Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalytic (3 + 2) annulation of donor–acceptor aminocyclopropane monoesters and indoles†
Vincent
Pirenne
 ,
Emma G. L.
Robert
,
Emma G. L.
Robert
 and
Jerome
Waser
and
Jerome
Waser
 *
*
Laboratory of Catalysis and Organic Synthesis, Institut des Sciences et Ingénierie Chimique, Ecole Polytechnique Fédérale de Lausanne, Ch-1015, Lausanne, Switzerland. E-mail: jerome.waser@epfl.ch
First published on 5th May 2021
Abstract
The efficient catalytic activation of donor–acceptor aminocyclopropanes lacking the commonly used diester acceptor is reported here in a (3 + 2) dearomative annulation with indoles. Bench-stable tosyl-protected aminocyclopropyl esters were converted into cycloadducts in 46–95% yields and up to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 diastereomeric ratio using catalytic amounts of triethylsilyl triflimide. Tricyclic indoline frameworks containing four stereogenic centers including all-carbon quaternary centers were obtained.
5 diastereomeric ratio using catalytic amounts of triethylsilyl triflimide. Tricyclic indoline frameworks containing four stereogenic centers including all-carbon quaternary centers were obtained.
1. Introduction
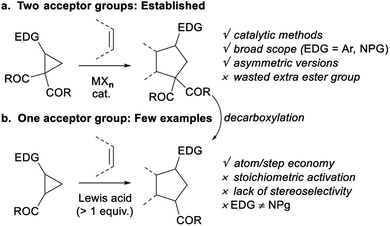
Vicinal donor–acceptor (D–A) cyclopropanes are useful three-carbon 1,3-zwitterion synthetic equivalents for the synthesis of carbocyclic scaffolds.1 The electronic properties of the donor and acceptor groups are essential to obtain stable yet reactive enough push–pull systems. Dicarbonyl motifs are acceptors of choice for metal-catalyzed ring opening reactions.1d Among the many possible transformations, (3 + 2) annulations giving access to five-membered rings are especially useful and have been thoroughly investigated with several donor substituents, with a particular focus on aryl2 and protected amines3 (Scheme 1a). Enantioselective methods have also been reported.4In contrast, D–A cyclopropanes with a single carbonyl acceptor have been less studied (Scheme 1b). Such substrates lead to the formation of one more stereocenter and do not require a decarboxylation step to remove the diester group.5 However, activation and control over diastereoselectivity is challenging for these less reactive cyclopropanes. Only rare examples of (3 + 2) annulations have been reported, and they are often neither catalytic nor stereoselective.1d,e Cyclopropanecarbaldehydes were mostly suitable in iminium–enamine catalysis for ring-opening reactions rather than annulations.6 Annulation reactions of cyclopropyl ketones were performed using stoichiometric Lewis acids such as SnCl4, TiCl4, BF3·Et2O and Me2AlCl.7 Reactions with less reactive cyclopropyl monoesters are limited to alkoxycyclopropanes using silyl triflates or organoaluminium reagents as stoichiometric activators.7a,8 Catalytic activation remains limited to ring expansion, intramolecular annulation and spirocyclic D–A cyclopropanes.9 Only one catalytic intermolecular (3 + 2) annulation of 2-butoxycyclopropanecarboxylate with silyl enol ethers was described by Ihara and co-workers using bistriflimide, but no stereoselectivity was observed.10 Furthermore, in contrast to the numerous reports for annulation of aminocyclopropane diesters,3 there is currently no report on the use of aminocyclopropane monoesters in annulation reactions, despite the importance of nitrogen-containing building blocks in synthetic and medicinal chemistry. Phthalimide and succinimide have the appropriate electronic properties in the case of aminocyclopropane diesters,3 but are not donating enough when a single ester group is present. With carbamate protecting groups, the ring-opening processes of aminocyclopropane monoesters are limited to hydrolysis and rearrangements.11 A carbamate-protected aminocyclopropyl ketone was also showed by our group to react intramolecularly in a formal homo-Nazarov cyclization.12
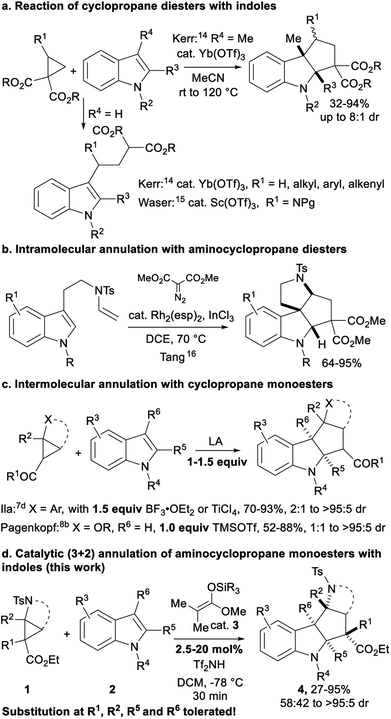
Annulations of D–A cyclopropanes with indole derivatives are particularly interesting, as they provide a quick access to polycyclic indoline scaffolds (Scheme 2). Aminocyclopropanes are especially attractive starting materials, as the obtained indoline-fused cyclopentylamines are present in the core of alkaloid natural products, such as vindolinine, pleiomutinine or huncaniterine A and B.13 3-Methylindoles were used by Kerr and co-workers in a ytterbium triflate catalyzed (3 + 2) annulation with cyclopropane diesters (Scheme 2a).14 However, in the absence of substituent at the C-3 position, ring-opening products were obtained. Ring-opening was also observed with aminocyclopropanes by our group.15 Ila and co-workers later showed that annulation products can be obtained not only with 3-alkylsubstituted, but also with unsubstituted indoles and arylcyclopropanes, but only using a stoichiometric amount of boron trifluoride etherate as activator.7d Recently, Tang and co-workers described the in situ formation of unstable tosyl-protected aminocyclopropane diesters and their use in intramolecular annulation with indoles leading to tetracyclic indolines (Scheme 2b).16 By comparison, arylcyclopropyl ketones7d and alkoxycyclopropyl monoesters8b gave annulation products in good yields, but these methods are not catalytic and are limited to the synthesis of tertiary stereocenters at the acceptor position (Scheme 2c). Low diastereoselectivities are obtained for several substitution patterns and in the case of alkoxycyclopropanes, annulation was successful only for C3-unsubstituted indoles.
