Recent progress in the syntheses and applications of multishelled hollow nanostructures
Maiyong
Zhu
 *,
Jingjing
Tang
,
Wenjing
Wei
and
Songjun
Li
*
*,
Jingjing
Tang
,
Wenjing
Wei
and
Songjun
Li
*
Research School of Polymeric Materials, School of Materials Science & Engineering, Jiangsu University, Zhenjiang, 212013, China. E-mail: maiyongzhu@ujs.edu.cn; lsjchem@ujs.edu.cn
First published on 23rd January 2020
Abstract
Multishelled hollow nanostructures have attracted considerable research interest owing to their unique structural features, promising properties, and fascinating performances in relevant applications. During the past few decades, considerable progress has been made in the synthesis of multishelled hollow nanostructures by accurately controlling their geometric morphology, chemical composition, thickness, number of shells, and their applications in various fields. In this review, we present a comprehensive overview on the recently used synthesis approaches for fabricating multishelled hollow nanostructures. For a comprehensive review, the synthesis approaches have been classified into four categories, namely, hard template, soft template, self-template, and template-free approaches based on the template/structure-directing agent used. The advantages and disadvantages of each approach are discussed by comparing with each other. Furthermore, the fascinating performances of multishelled hollow nanostructures, application in energy conversion and storage, environment remediation, chemical catalysis, and biomedicine are comprehensively summarized. Combining certain typical examples and related theoretical analysis, the relationship between the structure of multishelled hollow nanomaterials and their specific application performances in the related areas is highlighted. Finally, the emerging challenges and future prospects of multishelled hollow nanostructures in the research and development for the future are outlined.
1 Introduction
Multishelled hollow nanostructures particularly refer to hollow structures that have at least two separate shells spatially ordered from the outside to the inside. As compared to solid materials as well as traditional single-shelled hollow nanostructures, multishelled hollow nanostructures exhibit multiple advantages. Firstly, multishelled structures may be endowed with prominent properties for specific applications by the integration of various functional components into a single system. Secondly, multishelled hollow structures are expected to allow more abundant exposure of the available active surface, which facilitates the accommodation of drug molecules and biomolecules (such as enzymes). Thirdly, the voids between the neighboring shells may act as a depot to hold various cargoes and a reaction chamber during catalysis. Finally, because of the higher number of shells, their mechanical stability is often much better than that of single-shelled hollow structures, which is particularly important when used as electrodes for energy storage, since such a void in hollow configurations as well as the space between the shells can be expected to buffer volume changes.1,2Owing to their fascinating structural characteristics and superior properties, multishelled hollow nanostructures are expected to provide promising application potential in various fields.3–8 The development of multishelled hollow nanostructures can be divided into two stages. Before 2009, owing to the shortage of facile, scalable, and controllable synthesis approaches, the development of multishelled hollow nanostructures was very slow. Since Wang's group developed the sequential template approach in 2009, the development of multishelled hollow nanostructures has been considerably enhanced. It is worth noting that other strategies have also been developed during the past years to synthesize multishelled hollow nanostructures in spite of the fact that about 72.5% of multishelled hollow nanostructures have been prepared by this sequence template approach during 2018.9 Many leading research groups have made pioneering works in the synthesis of multishelled hollow nanostructures. These methods could be generally divided into the hard template, soft template (various surfactants), self-template, and template-free approaches. Each method has its own advantages and disadvantages. For example, the removal of a hard template without destroying the structure of the final product is usually a challenge for the hard template method. Meanwhile, the synthesis process for the hard template method is tedious and incurs a high cost. Soft templates (organic molecule assembly, such as micelles, emulsion droplets, and vesicles) are expected to yield products with uniform sizes and shapes. However, the purity of the final product might be seriously affected by the organic molecule assembly. Furthermore, organic molecule assemblies are thermodynamically unstable, which are highly sensitive to the environment; therefore, it is difficult to extend this method to a wide range of multishelled hollow structures. With regard to the self-template and template-free methods, the quality of the final product may be controlled by adjusting the reaction parameters, such as temperature and time. However, these methods have considerable dependence on the precursor systems and reaction conditions.10–15
Certain research groups have summarized the exploration and development of multishelled hollow nanostructures. For example, a comprehensive review article in 2015 formulated by Qi et al. discussed the progress made in the synthesis of multishelled hollow nanostructures and their applications in dye-sensitized solar cells, lithium-ion batteries (LIBs), supercapacitors, sensors, photocatalysis, and drug delivery.16 Qin et al. provided an overview on the recent advances made in the synthesis of multishelled hollow nanostructures with typical single-component metal oxides such as NiO, Co3O4, and ZnO, as well as their morphology-related applications.17 Ren et al. and Liu et al. summarized the research progress made in the synthesis of such materials and their promising applications in energy conversion and storage.18,19 Wang et al. gave a brief summary on the advancements made in multishelled hollow nanostructures for LIBs.20 Mao et al. highlighted the synthesis methods, particularly the sequential template approach, toward multishelled hollow nanostructures for various applications.9 Recently, Wang et al. also provided an in-depth understanding on the structure–performance correlation in multishelled hollow nanostructures for promising applications such as energy storage, electromagnetic wave absorption, catalysis, sensors, and drug delivery.21 Apparently, in spite of the above reviews on multishelled hollow nanostructures, most of which focused on certain special aspects, only Qi et al. provided a comprehensive review in 2015. It is well known that the research and development in this field has been very rapid. In this context, we have provided a more detailed discussion on the advancement of the syntheses and applications of multishelled hollow nanostructures. For the sake of convenience, the synthesis methods are divided into four categories: hard template method, soft template method, self-template method, and template-free method. For clearly showing the principle and operating procedures for each method, we provided many examples according to the template materials or precursors used for forming the multishelled hollow nanostructures. For instance, hard templates are further classified as silica, carbonaceous, polymer, metal oxide, and others. Soft templates involve single surfactant and dual/multi-surfactant systems. Three types of precursors were highlighted in the self-template approach section, namely, coordination polymers (CPs) or metal–organic frameworks (MOFs), metal glycerates/glycorates, and metal–inorganic precursors (oxide/hydroxide/carbonate). Here, it should be noted that the sequential template approach has not been listed as a specific synthesis method owing to its considerable overlap with the hard template and self-template methods. When compared with Qi's comprehensive review, additional recent application areas of multishelled hollow nanostructures have been incorporated into this review, such as the adsorptive removal of pollutants, catalytic conversion of gas pollutants, and high-performance batteries (sodium-ion batteries; lithium–sulfur batteries). Finally, a brief outlook and future challenges of this research field are also pointed out.
2 Synthesis strategies for multishelled hollow nanostructures
2.1 Hard template approach
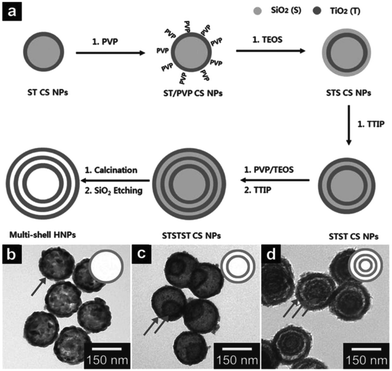
From a simplistic point of view, the hard template approach seems to be the most straightforward tool for synthesizing multishelled hollow nanostructures. In general, the conversion of a traditional hard template approach to multishelled hollow nanostructures includes the following: (i) the fabrication of templates with the desired shapes; (ii) in situ formation of the target architectures using the above templates, yielding template@target materials with core–shell structures; (iii) assembly of the template on the outer surface of the template@target composites; and (iv) repeating steps ii and iii, and ending with step ii, and finally resulting in the removal of the template (Fig. 1). To form a successful coating between the shell and template materials, a surface modification step might become necessary, using which the surface functionality can be adjusted, such as surface charge and polarity. Many techniques, such as solvothermal treatment, static electronic deposition, electrochemical deposition, chemical oxidation, or self-assembly process, might be useful for depositing the shell/template materials on the template/shell surface. With regard to the removal of the hard template, a specific procedure might considerably depend on the inherent properties of the hard template. Typically, chemical etching, thermal decomposition or calcination, and solvent dissolution in a specific solvent can be applied for the removal of the hard template. Until now, several rigid materials, such as silica, carbonaceous, polymers, and metallic oxides, have been successfully developed as hard templates to synthesize multishelled hollow nanostructures. In this section, we will introduce the hard template syntheses of multishelled hollow nanostructure based on their different compositions. | ||
| Fig. 1 Schematic illustration of the hard template principle for preparing multishelled hollow nanostructures. | ||
 | ||
| Fig. 2 (a) Schematic illustration of the synthesis of multishelled porous TiO2 hollow nanoparticles using SiO2 as the hard template. Typical TEM images of single-shelled (b), double-shelled (c), and triple-shelled (d) hollow TiO2 nanospheres. Reprinted from ref. 19 with permission. Copyright 2019 John Wiley & Sons. | ||
Lou and co-workers presented a shell-by-shell hydrothermal synthesis process to synthesize doubled-shelled hollow SnO2 using monodisperse SiO2 nanospheres (diameter: ∼320 nm) as the sacrificial hard template.23 The core silica template was coated with a double layer of polycrystalline SnO2 obtained via a hydrothermal process to form uniform double shells. Subsequently, the silica-cores-based as-formed double-shelled particles were etched with dilute HF to control its size (partially dissolved or completely dissolved). Interestingly, it was observed that the double-shelled hollow particles transformed into single-shelled particles on etching with the dilute HF solution.
In addition to solid silica-based materials, hollow silica structures have also been employed as the hard template to synthesize multishelled hollow nanostructures. When using a hollow template, both inner and outer surfaces could adsorb the precursors of the target materials. Hierarchical double-shelled metal sulfide (NiS, CuS, and MnS) hollow structures were successfully synthesized by Yu et al. by using silica nanoboxes as the hard template.24 The synthesis strategy for the formation of multishelled NiS box-in-box hollow structures started from silica boxes (shell thickness: ∼100 nm) that were presynthesized using Fe2O3 as the template. Initially, the silica boxes were dispersed in an alkaline solution containing nickel acetate, NH3·H2O, and NH4Cl. Under hydrothermal conditions, silica gets partially dissolved to generate silicate anions. These alkaline-induced silicate anions would further react with Ni2+, forming a thin layer of Ni silicate on the surface of the silica box. As the reaction proceeds, more silicate anions derived from the dissolution of silica may diffuse outward and promote the growth of the Ni silicate shell, simultaneously forming a void between the residual silica core and Ni silicate shell. When the size of the void reaches a critical value, the silicate anions may react with the inward-diffusing Ni2+, forming a secondary shell layer of Ni silicate. After the silica core is completely consumed, a double-shelled Ni silicate box-in-box hollow structure is formed. Finally, by treating with Na2S, the as-prepared Ni silicate box-in-box hollow structures with double shells are converted into NiS box-in-box hollow nanostructures. Such a synthesis procedure is expected to be extended to synthesize other metal sulfides with similar structures. Fig. 3a–c show the typical TEM images of the as-prepared box-in-box hollow nanostructures of NiS, CuS, and MnS, which exhibits uniform box-in-box hollow structures of all the synthesized materials. Owing to the different properties of these different metal ions, the primary building blocks of the three box-in-box hollow structures are ultrathin nanosheets (NiS), ultrafine nanoneedles (CuS), and nanoparticles (MnS). Du et al. prepared double-shelled NiS hollow nanostructures with different morphologies (cube, ellipsoid, and capsule) using similar procedures.25 Using hollow silica as the hard template, Li et al. prepared double-shelled SnO2@C hollow spheres.26
 | ||
| Fig. 3 Schematic illustration of the synthesis procedure of NiS box-in-box hollow structures. TEM images of the box-in-box hollow structures: NiS (a), CuS (b), and MnS (c). Reprinted from ref. 24 with kind permission. | ||
Sun et al. prepared double- and quadruple-shelled carbon hollow spheres by using single-shelled silica and double-shelled silica, respectively, as a permeable template. The entire preparation procedure is shown in Fig. 4.27 Evidently, step I enumerates the process to form double-shelled carbon hollow spheres, which involves several procedures. Initially, mesoporous silica hollow spheres were obtained by using a common vesicle template route, as explained in an earlier report. In order to improve the affinity between the carbon (precursor) and mesoporous silica hollow spheres, the amine-terminated functionalization properties of the mesoporous silica hollow spheres were applied. Subsequently, both the outer and inner surfaces of the mesoporous silica hollow spheres were coated with carbon using glucose as a precursor through a hydrothermal reaction followed by calcination. Finally, double-shelled carbon hollow spheres were obtained after removing the mesoporous silica hollow spheres with HF. The procedures for forming quadruple-shelled carbon hollow spheres (step II) are very similar to those for forming double-shelled carbon hollow spheres by replacing the mesoporous silica hollow spheres with double-shelled silica hollow spheres.
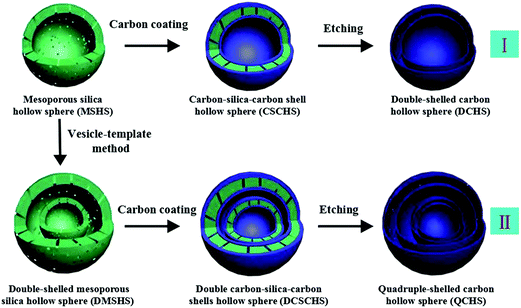 | ||
| Fig. 4 Schematic illustration of the preparation procedure of double- and quadruple-shelled carbon hollow spheres. Reproduced from ref. 27 with permission. | ||
Lai et al. developed a generic sequential template approach based on carbonaceous microspheres to prepare a series of multishelled metal oxide hollow microspheres, as shown in Fig. 5a. The driving force for the adsorption of metal cations into the carbonaceous template is the electrostatic attraction between the positively charged cations and negatively charged carbonaceous templates. After the adsorption of metallic ions, the carbonaceous spheres (CSs) could be sacrificed under thermal treatments, thereby yielding metal oxides with hollow structures. By repeating the adsorption of metal precursors and the heating process, the number of shells of the final metal oxide product may be controlled. As shown in Fig. 5b–d, the number of shells of α-Fe2O3 hollow microspheres can be easily controlled, ranging from 2 to 4.28 Wu et al. and Chu et al. demonstrated the shell-by-shell assembly formation mechanism of synthesizing multishelled Cr2O3, ZnO, NiO, and Co3O4 hollow microspheres by using glucose-derived carbonaceous microspheres as the hard template. Interestingly, the metallic precursor (Cr(NO3)3·9H2O, ZnSO4·7H2O, Ni(NO3)2·6H2O, or Co(NO3)2·6H2O) and carbonaceous precursor (D-glucose) were initially mixed together before the carbonaceous template formation and subsequent annealing treatment.29–33
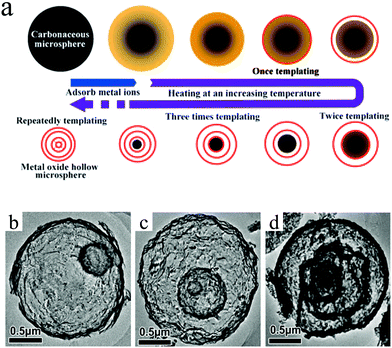 | ||
| Fig. 5 (a) Schematic diagram of the sequential template strategy to multishelled metal oxide hollow microspheres. TEM images of double-shelled (b), triple-shelled (c), and quadruple-shelled (d) α-Fe2O3 hollow microspheres. Reprinted from ref. 28 with kind permission. | ||
Dong et al. presented a programmable heating process to synthesize multishelled ZnO, TiO2, and α-Fe2O3 hollow microspheres using carbonaceous microspheres as the hard template.34–36 The formation mechanism of ZnO multishelled hollow microspheres is shown in Fig. 6. Hydroxide- or carboxylic-acid-modified CSs can easily absorb zinc ions when immersed in a zinc salt solution. Upon calcination, the CSs can evaporate and direct the fabrication of ZnO shells. The separation of the outer ZnO shell layer and contraction of the inner carbon core are the two key factors to generate multishelled ZnO hollow nanospheres. Moreover, the concentration of zinc precursors also has a significant influence on the structure of the final products. Typically, the higher concentration of zinc precursors may facilitate a deeper penetration of zinc ions into the carbonaceous template cores, which implies that additional zinc ions will get adsorbed on the inside of the inner core. As a consequence, the number of shells may increase with a higher concentration of zinc precursors. In addition, the diameter of the carbonaceous template is another important factor affecting the quality of the final products. It is well known that the larger carbonaceous microspheres are expected to provide more space to accommodate more zinc ions, thereby forming a hollow structure with more shells (quadruple or even more). Notably, other experimental parameters besides the above may also play a significant role in controlling the number of shells and thickness, such as the time for which the carbonaceous template is immersed in the zinc salt solution, acidity (pH value) of the solution, and experimental temperature, although the authors did not perform such investigations. In addition, suffering from the limited ability of the loading metal ion of the unmodified carbonaceous microsphere template, the synthesis approach could not be extended to prepare other multishelled semiconductor hollow structures with thick exterior shells. Using similar procedures, Niu et al. prepared SnO2 hollow spheres with double and triple shells.37
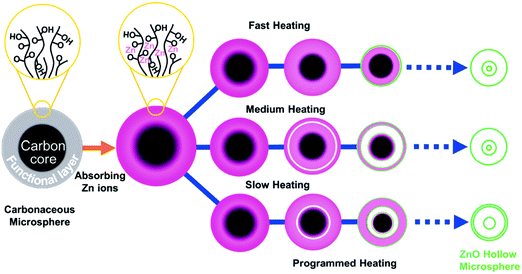 | ||
| Fig. 6 Schematic illustration of the synthesis process of multishelled ZnO hollow microspheres. Reprinted from ref. 34 with kind permission. | ||
In order to enhance the interaction between the carbonaceous template and metallic ions as well as improve the loading capability of the template, the pretreatment of a carbonaceous hard template is often applied. Dong et al. further reported the preparation of multishelled SnO2 hollow microspheres with controllable numbers of shells (∼1–5) using alkali-treated carbonaceous microspheres as the hard template through a direct one-step thermal treatment.38 The alkali-treated carbonaceous templates have abundant negatively charged hydroxyl surface functional groups, increasing the absorption of Sn4+ cation in the templates. Therefore, an increased number of SnO2 shells could be formed during such thermal treatments. Meanwhile, closed exterior double shells may be formed at higher absorption amounts of Sn4+. By varying the dosage of Sn4+, the number of multishells in the final product can be controlled to be within 1–5; the corresponding TEM images are shown in Fig. 7.
