 71, 164–175 CrossRef CAS PubMed.
- W. A. Stubbings and S. Harrad, Laboratory studies on leaching of HBCDD from building insulation foams, Emerging Contam., 2019, 5, 36–44 CrossRef.
- D. C. G. Muir and C. A. de Wit, Trends of legacy and new persistent organic pollutants in the circumpolar arctic: Overview, conclusions, and recommendations, Sci. Total Environ., 2010, 408(15), 3044–3051 CrossRef CAS PubMed.
- M. Ichihara, et al., Distribution and pollutant load of hexabromocyclododecane (HBCD) in sewage treatment plants and water from Japanese Rivers, Chemosphere, 2014, 110, 78–84 CrossRef CAS PubMed.
- J.-F. Lu, et al., Occurrence of tetrabromobisphenol a (TBBPA) and hexabromocyclododecane (HBCD) in soil and road dust in Chongqing, western China, with emphasis on diastereoisomer profiles, particle size distribution, and human exposure, Environ. Pollut., 2018, 242, 219–228 CrossRef CAS PubMed.
- X. Wang, B. F. Hales and B. Robaire, Effects of flame retardants on ovarian function, Reprod. Toxicol., 2021, 102, 10–23 CrossRef CAS PubMed.
- N. V. Heeb, et al., Structure elucidation of hexabromocyclododecanes—a class of compounds with a complex stereochemistry, Chemosphere, 2005, 61(1), 65–73 CrossRef CAS PubMed.
- UNEP, Guidance for the inventory, identification and substitution of Hexabromocyclododecane (HBCD), 2017, accessed 21st February 2022, https://chm.pops.int/Implementation/NIPs/Guidance/GuidanceforHBCD/tabid/5332/Default.aspx Search PubMed.
- J. E. Balmer, et al., Hexachlorobutadiene (HCBD) contamination in the Arctic environment: A review, Emerging Contam., 2019, 5, 116–122 CrossRef.
- C. Koch, et al., Review of hexabromocyclododecane (HBCD) with a focus on legislation and recent publications concerning toxicokinetics and-dynamics, Environ. Pollut., 2015, 199, 26–34 CrossRef CAS PubMed.
- C. Emond, M. J. DeVito and L. S. Birnbaum, A PBPK model describing the pharmacokinetics of γ-HBCD exposure in mice, Toxicol. Appl. Pharmacol., 2021, 428, 115678 CrossRef CAS PubMed.
- UNEP, Guidance on best available techniques and best environmental practices for the use of hexabromocyclododecane listed with specific exemptions under the Stockholm Convention, 2021, accessed 21st February 2022, https://chm.pops.int/Implementation/NationalImplementationPlans/GuidanceArchive/GuidanceonBATBEPforHBCD/tabid/5526/Default.aspx Search PubMed.
- Y. J. Li, M. H. Li and Y. H. Shih, Aerobic degradation and the effect of hexabromocyclododecane by soil microbial communities in Taiwan, Environ. Int., 2020, 145, 106128 CrossRef CAS PubMed.
- EFSA, Update of the risk assessment of hexabromocyclododecanes (HBCDDs) in food,2021, accessed 1st January 2022, https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2021.6421 Search PubMed.
- W. A. Stubbings, et al., Leaching behaviour of hexabromocyclododecane from treated curtains, Chemosphere, 2016, 144, 2091–2096 CrossRef CAS PubMed.
- K. Thuresson, J. A. Björklund and C. A. de Wit, Tri-decabrominated diphenyl ethers and hexabromocyclododecane in indoor air and dust from Stockholm microenvironments 1: Levels and profiles, Sci. Total Environ., 2012, 414, 713–721 CrossRef CAS PubMed.
- S. Newton, U. Sellström and C. A. de Wit, Emerging flame retardants, PBDEs, and HBCDDs in indoor and outdoor media in Stockholm, Sweden, Environ. Sci. Technol., 2015, 49(5), 2912–2920 CrossRef CAS.
- N. Wemken, et al., Concentrations of Brominated Flame Retardants in Indoor Air and Dust from Ireland Reveal Elevated Exposure to Decabromodiphenyl Ethane, Environ. Sci. Technol., 2019, 53(16), 9826–9836 CrossRef CAS PubMed.