Herein, we describe the first catalytic (3 + 2) annulation of bench-stable tosyl-protected aminocyclopropane monoesters 1 (Scheme 2d). With indoles 2 as partners, key for success was the use of a silyl bistriflimide as catalyst, generated in situ from silyl ketene acetal 3 through protodesilylation.17,18 In contrast to previous (3 + 2) annulations of cyclopropane monoesters that all required stoichiometric amounts of Lewis acid,7d,8b full conversion could be achieved with only 2.5 mol% TESNTf2 for several substrates. Furthermore, the method is unique for its tolerance towards substitution patterns, as it works for both C2- and C3-substituted indoles and can be used for the first time to introduce a non-symmetrical all carbon quaternary center at the acceptor position in good yield and high diastereoselectivity.
2. Results and discussion
2.1. Screening of aminocyclopropanes and optimization
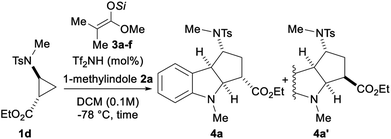
Our work started with identifying a suitable push–pull system (Scheme 3). Aminocyclopropanes 1a–f were used in the (3 + 2) annulation with 1-methylindole (2a) using Lewis acids as catalysts. Preliminary experiments using TMS triflate led to no reaction (see ESI†), although such conditions have been successful for alkoxycyclopropanes.8b Compared to silyl triflates, silyl triflimides have shown superior catalytic activity.17 TMS triflimide was formed through the protodesilylation of trimethylsilyl ketene acetal 3a with bistriflimide.18 Although the aminocyclopropyl esters protected by a phthalimide (1a), an amide (1b) or a nosyl group (1c) were not reactive, the tosyl protecting group (1d) furnished the cycloadduct 4a in 85% yield and 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 dr. The introduction of an oxazolidinone (1e) or a ketone (1f) as the electron withdrawing group led to stability issues and decomposition in presence of TMS triflimide.
9 dr. The introduction of an oxazolidinone (1e) or a ketone (1f) as the electron withdrawing group led to stability issues and decomposition in presence of TMS triflimide.
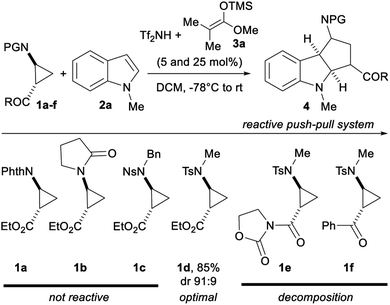 | ||
| Scheme 3 Screening of several push–pull systems for the TMS triflimide-catalyzed (3 + 2) annulation of aminocyclopropanes 1a–f with 1-methylindole (2a). | ||
With the optimal D–A aminocyclopropyl ester 1d, the influence of the substituents on the silicon was examined starting from silyl ketene acetals 3a–f (Table 1). Using trimethylsilyl ketene acetal 3a (entry 1), the reaction was completed in less than 30 minutes leading to cycloadduct 4a in 85% yield and 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 dr. NOESY experiments allowed the determination of the relative configuration of both isomers (see ESI†). The TES group (3b) and other tri-n-alkyl silyl groups (3c and 3d) improved the dr to 93
9 dr. NOESY experiments allowed the determination of the relative configuration of both isomers (see ESI†). The TES group (3b) and other tri-n-alkyl silyl groups (3c and 3d) improved the dr to 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (entries 2–4). Increasing the bulkiness of the silyl groups (TBS and TIPS, 3e and 3f) led to a decrease of the diastereoselectivity (entries 5 and 6). The yield was slightly improved by diminishing the catalyst loading to 2.5 mol% at 0.3 mmol scale without affecting the diastereoselectivity (entry 7). Finally, cycloadduct 4a was obtained in 87% yield and 92
7 (entries 2–4). Increasing the bulkiness of the silyl groups (TBS and TIPS, 3e and 3f) led to a decrease of the diastereoselectivity (entries 5 and 6). The yield was slightly improved by diminishing the catalyst loading to 2.5 mol% at 0.3 mmol scale without affecting the diastereoselectivity (entry 7). Finally, cycloadduct 4a was obtained in 87% yield and 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 dr when the reaction was performed at 0.3 M concentration (entry 8).