 | ||
| Fig. 7 TEM images of (a) single-, (b) double-, (c) triple-, (d) quadruple-, and (e) quintuple-shelled hollow SnO2 microspheres prepared by a one-step thermal treatment process using alkali-treated carbonaceous microspheres as the hard template. Reprinted from ref. 38 with kind permission. | ||
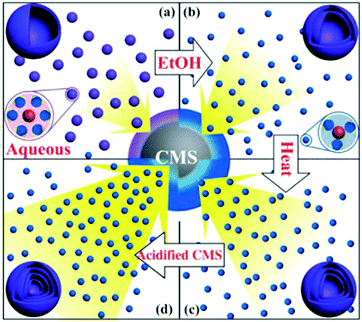
Despite the considerable achievement of controlling the number of shells, further improved measurements should be performed to synthesize a wide range of materials. When the radius of the hydrate metal ions is larger, such as cobalt, it might be difficult to control the number of shells in the product by using the above generic method. In order to circumvent this issue, on the basis of systematically investigating the relationship between the size and diffusion rate of the hydrate metal ions, Wang and co-workers successfully controlled the number of shells of Co3O4 hollow microspheres. According to their preliminary experiments, it was proposed that the rapid diffusion rate of hydrate metal ions facilitates the formation of more shelled structures, while both the size of the hydrate metal ions and temperature are important factors affecting their diffusion rates. Accordingly, a modified carbonaceous template approach in which the solvent composition could be adjusted was presented to control the number of shells in the Co3O4 hollow microspheres, as shown in Fig. 8.39,40 In an aqueous medium, Co(II) ions are expected to be in the form of [Co(H2O)6]2+ (Fig. 8a), which has a large radius as compared to normal ions and relatively low diffusion rates in a carbonaceous microsphere template; therefore, only single-shelled Co3O4 hollow microspheres (Fig. 9a) can be formed after the removal of the carbonaceous template via calcination. In a mixture of water and ethanol (e.g., ethanol/water in equal volume of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent, Co(II) ion would exist as [Co(H2O)(6−x)]2+, where x = 3–6 (Fig. 8b). With a decrease in the number of coordination water molecules, the radius of Co(II) decreases, offering a rapid diffusion rate than that in [Co(H2O)6]2+, which ensures that an increased amount of cobalt ions gets loaded into the carbonaceous template; this yields double-shelled Co3O4 hollow microspheres (Fig. 9b). By further increasing the ethanol content in the solvent (75%, v/v) along with simultaneously heating to a higher temperature (Fig. 8c), a higher diffusion rate can be achieved, thereby generating the ready adsorption of Co(II) by means of carbonaceous microspheres. As a consequence, a triple-shelled Co3O4 hollow microsphere is obtained (Fig. 9c). In addition to adjusting the solvent component, the authors also investigated the effect of acid-treated template on the adsorption of Co(II) as well as on the final structure of Co3O4 (Fig. 8d). Their results demonstrated that the carbonaceous microsphere template, after the treatment of acid, offered a much larger specific surface area and enhanced pore volume than those of the template that did not undergo any acid treatment. Larger surface areas and enhanced pore volumes are expected to increase the loading of metal ions, yielding quadruple-shelled hollow microspheres (Fig. 9d). In the subsequent work, they extended this sequence template approach to prepare multishelled hollow (Co2/3Mn1/3)(Co5/6Mn1/6)2O4. By precisely controlling the experimental parameters, the number of shells could be effectively controlled and the maximum number of shells reached up to a septuple, which is the highest number of shells obtained until now.41 Further, Wang et al. synthesized nanorod-assembled multishelled Co3O4 microspheres in a mixture of water and ethanol as the solvent using a carbon microsphere as the hard template, where carbon microspheres@Co2CO3(OH)2 core–shell composites were formed with a subsequent calcination process. After the calcination treatment, the carbon microspheres@Co2CO3(OH)2 core–shell composites could be converted into multishelled Co3O4 microspheres.42
1) as the solvent, Co(II) ion would exist as [Co(H2O)(6−x)]2+, where x = 3–6 (Fig. 8b). With a decrease in the number of coordination water molecules, the radius of Co(II) decreases, offering a rapid diffusion rate than that in [Co(H2O)6]2+, which ensures that an increased amount of cobalt ions gets loaded into the carbonaceous template; this yields double-shelled Co3O4 hollow microspheres (Fig. 9b). By further increasing the ethanol content in the solvent (75%, v/v) along with simultaneously heating to a higher temperature (Fig. 8c), a higher diffusion rate can be achieved, thereby generating the ready adsorption of Co(II) by means of carbonaceous microspheres. As a consequence, a triple-shelled Co3O4 hollow microsphere is obtained (Fig. 9c). In addition to adjusting the solvent component, the authors also investigated the effect of acid-treated template on the adsorption of Co(II) as well as on the final structure of Co3O4 (Fig. 8d). Their results demonstrated that the carbonaceous microsphere template, after the treatment of acid, offered a much larger specific surface area and enhanced pore volume than those of the template that did not undergo any acid treatment. Larger surface areas and enhanced pore volumes are expected to increase the loading of metal ions, yielding quadruple-shelled hollow microspheres (Fig. 9d). In the subsequent work, they extended this sequence template approach to prepare multishelled hollow (Co2/3Mn1/3)(Co5/6Mn1/6)2O4. By precisely controlling the experimental parameters, the number of shells could be effectively controlled and the maximum number of shells reached up to a septuple, which is the highest number of shells obtained until now.41 Further, Wang et al. synthesized nanorod-assembled multishelled Co3O4 microspheres in a mixture of water and ethanol as the solvent using a carbon microsphere as the hard template, where carbon microspheres@Co2CO3(OH)2 core–shell composites were formed with a subsequent calcination process. After the calcination treatment, the carbon microspheres@Co2CO3(OH)2 core–shell composites could be converted into multishelled Co3O4 microspheres.42
 | ||
| Fig. 8 Mechanism for the formation of multishelled Co3O4 hollow microspheres under different adsorption conditions. Reprinted from ref. 39 with permission. | ||
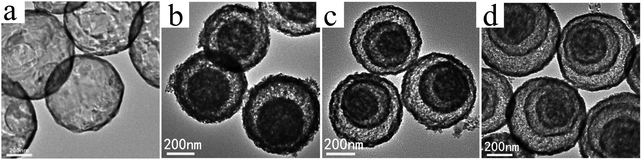 | ||
| Fig. 9 TEM images of (a) single-, (b) double-, (c) triple-, and (d) quadruple-shelled Co3O4 hollow microspheres. Reprinted from ref. 40 with kind permission. | ||
We have noted that the acid treatment on a carbonaceous template may promote the loading of cobalt ions into the template; in their earlier report, the treatment of alkali facilitated the adsorption of Sn4+ by the carbonaceous microsphere template. In fact, both acid treatment and alkali treatment may activate the carbonaceous template, generating more functional surface groups. According to the electronic theory of acids and alkalis, Co(II) ions can easily donate an electron due their large size and low nuclear charge, while the case for Sn(IV) is completely opposite. As a result, acid-treated carbonaceous microspheres are more preferable for loading Co(II), whereas alkali-treated carbonaceous microspheres are more suitable for adsorbing Sn(IV).
In another work, Wang et al. demonstrated that the pH value of a solution may also significantly affect the formation of multishelled Mn2O3 hollow nanostructures in addition to adjusting the solvent components and temperature.43 The higher pH of aqueous manganese acetate solutions can lead to the fabrication of hollow Mn2O3 spheres with a higher number of shells. The reason for such phenomena could be explained as follows. The higher concentration of H+ in the solution means that the functional groups (mainly hydroxide groups) get protonated. Therefore, a decrease in pH results in a decrease in the surface negative charge of the carbonaceous microsphere template, which could be confirmed by its zeta potential. As a consequence, the amount of Mn(II) adsorbed on the template also decreases, which is not beneficial for the formation of multiple-shelled hollow nanostructures. In particular, single-, double-, and triple-shelled Mn2O3 hollow nanospheres could be obtained at the pH values of 3.35, 4.43, and 6.43, respectively, of the solution. It should be noted that only a limited amount of Mn(II) could be adsorbed onto the carbonaceous microsphere templates, generating Mn2O3 nanoparticles, but not Mn2O3 hollow structures, when the pH of the solution was less than 1. Moreover, the form of Mn(II) may vary with the pH of the solution. It is well known that Mn(II) is likely to exist in the form of [Mn(H2O)6−xClx]2−x (0 < x < 2, when 0 ≤ pH ≤ 7) when HCl is used to adjust the pH of the solution. At lower pH, more Cl− can coordinate with Mn(II), which implies that the electrostatic attraction between Mn(II) and carbonaceous template becomes weaker and hence the amount of adsorbed Mn(II) onto the carbonaceous template decreases due to the generation of Mn2O3 hollow microspheres with a limited number of shells (even nanoparticle shape) after heating. Tian et al. proposed a general and versatile strategy to prepare a wide range of multishelled metal oxide hollow microspheres, including, but not limited to, Co3O4, CuO, Fe2O3, In2O3, PrO1.83, and ZnO, by using a commercial gluconate salt as both a carbon and metal precursor, where a similar effect of pH value could be observed.44
Using glucose as the carbon source and nickel nitrate as the nickel source, Chu et al. systematically investigated the effect of pH of the solution (ranging from 8.3 to 10.9) on the morphology of NiO using NH3·H2O as the pH regulator.45 In their work, glucose was initially dehydrated to generate the carbon spheres. The surface of such carbon is hydrophilic and attached to numerous OH− groups. Consequently, this carbon sphere can act as a substrate for ion penetration in the subsequent reaction processes. Their investigations demonstrated that multishelled NiO hollow spheres could be finally formed only at high pH values, such as 10.9. This result could be explained as follows. At high pH values, the OH− and NH3 concentrations, chelating interactions between NH3 and Ni2+, and growth rate of Ni(OH)2 nuclei would simultaneously increase, leading to the gradual formation of Ni(OH)2 nuclei, self-organized, and further reacted. Meanwhile, there would be a large number of OH− on the surface of the carbon spheres; therefore, it is likely to form Ni(OH)2 nuclei on the surface of the carbon spheres. These Ni(OH)2 nuclei would further assemble into nanoparticles on the basis of the coalescence mechanism. Finally, the inside-out Ostwald ripening growth mechanism would dominate, yielding multishelled structures. After the calcination treatment, the carbon spheres would decompose and Ni(OH)2 would dehydrate to afford NiO. The possible mechanism of the formation is shown in Fig. 10.
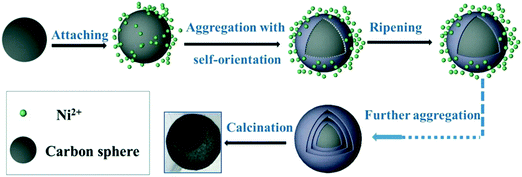 | ||
| Fig. 10 Plausible formation process of multishelled NiO microspheres. Reproduced from ref. 45 with permission. | ||
Considering most of the existing approaches are only available in the synthesis of simple binary multishelled metal oxide hollow spheres, Zhang et al. developed a general penetration–solidification–annealing approach to synthesize multicomponent metal oxide hollow spheres with multishelled structures using CSs as the hard template, as shown in Fig. 11a.46 The entire process comprises the following steps. Firstly, the carbonaceous hard template spheres were synthesized via the hydrothermal carbonization of glucose at ∼160–180 °C and then dispersed into ethylene-glycol-containing metallic acetate (such as nickel acetate, cobalt acetate, and/or manganese acetate). Secondly, heating treatment at 120 °C was performed to promote the penetration of metallic ions deep into the carbonaceous template. This procedure could last for 12 h. In the next step, the substance was further heated up to 170 °C for 2 h till the metal glycolate was formed on both the inner and outer surfaces of the carbonaceous template. With the consumption of ethylene glycol, the viscosity of the system considerably increased; therefore, the process was referred to as solidification. Finally, in order to obtain multishelled mixed metal oxide hollow spheres, the decomposition of metal glycolate and removal of carbonaceous template were achieved by annealing at 500 °C for 4 h. Fig. 11b–g shows the typical TEM images of some of the obtained samples. Evidently, triple-shelled hollow spheres could be fabricated for CoMn2O4, Co1.5Mn1.5O4, MnCo2O4, ZnMn2O4, ZnCo2O4, and NiCo2O4. The thickness of these mixed metal oxides slightly varied with a combination of metals within ∼30–50 nm. By varying the diameter of the employed carbonaceous template or adjusting the penetration time, the number of shells could be adjusted. Generally, the larger diameter of the utilized carbonaceous template was expected to provide more space to form more shells in the final hollow structures. Such a result is in good agreement with those obtained from earlier reports.34 Zhang et al. used a similar principle to prepare CoFe2O4 hollow spheres.47 Recently, Peng et al. proposed an adsorption–calcination–reduction approach to prepare various spinel oxides (NiCo2O4, CoMn2O4, and NiMn2O4) with necklace-like multishelled hollow structures using carbonaceous microspheres as the sacrificial template. It should be pointed out that the goal of the additional reduction treatment was to induce the formation of oxygen vacancies in the spinel crystals, which may boost the performance of the materials and various properties, such as catalytic activity.48
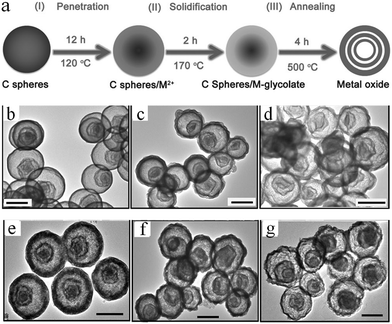 | ||
| Fig. 11 (a) Preparation schematics of multicomponent metal oxide multishelled hollow spheres via the penetration–solidification–annealing approach using carbon spheres as the hard template. Typical TEM images with scale bars of 500 nm for some of the samples of multicomponent metal oxide multishelled hollow spheres. (b) CoMn2O4, (c) Co1.5Mn1.5O4, (d) MnCo2O4, (e) ZnMn2O4, (f) ZnCo2O4, and (g) NiCo2O4. Reprinted from ref. 46 with kind permission. | ||
As a versatile template, carbonaceous microspheres could adsorb either metallic cations or metal-containing anions. The first example of using a carbonaceous microsphere to adsorb an anion for preparing multishelled hollow nanostructures was reported by Wang et al. In their epoch-making work, a series of multishelled (V2O5, MnO2, MoO3, Cr2O3, and WO3) hollow nanostructures were obtained. The formation mechanism involved the chemical adsorption of metal-containing anions (VO3−, MnO4−, MoO42−, CrO42−, and W7O246−) onto carbonaceous microsphere templates followed by a Trojan catalytic combustion process to remove the carbonaceous template. The driving force for the adsorption of metal anions into the microspheres is different from the traditional cation-adsorption process. Taking VO3− as the example, because oxygen (O) prefers to coordinate with V5+ by donating its p electrons to the empty d orbits of V5+, the electrostatic repulsion energy can be considered to be much smaller than the binding energy between VO3− with the OH group via a coordination interaction. As a result, the adsorption of VO3− onto the negatively charged carbonaceous template with abundant oxygen-containing groups is thermodynamically feasible via coordination bonds. Meanwhile, in real-world cases, the penetrated cations (such as NH4+) can partly neutralize the negative charges, promoting the further adsorption of metal-containing anions.49 On the basis of their systematic work, the same group recently extended this principle to the co-absorption of positive and negative ions to fabricate binary metal oxides (Fe2(MoO4)3, NiMoO4, MnMoO4, CoWO4, MnWO4, etc.) with multishelled hollow structures.50 Zong et al. prepared YVO4 multishelled hollow spheres using a yttrium CS as the hard template.51 By means of a hydrothermal route, yttrium CSs were firstly prepared, which possessed hydrophilic functional groups. Therefore, VO43− anions could easily absorb to form the YVO4 complex oxide. The multishelled hollow spheres of YVO4 with uniform morphology, high crystallinity, and controlled number of shells (up to three) could be successfully obtained by thermal annealing.
In certain cases, CSs may not only serve as a grid template to form multishelled hollow target materials, but also take part in the reaction process. For example, Zhang and co-workers prepared double-shelled MnO2/CeO2–MnO2 hollow spheres using colloidal CSs as the hard template, as shown in Fig. 12a.52 The negatively charged carbonaceous microspheres were firstly applied as the substrate to deposit Ce species by means of a layer-by-layer self-assembly process, forming core–shell CSs@CeO2 precursor spheres (Fig. 12b). Based on the fact that there were plenty of mesoporous pores in the shell layer of the CeO2 precursor, a KMnO4 solution was percolated through the CeO2 precursor that had deeper access to the spheres. Subsequently, the redox reactions between the carbonaceous core/KMnO4 and CeO2 precursor/KMnO4 occurred, generating core–shell CSs@MnO2/CeO2–MnO2 due to the close contact between the CSs core and Ce species. Owing to the Kirkendall effect caused by the diffusion couple of the faster inward-diffusing MnO4− and outward-diffusing organic carbon chains, the core–shell-structured CSs@MnO2/CeO2–MnO2 could be easily converted into yolk–shell CSs@MnO2/CeO2–MnO2 (Fig. 12c). Finally, annealing the above yolk–shell structure in air, the residual CS core would completely decompose, thereby generating MnO2/CeO2–MnO2 double-shelled hollow spheres (Fig. 12d).
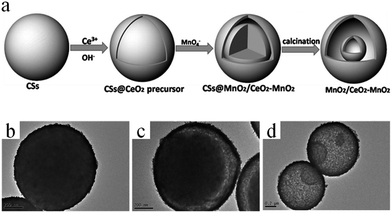 | ||
| Fig. 12 (a) Schematic illustration of the synthesis process of MnO2/CeO2–MnO2 double-shelled hollow spheres. TEM images of (b) CSs@CeO2 precursor core–shell spheres, (c) yolk–shell CSs@MnO2/CeO2–MnO2, and (d) MnO2/CeO2–MnO2 double-shelled hollow spheres. Reprinted from ref. 52 with kind permission. | ||
In addition to the abovementioned merits of a carbonaceous template that make it more resistant to acids and alkalis as compared to a silica template, the former consumes a lesser amount of solvent or additional chemicals, except precursors, in the entire preparation process. It should be noted that the carbonaceous template may not be available for producing metallic oxides in a sub-stable oxidation state (such as Cu2O, Fe3O4, and MnO), since in most cases, the removal of carbon is usually carried out by annealing in an air flow. These sub-stable compounds may be oxidized by O2 in air. Further, high energy consumption in the removal of the carbonaceous template for the simultaneous formation of multishelled hollow nanostructures may be another disadvantage of the carbonaceous template approach.
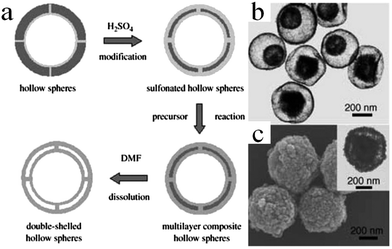 | ||
| Fig. 13 (a) Schematic illustration of the synthesis of double-shelled inorganic hollow spheres. (b) TEM images of double-shelled TiO2 hollow spheres and (c) SEM and TEM images (inset) of Fe3O4. Reprinted from ref. 53 with kind permission. | ||
In another report, Hou et al. described an interesting strategy to prepare double-shelled silica hollow particles (DSHPs) using single-hole hollow particles (SHHPs) of PS as the templates (Fig. 14).54 The entire preparation procedure could be divided into three steps. Initially, SHHPs were prepared by seed emulsion polymerization. Furthermore, via the Stöber method, both the inner and outer surfaces of SHHPs were coated with silica shells. Finally, the intermediate SHHP layer was removed by calcination, thereby forming DSHPs. In addition, other double-shelled hollow microspheres such as TiO2, Fe3O4, BaTiO3, and conducting polymer (polypyrrole and polyaniline) were also successfully prepared by making use of sulfonated PS hollow spheres as the templates.55–59 It should be noted that the PS template method seems to be a little tedious and dangerous since concentrated H2SO4 is often utilized to treat PS colloidal microspheres.
 | ||
| Fig. 14 Schematic of the preparation process of DSHPs. Reprinted from ref. 54 with kind permission. | ||
Resorcinol–formaldehyde (RF) resins are another important polymer that can serve as a template for the synthesis of various functional materials. Similar to a carbonaceous template, RF resins can often siphon metallic ions and then transform into oxides with multishelled hollow structures when the operating conditions are precisely controlled. Li et al. used a RF resin microsphere as a sacrificial template for preparing a series of triple-shelled Ni–Co–O hollow microspheres with a delicate ratio of Ni/Co (Fig. 15a).60,61 The RF resin microspheres were obtained from 2,4-dihydroxybenzoic acid reacted with formaldehyde under hydrothermal conditions. Owing to the residence of numerous –COOH and –OH groups in the precursors, RF could facilitate the adsorption and penetration of metal ions (Ni2+ and/or Co2+). It should be noted that the adsorption procedure was performed in ethylene glycol but not in water. The reason might be related to the larger radii of hydrated Ni and Co ions, which are difficult to deeply diffuse into the RF template. After heating to 550 °C at the rate of 5 °C min−1 and maintained for 4 h in an air atmosphere, the above Ni2+/Co2+-ion-infused RF resin could be converted into a triple-shelled oxide hollow microsphere with different Ni/Co ratios. Fig. 15b–g shows the TEM images of these Ni–Co–O samples, confirming that triple-shelled hollow structures were obtained. In addition, single- and double-shelled hollow structures may also be obtained by shortening the soaking time to 0.5 h and lowering the annealing rate to 2 °C min−1. By varying the initial concentrations of Ni2+ and Co2+, the thickness of the triple-shelled hollow structures may be tuned. Recently, Zhu et al. prepared multishelled CoFe2O4 using a RF colloidal sphere as the hard template.62
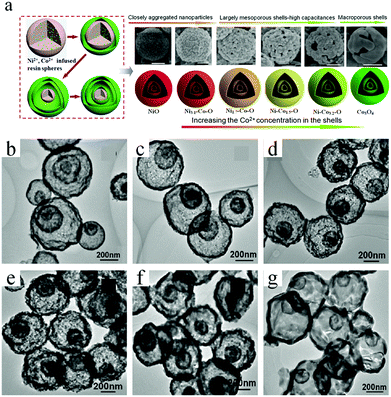 | ||
| Fig. 15 (a) Schematic illustration of the preparation of triple-shelled NiO, Co3O4, and their mixed oxide (Ni–Co–O) hollow spheres. (b–g) Typical TEM images of these samples. Reprinted from ref. 60 with kind permission. | ||
Polyacrylic acid (PAA) is an anionic polymer rich in carboxyls that can efficiently coordinate with metal ions. Therefore, PAA can also be used as a hard template to fabricate multishelled metal oxide hollow nanostructures. Qi et al. described the syntheses of CoFe2O4 (CFO) solid nanospheres (SNSs), hollow nanospheres (HNSs), and multishelled hollow nanospheres (MS-CFO-NHSs) using PAA-NH4 microspheres as the hard template. The synthesis mechanism is shown in Fig. 16.63 Initially, PAA-NH4 microspheres adsorb plenty of water containing Fe2+ and Co2+. Subsequently, Fe2+ and Co2+ are simultaneously hydrolyzed to form Fe(OH)2 and Co(OH)2, which are randomly dispersed in the PAA-NH4 microsphere. It is well known that Fe(OH)2 can be easily oxidized into Fe(OH)3; therefore, the CFO NCs precursor was generated. Finally, by precisely controlling the calcination process, CFO-SNSs, CFO-HNSs, and MS-CFO-HNSs were obtained.