- B. A. Parvizian, et al., Concentrations and Long-Term Temporal Trends of Hexabromocyclododecanes (HBCDD) in Lake Trout and Walleye from the Great Lakes, Environ. Sci. Technol., 2020, 54(10), 6134–6141 CrossRef CAS PubMed.
- S. Lee and H. B. Moon, Multi-matrix distribution and contamination profiles of HBCDD isomers in a man-made saltwater lake near industrial complexes with high flame retardant consumption in Korea, Mar. Pollut. Bull., 2021, 172, 112812 CrossRef CAS PubMed.
- J. A. De Guzman, Hexabromocyclododecane in municipal wastewater treatment plant: Occurrence, fate and potential environmental risks, in 5th International Conference on Measurement, Instrumentation and Automation (ICMIA 2016), Shenzhen, China, 2016 Search PubMed.
- H. Demirtepe and I. Imamoglu, Levels of polybrominated diphenyl ethers and hexabromocyclododecane in treatment plant sludge: Implications on sludge management, Chemosphere, 2019, 221, 606–615 CrossRef CAS PubMed.
- C. H. Marvin, et al., Distribution of hexabromocyclododecane in Detroit River suspended sediments, Chemosphere, 2006, 64(2), 268–275 CrossRef CAS PubMed.
- X. Wang, et al., Concentrations, Distributions, and Risk Assessment of HBCD in Sediment in the Weihe River Basin in Northwest China, Int. J. Environ. Res. Public Health, 2018, 15(11), 2340 CrossRef CAS PubMed.
- B. Harris and M. Abou-Elwafa Abdallah, Exploring variations of hexabromocyclododecane concentrations in riverine sediments along the River Medway, UK, Environ. Sci.: Processes Impacts, 2021, 23(5), 776–785 RSC.
- M. H. Wu, et al., Occurrence of Hexabromocyclododecane in soil and road dust from mixed-land-use areas of Shanghai, China, and its implications for human exposure, Sci. Total Environ., 2016, 559, 282–290 CrossRef CAS PubMed.
- M. Shen, et al., Occurrence of two novel triazine-based flame retardants in an E-waste recycling area in South China: Implication for human exposure, Sci. Total Environ., 2019, 683, 249–257 CrossRef CAS PubMed.
- S. Harrad, M. A. Abdallah and A. Covaci, Causes of variability in concentrations and diastereomer patterns of hexabromocyclododecanes in indoor dust, Environ. Int., 2009, 35(3), 573–579 CrossRef CAS PubMed.
- Z. Cao, et al., Seasonal and Particle Size-Dependent Variations of Hexabromocyclododecanes in Settled Dust: Implications for Sampling, Environ. Sci. Technol., 2015, 49(18), 11151–11157 CrossRef CAS PubMed.
- M. Barghi, et al., Hexabromocyclododecane (HBCD) in the Korean food basket and estimation of dietary exposure, Environ. Pollut., 2016, 213, 268–277 CrossRef CAS PubMed.
- R. Cariou, et al., Enantiomeric fraction of hexabromocyclododecanes in foodstuff from the Belgian market, Chemosphere, 2020, 260, 127607 CrossRef CAS PubMed.
- D. Zacs, et al., Polybrominated diphenyl ethers (PBDEs), hexabromocyclododecanes (HBCDD), dechlorane-related compounds (DRCs), and emerging brominated flame retardants (EBFRs) in foods: The levels, profiles, and dietary intake in Latvia, Sci. Total Environ., 2021, 752, 141996 CrossRef CAS.
- A. Schecter, et al., Hexabromocyclododecane (HBCD) stereoisomers in U.S. food from Dallas, Texas, Environ. Health Perspect., 2012, 120(9), 1260–1264 CrossRef CAS PubMed.
- H. Li, et al., Bioaccumulation and translocation of tetrabromobisphenol A and hexabromocyclododecanes in mangrove plants from a national nature reserve of Shenzhen City, South China, Environ. Int., 2019, 129, 239–246 CrossRef CAS PubMed.