8 dr when the reaction was performed at 0.3 M concentration (entry 8).
| Entry | Tf2NH | Si | Time (min) | Yieldb (%) | drc |
|---|---|---|---|---|---|
| a Reaction conditions: 0.1 mmol scale and 0.1 M, 1.05 equiv. of 1-methylindole (2a), 25 mol% of silyl ketene acetals 3a–f. b Isolated yield for the mixture of both isomers. c dr measured from the 1H NMR spectrum of the isolated mixture. d On 0.3 mmol scale. e On 0.3 mmol scale and at 0.3 M. | |||||
| 1 | 5 | SiMe3 (3a) | 30 | 85 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 2 | 5 | SiEt3 (3b) | 30 | 91 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 3 | 5 | Si-n-Pr3 (3c) | 30 | 87 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 4 | 5 | Si-n-Bu3 (3d) | 30 | 88 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 5 | 5 | SiMe2tBu (3e) | 80 | 90 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 6 | 5 | Si-i-Pr3 (3f) | 30 | 91 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
| 7 | 2.5 | SiEt 3 ( 3b ) | 30 | 95 |
93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 7 7 |
| 8e | 2.5 | SiEt3 (3b) | 30 | 87 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
2.2. Scope of indole derivatives19
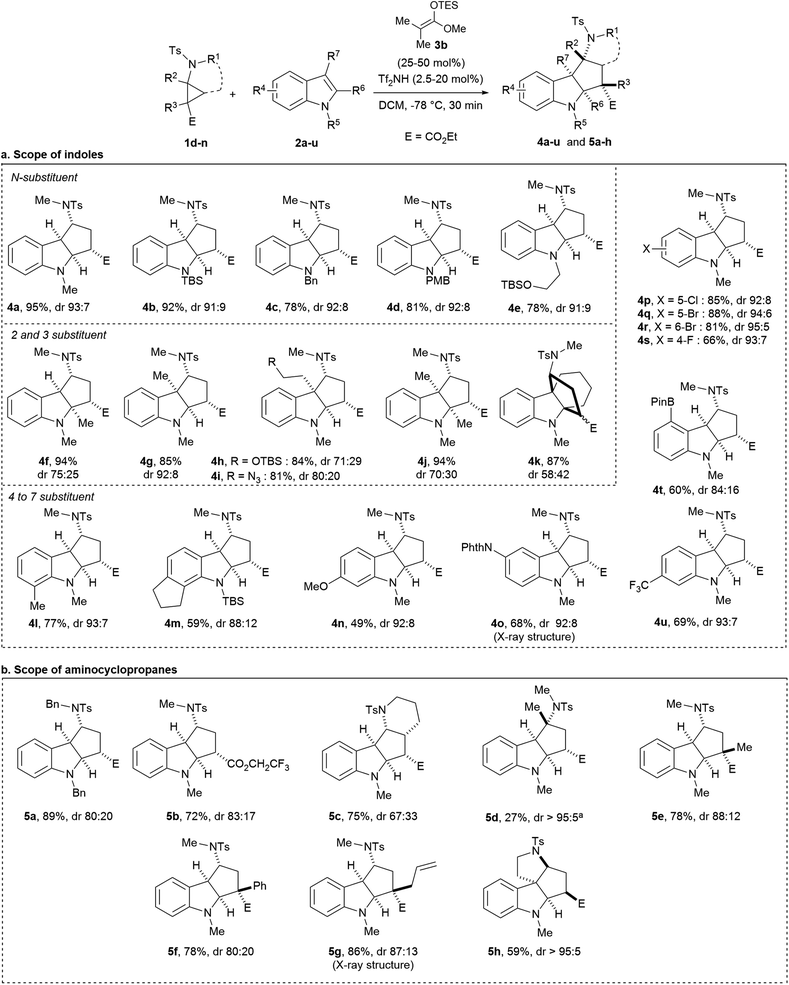
The optimal conditions of entry 7 were then applied to different indole derivatives 2a–u (Scheme 4a). Indoles 2b–d protected by a TBS, a benzyl or a PMB group were converted to cycloadducts 4b–d in 78–92% yield and diastereoselectivities ≥91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9. A functionalized N-alkyl substituent led to the formation of compound 4e in similar yield and dr. Free indole gave no reaction. Indoles 2f–k substituted at the 2 and/or 3-position leading to sterically more encumbered products 4f–k were successful. For all substitution patterns, annulation products were obtained without ring-opening side reactions. Protected tryptophol and tryptamine gave the desired cycloadducts 4h and 4i with a quaternary carbon center in 84%/81% yield and 71
9. A functionalized N-alkyl substituent led to the formation of compound 4e in similar yield and dr. Free indole gave no reaction. Indoles 2f–k substituted at the 2 and/or 3-position leading to sterically more encumbered products 4f–k were successful. For all substitution patterns, annulation products were obtained without ring-opening side reactions. Protected tryptophol and tryptamine gave the desired cycloadducts 4h and 4i with a quaternary carbon center in 84%/81% yield and 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29/80
29/80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 dr. Excellent yields and diastereoselectivities were obtained for alkyl substituents (4l and 4m), a methoxy (4n), a protected nitrogen (4o) and halogens (4p–s) on the aryl ring. Other functional groups such as pinacol borane and a trifluoromethyl group were also tolerated (4t and 4u). More electron withdrawing substituents such as an ester or a nitrile gave no reaction. X-ray crystal structure analysis of 4o20 confirmed the relative configuration of the cycloadducts. The all-cis substituted product was the major diastereoisomer in all cases. The diastereoselectivity decreased with increasing substitution at C2/C3 position (no substituent: 84
20 dr. Excellent yields and diastereoselectivities were obtained for alkyl substituents (4l and 4m), a methoxy (4n), a protected nitrogen (4o) and halogens (4p–s) on the aryl ring. Other functional groups such as pinacol borane and a trifluoromethyl group were also tolerated (4t and 4u). More electron withdrawing substituents such as an ester or a nitrile gave no reaction. X-ray crystal structure analysis of 4o20 confirmed the relative configuration of the cycloadducts. The all-cis substituted product was the major diastereoisomer in all cases. The diastereoselectivity decreased with increasing substitution at C2/C3 position (no substituent: 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16–95
16–95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, one substituent: 71
5, one substituent: 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29–92