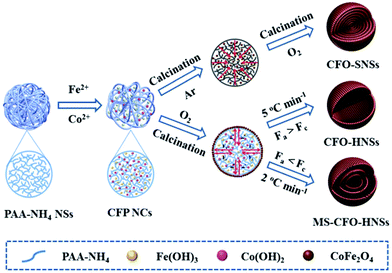 | ||
| Fig. 16 Schematic illustrations of the formation processes of CFO-SNSs, CFO-HNSs, and MS-CFO-HNSs using PAA-NH4 as the hard template. Reproduced from ref. 63 with permission. | ||
Besides certain synthetic polymers, some natural polymers have also been good candidates for use as templates for preparing various functional materials with desirable structures. Alginate, a natural polysaccharide rich in hydroxyl groups, also exhibits good affinity toward metal ions. It is expected that alginates with particular morphologies could direct the formation of transition metal oxides with multishelled hollow structures. Sun et al. developed a generic strategy to fabricate a class of multishelled hollow transition metal oxide nanofibers, such as NiO, Co3O4, and Fe2O3, based on immobilizing metal ions into the alginate fibers followed by annealing in air, as shown in Fig. 17. Similar to carbonaceous and other polymer hard templates, the gradient distribution of transition metal ions is crucial for the formation of multishelled structures. Here, the ratio of ethanol to water in the solvent plays a significant role in the distribution of metal ions in the alginate template. The more ethanol in the solvent, the lower is the surface tension and higher is the wettability, which leads to deeper and higher adsorption of metal ions, yielding metal–alginate fibers. Upon performing a controlled annealing treatment, the transition metal–alginate fibers could be converted into multishelled hollow fibers.64
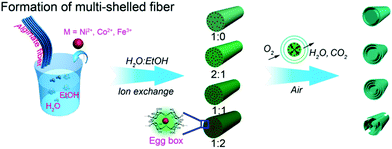 | ||
| Fig. 17 Schematic illustration of the preparation of multishelled transition metal (Ni, Co, and Fe) oxide hollow fibers using alginate as the template. Reproduced from ref. 64 with kind permission. | ||
In addition to the abovementioned polymers, other polymers, such as polyacrylamide,65 PVP gel microspheres,66etc., have also been used as hard templates for preparing multishelled hollow nanostructures. For many polymer resins as hard templates, there are many functional groups on their surfaces; therefore, no surface modification or activation steps are required in most cases. This considerably simplifies the processing steps and therefore consumes less time as compared to silica or carbonaceous template methods. On the basis of the properties of the polymer resin utilized, either specific solvent dissolution or calcination treatment could be employed for the removal of the polymer resin template. It should be noted that many polymer resins can get easily swollen in the liquid environment and expand at a relative high temperature; therefore, the reaction medium and temperature required to synthesize multishelled hollow materials using a polymer resin as the sacrificial template should be appropriately selected.
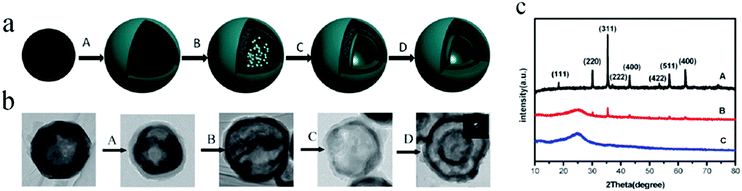 | ||
| Fig. 18 (a) Proposed formation mechanism of double-shelled conducting polymer hollow microspheres using hollow Fe3O4 spheres as the template; (A) coating of a conducting polymer on the outer surface of hollow Fe3O4 spheres; (B) penetration of the monomer into the inner surface of the hollow Fe3O4 spheres; (C) in situ polymerization of the monomer on the inner surface of the hollow Fe3O4 spheres; (D) removal of the hollow Fe3O4 spheres via slow etching using p-TSA. (b) TEM images of the product at each step. (c) XRD patterns of the product at different reaction times at 40 °C: (A) 0 h; (B) 12 h; and (C) 24 h. Reproduced from ref. 67 with kind permission. | ||
Apparently, the hard template method to synthesize multishelled hollow nanostructures exhibits several advantages. It is a versatile tool to prepare multishelled hollow structures with desirable morphology and show advantages in controlling the size and the diameter/cavity of the final product when compared with those obtainable via other techniques. Moreover, the final products obtained by the hard template strategy are expected to appear highly uniform with regard to particle size and morphology. The hard template method has at least three disadvantages. Firstly, presynthesized hard templates are necessary in most cases. The limited availability of raw materials, storage, and template transformation might be another bottleneck owing to the inherent properties of the template. Secondly, several other procedures, such as the coating of targeted materials or precursors on the surface of the template, as well as the ultimate removal of the template via etching or calcination, are often involved that may complicate the preparation process; posttreatment may destroy the final product to a certain extent. Thirdly, on the basis of tedious synthesis procedures, it is impractical to produce multishelled hollow nanostructures at a large scale with the hard template method, particularly for nanostructures with a higher number of shells (over 5 shells).
2.2 Soft template approach
In addition to the hard template method, soft template methods have also been versatile tools for the fabrication of multishelled hollow nanostructures. Generally, the soft template approach used to fabricate multishelled hollow nanostructures involves the self-assembly of surfactants/cosurfactants that can yield relatively flexible structures such as emulsion droplets, micelles, gas bubbles, and vesicles, etc. On the basis of the interactions between the soft template/solvent and precursors, the shell of the target materials may be initially produced, which could subsequently grow within the interfacial region during template removal. Until now, the soft template method has been widely applied in transcriptase synthesis with diverse hollow structures.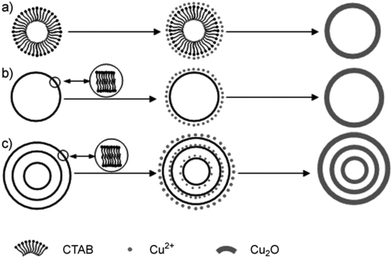 | ||
| Fig. 19 Schematic of the formation mechanism of single-shelled and multishelled Cu2O hollow spheres using CTAB-assembled vesicles as the soft template. Reprinted from ref. 75 with kind permission. | ||
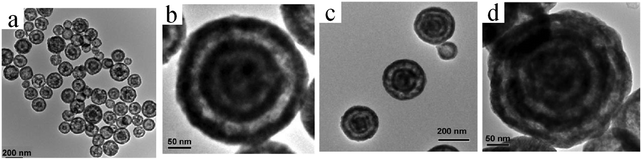 | ||
| Fig. 20 TEM images of (a) double-shelled, (b and c) triple-shelled, and (d) quadruple-shelled CuO2 hollow spheres for different CTAB concentrations: (a and b) 0.13 and (c and d) 0.15 M. Reprinted from ref. 75 with kind permission. | ||
Teng and co-workers extended CTAB as a soft template to fabricate multishelled periodic mesoporous organosilica (PMO) hollow spheres (Fig. 21).77 The entire process could be divided into four steps. First, TEOS and 1,4-bis(triethoxysilyl)benzene (BTSE) were transformed into mesostructured ethane-bridged organosilica spheres via a modified Stöber method using a CTAB-surfactant-directed sol–gel process. Subsequently, the repeated addition of TEOS and BTSE resulted in the formation of multilayered organosilica/CTAB spherical composites with enhanced sizes. The number of shells of the ultimately obtained multishelled PMO hollow spheres was dependent on the addition times of the TEOS and BTSE precursors. Thereafter, hydrothermal treatment was carried out to transform the subsequently grown solid organosilica spheres into hollow structures. Finally, CTAB surfactants were extracted by acidic ethanol, yielding multishelled PMO hollow spheres. By precisely controlling the synthesis conditions, such as CTAB concentration, amount of organosilica cores, and reaction temperature, the overall diameter, intershell space, and shell thickness of the finally obtained PMO hollow spheres could be easily tuned.
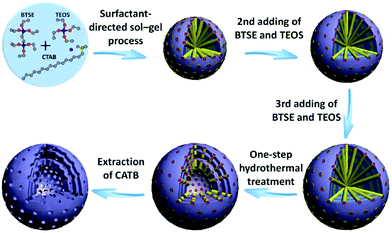 | ||
| Fig. 21 Schematic illustration of the synthesis procedure and successive growth process of triple-shelled PMO hollow spheres. Reprinted from ref. 77 with kind permission. | ||
Yang et al. developed a self-assembly procedure to prepare multishelled ZnO hollow spheres by using a triblock copolymer Pluronic P123 as the soft template.78 The preparation mechanism included the following steps, as shown in Fig. 22. Initially, P123 and a short-chain alcohol were self-assembled into spherical reverse micelles, which may provide space to accommodate inorganic oligomers. Subsequently, a solvothermal treatment was conducted to improve the organization, induce complete condensation, and crystallization. At this stage, by the Ostwald ripening of various intercrystallite spaces within these structures, a void space was created. When pristine noncompact aggregates were transformed into a smaller encapsulated aggregate, double-shelled ZnO hollow spheres were generated. Otherwise, if the ZnO aggregates were compact, ZnO hollow spheres with single-shelled structures were produced. Finally, when washed with water and ethanol, the surfactant template was removed, resulting in the formation of well-crystallized shelled ZnO hollow spheres. Taking full advantage of the characteristics of the self-assembly of micelles by surfactants, Zhang et al. reported the synthesis of organosiliceous multilamellar vesicles using the commercially available triblock copolymer Pluronic P85 as a single template and 1,2-bis(triethoxysilyl)ethane as the organosilica precursor. The number of shells of organosilica could be precisely controlled (within 1 to 7) by adjusting the pH of the solution from 4.8 to 5.2.79
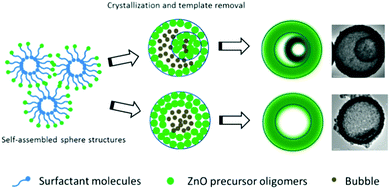 | ||
| Fig. 22 Synthesis mechanism of shelled ZnO hollow spheres using P123 as the soft template. Reprinted from ref. 78 with kind permission. | ||
Xu et al. described a soft template method to prepare double-shelled hollow mesoporous carbon nanospheres using (polystyrene-b-polyacrylic acid) (PS-b-PAA) micelles as the soft template. The entire formation process is shown as Fig. 23, which involves several stages.80 Firstly, on the basis of electronic interactions, positively charged DA molecules and negatively charged PS-b-PAA blocks assembled into large micelles, i.e., PS-b-PAA/DA, in which multiple reverse small PAA-b-PS/DA micelles were present. Subsequently, DA molecules and TEOS would undergo self-polymerization and hydrolysis/condensation in the PS-b-PAA/DA composite micelles with the assistance of NH3·H2O, forming polydopamine (PDA) and SiO2, respectively, on the surface of the micelles. As the reaction proceeded, the residual DA continued to form PDA on the outermost layer. Thereafter, the PDA was converted into N-doped carbon via carbonization at higher temperatures, generating three-layered C/SiO2/C nanostructures. Finally, the intermediate layer of SiO2 was removed by NaOH etching, which resulted in the formation of double-shelled hollow nitrogen-doped carbon mesoporous nanospheres. It should be noted that the dosage ratio of TEOS/DA had a significant influence on the formation of the final product owing to the competitive relationship between the DA self-polymerization and TEOS hydrolysis/condensation under alkaline conditions. These research results demonstrated that only an intermediate dosage of TEOS could lead to the formation of double-shelled carbon hollow structures as the main product. At lower dosages of TEOS, DA self-polymerization would assume the dominant role, while TEOS oligomers might act as a template to generate mesopores in the carbon spheres. At higher dosages of TEOS, silica networks may dominate in the SiO2/C intermediate; as a result, an inconsecutive outer carbon shell could be formed, while the outer carbon shell may disappear during the subsequent silica removal process. If the dosage of TEOS is extreme high, only certain carbon fragments can be obtained.
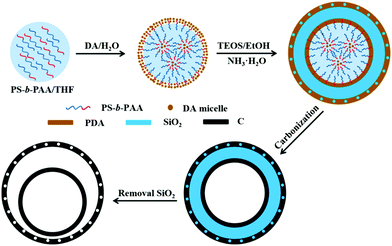 | ||
| Fig. 23 Schematic illustration of the formation process of double-shelled hollow mesoporous carbon nanospheres using PS-b-PAA micelles as the soft template. Reproduced from ref. 80 with permission. | ||
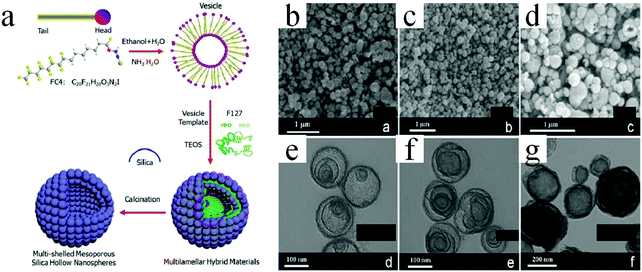 | ||
| Fig. 24 (a) Schematics of the synthesis procedures of multishelled mesoporous silica hollow nanospheres. (b–d) SEM and (e–g) TEM images of multishelled mesoporous silica hollow nanospheres obtained for different molar ratios of FC4 and F127. (b and e) FC4/F127 = 24, (c and f) FC4/F127 = 48, and (d and g) FC4/F127 = 72. Reprinted from ref. 81 with kind permission. | ||
Huang et al. developed a facile and sustainable strategy by using compressed CO2 to prepare multishelled hollow PMO nanospheres, in which the soft template is composed of the triblock copolymer Pluronic P123, CTAB, and sodium dodecyl sulfate (SDS).88 The formation mechanism of multishelled hollow PMOs comprises several steps, as shown in Fig. 25a. Similar to other soft template processes, the P123 + CTAB + SDS surfactants were initially self-assembled into vesicles in a solution. The penetration of CO2 into the hydrocarbon chain region of the surfactant molecules may cause volume expansion and increase in the blending energy when compressed CO2 was passed into the solution. Consequently, the mixed surfactants of CTAB and SDS formed multilamellar vesicles. Thereafter, the precursor, i.e., BTSE, underwent a hydrolysis reaction to form organosilica/vesicle composites. After the extraction of the surfactants by ethanol–HCl (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v), multishelled PMO hollow nanospheres were obtained. The number of shells/thickness and the overall diameter of the final products could be easily modulated by precisely adjusting the CO2 pressure. Fig. 25b–f show the typical TEM images of these products with different numbers of shells by tuning the CO2 pressure.
3, v/v), multishelled PMO hollow nanospheres were obtained. The number of shells/thickness and the overall diameter of the final products could be easily modulated by precisely adjusting the CO2 pressure. Fig. 25b–f show the typical TEM images of these products with different numbers of shells by tuning the CO2 pressure.
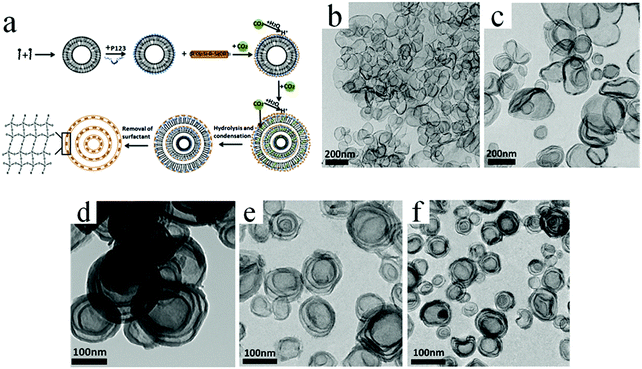 | ||
| Fig. 25 (a) Formation mechanism of multishelled hollow PMO nanospheres using P123, CTAB, and SDS as a dual template with the assistance of compressed CO2. (b–f) TEM images of multishelled PMO hollow nanospheres obtained at CO2 pressures of 3.90 (b), 4.40 (c), 4.90 (d), 5.60 (e), and 5.90 (f) MPa. Reproduced from ref. 88 with kind permission. | ||
As discussed above, soft template approaches possess the advantages of template removal since the process is expected to consume less energy than those by hard template approaches. Although tremendous progress has been made in the soft template synthesis of multishelled hollow nanostructures, the utilization of soft templates for synthesizing multishelled hollow nanostructures is not trivial since the soft templates themselves, including micelles, emulsion droplets, vesicles, gas bubbles, and other organized molecular assemblies, are usually not very stable under varying environments. Any marginal changes in pH, temperature, solvent, ionic strength, or any additives may destroy the structure of the soft template, resulting in failure in the preparation of the final products. As a consequence, the soft template method provides less control over particle uniformity. Meanwhile, it is difficult to completely remove the soft template, and the residual organic molecular assembly might affect the purity of the final products. Furthermore, in light of being environmentally benign, the soft template approach consumes large amounts of organic solvents and reagents, which may be harmful to the environment as well as living organisms. In addition, different from the hard template approaches, it is difficult to extend the soft template approach from one system to another.
2.3 Self-template approaches
Self-template methods without requiring an additional template material are more preferable in practical applications for creating hollow materials. Different from the hard-/soft-template-based approaches, the templates utilized in the self-template approaches may play multiple roles in the synthesis of multishelled hollow materials.89,90 The first one is that the template might comprise the final multishelled hollow architecture. The second one is that the template may act as a precursor to generate multishelled hollow materials. In addition, the template might also react during the formation of multishelled hollow nanomaterials, thereby generating a void structure in the final products. Until now, several materials have been demonstrated to be promising self-template candidates for the fabrication of multishelled hollow nanostructures. In this section, we will discuss certain examples and related mechanisms.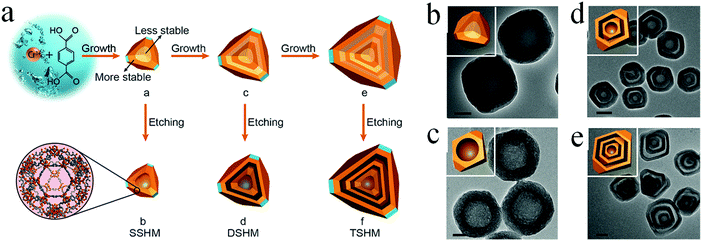 | ||
| Fig. 26 (a) Schematic illustration of the preparation procedure of multishelled hollow MIL-101 by step-by-step and post-etching processes. TEM images of solid MIL-101 (b), SSHM (c), DSHM (d), and TSHM (e). Scale bars: (b and c) 50 nm; (d and f) 200 nm. Insets in (b–e) show the corresponding FFT patterns. Reprinted from ref. 94 with kind permission. | ||
Researchers have noted that MOFs are robust precursors in the preparation of various functional materials with different structures, particularly complex hollow structures. Cho et al. reported the synthesis of multishelled hybrid metal oxide hollow microspheres by using coordination polymer microspheres as the template and combining cation exchange followed by controlled thermal treatment.97 The entire preparation process involves several procedures, as shown in Fig. 27. Firstly, solid spherical CPs containing Zn2+ or Co2+ were synthesized through a precipitation route. Subsequently, secondary metal ions (such as Ni2+ or Cu2+) were introduced via the ionic exchange process. Finally, calcination treatment was carried out to generate multishelled mixed metal oxide hollow microspheres. On eliminating the ionic exchange procedure, pure ZnO or Co3O4 multishelled hollow microspheres could be obtained. Initially, the outermost part of the solid spherical CPs was transformed into a ZnO shell when thermal treatment was applied. Thereafter, the internal CP component shrunk, accompanied by its decomposition into metal oxide species. Such a stepwise fabrication process of the metal oxide layers yielded the formation of multishelled metal oxide hollow microspheres.