- M. A.-E. Abdallah and S. Harrad, Tetrabromobisphenol-A, hexabromocyclododecane and its degradation products in UK human milk: Relationship to external exposure, Environ. Int., 2011, 37(2), 443–448 CrossRef CAS PubMed.
- F. Tao, et al., Emerging and legacy flame retardants in UK human milk and food suggest slow response to restrictions on use of PBDEs and HBCDD, Environ. Int., 2017, 105, 95–104 CrossRef CAS PubMed.
- Y. Fujii, et al., Contamination trends and factors affecting the transfer of hexabromocyclododecane diastereomers, tetrabromobisphenol A, and 2,4,6-tribromophenol to breast milk in Japan, Environ. Pollut., 2018, 237, 936–943 CrossRef CAS PubMed.
- U. J. Kim and J. E. Oh, Tetrabromobisphenol A and hexabromocyclododecane flame retardants in infant-mother paired serum samples, and their relationships with thyroid hormones and environmental factors, Environ. Pollut., 2014, 184, 193–200 CrossRef CAS PubMed.
- D. S. Drage, et al., Demographic and temporal trends of hexabromocyclododecanes (HBCDD) in an Australian population, Environ. Res., 2017, 152, 192–198 CrossRef CAS PubMed.
- M. Barghi, et al., HBCD and TBBPA in human scalp hair: Evidence of internal exposure, Chemosphere, 2018, 207, 70–77 CrossRef CAS PubMed.
- B. Tang, et al., Analysis of polybrominated diphenyl ethers, hexabromocyclododecanes, and legacy and emerging phosphorus flame retardants in human hair, Chemosphere, 2021, 262, 127807 CrossRef CAS PubMed.
- B. Barhoumi, et al., Halogenated flame retardants in atmospheric particles from a North African coastal city (Bizerte, Tunisia): Pollution characteristics and human exposure, Atmos. Pollut. Res., 2020, 11(4), 831–840 CrossRef CAS.
- R. Nakagawa, et al., Hexabromocyclododecane determination in seafood samples collected from Japanese coastal areas, Chemosphere, 2010, 81(4), 445–452 CrossRef CAS PubMed.
- S. Harrad and M. A. Abdallah, Brominated flame retardants in dust from UK cars—within-vehicle spatial variability, evidence for degradation and exposure implications, Chemosphere, 2011, 82(9), 1240–1245 CrossRef CAS PubMed.
- S. Mizouchi, et al., Exposure assessment of organophosphorus and organobromine flame retardants via indoor dust from elementary schools and domestic houses, Chemosphere, 2015, 123, 17–25 CrossRef CAS PubMed.
- A. Besis, et al., Legacy and novel brominated flame retardants in interior car dust-Implications for human exposure, Environ. Pollut., 2017, 230, 871–881 CrossRef CAS PubMed.
- G. Pawar, et al., Dermal bioaccessibility of flame retardants from indoor dust and the influence of topically applied cosmetics, J. Exposure Sci. Environ. Epidemiol., 2017, 27(1), 100–105 CrossRef CAS PubMed.
- M. A. Abdallah and S. Harrad, Dermal contact with furniture fabrics is a significant pathway of human exposure to brominated flame retardants, Environ. Int., 2018, 118, 26–33 CrossRef CAS PubMed.
- M. Ema, et al., Two-generation reproductive toxicity study of the flame retardant hexabromocyclododecane in rats, Reprod. Toxicol., 2008, 25(3), 335–351 CrossRef CAS PubMed.
- L. T. van der Ven, et al., Endocrine effects of hexabromocyclododecane (HBCD) in a one-generation reproduction study in Wistar rats, Toxicol. Lett., 2009, 185(1), 51–62 CrossRef CAS.
- A. Bernhard, et al., Marine fatty acids aggravate hepatotoxicity of α-HBCD in juvenile female BALB/c mice, Food Chem. Toxicol., 2016, 97, 411–423 CrossRef CAS PubMed.
- M. A.-E. Abdallah, et al., Hexabromocyclododecanes In Indoor Dust From Canada, the United Kingdom, and the United States, Environ. Sci. Technol., 2008a, 42(2), 459–464 Search PubMed.