29–92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, two substituents: 58
8, two substituents: 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42–70
42–70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30).
30).
2.3. Scope of aminocyclopropanes
The scope of aminocyclopropanes 1 was then examined (Scheme 4b). These substrates were easily obtained by copper-catalyzed cyclopropanation of the corresponding enamides and diazo compounds.21 First, aminocyclopropane 1g bearing a tosyl and a benzyl on the nitrogen as orthogonal protecting groups afforded cycloadduct 5a in 89% yield and 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 dr. Replacement of the ethyl ester (E) by a trifluoroethyl ester group was tolerated (5b). More substituted aminocyclopropanes 1i–n bearing quaternary stereocenters were then prepared. Bicyclo[4.1.0] aminocyclopropane 1i led to the formation of tetracyclic compound 5c in 75% yield and 67
20 dr. Replacement of the ethyl ester (E) by a trifluoroethyl ester group was tolerated (5b). More substituted aminocyclopropanes 1i–n bearing quaternary stereocenters were then prepared. Bicyclo[4.1.0] aminocyclopropane 1i led to the formation of tetracyclic compound 5c in 75% yield and 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 dr. Aminocyclopropane 1j bearing a fully substituted center at the donor position showed some reactivity only at room temperature, leading to product 5d in 27% yield and >95
33 dr. Aminocyclopropane 1j bearing a fully substituted center at the donor position showed some reactivity only at room temperature, leading to product 5d in 27% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr. Aminocyclopropanes fully substituted at the carbon center next to the ester group were more reactive. Alkyl, aryl and allyl substituents led to the formation of (3 + 2) cycloadducts 5e–g bearing a carbon quaternary center in 78–86% yield and 80
5 dr. Aminocyclopropanes fully substituted at the carbon center next to the ester group were more reactive. Alkyl, aryl and allyl substituents led to the formation of (3 + 2) cycloadducts 5e–g bearing a carbon quaternary center in 78–86% yield and 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20–88
20–88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 dr.18 To the best of our knowledge, such indoline products bearing a quaternary stereocenter have never been accessed before via an annulation of D–A cyclopropanes. When an intramolecular reaction was performed with aminocyclopropane 1n, the desired product 5h was obtained in >95
12 dr.18 To the best of our knowledge, such indoline products bearing a quaternary stereocenter have never been accessed before via an annulation of D–A cyclopropanes. When an intramolecular reaction was performed with aminocyclopropane 1n, the desired product 5h was obtained in >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, but with another relative configuration (supported by NOESY experiments, see ESI†), in agreement with the results reported by Tang and co-workers using aminocyclopropane diesters.16
5 dr, but with another relative configuration (supported by NOESY experiments, see ESI†), in agreement with the results reported by Tang and co-workers using aminocyclopropane diesters.16
We further performed the reaction with 1 mmol of aminocyclopropane 1d with protected indoles 2b–d and obtained similar yields and dr (Scheme 5). With 2a, a further scale up to 1.00 g (3.36 mmol) was done, giving 4a in 90% yield and 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 dr. After reduction of the ester on 4c with DIBALH, the tosyl group was removed using reductive naphthalene/lithium conditions leading to amino alcohol 6. Due to the cis orientation of the nitrogen and the ester, a bridgehead lactam 7 was produced in 43% yield when tosyl removal was performed directly on 4c. Finally, the TBS protecting group was removed with TBAF producing free indole 8 in 82% yield. Unfortunately, attempts to epimerize the ester center through enolate formation followed by reprotonation were not successful.