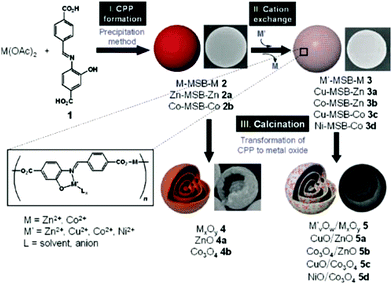 | ||
| Fig. 27 Schematic illustration of the preparation of multishelled hybrid metal oxide hollow microspheres. Reproduced from ref. 97 with permission. | ||
Zhang et al. demonstrated an effective strategy to prepare multishelled ZnS–CdS rhombic dodecahedral cages (RDC) using zinc zeolitic imidazole framework ZIF-8 as the precursor. As shown in Fig. 28, the entire process includes three steps. Firstly, ZIF-8 RD was converted into yolk–shell RDC when etched with tannic acid. Secondly, the yolk–shell ZIF-8 RDC would react with thioacetamide as the sulfidation agent under solvothermal conditions to form multishelled ZnS RDC. Finally, multishelled ZnS–CdS RDC could be obtained by a hydrothermal cation exchange reaction. It should be noted that the composition of multishelled ZnS–CdS RDC could be effectively tuned by adjusting the cation exchange reaction time. Furthermore, the number of shells of the final ZnS–CdS RDC was considerably dependent on the size of the ZIF RD precursor.98 By using similar procedures, Lou and co-workers undertook pioneering work in transforming CPS or MOFs into various multishelled hollow nanostructures, including metal oxides,99 metal oxyphosphides,100 and some complex metal oxides.101,102
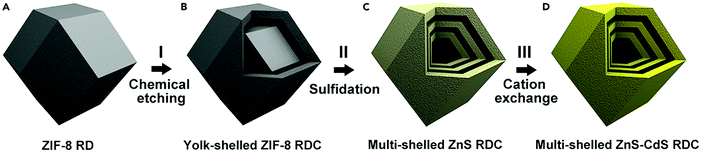 | ||
| Fig. 28 Schematic illustration of the synthesis procedure for multishelled ZnS–CdS RDC. Reproduced from ref. 98 with kind permission. | ||
Shen et al. reported the synthesis of NiCo2S4 ball-in-ball (double-shelled) hollow spheres using NiCo glycerate as the precursor and thioacetamide (TAA) as the sulfur source, as shown in Fig. 29.106 Initially, TAA decomposed to generate sulfide (S2−) under solvothermal conditions. S2− ions easily reacted with metal ions on the surface of NiCo glycerate through anion exchange, forming core–shell-structured NiCo glycerate@NiCo2S4. As the reaction proceeded, a well-defined gap between the shell and NiCo glycerate core was observed owing to the low rate of inward diffusion of S2− ions and fast rate of outward diffusion of metal cations. When the reaction proceeded to a certain degree, the outward diffusion of metal cations would become restricted; therefore, a new NiCo2S4 shell might be formed on the inner surface of the remaining NiCo glycerate core, forming yolk–shell-structured intermediates. Finally, unique NiCo2S4 ball-in-ball hollow spheres were obtained because of the competition of the anion exchange reaction. Recently, they extended this procedure to prepare NiCo2V2O8 yolk–double-shelled spheres using NiCo glycerate as the precursor.107 Making full use of this concept, starting from solid spheres of Mo glycerate, highly uniform Mo-PDA with triple-shelled hollow structures can be obtained through a facile solvothermal process. After thermal treatment, the triple-shelled Mo-PDA hollow spheres could be further converted into triple-shelled MoO2/C composite hollow spheres.108
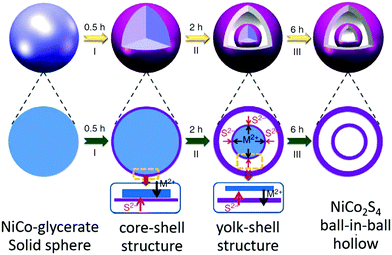 | ||
| Fig. 29 Schematic illustration of the preparation of double-shelled NiCo2S4 ball-in-ball hollow spheres via the sulfidation of NiCo glycerate solid spheres. Reprinted from ref. 39 with permission. | ||
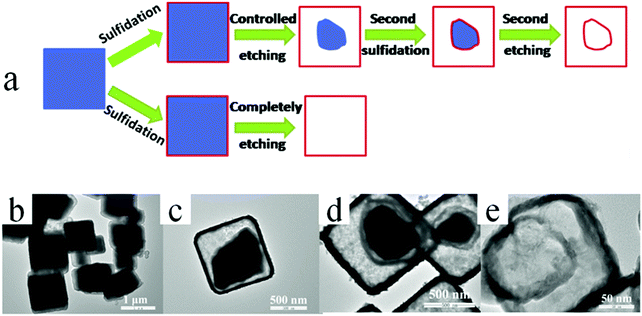 | ||
| Fig. 30 (a) Schematic illustration of the synthesis procedure for the formation of double-shelled CuS hollow nanocages via the Cu2O self-template approach. TEM images of Cu2O@CuS: (b) before and (c) after the initial partial etching treatment. TEM images of (d) Cu2O@CuS@CuS and (e) double-shelled CuS hollow nanocages. Reprinted from ref. 109 with kind permission. | ||
Wu et al. reported the synthesis of multishelled VOOH hollow nanospheres by the thermal decomposition of V(OH)2NH2 by exercising precise control over the external reaction temperature.110 As shown in Fig. 31, the formation process may yield a solid sphere, core–shell structure, and multishelled hollow structure on the basis of the Kirkendall and Ostwald ripening effects. By adjusting the experimental parameters, the number of shells could be precisely controlled. In addition to oxides/hydroxides, metal carbonates were also reported to be versatile precursors to form multishelled hollow nanostructures. For example, Lin et al. prepared triple-shelled Mn2O3 hollow nanocubes using a directly programmable annealing treatment with MnCO3. Expectedly, the interaction between the contraction force from the decomposition of MnCO3 and the adhesion force from the formation of Mn2O3 resulted in the formation of a hierarchical structure.111
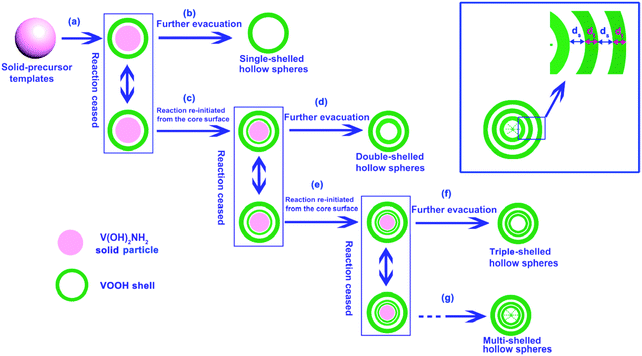 | ||
| Fig. 31 Schematic illustration of the formation of VOOH hollow nanospheres with controlled numbers of shells via the direct thermal treatment of solid V(OH)2NH2 nanospheres. Reprinted from ref. 110 with kind permission. | ||
2.4 Template-free approach
Despite the considerable progress made in template approaches that have been used to fabricate multishelled hollow nanostructures, due to the complex nature and composition of the template, template preparation always remains challenging for researchers. Therefore, template-free approaches for preparing multishelled hollow nanostructures with controlled sizes are preferable than other methods.112,113 Spray pyrolysis is a typical template-free method for preparing nanomaterials with unique structures. Zhou et al. described a spray-drying technique by adding glucose as a carbon source to prepare multishelled α-Fe2O3 hollow spheres.114 The fabrication mechanism of multishelled α-Fe2O3 hollow spheres may be explained as follows: the iron nitrate–sucrose system possessed several disadvantages when used as a precursor to prepare multishelled α-Fe2O3 hollow spheres. Due to the nitrogen-rich contents, the thermal treatment of iron nitrate could generate nitrogen oxides (such as NO and NO2), which are harmful to the environment as well as human beings. Further, iron nitrate readily absorbs moisture from air, complicating the storage of the iron nitrate–sucrose composite. Furthermore, the strong oxidative property of iron nitrate and strong reductive property of sucrose in a single system may result in safety hazards, such as explosions. In addition, sucrose/iron nitrate ratio ranging only within ∼0.75–1 may lead to multishelled α-Fe2O3 hollow spheres, thereby restricting mass production. Considering the above concerns, Padashbarmchi et al. proposed another spray-drying method by replacing iron nitrate with iron citrate.115 It is well known that iron citrate is a mild oxidant and avoids the abovementioned limitations since the nitrogen content of the compound is zero. Using different metallic salts and additive carbon sources, other metallic oxides with multishelled hollow structures may be obtained. For example, Park et al. successfully used cobalt nitrate and ethylene glycol to prepare multishelled Co3O4 powders.116From the literature, it is evident that the triaxial electrospinning technique is a versatile strategy to create multilayered structures. Inspired by this principle, Zanjani and co-workers fabricated multiwalled hollow fibers using a direct, one-step triaxial electrospinning process. In order to control the hydrophilicity of the inner and outer layers of the fiber, the polarities and viscosities of two spinnable polymer solutions used as the inner and outer layers were seriously considered. Consequently, the polymer concentrations play important roles in providing composite properties toward a wide range of applications. Furthermore, by adjusting the solvent properties and the degree of miscibility of solutions as well as the multiaxial electrospinning parameters (including applied voltage, flow rate, and electrospinning distance), multiwalled hollow fibers with the desired diameters, surface morphology, and layered structures could be obtained.117
The largest advantage of the template-free approach might be the fact that no tedious procedures are required, which is the necessary procedure for hard template approaches. When compared with soft template approaches, template-free ones might be preferred in controlling the particle size, number of shells/shell thickness, and morphology. With regard to the disadvantages, the exact formation mechanism of multishelled hollow structures by various template-free approaches is still unclear and needs to be further studied because of the difficulty in obtaining direct evidence by experiments.
2.5 Multimethod combination approaches
With the development of nanotechnology, some novel strategies may not be included in the four categories discussed above. Table 1 summarizes the advantages and disadvantages of the abovementioned methods. In the future, we can expect an integrated approach for synthesizing multishelled hollow nanostructures with desirable compositions and structural features, which cannot be directly fabricated by any single technique. When Zhang's work is considered as an example, both carbon nanofibers (CNFs) and metal glycolates were employed to form a series of multishelled metal oxide nanotubes. Apparently, CNFs and metal glycolates were used as the hard template and self-template, respectively.118 Meanwhile, owing to the inherent complications in a multishelled hollow structure, a combination of multiple methods may involve several mechanisms in a single system, such as ionic exchange, Ostwald ripening, Kirkendall effect, self-assembly, successive solid deposition,119 seed-mediated successive Ostwald ripening,120,121 and so on. Readers interested in these cases are encouraged to refer to the specific examples.122| Methods | Advantages | Disadvantages |
|---|---|---|
| Hard template method | Efficient in controlling dimensions and functionalities, available for numerous systems. | Tedious operation procedures, shell number often limited less than 5, template removal needed, time- and/or energy-consuming, difficulty in large scale production. |
| Soft template method | Leading to uniform shape and sizes of the product. Usually performed under mild condition. | Organic molecule assembly affects the purity of the product. Consuming plenty of organic solvent/compound. |
| Self-template method | Shell number reach up to or over 5, simple procedure, time and cost efficient. | Much dependent on the properties of precursors, need precise control over experimental condition, incapable of wide utilization, the shell number of the product cannot be accurately controlled. |
| Template-free method | No complicated operation needed, usually simple heat treatment or spray drying does work, shell number/thickness and morphology of the final products can be well controlled. | The exact mechanism is subject to further studies. Fewer precursor systems available for this method. |
3 Applications of multishelled hollow nanostructures
Multishelled hollow nanostructures, integrating the advantages of both hollow materials and multiple-shelled architectures, are expected to realize their optimized physical/chemical properties for specific applications. The unique characteristics of multishelled hollow structures render them to have widespread applications in energy conversion and storage, environmental treatment, catalysis, and biomedicine. The applications of multishelled hollow nanostructure in various areas are summarized in this section.3.1 Energy-related applications
With the depletion of fossil fuels and exploration of renewable sustainable clean energy resources, the electrical energy storage devices, such as supercapacitors, different ionic batteries, dye-sensitized solar cells, etc., have received extensive attention. As a result, there is an urgent need to develop high-performance electrodes for use in such devices. Superior over traditional single-shelled hollow nanostructures with regard to specific surface area and surface-to-mass/volume ratios, multishelled hollow nanostructures have the ability to increase the weight fraction of the active species, boosting the volumetric energy/power density, and prolonging cycling life.Considering their superior environmental compatibility, specific capacitance, and cost-effectiveness, some transition metal oxides, such as copper oxide, iron oxide, nickel oxide, cobalt oxide, and manganese oxide, have been widely investigated as supercapacitor electrode materials.140–147 In order to improve the amount of electrochemically active sites, enhance electron and ion conductivities, as well as inhibit the dissolution of active species into the electrolytes, various hollow metal oxide nanostructures have been preferred. For example, Wang et al. compared the electrochemical performances of Mn2O3 nanoparticles, as well as single-, double-, triple-, and quadruple-shelled Mn2O3 hollow nanospheres when used as supercapacitor electrode materials. Their research results revealed that triple-shelled Mn2O3 hollow nanospheres exhibited better supercapacitive performance than Mn2O3 nanoparticles and single-, double-, and quadruple-shelled counterparts.43 Yang et al. prepared single-, double-, and triple-shelled NiO hollow nanospheres through layer-by-layer self-assembly and subsequent calcination; their electrochemical performances were studied when used as supercapacitor electrodes.148 Their results showed that the NiO hollow nanosphere sample with double shells exhibited the best electrochemical properties among the three NiO hollow nanosphere samples, such as the highest specific capacitance of 612.5 F g−1 at 0.5 A g−1 and long-term cycling stability with retention of over 90% of the initial specific capacitance after 1000 charge–discharge cycles. They attributed such distinguishable performance to two factors: larger active surface area of NiO with the double-shelled structure assured the bulk accessibility of faradaic reactions and the loosely assembled thin nanoflakes of the double-shelled hollow NiO exhibited a porous structure that shortened the ion diffusion path for effective electrolyte diffusion.
Besides single-metal oxides, numerous studies have demonstrated that mixed metallic oxides are expected to deliver superior electrochemical performance as compared to single-metal oxides.149–151 Qi et al. reported thin/thick single-/double-/triple-shelled NiCo2O4 hollow microspheres used as electrodes for electrochemical supercapacitors.61 Moosavifard et al. demonstrated that the coordination polymer of isophthalic acid and various metallic ions are versatile precursors to obtain multishelled hollow nanostructures, including CuCo2O4 and mixed copper cobalt phosphide. These multishelled mixed metal oxides and phosphides were demonstrated to be promising electrode material candidates for asymmetric supercapacitor (ASC) devices.152,153
In addition to metal oxides, metal sulfides have also been widely applied as supercapacitor electrodes. When compared to oxides, transition metal sulfides are expected to exhibit greater electric conductivity and better redox chemistry.154 In order to improve the rate capability and cycling stability, the design and fabrication of the hierarchical nanostructure is demonstrated to be an effective way. Du et al. prepared three types of double-shelled NiS hollow nanostructures with different morphologies (cube-, ellipsoid-, and capsule-shaped), and they investigated their electrochemical performances when used as supercapacitor electrodes. Their results revealed that the double-shelled NiS hollow nanostructures in the shape of a capsule, having the highest specific surface area of 100.2 m2 g−1 and the lowest equivalent series resistance of 0.8 Ω, offered the largest specific capacitance of 1159 F g−1 at a current density of 2 A g−1 among all the three as-prepared samples.25
Another study by Lou et al. reported the enhanced pseudocapacitive properties of nickel cobalt sulfide ball-in-ball hollow spheres.106 When used as an electrode in electrochemical capacitors, the as-obtained NiCoS4 ball-in-ball hollow spheres delivered high specific capacitance of 1036 F g−1 at 1 A g−1. Furthermore, they constructed an ASC device using the as-obtained NiCoS4 hollow sample as the cathode and graphene/carbon sphere paper as the anode (Fig. 32a).19 The performance of this ASC device was systematically investigated by using an array of techniques, such as cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD). The authors found that the entire capacitance of the NiCoS4 ball-in-ball hollow sample was contributed by both electric double-layer capacitance and pseudocapacitance. Meanwhile, higher scan rates resulted in higher current densities, which is a common behavior of a typical electrochemical system. The shape of the CV curves is independent of the scan rate, showing no obvious distortion; this implied good and fast charge–discharge properties of the device. The specific capacitance values at various scan rates indicated the excellent rate capacity of the constructed ASC device. The cycling performance of the ASC device is shown in Fig. 32b. It is apparent that the ASC device offers very high cycling stability with retention of 78.6% of its initial capacitance at the end of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The authors also evaluated the energy and power densities of the ASC device and demonstrated that the ASC device exhibited superior balance between the energy density and power density as compared to many of the reported cobalt-/nickel-based ASCs.155–158 By changing the species of metal cations, Wei et al. prepared double-shelled ZnS–NiS1.97 hollow spheres by using a similar procedure, which possessed a porous structure and high specific surface area (105.26 m2 g−1). When used as an electrode material for a supercapacitor, the double-shelled ZnS–NiS1.97 hollow spheres delivered excellent electrochemical performance in terms of specific capacitance (696.8 C g−1 at 5 A g−1), cycling stability (less than 5.5% loss after 6000 cycles), and energy density of 25.6 W h kg−1 at a power density of 2173.8 W kg−1.159 Similar to oxides, ternary metal sulfides with complex double-shelled structures were also expected to provide a superior electrochemical performance over single-metal oxides. Considering the work of Lou's group as an example, they reported that NiCo2O4 double-shelled hollow spheres, as a typical battery-like electrode, offered specific capacitance as high as 1036 F g−1 at 1 A g−1. Significantly, the ASC device (Fig. 32), assembled by using NiCo2S4 hollow spheres and graphene/mesoporous carbon sphere composite, delivered a high energy density of 42.3 W h kg−1 at a power density of 476 W kg−1. Furthermore, the assembled ASC device also showed excellent cycling stability, retaining 78.6% of the initial capacitance after 10
000 cycles. The authors also evaluated the energy and power densities of the ASC device and demonstrated that the ASC device exhibited superior balance between the energy density and power density as compared to many of the reported cobalt-/nickel-based ASCs.155–158 By changing the species of metal cations, Wei et al. prepared double-shelled ZnS–NiS1.97 hollow spheres by using a similar procedure, which possessed a porous structure and high specific surface area (105.26 m2 g−1). When used as an electrode material for a supercapacitor, the double-shelled ZnS–NiS1.97 hollow spheres delivered excellent electrochemical performance in terms of specific capacitance (696.8 C g−1 at 5 A g−1), cycling stability (less than 5.5% loss after 6000 cycles), and energy density of 25.6 W h kg−1 at a power density of 2173.8 W kg−1.159 Similar to oxides, ternary metal sulfides with complex double-shelled structures were also expected to provide a superior electrochemical performance over single-metal oxides. Considering the work of Lou's group as an example, they reported that NiCo2O4 double-shelled hollow spheres, as a typical battery-like electrode, offered specific capacitance as high as 1036 F g−1 at 1 A g−1. Significantly, the ASC device (Fig. 32), assembled by using NiCo2S4 hollow spheres and graphene/mesoporous carbon sphere composite, delivered a high energy density of 42.3 W h kg−1 at a power density of 476 W kg−1. Furthermore, the assembled ASC device also showed excellent cycling stability, retaining 78.6% of the initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1.