- S. Harrad, et al., Indoor Contamination with Hexabromocyclododecanes, Polybrominated Diphenyl Ethers, and Perfluoroalkyl Compounds: An Important Exposure Pathway for People?, Environ. Sci. Technol., 2010, 44(9), 3221–3231 CrossRef CAS PubMed.
- H. Jiang, et al., Daily intake of polybrominated diphenyl ethers via dust and diet from an e-waste recycling area in China, J. Hazard. Mater., 2014, 276, 35–42 CrossRef CAS PubMed.
- E. Malliari and O. I. Kalantzi, Children's exposure to brominated flame retardants in indoor environments – A review, Environ. Int., 2017, 108, 146–169 CrossRef CAS PubMed.
- C. A. de Wit, J. A. Björklund and K. Thuresson, Tri-decabrominated diphenyl ethers and hexabromocyclododecane in indoor air and dust from Stockholm microenvironments 2: indoor sources and human exposure, Environ. Int., 2012, 39(1), 141–147 CrossRef CAS PubMed.
- L. Roosens, et al., Exposure to hexabromocyclododecanes (HBCDs) via dust ingestion, but not diet, correlates with concentrations in human serum: preliminary results, Environ. Health Perspect., 2009, 117(11), 1707–1712 CrossRef CAS PubMed.
- C. Rauert, et al., Direct contact between dust and HBCD-treated fabrics is an important pathway of source-to-dust transfer, Sci. Total Environ., 2016, 545–546, 77–83 CrossRef CAS PubMed.
- Z. Qian, et al., Enhanced emissions of brominated flame retardants from indoor sources by direct contact with dust, Environ. Monit. Assess., 2019, 191(3), 170 CrossRef PubMed.
- N. Ali, et al., Occurrence of alternative flame retardants in indoor dust from New Zealand: indoor sources and human exposure assessment, Chemosphere, 2012, 88(11), 1276–1282 CrossRef CAS PubMed.
- H. M. Stapleton, et al., Flame retardant associations between children’s handwipes and house dust, Chemosphere, 2014, 116, 54–60 CrossRef CAS PubMed.
- D.-J. Kweon, M.-K. Kim and K.-D. Zoh, Distribution of brominated flame retardants and phthalate esters in house dust in Korea, Environ. Eng. Res., 2018, 23(4), 354–363 CrossRef.
- D. S. Drage, et al., Temporal trends in concentrations of legacy and novel brominated flame retardants in house dust from Birmingham in the United Kingdom, Emerging Contam., 2020, 6, 323–329 CrossRef.
- H.-G. Ni and H. Zeng, HBCD and TBBPA in particulate phase of indoor air in Shenzhen, China, Sci. Total Environ., 2013, 458–460, 15–19 CrossRef CAS PubMed.
- F. Tao, U. Sellström and C. A. de Wit, Organohalogenated Flame Retardants and Organophosphate Esters in Office Air and Dust from Sweden, Environ. Sci. Technol., 2019, 53(4), 2124–2133 CrossRef CAS PubMed.
- K. Larsson, et al., Brominated Flame Retardants and Organophosphate Esters in Preschool Dust and Children’s Hand Wipes, Environ. Sci. Technol., 2018, 52(8), 4878–4888 CrossRef CAS PubMed.
- R. Esplugas, et al., Emerging and legacy flame retardants in indoor air and dust samples of Tarragona Province (Catalonia, Spain), Sci. Total Environ., 2022, 806, 150494 CrossRef CAS PubMed.
- K. Kalachova, et al., Occurrence of brominated flame retardants in household and car dust from the Czech Republic, Sci. Total Environ., 2012, 441, 182–193 CrossRef CAS PubMed.
- M. A.-E. Abdallah, et al., Hexabromocyclododecane and tetrabromobisphenol-A in indoor dust from France, Kazakhstan and Nigeria: Implications for human exposure, Emerging Contam., 2016, 2(2), 73–79 CrossRef.
- E. Cequier, et al., Occurrence of a Broad Range of Legacy and Emerging Flame Retardants in Indoor Environments in Norway, Environ. Sci. Technol., 2014, 48(12), 6827–6835 CrossRef CAS PubMed.
- A. Araki, et al., Phosphorus flame retardants in indoor dust and their relation to asthma and allergies of inhabitants, Indoor Air, 2014, 24(1), 3–15 CrossRef CAS PubMed.