7 dr. After reduction of the ester on 4c with DIBALH, the tosyl group was removed using reductive naphthalene/lithium conditions leading to amino alcohol 6. Due to the cis orientation of the nitrogen and the ester, a bridgehead lactam 7 was produced in 43% yield when tosyl removal was performed directly on 4c. Finally, the TBS protecting group was removed with TBAF producing free indole 8 in 82% yield. Unfortunately, attempts to epimerize the ester center through enolate formation followed by reprotonation were not successful.
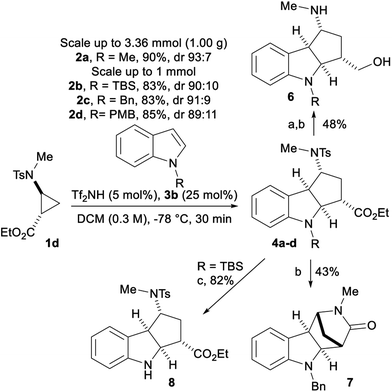 | ||
| Scheme 5 Scale up experiments and product modifications. Reaction conditions: (a) DIBALH, THF, 0 °C; (b) Li/naphthalene, THF, rt; (c) TBAF, THF, 0 °C. | ||
We then attempted to gain information about the reaction mechanism by starting with enantiopure aminocyclopropane ent-1d (Scheme 6a, eqn (1)).22 Racemic cycloadduct 4a was obtained in the TES triflimide-catalyzed (3 + 2) annulation with 2a (eqn (1)). Moreover, using cis-substituted cyclopropane cis-1d led to the formation of 4a with the same diastereoselectivity as observed for trans-substituted cyclopropane 1d (eqn (2)). Considering these results, the formation of an open-chain reactive intermediate is probable (Scheme 6b). The protodesilylation of silyl ketene acetal 3b produces the active TES triflimide catalyst,17,18 which then activates aminocyclopropane 1d through silylation of the ester. Ring-opening leads to iminium I, which is attacked by indole 2a at the most nucleophilic position to give iminium II. A Mannich reaction closes then the ring delivering the (3 + 2) cycloadduct 4a. The diastereoselectivity is controlled by minimizing steric repulsions between the silyl enol ether and the indole ring (IIvs.III).
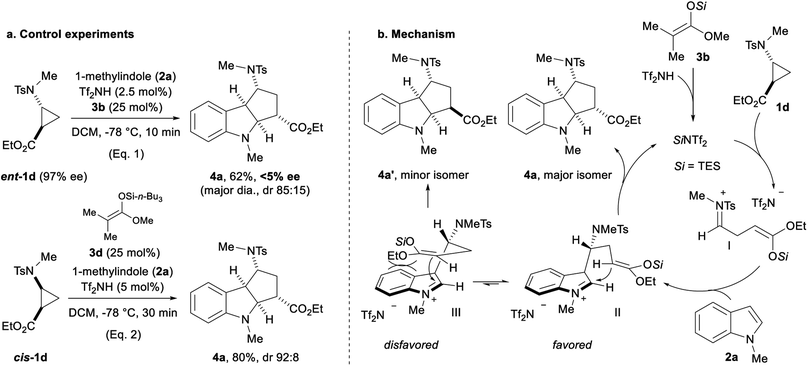 | ||
| Scheme 6 Influence of the absolute and relative configuration of the starting aminocyclopropane 1d on the (3 + 2) annulation with 1-methylindole (2a) (a); speculative mechanism proposal (b). | ||
3. Conclusion
In conclusion, a (3 + 2) annulation reaction of tosyl-protected aminocyclopropane monoesters with indoles catalyzed by triethylsilyl triflimide was disclosed. The tricyclic indoline products were obtained in excellent yields, high degrees of stereoselectivity and short reaction times (less than 30 minutes) with the formation of four stereocenters in one operation, including quaternary centers. The method gives access to complex nitrogen-substituted polycyclic indoline scaffolds of high interest for synthetic and medicinal chemistry.Author contributions
V. P. discovered and optimized the reaction, studied the scope, performed the functionalization of the products and the studies on the mechanism, prepared the experimental part and the first draft of the manuscript. E. G. L. R. did the required revisions after the departure of V. P. She performed the scale up of the transformation and further scope extension and functionalization attempts on the products and prepared the related experimental part. J. W. designed the overall research, supervised the work, finalized the manuscript, proofread the experimental part and coordinated the overall project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank EPFL for financial support and Dr R. Scopelliti from ISIC at EPFL for X-ray analysis.Notes and references
- Selected reviews: (a) H.-U. Reissig and R. Zimmer, Chem. Rev., 2003, 103, 1151–1196 CrossRef CAS PubMed; (b) M. Yu and B. L. Pagenkopf, Tetrahedron, 2005, 61, 321 CrossRef CAS; (c) M. A. Cavitt, L. H. Phun and S. France, Chem. Soc. Rev., 2014, 43, 804–818 RSC; (d) T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504–5523 CrossRef CAS PubMed; (e) H. K. Grover, M. R. Emmett and M. A. Kerr, Org. Biomol. Chem., 2015, 13, 655–671 RSC; (f) A. K. Pandey, A. Ghosh and P. Banerjee, Isr. J. Chem., 2016, 56, 512–521 CrossRef CAS; (g) R. Talukdar, A. Saha and M. K. Ghorai, Isr. J. Chem., 2016, 56, 445–453 CrossRef CAS; (h) O. Reiser, Isr. J. Chem., 2016, 56, 531–539 CrossRef CAS; (i) E. M. Budynina, K. L. Ivanov, I. D. Sorokin and M. Y. Melnikov, Synthesis, 2017, 49, 3035–3068 CrossRef CAS.