000 cycles at 5 A g−1.
 | ||
| Fig. 32 (a) Schematic illustration of the ASC device based on NiCo2S4 and graphene/carbon sphere paper. (b) Cycling performance of the ASC device at 5 A g−1. Reproduced from ref. 19 with permission. | ||
It is easy to consider the construction of the heterostructures of oxides/sulfides for use as supercapacitor electrodes since both oxides and sulfides are promising electrode materials owing to the high theoretical specific capacitance and high electrochemical activity. By the calcination of hollow Co3O4 hollow spheres with S powder in Ar gas, Wang et al. successfully prepared multishelled CoO@Co9S8 hollow microspheres.160 These multishelled CoO@Co9S8 hollow microspheres delivered high specific capacitance of 1100 F g−1 at 2 A g−1, good rate capability (809 F g−1 at 50 A g−1), and excellent cycling stability (from 1035 to 785 F g−1 after 2000 cycles at 2 A g−1) when used as an electrode material. Such performances are superior to those of previously reported cobalt-based oxide and sulfide electrodes. When coupled with activated carbon to construct an ASC device, the device shows faradaic characteristics in the CV curves and delivers good rate capability and high capability retention of 95.27% after 5000 cycles. Recently, they extended this method to prepare multishelled NiS@NiO hollow heterostructures, which also exhibited excellent electrochemical performance when used as the electrode in ASC devices.161 The excellent performance of these multishelled oxide@sulfide hollow heterostructures could be effectively indexed into the following aspects. First, the introduction of sulfur species could enhance the electrical conductivity, leading to improved charge transport. Second, oxide/sulfide heterostructures provide different bandgaps that facilitate the surface reaction kinetics and charge transfer between the electrode/electrolyte interfaces. Last, but not the least, the interlayer spaces in the multishelled hollow structures can act as reservoirs to accommodate more electrolytes and increase the contact area between the electrode and electrolyte.
As mentioned earlier, carbon is also a promising electrode material for use in supercapacitors. Using crosslinked PS and silica as a dual hard template and DA as a N-containing carbon precursor, Fang et al. fabricated uniform discrete double-shelled N-doped carbon hollow cage-like spheres. When used as a supercapacitor electrode, the specific capacitance values of the double-shelled N-doped carbon hollow cage-like spheres reached as high as 229.3 and 160 F g−1 at 1 and 10 A g−1, respectively. Meanwhile, owing to the superior 3D hierarchically porous structure, both rate capability and cycling stability were excellent.162
It is well known that three parameters, namely, energy density, power density, and cycling life, are the most important. On the basis of the above examples, it can be concluded that the structural characteristics of multishelled hollow nanostructures are expected to have a considerable influence on the performance of supercapacitors. Generally, multiple thin shells usually provide high volumetric energy density owing to the large surface-to-volume ratio, good electrode/electrolyte contact, and abundant active sites. The porous shells facilitate electronic/ionic transformation, leading to high power density. The intershell space with optimized void-to-solid ratio is a significant factor that determines the cycling stability of multishelled hollow nanostructures when used as supercapacitor electrodes.
Recently, a considerable amount of effort has been devoted toward exploring alternative electrode materials that can deliver high capacity and good rate capability. In this context, metal oxides have emerged as promising candidates for use as anode materials for LIBs in view of their relatively low cost, nontoxicity, higher theoretical capacity, and intrinsically enhanced safety.166,167 Multishelled hollow nanostructures, possessing advantages similar to other nanomaterials and unique advantages of their hollow cavities, may offer not only plentiful electrode–electrolyte reaction sites but also a “buffer” to restrict the volume expansion during the Li+ charge–discharge process. Using histidine as a structure-directing agent, Wu et al. developed a facile route based on a hydrothermal process and subsequent thermal treatment in air to prepare porous multishelled α-Fe2O3 hollow spheres. The unique porous multishelled α-Fe2O3 hollow spheres possessed a large specific surface area of 14.2 m2 g−1 and they offered a high specific capacity, excellent cycling stability, and high rate capability when applied as anode for LIBs. The electrochemical performance was demonstrated to be superior as compared to those of various Fe2O3-based anode materials reported earlier. This is due to their unique structural advantages, such as easy to be filtrated in the electrolyte, more locations to store lithium ions, and short ion diffusion path.168 Xu et al. prepared multishelled α-Fe2O3 hollow nanospheres with controlled number of shells and thickness by adjusting the solvent composition through a carbonaceous template method.36 As shown in Fig. 33, the number and thickness of shells could be controlled by adjusting the experimental parameters. When used as an anode for LIBs, the authors found that both number of shells and shell thickness have a significant impact on the energy storage performance. Apparently, thin triple-shelled samples deliver the best performance.
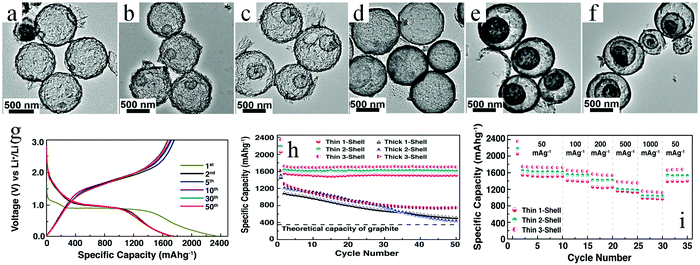 | ||
| Fig. 33 (a–f) TEM images of multishelled α-Fe2O3 hollow nanospheres with different numbers of shells and shell thicknesses: (a) thin single-shelled, (b) thin double-shelled, (c) thin triple-shelled, (d) thick single-shelled, (e) thick double-shelled, and (f) thick triple-shelled samples. (g–i) Electrochemical performance of above samples. Reprinted from ref. 36 with kind permission. | ||
Cobalt oxide has also been demonstrated as a promising electrode candidate for LIBs. As shown in Fig. 34a, the initial charge–discharge profiles show a distinct voltage plateau and long tailing. Different numbers of shells of the Co3O4 hollow spheres lead to different charge–discharge times. Apparently, triple-shelled structures deliver the highest energy density. With regard to the cycling performance (Fig. 34b), multishelled Co3O4 hollow spheres exhibit superior specific capacity than that of a commercial sample. The reversible capacities of single-, double-, triple-, and quadruple-shelled Co3O4 microspheres are much higher than that of commercial Co3O4.
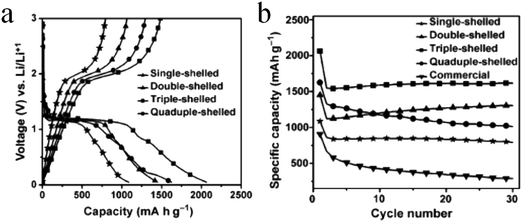 | ||
| Fig. 34 (a) Initial discharge–charge curves for Co3O4 hollow microspheres with different numbers of shells. (b) Discharge capacity versus number of cycles for Co3O4 hollow microspheres with different numbers of shells and commercial Co3O4. Reprinted from ref. 40 with permission. Copyright 2013 John Wiley & Sons. | ||
In another work, Yin and co-workers prepared Co3O4 hollow spheres with tunable numbers of shells (from yolk–shell to multishell) by forming cobalt complex microspheres via a solution-based route under mild conditions and subsequent controlled calcination processes in air (Fig. 35).169Fig. 36 shows the electrochemical performance of the three as-obtained Co3O4 samples. It has been demonstrated that multishelled Co3O4 hollow spheres possessed excellent storage properties with a high specific capacity (1379.6 mA h g−1), good rate capability (1026 mA h g−1 at 0.1 A g−1 and 769 mA h g−1 at 5 A g−1), and excellent cycling stability (1058 mA h g−1 at 1 A g−1 after 1000 cycles). Such values are better than fewer-shelled and yolk–shell structures.
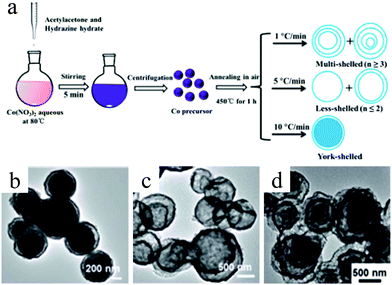 | ||
| Fig. 35 (a) Schematic illustration of the synthesis process and (b–d) typical TEM images of Co3O4 hollow spheres in yolk–shell, fewer-shelled, and multishelled structures. Reproduced from ref. 169 with permission. | ||
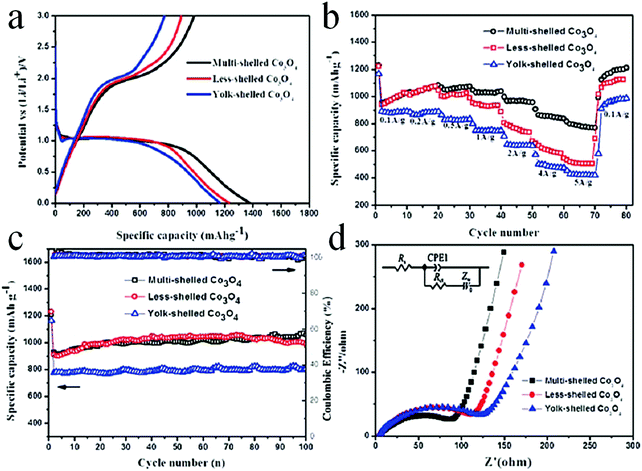 | ||
| Fig. 36 Electrochemical performance of Co3O4 hollow spheres in yolk–shell, fewer-shelled, and multishelled structures when used as the anode for LIBs. (a) Initial charge–discharge curves, (b) rate capability for different current densities, (c) cycling performance at 1 A g−1, and (d) electrochemical impedance spectra. Reproduced from ref. 169 with permission. | ||
In order to further improve the lithium storage properties of Co3O4, Yu et al. demonstrated an effective strategy to synthesize hollow Co3O4@Co3V2O8 hybrid nanoboxes using the reactivity of ZIF-67 with the vanadium source of vanadium oxytriisopropoxide. When tested as an anode for LIBs, triple-shelled hollow Co3O4@Co3V2O8 hybrid nanoboxes demonstrated a remarkable initial discharge capacity of 1909 mA h g−1, reversible charge capacity of 1186 mA g−1, and good stability with capacity retention of 948 mA h g−1 after 100 cycles at 100 mA g−1.170
Similar to iron oxides and cobalt oxides, nickel oxides have also received considerable attention as anode materials for LIBs because of their high theoretical capacity (718 mA h g−1), natural abundance, and nontoxicity.171,172 Li et al. improved the lithium storage properties by constructing multishelled NiO hollow microspheres for use as the anode. They found that triple-shelled NiO hollow microspheres exhibited high specific capacity, outstandingly high rate capability, as well as excellent cycling performance. After 100 discharge–charge cycles, the triple-shelled NiO hollow spheres could deliver a reversible capacity of ∼789 mA h g−1 at a current density of 500 mA g−1. Significantly, the specific discharge capacity of porous triple-shelled NiO hollow spheres retained up to 721 mA h g−1 even at a high current density of 2000 mA g−1. The excellent electrochemical performance could be indexed to the self-supporting multishelled hollow microstructure, guaranteeing ample sites for storing lithium, shortening the lithium-ion diffusion length, and providing adequate void space for buffering the volume expansion.173
Vanadium-based oxides possessing diverse oxidation states, such as V2O5, VO2, V4O7, and V2O3, have also been demonstrated as versatile lithium-ion intercalation hosts.174 Among vanadium-based oxides, V2O5 has been the most extensively investigated anode candidate for LIBs in the past decade. Recently, Wang et al. prepared multiple double-walled V2O5 hollow nanospheres from a vanadium oxide precursor via an ascorbic-acid-assisted solvothermal process followed by an annealing treatment (Fig. 37).175 When evaluated as an electrode for LIBs, the obtained multishelled V2O5 hollow nanospheres exhibited excellent performance (Fig. 38). The data shown in Fig. 38a have two cathodic peaks at 3.36 and 3.17 V, indicating the intercalation of lithium ions into the electrode materials at two steps and yielding two new phases, namely, Li0.5V2O5 and LiV2O5. The anodic peaks at 3.26 and 3.34 V can be attributed to the lithium deintercalation process and the phase changing back from Li0.5V2O5 and LiV2O5 to V2O5. The CV results reveal that the lithium insertion process in the as-obtained multishelled V2O5 hollow nanospheres is completely reversible. The rate performance of the as-obtained multishelled V2O5 hollow nanospheres is shown in Fig. 38b. Evidently, the initial discharge capacity could reach up to 146 mA h g−1 at 50 mA g−1. This value is very close to the theoretical capacity of V2O5. When the current density increased to 400 mA g−1, the capacity retained was 79 mA h g−1, indicating good rate capability of these multishelled V2O5 hollow nanospheres. Significantly, after 120 cycles, the capacity decay could be negligible (from 139 to 133 mA h g−1) at 100 mA g−1. Two plateaus observed in the charge–discharge curves (Fig. 38c) further demonstrated the good rate performance of multishelled V2O5 hollow nanospheres. When compared with single-shelled V2O5 hollow nanospheres, multishelled V2O5 hollow nanospheres exhibit superior cycling stability (Fig. 38d), which can be ascribed to the enlarged contact area between the electrode and electrolyte, resulting in the efficient penetration of the electrolyte.
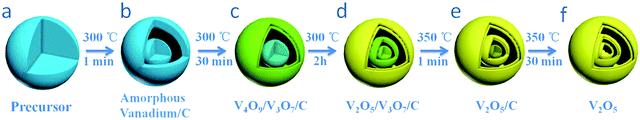 | ||
| Fig. 37 Schematic illustration of the formation process of hollow V2O5 spheres with different interiors. Reproduced from ref. 175 with permission. | ||
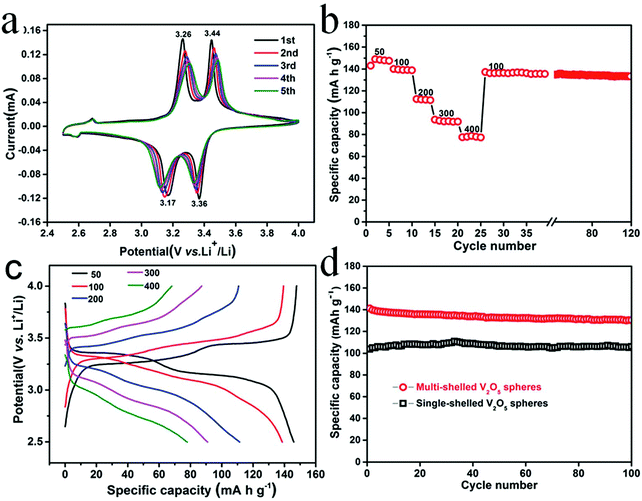 | ||
| Fig. 38 Electrochemical performance of multishelled V2O5 hollow microspheres as the anode for LIBs. (a) CV profiles at 0.05 mV s−1, (b) rate performance, (c) charge–discharge curves at different current densities, and (d) cycling stability when compared with traditional single-shelled hollow samples at a current density of 100 mA g−1. Reproduced from ref. 175 with permission. | ||
Guo et al. exhaustively compared the electrochemical performances of V2O5 double-shelled and single-shelled hollow nanospheres as well as V2O5 nanoparticles prepared by thermally treating vanadyl glycerolate solid spheres. Their results reveal that the as-prepared double-shelled V2O5 hollow nanospheres can deliver an initial capacity of 256.7 mA h g−1 with Coulombic efficiency of nearly 100%, while the single-shelled and nanoparticle samples offer only 226.6 and 217 mA h g−1, respectively. Furthermore, the reversible residual capacity retention is 197.6 mA h g−1 after 50 cycles, which is superior as compared to those of single-shelled hollow nanospheres (160.3 mA h g−1) and solid nanoparticles (145.5 mA h g−1).176 Apart from the above discussed transition metal oxides, improved lithium storage performance has also been achieved when using multishelled hollow structures of ZnO, La2O3, and so on.177,178
Several main group metal oxides could also be used as anode materials for LIBs.179 For instance, Wu et al. intensively investigated a carbon source and Sn salt precursor with regard to the interior and size of multishelled SnO2 hollow spheres. They found that the ratio of glucose to metal precursor plays a critical role. Furthermore, the electrochemical performance of multishelled SnO2 hollow nanospheres was also evaluated when used as an anode for LIBs. Their results demonstrated that the initial discharge and charge capacities were 861 and 442 mA h g−1, respectively. The considerable irreversible capacity loss indicated an irreversible process in the system, such as electrolyte decomposition or the formation of a solid electrolyte interphase (SEI) layer. With regard to the cycling performance, the charge–discharge capacity remained 214 mA h g−1 after 100 cycles, demonstrating good capacity retention. In addition, the as-prepared multishelled SnO2 hollow spheres were relatively tolerant to the various charge–discharge currents. The average capacities were ∼410, 358, 298, 202, and 112 mA h g−1 at current densities of 100, 200, 500, 1000, and 2000 mA g−1, respectively.180 Zhou et al. synthesized a trilayered microsphere composite of SiO2@SnO2@P(EGDMA-co-MAA) via a combination of Stöber method and in situ coating of SnO2 and P(EGDMA-co-MAA) shells. After the selective removal of the silica core via HF etching, double-shelled SnO2@P(EGDMA-co-MAA) hollow microspheres were obtained. Benefiting from the specific hollow structure with a large void space to buffer any volume expansion during the alloying and dealloying processes with lithium ions, the as-obtained double-shelled SnO2@P(EGDMA-co-MAA) hollow microspheres offered a high capacity toward lithium storage. The direct contact of lithium ion and SnO2 component may be effectively avoided since the conductive and flexible P(EGDMA-co-MAA) outer shell as a protective layer was permeable to lithium-ion exchange. Because of the above merits, the obtained SnO2@P(EGDMA-co-MAA) sample delivered a high discharge capacity of 1170 mA h g−1 at 200 mA g−1, good rate capacity of 322 mA h g−1 at 4000 mA g−1, and excellent cycling performance with capacity retention of 711.9 mA h g−1 after 400 cycles at 200 mA g−1.181 In order to further increase the lithium storage performance of SnO2, Zhang et al. fabricated heterogeneous SnO2@Fe2O3(MOF) and SnO2@FeOx-C(MOF) hollow multishelled structures by accurately controlling the transformation of MOF casing. When tested as anode materials for LIBs, these heterogeneous hollow multishelled structures demonstrated superior lithium storage capacity and cycling stability as compared to that of the original SnO2 hollow multishelled structures.182
Besides single-metal oxides, some ternary metal oxides have also been used as anode materials for LIBs. For example, Li et al. prepared triple-shelled CuCo2O4 hollow microspheres (T-CuCo2O4) via a solvothermal/calcination method (Fig. 39a). By controlling the solvothermal time, the number of shells in the final product could be adjusted. When used as an anode for LIBs, T-CuCo2O4 delivered a higher rate capability and superior cycling stability as compared to those obtained from single-shelled and double-shelled CuCo2O4 hollow microspheres, as shown in Fig. 39b and c.183 Qi et al. demonstrated that multishelled CoFe2O4 hollow nanospheres delivered a worthwhile lithium storage capacity when compared with those obtained from CoFe2O4 solid nanospheres and CoFe2O4 hollow nanospheres with traditional single shells.63 Shin et al. used colloidal carbon spheres as the template and prepared multishelled MgCo2O4 (MCO) hollow microspheres to achieve high rate capability and improved electrochemical stability. Recently, Jiao et al. prepared triple-shelled Mn–Co–O hollow dodecahedra using manganese-doped zeolitic imidazole framework ZIF-67 as a precursor via continuous two-step calcination. Benefiting from the residual C and N and the unique features of this hollow structure, the obtained triple-shelled Mn–Co–O hollow dodecahedra exhibited promising electrochemical performance with highly reversible high rate performance, excellent cycling stability, and high capacity when applied as the cathode in rechargeable alkaline batteries.184 Xu et al. constructed various multishelled Zn–Mn–O hollow microspheres, namely, ZnMnO3, ZnMn2O4, and ZnMn2O4/Mn2O3, via a simple programmable heating treatment on a coordination polymer precursor and found that these multishelled hollow microspheres afforded excellent activity and stability when used as the anode for LIBs.185
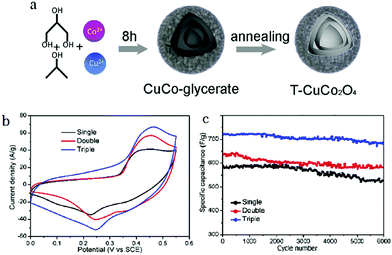 | ||
| Fig. 39 (a) Schematic illustration of the preparation of T-CuCo2O4. (b) CV curves at 50 mV s−1 and (c) cycling performance of CuCo2O4 hollow microspheres with different numbers of shells as an anode for LIBs. Reproduced from ref. 183 with permission. | ||
When compared with oxides, metal sulfides, often offering more abundant redox centers and electrochemically active sites, have emerged as a promising electrode candidate for use in rechargeable alkaline batteries. Li et al. designed and synthesized a series of sulfur-rich nickel sulfide multishelled hollow nanospheres, such as Ni3S2, NiS, and NiS2, using multishelled NiO hollow spheres as the precursor. These multishelled nickel sulfide hollow spheres possessed excellent electrochemical performance when used as the electrode materials for a rechargeable alkaline battery.186
In addition to metallic compounds, carbon materials have also been widely investigated as electrode materials for LIBs.187–190 Sun et al. reported the electrochemical performance of multishelled carbon hollow spheres prepared by using silica as the hard template.27Fig. 40a shows the CV curves of quadruple-shelled carbon hollow spheres in the first three cycles at a sweep rate of 0.1 mV s−1. Evidently, the cathodic peak at ∼0.5–0.8 V was observed only in the first cycle, which is related to the formation of a SEI film. The disappearance of this peak after the first cycle indicates a large initial irreversible capability in the subsequent cycles. Fig. 40b shows the charge–discharge profiles of the first three cycles. It has been observed that the voltage drop is very quick initially followed by a plateau at ∼0.5–0.8 V during the first cycle. The plateau could be attributed to the cathodic peak in the CV curves. Based on the charge curve, the first charge capacity was calculated to be 1254 mA h g−1, which is much higher than that of the other hollow carbon spheres reported elsewhere and nearly 4 times the theoretical value of graphite (372 mA h g−1). Fig. 40c shows the cycling performance of hollow carbon spheres with different numbers of shells (single, double, and quadruple). Notably, among these three hollow samples, quadruple-shelled carbon spheres show the most promising stability. The specific capacity of quadruple-shelled carbon hollow spheres was retained at 977.6 mA h g−1 after 40 cycles, while those of single- and double-shelled ones were 303.5 and 712.1 mA h g−1, respectively. The improved cycling performance of quadruple-shelled carbon hollow spheres is due to the high rate of volume occupation. In addition, the rate capabilities of single- and quadruple-shelled carbon hollow spheres were investigated, as shown in Fig. 40d. Obviously, the capacity of quadruple samples was determined to be 317 mA h g−1 at a high current density of 800 mA g−1, while it was only 85 mA h g−1 for the single-shelled samples. Recently, Xu et al. prepared N-doped double-shelled hollow mesoporous carbon nanospheres, possessing promising electrochemical performance when used as the anode materials for LIBs.80 In particular, the sample obtained by carbonization at 800 °C was demonstrated to offer a discharge specific capacity of 920.3 mA h g−1 at a current density of 0.1 A g−1 after 100 cycles and good rate capability when the current density increased in a step-by-step manner from 0.1 to 5 A g−1. The remarkably improved rate capability could be attributed to the increased contact area between the electrode and electrolyte, improved lithium-ion and electron transport, as well as an appropriate amount of N doping.