- S. Tajima, et al., Detection and intake assessment of organophosphate flame retardants in house dust in Japanese dwellings, Sci. Total Environ., 2014, 478, 190–199 CrossRef CAS PubMed.
- F. Xu, et al., Comprehensive Study of Human External Exposure to Organophosphate Flame Retardants via Air, Dust, and Hand Wipes: The Importance of Sampling and Assessment Strategy, Environ. Sci. Technol., 2016, 50(14), 7752–7760 CrossRef CAS PubMed.
- L. S. Al-Omran and S. Harrad, Polybrominated diphenyl ethers and “novel” brominated flame retardants in floor and elevated surface house dust from Iraq: Implications for human exposure assessment, Emerging Contam., 2016, 2(1), 7–13 CrossRef.
- L. S. Al-Omran and S. Harrad, Within-room and within-home spatial and temporal variability in concentrations of legacy and “novel” brominated flame retardants in indoor dust, Chemosphere, 2018, 193, 1105–1112 CrossRef CAS PubMed.
- M. A. Khairy and R. Lohmann, Selected organohalogenated flame retardants in Egyptian indoor and outdoor environments: Levels, sources and implications for human exposure, Sci. Total Environ., 2018, 633, 1536–1548 CrossRef CAS PubMed.
- M. A. Khairy and R. Lohmann, Organophosphate flame retardants in the indoor and outdoor dust and gas-phase of Alexandria, Egypt, Chemosphere, 2019, 220, 275–285 CrossRef CAS PubMed.
- L. S. Al-Omran, S. Harrad and M. Abou-Elwafa Abdallah, A meta-analysis of factors influencing concentrations of brominated flame retardants and organophosphate esters in indoor dust, Environ. Pollut., 2021, 285, 117262 CrossRef CAS PubMed.
- J. M. Allgood, et al., Potential human exposure to halogenated flame-retardants in elevated surface dust and floor dust in an academic environment, Environ. Res., 2017, 153, 55–62 CrossRef CAS PubMed.
- Z. Cao, et al., Distribution patterns of brominated, chlorinated, and phosphorus flame retardants with particle size in indoor and outdoor dust and implications for human exposure, Environ. Sci. Technol., 2014, 48(15), 8839–8846 CrossRef CAS PubMed.
- Y. Wang, et al., Organophosphate di- and tri-esters in indoor and outdoor dust from China and its implications for human exposure, Sci. Total Environ., 2020, 700, 134502 CrossRef CAS PubMed.
- L. S. Al-Omran, et al., Organophosphate esters in indoor and outdoor dust from Iraq: Implications for human exposure, Emerging Contam., 2021, 7, 204–212 CrossRef.
- Y. Hassan and T. Shoeib, Levels of polybrominated diphenyl ethers and novel flame retardants in microenvironment dust from Egypt: An assessment of human exposure, Sci. Total Environ., 2015, 505, 47–55 CrossRef CAS PubMed.
- P. B. Kurt-Karakus, et al., Polybrominated diphenyl ethers (PBDEs) and alternative flame retardants (NFRs) in indoor and outdoor air and indoor dust from Istanbul-Turkey: Levels and an assessment of human exposure, Atmos. Pollut. Res., 2017, 8(5), 801–815 CrossRef.
- S. Harrad, et al., Polybrominated diphenyl ethers in domestic indoor dust from Canada, New Zealand, United Kingdom and United States, Environ. Int., 2008, 34(2), 232–238 CrossRef CAS PubMed.
- N. Van den Eede, et al., Multi-residue method for the determination of brominated and organophosphate flame retardants in indoor dust, Talanta, 2012, 89, 292–300 CrossRef CAS PubMed.
- A. Keller, et al., Genetic variation in a human odorant receptor alters odour perception, Nature, 2007, 449(7161), 468–472 CrossRef CAS PubMed.
- N. M. Tue, et al., Contamination of indoor dust and air by polychlorinated biphenyls and brominated flame retardants and relevance of non-dietary exposure in Vietnamese informal e-waste recycling sites, Environ. Int., 2013, 51, 160–167 CrossRef PubMed.