- Selected examples: (a) P. D. Pohlhaus and J. S. Johnson, J. Am. Chem. Soc., 2005, 127, 16014–16015 CrossRef CAS PubMed; (b) P. D. Pohlhaus and J. S. Johnson, J. Org. Chem., 2005, 70, 1057–1059 CrossRef CAS PubMed; (c) A. T. Parsons and J. S. Johnson, J. Am. Chem. Soc., 2009, 131, 3122–3123 CrossRef CAS PubMed; (d) A. T. Parsons, A. G. Smith, A. J. Neel and J. S. Johnson, J. Am. Chem. Soc., 2010, 132, 9688–9692 CrossRef CAS PubMed; (e) C. A. Carson and M. A. Kerr, J. Org. Chem., 2005, 70, 8242–8244 CrossRef CAS PubMed; (f) J. E. Curiel Tejeda, L. C. Irwin and M. A. Kerr, Org. Lett., 2016, 18, 4738–4741 CrossRef CAS; (g) J.-P. Qu, C. Deng, J. Zhou, X.-L. Sun and Y. Tang, J. Org. Chem., 2009, 74, 7684–7689 CrossRef CAS PubMed; (h) H. Xu, J.-P. Qu, S. Liao, H. Xiong and Y. Tang, Angew. Chem., Int. Ed., 2013, 52, 4004–4007 CrossRef CAS PubMed; (i) H. Xiong, H. Xu, S. Liao, Z. Xie and Y. Tang, J. Am. Chem. Soc., 2013, 135, 7851–7854 CrossRef CAS PubMed; (j) G. Yang, Y. Shen, K. Li, Y. Sun and Y. Hua, J. Org. Chem., 2011, 76, 229–233 CrossRef CAS PubMed; (k) G. Sathishkannan and K. Srinivasan, Org. Lett., 2011, 13, 6002–6005 CrossRef CAS PubMed; (l) A. F. G. Goldberg, N. R. O. Connor, R. A. Craig and B. M. Stoltz, Org. Lett., 2012, 14, 5314–5317 CrossRef CAS PubMed; (m) H. Wang, W. Yang, H. Liu, W. Wang and H. Li, Org. Biomol. Chem., 2012, 10, 5032–5035 RSC; (n) S. Chakrabarty, I. Chatterjee, B. Wibbeling, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2014, 53, 5964–5968 CrossRef CAS PubMed; (o) D.-C. Wang, M.-S. Xie, H.-M. Guo, G.-R. Qu, M.-C. Zhang and S.-L. You, Angew. Chem., Int. Ed., 2016, 55, 14111–14115 CrossRef CAS PubMed; (p) K. Verma and P. Banerjee, Adv. Synth. Catal., 2016, 358, 2053–2058 CrossRef CAS; (q) A. U. Augustin, M. Sensse, P. G. Jones and D. B. Werz, Angew. Chem., Int. Ed., 2017, 56, 14293–14296 CrossRef CAS PubMed; (r) A. U. Augustin, M. Busse, P. G. Jones and D. B. Werz, Org. Lett., 2018, 20, 820–823 CrossRef CAS PubMed; (s) N. L. Ahlburg, P. G. Jones and D. B. Werz, Org. Lett., 2020, 22, 6404–6408 CrossRef CAS PubMed; (t) A. Kaga, D. A. Gandamana, S. Tamura, M. Demirelli and S. Chiba, Synlett, 2017, 28, 1091–1095 CrossRef CAS; (u) Y. Matsumoto, D. Nakatake, R. Yazaki and T. Ohshima, Chem.–Eur. J., 2018, 24, 6062–6066 CrossRef CAS PubMed; (v) M. Mondal, M. Panda, N. W. Davis, V. McKee and N. J. Kerrigan, Chem. Commun., 2019, 55, 13558–13561 RSC.