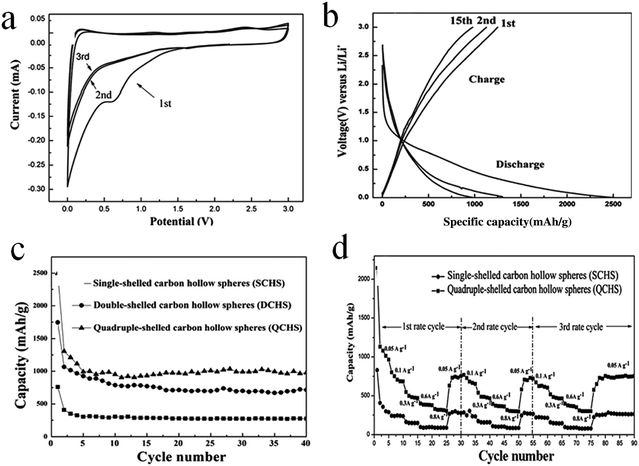 | ||
| Fig. 40 (a) CV curves of the first 3 cycles and (b) GCD curves at 50 mA g−1 for the quadruple-shelled carbon hollow spheres. (c) Cycling performances of carbon hollow spheres for different numbers of shells. (d) Rate capability of quadruple- and single-shelled carbon hollow spheres at various current densities. Reproduced from ref. 27 with permission. | ||
Besides anode materials, multishelled hollow structures can also be applied as cathode materials for LIBs. Spinel LiMn2O4 is one of the most promising alternatives to commercial LiCoO2 when used as the cathode for LIBs because it is environmentally benign, low cost, and highly abundant. Niu et al. presented a facile aerosol ultrasonic spray pyrolysis approach to prepare multishelled LiMn2O4 hollow nanospheres that were assembled by nanosized LiMn2O4 particles (∼10–30 nm). The electrochemical properties of these multishelled LiMn2O4 hollow nanospheres when used as a cathode for LIBs were comprehensively investigated. They found that the capacity of the prepared multishelled LiMn2O4 hollow nanospheres could reach up to 110 mA h g−1 (very near to its theoretical value of 148 mA h g−1) with capacity retention of 91.9% after 400 cycles at a current density of 0.2 A g−1. Such excellent properties are directly related to the aggregation of ultrafine LiMn2O4 particles and the presence of unique mesopores and voids in the sample.191 Wang et al. prepared multishelled LiMn2O4 hollow microspheres using carbonaceous materials as the hard template and systematically investigated their electrochemical performance as a cathode for LIBs. They found that the samples with three shells exhibited relative superior lithium storage capacity as compared to multishelled hollow structures.192
Similar to LIBs, sodium-ion batteries have also gained extensive interest in the field of scientific research and engineering applications during the recent years.193–195 Recently, metal sulfides/phosphides have also been extensively investigated for applications in rechargeable batteries. Xie et al. prepared multishelled hollow structures of Sb2S3 from a MOF template. Owing to the better utilization of the large internal voids, multishelled hollow Sb2S3 showed outstanding volumetric energy density and enhanced durability when used as the anode material for sodium-ion batteries.196 Huang et al. developed a facile and low-temperature solvothermal process to prepare multishelled Sn4P3 nanostructures, exhibiting a large specific area and interlayer space. When applied as an anode for a sodium-ion battery, the specific capacity of the as-prepared multishelled Sn4P3 nanostructures could reach up to 770 mA h g−1 with capacity retention of 96% after 50 cycles at a current density of 50 mA g−1.197
Multishelled hollow nanostructures are also advantageous for use in lithium–sulfur batteries. It is well known that the loading of sulfur in a cathode is critical for the comprehensive performance of the constructed lithium–sulfur battery. Multishelled hollow nanostructures as sulfur carriers are expected to exhibit the following advantages. First, electrolyte penetration into the cavities may be obviously increased when the shells are porous. Secondly, the thin shells comprising tiny nanoparticles may shorten the charge/mass diffusion paths, resulting in high rate capacity. Finally, the interlayer spaces can buffer the volume expansion during the lithiation/delithiation process. Salhabi et al. employed hollow multishelled TiO2−x nanospheres as a sulfur carrier that exhibited a high capacity of 903 mA h g−1 with good capacity retention of 79% at 0.5C and impressive cycling stability (97.5% over 1000 cycles).198 Chen et al. prepared multishelled CoP hollow nanospheres with high electrical conductivity by calcining the Co(II) complex precursor in the presence of NaH2PO2. The as-prepared multishelled CoP hollow nanospheres are expected to possess a polar chemisorptive ability toward polysulfides and provide sufficient active sites to trap polysulfides. When applied as the upper current collector in lithium–sulfur batteries, the constructed battery delivers good cycling performance and good rate capability. Significantly, the mass loading of sulfur in such CoP samples can reach up to 3.24 mg cm−2, yielding high capacity and excellent cycling stability.199 Chen et al. prepared multishelled carbon hollow nanospheres using an emulsion process. Sulfur could be effectively encapsulated into the multishelled carbon hollow nanosphere by means of an in situ method. The percentage of sulfur in the cathode could reach up to 86%. The prepared multishelled carbon hollow spheres offered enhanced cyclability and good capacity retention when applied as cathodes in lithium–sulfur batteries.200
Similar to supercapacitors, the number and thickness of multiple thin shells have a considerable impact on the energy density of alkali metal-ion batteries. Appropriate intershell layer spacing is crucial for buffering volume expansion and short transport pathways facilitate mass transfer. Multishelled hollow nanostructures with a designed composition for each shell show promising prospects for fascinating alkali metal ion and electron transfer, thereby accelerating redox kinetics. Consequently, it is feasible to design multishelled hollow nanostructures as high-performance electrodes to satisfy the increasing demands of energy storage devices.
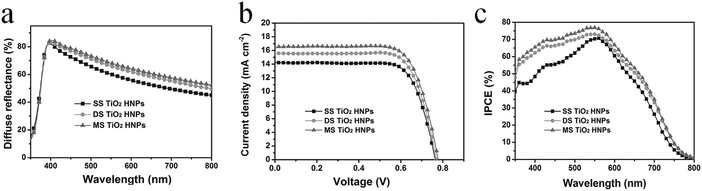 | ||
| Fig. 41 (a) Diffuse reflectance spectra, (b) current density–voltage characteristics (J–V curves), and (c) IPCEs of SS-TiO2-HNPs (square), DS-TiO2-HNPs (circle), and MS-TiO2-HNPs (triangle). Reprinted from ref. 22 with kind permission. | ||
Besides TiO2, ZnO has also been regarded as a promising photoanode candidate for use in dye-sensitized solar cells.210–212 Tian et al. tested the performance of multishelled ZnO hollow microspheres for this purpose, which was obtained by the hydrothermal treatment of a zinc gluconate solution with the desired acidity followed by a calcination treatment.44 Cells based on multishelled ZnO hollow microspheres deliver a higher current density of 13.04 mA cm−2 with conversion efficiency of 4.13%, which is much better than the cells based on commercial ZnO solid spheres. The improved photoanode performance of the as-prepared multishelled ZnO hollow microspheres is undoubtedly related to the features of a multishelled structure, where the large surface area can load abundant dye molecules and relatively large size can scatter the incident light.
In another study, Wang et al. demonstrated that quadruple-shelled ZnO hollow microspheres could deliver conversion efficiency of 5.60%, which is an increase of 20% of the overall conversion efficiency as compared to that obtained from a standard solid-particle-shaped ZnO photoanode. They also noted that the thickness of the exterior shells had a significant impact on the light-harvesting capability and the generated photocurrent. Typically, a thicker exterior shell may scatter more light and afford a higher photocurrent. Besides ZnO, they also investigated the performance of multishelled SnO2 hollow microspheres as a photoanode for use in dye-sensitized solar cells.34,38
CeO2, a rare-earth oxide with a bandgap of 3.2 eV, has also been suggested as a promising photoanode material for use in solar cells. Umable et al. synthesized CeO2 nanoparticles using a solution combustion process. The characteristic of spongy and porous morphology rendered them with high incident photon current efficiency of 68%.213 Nevertheless, CeO2 nanoparticles often suffer from the tradeoff between the scattering effect and loss of incident light owing to the transmission of light. In order to overcome this issue, double-shelled CeO2@TiO2 hollow sphere composites were proposed by Diao's group, which were prepared by a one-pot hydrothermal method followed by coating with a TiO2 shell.214 They found that the dye-loading capacity could be improved owing to the double-shelled CeO2 hollow sphere core; therefore, the hybrid double-shelled CeO2@TiO2 hollow sphere exhibited higher conversion efficiency (7.95%) than that of bare TiO2 electrodes (5.72%). In addition, Diao's group also did a series of works on CeO2-based photoanode materials for use in dye-sensitized solar cells, namely, CeO2:Yb,Er@SiO2@Ag upconversion composite;215 Er, Yb–CeO2 hollow spheres;216 and Yb, Er-doped CeO2 nanotubes.217
Sunlight absorption and dye loading are two crucial factors that determine the performance of dye-sensitized solar cells. The number of shells and intershell spacing are the two most important characteristics of multishelled hollow nanostructures. Typically, larger number of shells and smaller intershell spacing are expected to offer superior sunlight-harvesting capability and higher energy conversion efficiency. The porosity of shells as well as grain size of multishelled hollow nanostructures play a significant role in influencing the loading capacity of dyes. Normally, shells composed of smaller grains can provide a larger surface area to accommodate dyes. Meanwhile, porous shells can yield multiple benefits. Firstly, they can provide a larger specific surface area for dye loading. Lastly, porous shells are expected to improve the light-absorbing efficiency by facilitating multiple sunlight reflections and scattering between the shells.
Qi et al. reported a self-template route to synthesize triple-shelled CeO2 hollow microspheres, involving hydrothermally treating the mixed aqueous solution containing glucose, CeCl3 precursors and urea, as well as a subsequent calcination treatment, as shown in Fig. 42a.219 The following chemical processes are expected to occur in this system. Firstly, glucose is converted into carbonaceous microspheres under the hydrothermal condition, which may act as a template to take up Ce3+ through electrostatic attractions. The main role of urea is to deprotonate the functional groups inside the carbonaceous microspheres, thereby enabling interactions between the carbonaceous material and Ce3+. Finally, the carbonaceous microspheres containing Ce3+ can generate triple-shelled CeO2 hollow spheres by calcination in air. In this process, the carbonaceous microsphere is decomposed into gases and Ce3+ gets oxidized into CeO2. Impressively, the morphology and structure of the final product can vary with the experimental parameters, including the dosage of each reactant, crystalline reaction, and calcination temperature. Furthermore, the results of the four CeO2 samples, namely, commercial CeO2 nanoparticles (CeO2 NPs), single-shelled CeO2 hollow spheres (SSCeHSs), double-shelled CeO2 hollow spheres (DSCeHSs), and triple-shelled CeO2 hollow spheres (TSCeHSs), are shown in Fig. 42b. Evidently, all the hollow CeO2 spheres are superior over CeO2 NPs with regard to catalytic activity. Among the three hollow samples, TSCeHSs exhibit the best performance. The average oxygen evolution rate from the oxidation of water was 78 μmol (gcat h)−1. Such a remarkably improved photocatalytic activity of TSCeHSs was directly associated with the considerably increased surface area and number of active sites generated by multishelled features, improving the separation of electron–hole pairs.
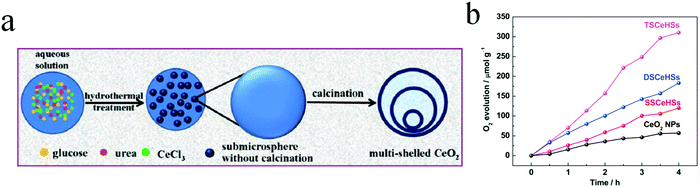 | ||
| Fig. 42 (a) Proposed formation process of multishelled CeO2 hollow microspheres. (b) Comparison of photocatalytic oxygen evolution performance of CeO2 NPs and as-prepared multishelled CeO2 hollow microspheres with different numbers of shells. Reproduced from ref. 219 with kind permission. | ||
Wei et al. prepared SrTiO3–TiO2 heterogeneous hollow multishelled structures (STHoMs) by the hydrothermal crystallization of SrTiO3 on the surface of TiO2 hollow multishelled structures, resulted in outstanding solar water-splitting performance of 10.6 mmol h−1 for H2 production and 5.1 mmol h−1 for oxygen evolution. They explained that the synergistic effect of SrTiO3 and TiO2 afforded better separation efficiency of the photogenerated charge carriers and hollow multishelled structures increased the light-absorption ability of the SrTiO3–TiO2 heterogeneous catalyst.220
3.2 Environmental-remediation-related applications
Wang et al. prepared multishelled ZnO core–shell hollow microspheres with a diameter of ∼0.4–3.5 μm by using carbonaceous materials as the hard template. A chemical gas sensor based on the as-prepared multishelled ZnO core–shell hollow microspheres exhibited rapid, high, and selective response toward toluene.221 At the optimal operating temperature of 300 °C, the response and recovery times of the sensor for toluene at 20 ppm were 0.3 and 3 s, respectively. The detection limit was as low as 1 ppm and the detectable concentration range was up to 2000 ppm. Qu et al. synthesized multishelled mixed Ni–Co oxide microspheres, which showed substantial selectivity and remarkable sensitivity (11.5–5 ppm at 255 °C) toward xylene. Significantly, the selectivity and response toward xylene could be maintained with excellent humidity resistance, suggesting the potential of such multishelled Ni–Co oxide microspheres in the detection of ground-level xylene gas in the environment.222
With the development of industries and widespread utilization of vehicles, the increasing consumption of fossil fuels has generated a large amount of NOx (NO and NO2), which is harmful to the domestic environment as well as the human body. The accurate content of NO2 in air even at a very low concentration is an important parameter in order to evaluate air quality. Kim et al. developed a highly sensitive and selective NO2 sensor based on multishelled WO3 microspheres.223 As shown in Fig. 43, the number of shells of the WO3 hollow microspheres has a significant impact on the sensor performance. Such detection ability can be attributed to the enhanced gas accessibility of the yolk–shell morphology with thin and permeable multiple shells.
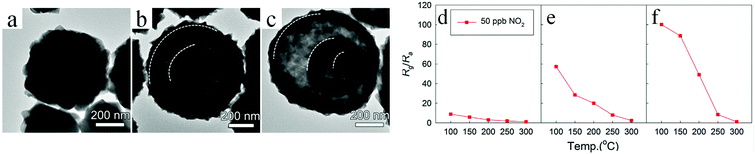 | ||
| Fig. 43 TEM images of (a) D-WO3, (b) 2S-WO3, and (c) 3S-WO3. Gas responses (Rg/Ra) to 50 ppb NO2 of (d) D-WO3 spheres, (e) 2S-WO3 spheres, and (f) 3S-WO3 spheres. Reprinted from ref. 223 with kind permission. | ||
As a commonly applied organic solvent in the industry and research laboratory, acetone is characterized as a volatile and deleterious chemical. If the acetone content in the environment reaches up to 450 mg m−3, the health of human beings can be considerably threatened. Meanwhile, as reported by medical research studies, acetone concentration can be a signal for diabetes diagnoses since the acetone concentration exhaled from a healthy person is usually lower than 0.8 ppm, while it equals to/exceeds 1.8 ppm for diabetic patients. Therefore, developing sensitive and selective acetone sensors to detect acetone concentration in the public or human body is worthwhile. Li et al. developed an acetone gas sensor with high sensitivity and selectivity based on double-shelled ZnO hollow microspheres.224 This device yielded high response toward 100 ppm acetone (101.1), achieving a rapid response rate and recovery process (within 1/7 s). Moreover, the substantially low detection limit (0.5 ppm), low operating temperature (40 °C), high selectivity, and long-term stability showed that the double-shelled ZnO hollow microspheres are a reliable solution to the problem of acetone detection.
Since the sensing mechanism depends on the interaction between the target gas and surface of the sensing layer, the sensibility of metal oxide gas sensors relies on the available sensing surface and target gas concentration. It is well known that the different facets of nanomaterials may show different affinities toward certain gases. Recently, Zheng et al. reported Y2O3 multishelled hollow structures with exposed {220} facets, which makes a significant contribution to the detection of methanol with ultrahigh sensitivity and selectivity.225
It has been reported that the performance of semiconductor-based gas sensors may be further enhanced by loading uniform noble metal nanoparticles.226–228 Yoon and co-workers prepared Pd-loaded quintuple-shelled Co3O4 microspheres via spray drying followed by thermal treatment.229 These Pd-loaded quintuple-shelled Co3O4 microspheres exhibited unprecedented response (resistance ratio) and selectivity toward toluene and p-xylene against various interfering gases such as ethanol, benzene, formaldehyde, ammonia, nitrogen monoxide, carbon monoxide, and hydrogen. They also compared their performance with Pd–Co3O4 nanoparticles, confirming that multishelled Pd–Co3O4 is much superior to other materials. The excellent gas sensing performance could be attributed to several aspects. On one hand, the serious agglomeration characteristics of the Pd–Co3O4 nanoparticles yield absolute results in lowering the gas response. For multishelled Pd–Co3O4 microspheres, the outermost shell may be the determining factor that affects the gas response. On the other hand, it is the effective dissociation of methylbenzenes into active small species that can promote the selectivity of the fabricated sensors. Undoubtedly, loading metallic nanoparticles can improve the catalytic activity of oxide semiconductors. Meanwhile, multishelled structures may favor the dissociation of methylbenzene by providing more active sites and large space as compared to those afforded by solid nanoparticles. Apart from the above study, Sun et al. demonstrated that PdO-functionalized double-shelled Fe2O3 hollow nanospheres exhibit superior sensing properties toward acetone owing to the larger surface area and high catalytic activity of PdO.230 Ma et al. recently demonstrated that Au nanoparticles could also further improve the sensor's response of α-Fe2O3 double-shelled hollow spheres owing to the catalytic sensitization from Au nanoparticles.231
The sensing selectivity, sensitivity, and stability are considerably dependent on the interaction between the detected molecules and multishelled hollow nanostructures. Both porosity and hydrophilic characteristics affect the substrate with suitable size/configuration and specific groups accessing the interior of the multishelled hollow nanostructures, leading to worthwhile sensing selectivity. Multiple thin shells often provide abundant responsive sites and the transportation of detected molecules may be facilitated if the shells are porous owing to the capillary effect. In addition, the stability of sensors based on a higher number of shells is expected to be better than those having fewer shells owing to the supporting effect of multiple shells.