- H. Fromme, et al., Polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD) and “novel” brominated flame retardants in house dust in Germany, Environ. Int., 2014, 64, 61–68 CrossRef CAS PubMed.
- H. Zhu, et al., Legacy and alternative brominated flame retardants in outdoor dust and pine needles in mainland China: Spatial trends, dust-plant partitioning and human exposure, Environ. Pollut., 2018, 243, 758–765 CrossRef CAS PubMed.
- R. Köppen, et al., On the thermally induced isomerisation of hexabromocyclododecane stereoisomers, Chemosphere, 2008, 71(4), 656–662 CrossRef PubMed.
- Q. Bu, et al., Polybrominated diphenyl ethers and novel brominated flame retardants in indoor dust of different microenvironments in Beijing, China, Environ. Int., 2019, 122, 159–167 CrossRef CAS PubMed.
- C. J. Weschler and W. W. Nazaroff, SVOC partitioning between the gas phase and settled dust indoors, Atmos. Environ., 2010, 44(30), 3609–3620 CrossRef CAS.
- C. Rauert, et al., Test chamber and forensic microscopy investigation of the transfer of brominated flame retardants into indoor dust via abrasion of source materials, Sci. Total Environ., 2014, 493, 639–648 CrossRef CAS PubMed.
- J. Desborough, et al., Polychlorinated biphenyls (PCBs), hexabromocyclododecanes (HBCDDs) and degradation products in topsoil from Australia and the United Kingdom, Emerging Contam., 2016, 2(1), 37–41 CrossRef.
- M. A. Abdallah, S. Harrad and A. Covaci, Hexabromocyclododecanes and tetrabromobisphenol-A in indoor air and dust in Birmingham, U.K: implications for human exposure, Environ. Sci. Technol., 2008, 42(18), 6855–6861 CrossRef CAS PubMed.
- M. Barghi, et al., Human exposure to HBCD and TBBPA via indoor dust in Korea: Estimation of external exposure and body burden, Sci. Total Environ., 2017, 593–594, 779–786 CrossRef CAS PubMed.
- U.S. EPA, Update for Chapter 5 of the Exposure Factors Handbook – Soil and Dust Ingestion, 2017, accessed 1st January 2022, https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=337521 Search PubMed.
- A. de la Torre, et al., Organophosphate compounds, polybrominated diphenyl ethers and novel brominated flame retardants in European indoor house dust: Use, evidence for replacements and assessment of human exposure, J. Hazard. Mater., 2020, 382, 121009 CrossRef CAS PubMed.
- H. M. Stapleton, et al., Alternate and New Brominated Flame Retardants Detected in U.S. House Dust, Environ. Sci. Technol., 2008, 42(18), 6910–6916 CrossRef CAS PubMed.
- COT, Addendum to the 2015 COT Statement on potential risks from hexabromocyclododecanes (HBCDDs) in the infant diet, 2016, accessed 21st February 2022, https://cot.food.gov.uk/sites/default/files/finaladdendumonhbcdds.pdf Search PubMed.
- L. M. O. Sahlström, et al., Estimated intakes of brominated flame retardants via diet and dust compared to internal concentrations in a Swedish mother–toddler cohort, Int. J. Hyg. Environ. Health, 2015, 218(4), 422–432 CrossRef.
- S. D. Coelho, et al., Brominated, chlorinated and phosphate organic contaminants in house dust from Portugal, Sci. Total Environ., 2016, 569–570, 442–449 CrossRef CAS PubMed.
- J. H. Tay, et al., Human Exposure to Legacy and Emerging Halogenated Flame Retardants via Inhalation and Dust Ingestion in a Norwegian Cohort, Environ. Sci. Technol., 2017, 51(14), 8176–8184 CrossRef CAS PubMed.
- A. C. Dirtu, et al., Country specific comparison for profile of chlorinated, brominated and phosphate organic contaminants in indoor dust. Case study for Eastern Romania, 2010, Environ. Int., 2012, 49, 1–8 CrossRef CAS PubMed.
- D. Chen, et al., Hexabromocyclododecane flame retardant in Antarctica: Research stations as sources, Environ. Pollut., 2015, 206, 611–618 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2em00133k |
| This journal is © The Royal Society of Chemistry 2022 |