- For a review see: (a) F. de Nanteuil, F. De Simone, R. Frei, F. Benfatti, E. Serrano and J. Waser, Chem. Commun., 2014, 50, 10912–10928 RSC; selected examples: (b) F. de Nanteuil and J. Waser, Angew. Chem., Int. Ed., 2011, 50, 12075–12079 CrossRef CAS; (c) F. Benfatti, F. de Nanteuil and J. Waser, Org. Lett., 2012, 14, 386–389 CrossRef CAS; (d) F. Benfatti, F. de Nanteuil and J. Waser, Chem.–Eur. J., 2012, 18, 4844–4849 CrossRef CAS PubMed; (e) S. Racine, F. de Nanteuil, E. Serrano and J. Waser, Angew. Chem., Int. Ed., 2014, 53, 8484–8487 CrossRef CAS PubMed; (f) F. de Nanteuil, E. Serrano, D. Perrotta and J. Waser, J. Am. Chem. Soc., 2014, 136, 6239–6242 CrossRef CAS PubMed; (g) S. Racine, B. Hegedüs, R. Scopelliti and J. Waser, Chem.–Eur. J., 2016, 22, 11997–12001 CrossRef CAS PubMed; (h) J. Preindl, S. Chakrabarty and J. Waser, Chem. Sci., 2017, 8, 7112–7118 RSC; (i) A. A. Suleymanov, E. Le Du, Z. Dong, B. Muriel, R. Scopelliti, F. Fadaei-Tirani, J. Waser and K. Severin, Org. Lett., 2020, 22, 4517–4522 CrossRef CAS PubMed; (j) A. R. Rivero, I. Fernandez and M. Sierra, Org. Lett., 2013, 15, 4928–4931 CrossRef CAS PubMed; (k) M.-C. Zhang, D.-C. Wang, M.-S. Xie, G.-R. Qu, H.-M. Guo and S.-L. You, Chem, 2019, 5, 156–167 CrossRef CAS; (l) E.-J. Hao, D.-D. Fu, D.-C. Wang, T. Zhang, G.-R. Qu, G.-X. Li, Y. Lan and H.-M. Guo, Org. Chem. Front., 2019, 6, 863–867 RSC; (m) H.-X. Wang, W.-P. Li, M.-M. Zhang, M.-S. Xie, G.-R. Qu and H.-M. Guo, Chem. Commun., 2020, 56, 11649–11652 RSC.
- (a) L. Wang and Y. Tang, Isr. J. Chem., 2016, 56, 463–475 CrossRef CAS; (b) V. Pirenne, B. Muriel and J. Waser, Chem. Rev., 2021, 121, 227–263 CrossRef CAS PubMed; (c) Y. Xia, X. H. Liu and X. M. Feng, Angew. Chem., Int. Ed., 2021, 60, 9192–9204 CrossRef CAS PubMed.
- P.-P. Zhang, Z.-M. Yan, Y.-H. Li, J.-X. Gong and Z. Yang, J. Am. Chem. Soc., 2017, 139, 13989–13992 CrossRef CAS PubMed.
- Selected examples: (a) L. Li, Z. Li and Q. Wang, Synlett, 2009, 1830–1834 CAS; (b) C. Sparr and R. Gilmour, Angew. Chem., Int. Ed., 2011, 50, 8391–8395 CrossRef CAS PubMed; (c) J. Wallbaum, L. K. B. Garve, P. G. Jones and D. B. Werz, Chem.–Eur. J., 2016, 22, 18756–18759 CrossRef CAS PubMed; (d) E. Diaz, E. Reyes, U. Uria, L. Carrillo, T. Tejero, P. Merino and J. L. Vicario, Chem.–Eur. J., 2018, 24, 8764–8768 CrossRef CAS; (e) R. Dey and P. Banerjee, Adv. Synth. Catal., 2019, 361, 2849–2854 CrossRef CAS.
- Selected examples: (a) M. Komatsu, I. Suehiro, Y. Horiguchi and I. Kuwajima, Synlett, 1991, 771–773 CrossRef CAS; (b) Y. Horiguchi, I. Suehiro, A. Sasaki and I. Kuwajima, Tetrahedron Lett., 1993, 34, 6077–6080 CrossRef CAS; (c) V. K. Yadav and V. Sriramurthy, Angew. Chem., Int. Ed., 2004, 43, 2669–2671 CrossRef CAS PubMed; (d) C. Venkatesh, P. P. Singh, H. Ila and H. Junjappa, Eur. J. Org. Chem., 2006, 5378–5386 CrossRef CAS.
- Selected examples: (a) N. A. Morra, C. L. Morales, B. Bajtos, X. Wang, H. Jang, J. Wang, M. Yu and B. L. Pagenkopf, Adv. Synth. Catal., 2006, 348, 2385–2390 CrossRef CAS; (b) B. Bajtos, M. Yu, H. Zhao and B. L. Pagenkopf, J. Am. Chem. Soc., 2007, 129, 9631–9634 CrossRef CAS PubMed; (c) X. Qi and J. M. Ready, Angew. Chem., Int. Ed., 2008, 47, 7068–7070 CrossRef CAS PubMed.
- (a) Y. Sugita, K. Kawai, H. Hosoya and I. Yokoe, Heterocycles, 1999, 51, 2029–2033 CrossRef CAS; (b) Y. Sugita, S. Yamadoi, H. Hosoya and I. Yokoe, Chem. Pharm. Bull., 2001, 49, 657–658 CrossRef CAS PubMed; (c) S. Xing, Y. Li, Z. Li, C. Liu, J. Ren and Z. Wang, Angew. Chem., Int. Ed., 2011, 50, 12605–12609 CrossRef CAS PubMed; (d) P.-W. Xu, J.-K. Liu, L. Shen, Z.-Y. Cao, X.-L. Zhao, J. Yan and J. Zhou, Nat. Commun., 2017, 8, 1619 CrossRef.