When compared with simple metal oxides, bimetal oxides usually exhibit superior photocatalytic performance. Zong et al. designed a modified carbonaceous microsphere template method to prepare multishelled hollow spheres of heterostructured BiVO4 (Fig. 44a). The multishelled heterostructured BiVO4 hollow spheres exhibited high photocatalytic activity toward the degradation of methylene blue under visible light, and the double-shell spheres with the highest Bi4V2O11 content delivered the highest photocatalytic activity (Fig. 44b). The excellent photocatalytic performance could be attributed to the effective utilization of visible light induced by multiple reflections of their special multishelled hollow spheres, as shown in the schematic in Fig. 44c.234 By taking full advantage of this concept, the excellent photocatalytic degradation of organic pollutants could also be achieved using multishelled Bi2WO6 hollow microspheres.235
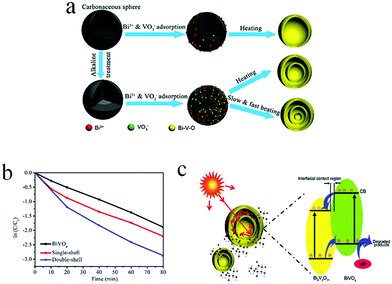 | ||
| Fig. 44 (a) Schematic illustration of the synthesis procedure of multishelled Bi–V–O hollow microspheres. (b) Catalytic performance of solid BiVO4 and single- and double-shelled Bi–V–O hollow microspheres. (c) Schematic diagram of the photocatalytic mechanism of the heterostructures of multishelled Bi–V–O hollow microspheres. Reprinted from ref. 234 with kind permission. | ||
Liu et al. demonstrated that double-shelled ZnFe2O4 hollow microspheres exhibited excellent photocatalytic activity toward the degradation of gaseous o-dichlorobenzene (o-DCB).236 The degradation ratio could reach up to 74% within 7 h, which is higher than that of solid spheres (51%) and yolk–shell spheres (65%). Moreover, the double-shelled ZnFe2O4 hollow microsphere catalyst possesses high stability (the degradation ratio retaining 70% after 5 repetitions). Such outstanding photocatalytic performance could be attributed to the advantages of double-shelled hollow structures in the adsorption of reactants and sunlight-harvesting capability.
Fig. 45a shows the diffusion characteristics of gaseous o-DCB over solid spheres and yolk–shell and double-shelled spheres. Notably, the diffusion rate of ZnFe2O4 double-shelled spheres is much higher than the rates for the other structures during the adsorption period. This can be directly attributed to the fact that double-shelled hollow spheres can provide a higher surface area and more intricate pore structures than those provided by solid and yolk–shell spheres. Importantly, the superior photocatalytic performance of double-shelled structures is also likely to enhance the effective light absorption for multiple scatterings, as shown in Fig. 45b.
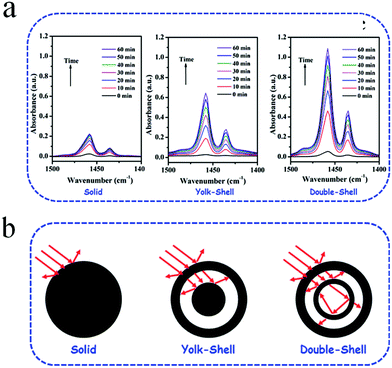 | ||
| Fig. 45 Comparison of the diffusion characteristics of o-DCB (a) and light diffusion (b) over solid, yolk–shell, and double-shelled ZnFe2O4 spheres. Reprinted from ref. 236 with permission. | ||
Besides the catalytic degradation of organic pollutants in water, multishelled hollow nanostructures also show promising potential in the catalytic reduction of gas pollutants, such as NO, CO, and CO2. For example, Ma et al. prepared triple-shelled CeO2–MnOx hollow hybrid spheres by using a carbonaceous microsphere as the sacrificial hard template. The as-prepared triple-shelled CeO2–MnOx hollow spheres showed apparent advantages when compared with traditional CeO2–MnOx nanoparticles, as well as single-shell and double-shell hollow spheres, when applied as a catalyst for the selective catalytic reduction of NO with NH3.237 As shown in Fig. 46a, the catalytic performances of the samples can be ranked in the following order: triple-shelled > double-shelled > single-shelled > nanoparticles. In particular, triple-shelled CeO2–MnOx hollow spheres yielded the sustained conversion of 100% in the temperature range of 150–250 °C. Such superior catalytic performance of triple-shelled CeO2–MnOx hollow spheres can be attributed to the synergistic effect between elemental Ce and Mn. Furthermore, the water resistance performance of these catalysts was also investigated. As shown in Fig. 46b, the addition of water into a mixture of the reactant gases can decrease the conversion of NO for all the catalysts. In particular, the NO conversions of single-, double-, and triple-shelled CeO2–MnO2 hollow catalyst decreased from their initial conversion to about 70% in the first hour and then maintained the conversion well up to 14 h in the presence of water when the reaction temperature was set at 150 °C. However, a decrease from 80% to below 40% of the NO conversion was observed for the CeO2–MnO2 nanoparticle catalyst. The deactivation of the CeO2–MnO2 catalyst may be related to the formation of hydroxyls on the surface of the catalyst owing to the adsorption of H2O, leading to a decline in the activity. A typical example is given by Wang et al. They prepared hollow multishelled Co3O4–CeO2−x composites via a general strategy using the sequential template approach with a facile and efficient electrostatic spray process. When compared with the simple components of Co3O4, CeO2, and heterogeneous Co3O4–CeO2−x nanoparticles, the multishelled hollow Co3O4–CeO2−x composites exhibited much superior catalytic activity toward the oxidation of CO (complete conversion temperature is 166.9 °C) and stability (100 h). They claimed that the worthwhile catalytic performance can be attributed to two factors. For one thing, the synergistic effects generated by the combination of Co3O4 and CeO2−x facilitate the activation of oxygen. For another thing, abundant active sites and gas transformation channels of such multishelled hollow structures facilitate gas diffusion and maintain structural stability.238
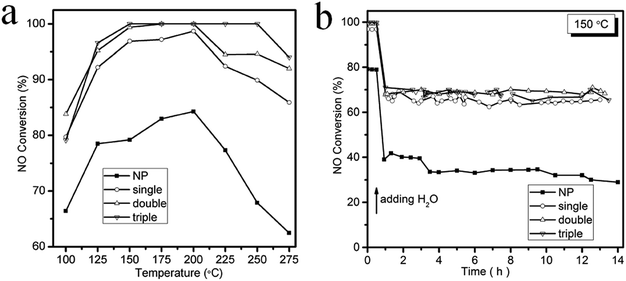 | ||
| Fig. 46 NO conversion of the CeO2–MnOx catalysts as a function of temperature in the absence of water (a) and in the presence of water at 150 °C (b). Reproduced from ref. 237 with kind permission. | ||
Nowadays, the excessive emissions of CO2 from the accelerated consumption of fossil fuels have increased the threat to the climate. Therefore, the transformation of CO2 has attracted extensive attention. Multishelled hollow nanostructures show promising potential in the photocatalytic reduction of CO2. Recently, Wang and co-workers developed multishelled Co3O4 hollow dodecahedra using a sequential template approach by adopting MOFs as the template.239 Co3O4 nanocrystals with multishelled hollow structures were assembled in the desired orientation, forming a unique shell with the dominant exposure of the (111) facets. It was observed that the catalytic activity of quadruple-shelled Co3O4 hollow dodecahedra was superior over those obtained from Co3O4 nanoparticles and Co3O4 hollow structures without the controlled facet in CO2 photoreduction.
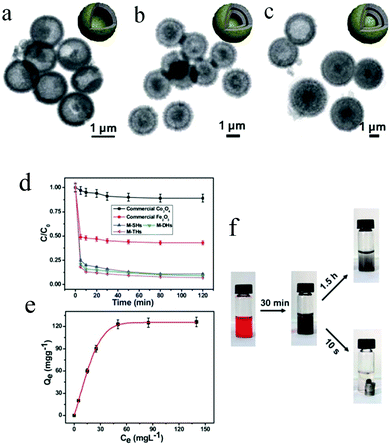 | ||
| Fig. 47 TEM images of M-MHs: (a) M-SHs, (b) M-DHs, and (c) M-THs. (d) Adsorption rates of Congo red on the three M-MHs, commercial γ-Fe2O3, and commercial Co3O4. (e) Adsorption isothermal curves of Congo red on M-THs. (f) Photographs confirming the absorption of Congo red by M-THs with time. Reproduced from ref. 240 with kind permission. | ||
Nowadays, nuclear energy has become increasingly popular in many countries, which is unavoidably accompanied by excessive wastewater containing radionuclides. Because of their mobility and high toxicity, radionuclide contaminants can harm human beings as well as the ecosystem. Therefore, it has become imperative to develop efficient techniques to remove radionuclide pollutants.241,242 Adsorption has proven to be one of the most promising methods in the treatment of radionuclides-contaminated water owing to its convenient operation and effectiveness.243,244 Recently, Song et al. reported multishelled Fe3O4@MnOx hollow spheres, which were synthesized via a three-stepped process, namely, the in situ coating of Fe3O4@C on the surface SiO2 cores, self-template formation of MnOx by reacting between Fe3O4@C and KMnO4, and SiO2 removal via etching with NaOH.245 When used as an adsorbent for the removal of uranium (U(VI)) and europium (Eu(III)), the isotherms of these two radionuclides onto the adsorbents effectively matched with the Langmuir model. The as-obtained multishelled Fe3O4@MnOx hollow spheres offered high adsorption capacities (maximum absorption capacities of 106.72 mg g−1 for U(VI) and 138.13 mg g−1 for Eu(III) could be obtained at 298 K), revealing the advantages of high-performance adsorbents. The adsorption thermodynamics are endothermic processes, while the adsorption kinetics conform to the pseudo-second-order model. It is well known that radionuclides can often coexist with humic acid. The investigation results demonstrate exciting information that the presence of humic acid may provide higher simulation yields of the maximum adsorption capacities toward U(VI) and Eu(III), namely, 169.17 and 146.81 mg g−1, respectively. Significantly, the Fe3O4@MnO2 adsorbent could be easily recovered by using an external magnet for successive usage.
Apart from heavy metal ions and organic pollutants, multishelled hollow nanostructures can also be used for the removal of gaseous pollutants. Utilizing carbonaceous microspheres as the hard template, Feng et al. fabricated nanostructures with controlled Ca/Mg molar ratio, number of shells, and size.246 The Mg-modified CaCO3 multishelled hollow microspheres exhibited remarkable CO2 adsorption performance. Under optimized conditions, triple-shelled Mg-modified CaCO3 provides adsorption capacity of 0.51 g CO2 g−1 adsorbent, which is marginally less than that of commercial CaCO3–C (0.53 g CO2 g−1 adsorbent). Nevertheless, owing to the conversion of CaCO3 into CaO at higher temperatures, the serious sintering of commercial CaCO3–C results in a rapid decay in the adsorption capacity for CO2 from the second cycle. Because of the introduction of Mg, the sintering and aggregation of CaO nanoparticles at high temperature could be efficiently prevented due to the high Tammann temperature of MgO. As a result, these multishelled Mg-modified CaCO3 microspheres could maintain the CO2 adsorption capacity after repeated cycles.
3.3 Chemical catalysis for organic transformation
Via a layer-by-layer deposition process followed by alkali etching, Liu and co-workers prepared double-shelled CeO2/M@M/TiO2 (M = Au and/or Pd) nanospheres, which exhibited excellent catalytic performance toward several organic reactions, such as Suzuki reaction, aerobic oxidation of benzyl alcohol, and reduction of 4-nitrophene.247 Xu et al. prepared CSs containing Ce3+ by a two-stepped hydrothermal method using glucose as the carbon precursor and Ce(NO3)3 as the Ce precursor and then converted them into multishelled CeO2 hollow spheres via controlled calcination in an air/gas flow. Au nanoparticles were deposited and dispersed on these multishelled CeO2 hollow spheres at a high content. The Au/CeO2 hollow spheres exhibited higher catalytic activity and more stability toward the reduction of 4-nitrophenol with NaBH4 than those from other noble-metal-loaded catalysts in other structures. Significantly, the Au/CeO2 catalyst showed no changes in both morphology and activity after eight cycles. This can be attributed to the hierarchically nanoscale sizes of the multishelled CeO2 hollow sphere support and the highly dispersed ultrathin Au nanoparticles.248 Liao et al. synthesized Ce-based coordination polymer by a mixed-solvothermal process and then converted it into multishelled CeO2 hollow nanospheres via a simple thermal treatment in air. The prepared multishelled CeO2 hollow nanospheres not only exhibited efficient photocatalytic activity for the degradation of rhodamine B under visible light but also could be used as a support to load noble metal nanoparticles (such as Au and AuPd). When used as a catalyst, Au/CeO2 showed high activity toward the reduction of 4-nitrophenol at a reaction rate constant of 0.416 min−1, and AuPd/CeO2 are efficient catalysts for CO oxidation.249 Zhang et al. introduced Pt species into double-shelled TiO2 hollow spheres, which was prepared via a simple hydrothermal treatment of SiO2@TiO2 (TiO2-coated solid SiO2 spheres).250 The as-prepared Pt (DHS–TiPt) composite showed excellent catalytic activity toward the reduction of 4-NP with NaBH4.Similar to gas sensor applications, by precisely controlling the pore size distribution, multishelled hollow nanostructures could selectively permit reactant/product diffusion through the shells, resulting in outstanding selective catalysis processes. In aqueous solutions, the hydrophilic characteristics of the shell influence the reactants with specific groups accessing the interior of such multishelled hollow nanostructures, further improving selectivity. In addition, multiple thin shells with large intershell spacings can provide a large surface area, resulting in more reactants contacting the active site and therefore enhancing the catalytic performance.
3.4 Drug delivery
Drug delivery, aimed at improving the aqueous solubility, chemical stability, and pharmacological activity, as well as reducing the side-effects of drugs, can be used to transport therapeutic drugs in the body as needed to safely achieve the desired therapeutic effect. The goal of any drug delivery system is to provide and maintain the therapeutic concentrations of drug at the target biological site.251,252Multishelled hollow nanostructures with tunable pore and shell structures have been widely employed for drug delivery. For drug delivery systems, three important parameters are the most significant: loading capacity, good targeting, and sustained drug release. These evaluation parameters can be influenced by the number, thickness, and porosity of shells. Multiple thin shells can provide abundant loading sites, leading to high drug loading capacity. Porous shells allow multishelled hollow nanocarriers to reach the target drug molecules with preferred sizes. In addition, multishelled hollow nanostructures with adjustable hydrophilic and hydrophobic properties can allow the co-loading/release of various drugs.
For example, Wu et al. synthesized multishelled mesoporous silica spheres by the vesicle template method. When used as the carriers of anticancer drugs, the loading efficiency of doxorubicin hydrochloride could reach up to 83% at the optimum loading concentration of 1.2 mg L−1. More significantly, the loaded anticancer drug exhibited the desired pH-responsive release behavior. In particular, the release rate under acidic conditions is much higher than that under basic conditions, which is beneficial for killing cancer cells since almost all the cancer cells have an acidic intracellular environment. Huang et al. demonstrated that hollow triple-shelled mesoporous silica spheres with tunable shell-to-shell distances loaded with doxorubicin hydrochloride yielded excellent performance in killing cancer cells by a slow, controlled, and sustained release.253
4 Conclusion and outlook
In summary, multishelled hollow nanostructures have become a hot topic in both the scientific arena and engineering applications. Substantial progress has been made in the past few decades with the development of efficient methods to construct multishelled hollow nanostructures. In this review, we have provided a comprehensive overview of the synthesis strategies and applications of multishelled hollow nanostructures. For further research in the field of multishelled hollow nanostructures, the focus should be on the following aspects. As far as synthesis is concerned, it might always be challenging to precisely control and manipulate multishelled hollow nanostructures by means of simple and cost-effective approaches such that they can have the desired compositions and morphologies. Several solutions can be considered. For one thing, further expanding and modifying the existing template-based/template-free approaches are expected to be developed. For another thing, the combination of various approaches containing multiple hollowing mechanisms in a single synthesis process can undoubtedly be the trend for fabricating certain multishelled hollow nanomaterials with special structures and properties. Furthermore, despite several challenges, novel strategies for generating multishelled hollow structures can be developed, bringing revolutionary advances to this area. For the applications of multishelled hollow nanostructures, engineers and scientists can investigate the relationship among the structures of such complex hollow materials as well as enhanced performances. Meanwhile, for practical applications of such promising materials, the situation for multishelled hollow nanostructures might be fairly different, which should be effectively considered, too. In particular, energy-related applications often consider ion/electron and charge/mass transports as well as the capability of immobilizing sulfide/lithium or electrolyte ions by chemical/physical means. For catalyst/adsorbent materials, the activity and reusability should be seriously considered. Carrier materials for certain drugs heavily rely on biocompatibility as well as interaction between the drug molecules. Consequently, a comprehensive understanding of the requirements of specific applications is expected to, in turn, direct the fabrication of multishelled hollow nanomaterials. It is believed that multishelled hollow nanostructures, as a type of versatile functional materials, can have a bright future in many fields.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21403091, and 51473070).References
- X.-J. Wang, J. Feng, Y.-C. Bai, Q. Zhang and Y.-D. Yin, Synthesis, Properties, and Applications of Hollow Micro-/Nanostructures, Chem. Rev., 2016, 116, 10983–11060 CrossRef CAS PubMed
.
- Q. Zhang, W.-S. Wang, J. Goebl and Y.-D. Yin, Self-templated Synthesis of Hollow Nanostructures, Nano Today, 2009, 4, 494–507 CrossRef CAS
.
- T. Liu, L. Zhang, B. Cheng and J. Yu, Hollow Carbon Spheres and Their Hybrid Nanomaterials in Electrochemical Energy Storage, Adv. Energy Mater., 2019, 9, 1803900 CrossRef
.
- L. Zhou, Z.-C. Zhuang, H.-H. Zhao, M.-T. Lin, D.-Y. Zhao and L.-Q. Mai, Intricate Hollow Structures: Controlled Synthesis and Applications in Energy Storage and Conversion, Adv. Mater., 2017, 29, 1602914 CrossRef PubMed
.
- X.-Y. Yu, L. Yu and X.-W. Lou, Metal Sulfide Hollow Nanostructures for Electrochemical Energy Storage, Adv. Energy Mater., 2016, 6, 1501333 CrossRef
.
- L. Yu., X.-Y. Yu and X.-W. Lou, The Design and Synthesis of Hollow Micro-/Nanostructures: Present and Future Trend, Adv. Mater., 2018, 30, 1800939 CrossRef PubMed
.
- L. Yu, H. Hu, H.-B. Wu and X.-W. Lou, Complex Hollow Nanostructures: Synthesis and Energy-Related Applications, Adv. Mater., 2017, 29, 1604563 CrossRef PubMed
.
- X.-Y. Yu, L. Yu and X.-W. Lou, Hollow Nanostructures of Molybdenum Sulfides for Electrochemical Energy Storage and Conversion, Small Methods, 2017, 1, 1600020 CrossRef
.
- D. Mao, J.-W. Wan, J.-Y. Wang and D. Wang, Sequential Templating Approach: A Groundbreaking Strategy to Create Hollow Multishelled Structures, Adv. Mater., 2018, 1802874 Search PubMed
.
- H.-X. Yang, J.-F. Qian, Z.-X. Chen, X.-P. Ai and Y.-L. Cao, Multilayered Nanocrystalline SnO2 Hollow Microspheres Synthesized by Chemically Induced Self-Assembly in the Hydrothermal Environment, J. Phys. Chem. C, 2007, 111, 14067–14071 CrossRef CAS
.
- X.-Y. Lai, J. E. Halpert and D. Wang, Recent Advances in Micro-/Nano-tructured Hollow Spheres for Energy Applications: From Simple to Complex Systems, Energy Environ. Sci., 2012, 5, 5604–5618 RSC
.
- A.-B. Chen, W.-P. Zhang, Y. Liu, X.-W. Han and X.-H. Bao,
In Situ Introduction of Dispersed Metallic Ag Nanoparticles into the Channels of Mesoporous Carbon CMK-3, Chin. Chem. Lett., 2007, 18, 1017–1020 CrossRef CAS
.