- K. Takasu, S. Nagao and M. Ihara, Adv. Synth. Catal., 2006, 348, 2376–2380 CrossRef CAS.
- For the synthesis of carbamate-protected aminocyclopropanes, see: (a) S. Chanthamath, D. T. Nguyen, K. Shibatomi and S. Iwasa, Org. Lett., 2013, 15, 772–775 CrossRef CAS PubMed; for ring-opening processes of aminocyclopropanes see: (b) G. Oezueduru, T. Schubach and M. M. K. Boysen, Org. Lett., 2012, 14, 4990–4993 CrossRef CAS PubMed; (c) Y. Jiang, V. Z. Y. Khong, E. Lourdusamy and C.-M. Park, Chem. Commun., 2012, 48, 3133–3135 RSC.
- (a) F. De Simone, J. Gertsch and J. Waser, Angew. Chem., Int. Ed., 2010, 49, 5767–5770 CrossRef CAS PubMed; (b) R. Frei, D. Staedler, A. Raja, R. Franke, F. Sasse, S. Gerber-Lemaire and J. Waser, Angew. Chem., Int. Ed., 2013, 52, 13373–13376 CrossRef CAS PubMed.
- (a) M. Gorman, N. Neuss, G. H. Svoboda, A. J. Barnes and N. J. Cone, J. Am. Pharm. Assoc., 1959, 48, 256–257 CrossRef CAS; (b) A. Ahond, M. M. Janot, N. Langlois, G. Lukacs, P. Potier, P. Rasoanaivo, M. Sangare, N. Neuss, N. Platt, J. Lemen, E. W. Hagaman and E. Wenkert, J. Am. Chem. Soc., 1974, 96, 633–634 CrossRef CAS; (c) J. Addae-Kyereme, S. L. Croft, H. Kendrick and C. W. Wright, J. Ethnopharmacol., 2001, 76, 99–103 CrossRef CAS; (d) J. T. Ndongo, J. N. Mbing, A. Monteillier, M. F. Tala, M. Rütten, D. Mombers, M. Cuendet, D. E. Pegnyemb, B. Dittrich and H. Laatsch, J. Nat. Prod., 2018, 81, 1193–1202 CrossRef CAS PubMed.
- (a) P. Harrington and M. A. Kerr, Tetrahedron Lett., 1997, 38, 5949–5952 CrossRef CAS; (b) M. A. Kerr and R. G. Keddy, Tetrahedron Lett., 1999, 40, 5671–5675 CrossRef CAS; (c) D. B. England, T. D. O. Kuss, R. G. Keddy and M. A. Kerr, J. Org. Chem., 2001, 66, 4704–4709 CrossRef CAS PubMed.
- F. de Nanteuil, J. Loup and J. Waser, Org. Lett., 2013, 15, 3738–3741 CrossRef CAS PubMed.
- H.-K. Liu, S. R. Wang, X.-Y. Song, L.-P. Zhao, L. Wang and Y. Tang, Angew. Chem., Int. Ed., 2019, 58, 4345–4349 CrossRef CAS.
- (a) B. Mathieu and L. Ghosez, Tetrahedron Lett., 1997, 38, 5497–5500 CrossRef CAS; (b) B. Mathieu and L. Ghosez, Tetrahedron, 2002, 58, 8219–8226 CrossRef CAS.
- Although TMS trifimide can be formed in situ by protodesilylation of allyltrimethylsilane, vinyltrimethylsilane, aryltrimethylsilane and trimethylsilane,16 silyl ketene acetals were selected for the generation of silyl triflimide catalysts because of their versatility and easy preparation: (a) R. Kakuchi, K. Chiba, K. Fuchise, R. Sakai, T. Satoh and T. Kakuchi, Macromolecules, 2009, 42, 8747–8750 CrossRef CAS; (b) T. Gatzenmeier, P. S. J. Kaib, J. B. Lingnau, R. Goddard and B. List, Angew. Chem., Int. Ed., 2018, 57, 2464–2468 CrossRef CAS PubMed.
- Benzofurans, furans, pyrroles, quinolines, 1,4-dimethoxybenzene and enamides could not be used as reaction partners. See ESI† for details.
- The X-ray data for 4o and 5g (CCDC numbers 2054864 and 2054865 respectively†).
- The cyclopropanation usually proceeded in high yield, but very low diastereoselectivity. The trans isomer was isolated to perform the annulation. However, both isomers performed equally well in the (3 + 2) process to give the same product with identical diastereoselectivity (see Scheme 6 and ESI†).
- The enantioselective cyclopropanation of vinyl carbamates described by Iwasa and co-workers11a was also suitable for vinyl sulfonamides delivering 1d in 97% ee (see ESI†).
Footnote |
| † Electronic supplementary information (ESI) available: Experimental data. Raw data is available at zenodo.org: https://doi.org/10.5281/zenodo.4705362. CCDC 2054864 and 2054865. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc01127h |
| This journal is © The Royal Society of Chemistry 2021 |