- J.-S. Wu and D.-F. Xue, Hierarchical Integration of ZnO Nanocrystals into Multishelled Superstructures, Nanosci. Nanotechnol. Lett., 2011, 3, 371–377 CrossRef CAS
.
- Z.-M. Li, X.-Y. Lai, H. Wang, D. Mao, C.-J. Xing and D. Wang, General Synthesis of Homogeneous Hollow Core–Shell Ferrite Microspheres, J. Phys. Chem. C, 2009, 113, 2792–2797 CrossRef CAS
.
- H.-L. Xu and W.-Z. Wang, Template Synthesis of Multishelled Cu2O Hollow Spheres with a Single-Crystalline Shell Wall, Angew. Chem., Int. Ed., 2007, 46, 1489–1492 CrossRef CAS PubMed
.
- J. Qi, X.-Y. Lai, J.-Y. Wang, H.-J. Tang, H. Ren, Y. Yang, Q. Jin, L.-J. Zhang, R.-B. Yu, G.-H. Ma, Z.-G. Su, H.-J. Zhao and D. Wang, Multi-shelled Hollow Micro-/Nanostructures, Chem. Soc. Rev., 2015, 44, 6749–6773 RSC
.
- M. Qin, D. Lan, J.-L. Liu, H.-S. Liang, L.-M. Zhang, H. Xing, T.-T. Xu and H.-J. Wu, Synthesis of Single-component Metal Oxides with Controllable Multi-shelled Structure and their Morphology-related Applications, Chem. Rec., 2019, 19, 1–19 CrossRef
.
- H. Ren and R.-B. Yu, Hollow Multi-shelled Structures for Energy Conversion and Storage Applications, Inorg. Chem. Front., 2019, 6, 2239–2259 RSC
.
- Y. Liu, X.-C. Li, W.-M. Shen, Y. Dai, W. Kou, W.-J. Zheng, X.-B. Jiang and G.-H. He, Multishelled Transition Metal-based Microspheres: Synthesis and Applications for Batteries and Supercapacitors, Small, 2019, 15, 1804737 CrossRef PubMed
.
- J.-Y. Wang, H.-J. Tang, H. Wang, R.-B. Yu and D. Wang, Multi-shelled Hollow Micro-/anostructures: Promising Platforms for Lithium-ion Batteries, Mater. Chem. Front., 2017, 1, 414–430 RSC
.
- J.-Y. Wang, J.-W. Wan and D. Wang, Hollow Multishelled Structures for Promising Applications: Understanding the Structure-Performance Correlation, Acc. Chem. Res., 2019, 52, 2169–2178 CrossRef CAS PubMed
.
- S. H. Hwang, J. Yun and J. Jang, Multi-Shell Porous TiO2 Hollow Nanoparticles for Enhanced Light Harvesting in Dye-sensitized Solar Cells, Adv. Funct. Mater., 2014, 24, 7619–7626 CrossRef CAS
.
- X.-W. Lou, C.-L. Yuan and L. A. Archer, Shell-by-Shell Synthesis of Tin Oxide Hollow Colloids with Nanoarchitectured Walls: Cavity Size Tuning and Functionalization, Small, 2007, 3, 261–265 CrossRef CAS PubMed
.
- X.-Y. Yu, L. Yu, L.-F. Shen, X.-H. Song, H.-Y. Chen and X.-W. Lou, General Formation of MS (M = Ni, Cu, Mn) Box-in-Box Hollow Structures with Enhanced Pseudocapacitive Properties, Adv. Funct. Mater., 2014, 24, 7440–7446 CrossRef CAS
.
- N.-X. Du, W.-J. Zheng, X.-C. Li, G.-H. He, L. Wang and J.-H. Shi, Nanosheet-assembled NiS Hollow Structures with Double Shells and Controlled Shapes for High-performance Supercapacitors, Chem. Eng. J., 2017, 323, 415–424 CrossRef CAS
.
- X. Li, Y. Zhang, H.-J. Zhang, Y.-Y. Feng and Y. Wang, Porous Double-shelled SnO2@C Hollow Spheres as High-Performance Anode Material for Lithium Ion Batteries, Electrochim. Acta, 2016, 195, 208–215 CrossRef CAS
.
- Z. Sun, X.-F. Song, P. Zhang and L. Gao, Template-assisted Synthesis of Multi-shelled Carbon Hollow Spheres with an Ultralarge Pore Volume as Anode Materials in Li-ion Batteries, RSC Adv., 2015, 5, 3657–3664 RSC
.
- X.-Y. Lai, J. Li, B. A. Korgel, Z.-H. Dong, Z.-M. Li, F.-B. Su, J. Du and D. Wang, General Synthesis and Gas-Sensing Properties of Multiple-Shell Metal Oxide Hollow Microspheres, Angew. Chem., Int. Ed., 2011, 50, 2738–2741 CrossRef CAS PubMed
.
- H.-M. Sun, L.-M. Wang, D.-Q. Chu, Z.-C. Ma and A.-X. Wang, Facile Fabrication of Multishelled Cr2O3 Hollow Microspheres with Enhanced Gas Sensitivity, Mater. Lett., 2015, 140, 158–161 CrossRef CAS
.
- T. Gan, A.-X. Zhao, S.-H. Wang, Z. Lv and J.-Y. Sun, Hierarchical Triple-shelled Porous Hollow Zinc Oxide Spheres Wrapped in Graphene Oxide as Efficient Sensor Material for Simultaneous Electrochemical Determination of Synthetic Antioxidants in Vegetable Oil, Sens. Actuators, B, 2016, 235, 707–716 CrossRef CAS
.
- H.-J. Wu, G.-L. Wu, Q.-F. Wu and L.-D. Wang, Facile Synthesis and Microwave Absorbability of C@Ni–NiO Core–shell Hybrid Solid Sphere and Multi-shelled NiO Hollow Sphere, Mater. Charact., 2014, 97, 18–26 CrossRef CAS
.
- H.-J. Wu, Y.-Q. Wang, C.-H. Zheng, J.-M. Zhu, G.-L. Wu and X.-H. Li, Multi-shelled NiO Hollow Spheres: Easy Hydrothermal Synthesis and Lithium Storage Performances, J. Alloys Compd., 2016, 685, 8–14 CrossRef CAS
.
- Z.-D. Wang, S.-H. Qu, Y.-H. Cheng, C.-H. Zheng, S.-Y. Chen and H.-J. Wu, Facile Synthesis of Co3O4 Spheres and their Unexpected High Specific Discharge Capacity for Lithium-ion Batteries, Appl. Surf. Sci., 2017, 416, 338–343 CrossRef CAS
.
- Z.-H. Dong, X.-Y. Lai, J. E. Halpert, N.-L. Yang, L.-X. Yi, J. Zhai, D. Wang, Z.-Y. Tang and L. Jiang, Accurate Control of Multishelled ZnO Hollow Microspheres for Dye-Sensitized Solar Cells with High Efficiency, Adv. Mater., 2012, 24, 1046–1049 CrossRef CAS PubMed
.
- H. Ren, R.-B. Yu, J.-Y. Wang, Q. Jin, M. Yang, D. Mao, D. Kisailus, H.-J. Zhao and D. Wang, Multishelled TiO2 Hollow Microspheres as Anodes with Superior Reversible Capacity for Lithium Ion Batteries, Nano Lett., 2014, 14, 6679–6684 CrossRef CAS PubMed
.
- S.-M. Xu, C. M. Hessel, H. Ren, R.-B. Yu, Q. Jin, M. Yang, H.-J. Zhao and D. Wang, α-Fe2O3 Multi-shelled Hollow Microspheres for Lithium ion Battery Anodes with Superior Capacity and Charge Retention, Energy Environ. Sci., 2014, 7, 632–637 RSC
.
- S.-S. Niu, Y. Wang, S. Lu, D.-X. Wang and P. Wang, Fabrication and Photocatalytic Properties of SnO2 Double-shelled and Triple-shelled Hollow Spheres, Solid State Sci., 2016, 56, 63–67 CrossRef CAS
.
- Z.-H. Dong, H. Ren, C. M. Hessel, J.-Y. Wang, R.-B. Yu, Q. Jin, M. Yang, Z.-D. Hu, Y.-F. Chen, Z.-Y. Tang, H.-J. Zhao and D. Wang, Quintuple-Shelled SnO2 Hollow Microspheres with Superior Light Scattering for High-Performance Dye-Sensitized Solar Cells, Adv. Mater., 2014, 26, 905–909 CrossRef CAS PubMed
.
- J.-Y. Wang, Y. Cui and D. Wang, Design of Hollow Nanostructures for Energy Storage, Conversion and Production, Adv. Mater., 2019, 31, 1801993 CrossRef PubMed
.
- J. Wang, N. Yang, H. Tang, Z. Dong, Q. Jin, M. Yang, D. Kisailus, H. Zhao, Z. Tang and D. Wang, Accurate Control of Multishelled Co3O4 Hollow Microspheres as High Performance Anode Materials in Lithium-Ion Batteries, Angew. Chem., Int. Ed., 2013, 52, 6417–6420 CrossRef CAS PubMed
.
- X.-X. Zhao, R.-B. Yu, H.-J. Tang, D. Mao, J. Qi, B. Wang, Y. Zhang, H.-J. Zhao, W.-P. Hu and D. Wang, Formation of Septuple-Shelled (Co2/3Mn1/3)(Co5/6Mn1/6)2O4 Hollow Spheres as Electrode Material for Alkaline Rechargeable Battery, Adv. Mater., 2017, 29, 1700550 CrossRef PubMed
.
- Y.-P. Wang, A.-Q. Pan, Q.-Y. Zhu, Z.-W. Nie, Y.-F. Zhang, Y. Tang, S.-Q. Liang and G.-Z. Cao, Facile Synthesis of Nanorod-assembled Multi-shelled Co3O4 Hollow Microspheres for High-performance Supercapacitors, J. Power Sources, 2014, 272, 107–112 CrossRef CAS
.
- J.-Y. Wang, H.-J. Tang, H. Ren, R.-B. Yu, J. Qi, D. Mao, H.-J. Zhao and D. Wang, pH-Regulated Synthesis of Multi-Shelled Manganese Oxide Hollow Microspheres as Supercapacitor Electrodes Using Carbonaceous Microspheres as Templates, Adv. Sci., 2014, 1, 1400011 CrossRef PubMed
.
- Z.-P. Tian, Y. Zhou, Z.-D. Li, Q. Liu and Z.-G. Zou, Generalized Synthesis of a Family of Multishelled Metal Oxide Hollow Microspheres, J. Mater. Chem. A, 2013, 1, 3575–3579 RSC
.
- L.-H. Chu, M.-C. Li, Z.-P. Wan, L. Ding, D.-D. Song, S.-Y. Dou, J.-W. Chen and Y. Wang, Morphology Control and Fabrication of Multi-shelled NiO Spheres by Tuning the pH Value via a Hydrothermal Process, CrystEngComm, 2014, 16, 11096–11101 RSC
.
- G.-Q. Zhang and X.-W. Lou, General Synthesis of Multi-Shelled Mixed Metal Oxide Hollow Spheres with Superior Lithium Storage Properties, Angew. Chem., Int. Ed., 2014, 53, 9041–9044 CrossRef CAS PubMed
.
- L.-X. Zhang, Y.-X. Sun, W.-B. Jia, S.-S. Ma, B. Song, Y. Li, H.-F. Jiu and J.-W. Liu, Multiple Shell Hollow CoFe2O4 Spheres: Synthesis, Formation Mechanism and Properties, Ceram. Int., 2014, 40, 8997–9002 CrossRef CAS
.
- S.-J. Peng, F. Gong, L.-L. Li, D.-S. Yu, D.-X. Ji, T.-R. Zhang, Z. Hu, Z.-Q. Zhang, S.-L. Chou, Y.-H. Du and S. Ramakrishna, Necklace-like Multishelled Hollow Spinel Oxides with Oxygen Vacancies for Efficient Water Electrolysis, J. Am. Chem. Soc., 2018, 140, 13644–13653 CrossRef CAS PubMed
.
- J.-Y. Wang, H.-J. Tang, L.-J. Zhang, H. Ren, R.-B. Yu, Q. Jin, J. Qi, D. Mao, M. Yang, Y. Wang, P.-R. Liu, Y. Zhang, Y.-R. Wen, L. Gu, G.-H. Ma, Z.-G. Su, Z.-Y. Tang, H.-J. Zhao and D. Wang, Multi-shelled Metal Oxides Prepared via an Anion-adsorption Mechanism for Lithium-ion Batteries, Nat. Energy, 2016, 1, 16050 CrossRef CAS
.
- X.-X. Zhao, J.-Y. Wang, R.-B. Yu and D. Wang, Construction of Multishelled Binary Metal Oxides via Coabsorption of Positive and Negative Ions as a Superior Cathode for Sodium-Ion Batteries, J. Am. Chem. Soc., 2018, 140, 17114–17119 CrossRef CAS PubMed
.
- L.-B. Zong, J. Xu, S.-Y. Jiang, K. Zhao, Z.-M. Wang, P.-R. Liu, H.-J. Zhao, J. Chen, X.-R. Xing and R.-B. Yu, Composite Yttrium-carbonaceous Spheres Templated Multi-Shell YVO4 Hollow Spheres with Superior Upconversion Photoluminescence, Adv. Mater., 2017, 29, 1604377 CrossRef PubMed
.
- J. Zhang, Y.-D. Cao, C.-A. Wang and R. Ran, Design and Preparation of MnO2/CeO2–MnO2 Double-Shelled Binary Oxide Hollow Spheres and their Application in CO Oxidation, ACS Appl. Mater. Interfaces, 2016, 8, 8670–8677 CrossRef CAS PubMed
.
- M. Yang, J. Ma, C.-L. Zhang, Z.-Z. Yang and Y.-F. Lu, General Synthetic Route toward Functional Hollow Spheres with Double-Shelled Structures, Angew. Chem., Int. Ed., 2005, 44, 6727–6730 CrossRef CAS PubMed
.
- H.-B. Hou, L.-P. Zhang, T.-M. Liu, J.-M. Cheng, L.-Q. Sun, C.-D. Wang, M.-M. Jin, Z.-X. Song, J.-Y. Cheng, Q.-G. Wang, H.-G. Sun, X. Chen and G.-L. Cui, A Facile Approach to Preparation of Silica Double-Shell Hollow Particles, and their Application in Gel Composite Electrolytes, J. Colloid Interface Sci., 2018, 529, 130–138 CrossRef CAS PubMed
.
- M. Yang, J. Ma, Z. Niu, X. Dong, H. Xu, Z. Meng, Z. Jin, Y. Lu, Z. Hu and Z. Yang, Synthesis of Spheres with Complex Structures Using Hollow Latex Cages as Templates, Adv. Funct. Mater., 2005, 15, 1523–1528 CrossRef CAS
.
- M. Yang, J. Ma, C.-L. Zhang, Z.-Z. Yang and Y.-F. Lu, General Synthetic Route toward Functional Hollow Spheres with Double-Shelled Structures, Angew. Chem., Int. Ed., 2005, 44, 6727–6730 CrossRef CAS PubMed
.
- M.-Y. Bai, Y.-J. Cheng, S. A. Wickline and Y.-N. Xia, Colloidal Hollow Spheres of Conducting Polymers with Smooth Surface and Uniform, Controllable Sizes, Small, 2009, 5, 1747–1752 CrossRef CAS PubMed
.
- M.-Y. Bai and Y.-N. Xia, Facile Synthesis of Double-Shelled Polypyrrole Hollow Particles with a Structure Similar to that of a Thermal Bottle, Macromol. Rapid Commun., 2010, 31, 1863–1868 CrossRef CAS PubMed
.
- Y. Zeng, X. Wang, H. Wang, Y. Dong, Y. Ma and J.-N. Yao, Multi-shelled Titania Hollow Spheres Fabricated by a Hard Template Strategy: Enhanced Photocatalytic Activity, Chem. Commun., 2010, 46, 4312–4314 RSC
.
- X.-C. Li, L. Wang, J.-H. Shi, N.-X. Du and G.-H. He, Multishelled Nickel-Cobalt Oxide Hollow Microspheres with Optimized Compositions and Shell Porosity for High-Performance Pseudocapacitors, ACS Appl. Mater. Interfaces, 2016, 8, 17276–17283 CrossRef CAS PubMed
.
- X.-H. Qi, W.-J. Zheng, G.-H. He, T.-F. Tian, N.-X. Du and L. Wang, NiCo2O4 Hollow Microspheres with Tunable Numbers and Thickness of Shell for Supercapacitors, Chem. Eng. J., 2017, 309, 426–434 CrossRef CAS
.
- K.-X. Zhu, C.-Z. Jin, C.-X. Zhao, R.-S. Hu, Z. Klencsár, G. A. Sundaram, D. F. Srankóf, R. Ge and J.-H. Wang, Modulation Synthesis of Multi-shelled Cobalt-iron Oxides as Efficient Catalysts for Peroxymonosulfate-mediated Organics Degradation, Chem. Eng. J., 2019, 359, 1537–1549 CrossRef CAS
.
- Y. Qi, B.-Q. Liu, L.-Y. Zhang, Y.-Q. Huo, L. Li, H.-M. Xie, C.-G. Wang and Z.-M. Su, One-pot Controllable Synthesis of CoFe2O4 Solid, Hollow and Multi-shell Hollow Nanospheres as Superior Anode Materials for Lithium ion Batteries, J. Mater. Chem. A, 2017, 5, 21994–22003 RSC
.
- J. Sun, C.-X. Lv, F. Lv, S. Chen, D.-H. Li, Z.-Q. Guo, W. Han, D.-J. Yang and S.-J. Guo, Tuning the Shell Number of Multishelled Metal Oxide Hollow Fibers for Optimized Lithium-Ion Storage, ACS Nano, 2017, 11, 6186–6193 CrossRef CAS PubMed
.
- L.-F. Wang, F.-J. Liu, W.-T. Yang, H.-Y. Zhao, Y.-Y. Zheng, X.-B. Chen and W.-J. Dong, Synthesis of Multiple-shell Porous CeO2 Hollow Spheres by a Hydrogel Template Method, Mater. Lett., 2013, 107, 42–45 CrossRef CAS
.
- F.-Z. Mou, J.-G. Guan, W.-D. Shi, Z.-G. Sun and S.-H. Wang, Oriented Contraction: A Facile Nonequilibrium Heat-Treatment Approach for Fabrication of Maghemite Fiber-in-Tube and Tube-in-Tube Nanostructures, Langmuir, 2010, 26, 15580–15585 CrossRef CAS PubMed
.
- R. Pang, X.-J. Hu, S.-Y. Zhou, C.-H. Sun, J. Yan, X.-M. Sun, S.-Z. Xiao and P. Chen, Preparation of Multi-shelled Conductive Polymer Hollow Microspheres by Using Fe3O4 Hollow Spheres as Sacrificial Templates, Chem. Commun., 2014, 50, 12493–12496 RSC
.
- C.-Y. Niu, B.-F. Zou, Y.-Q. Wang, L. Chen, H.-H. Zheng and S.-M. Zhou, The Template-assisted Aynthesis of Polypyrrole Hollow Microspheres with a Double-shelled Structure, Chem. Commun., 2015, 51, 5009–5012 RSC
.
- K.-L. Zhang, X.-N. Li, J.-W. Liang, Y.-C. Zhu, L. Hu, Q.-S. Cheng, C. Guo, N. Lin and Y.-T. Qian, Nitrogen-doped Porous Interconnected Double-shelled Hollow Carbon Spheres with High Capacity for Lithium Ion Batteries and Sodium Ion Batteries, Electrochim. Acta, 2015, 155, 174–182 CrossRef CAS
.
- G.-M. Zhou, Y.-B. Zhao and A. Manthiram, Dual-Confined Flexible Sulfur Cathodes Encapsulated in Nitrogen-Doped Doubl-Shelled Hollow Carbon Spheres and Wrapped with Graphene for Li-S Batteries, Adv. Energy Mater., 2015, 5, 1402263 CrossRef
.
- R. G. Chaudhuri and S. Paria, Au and Ag/Au Double-shells Hollow Nanoparticles with Improved near Infrared Surface Plasmon and Photoluminescence Properties, J. Colloid Interface Sci., 2016, 461, 15–19 CrossRef PubMed
.
- Y. J. Wong, L.-F. Zhu, W. S. Teo, Y. W. Tan, Y.-H. Yang, C. Wang and H.-Y. Chen, Revisiting the Stöber Method: Inhomogeneity in Silica Shells, J. Am. Chem. Soc., 2011, 133, 11422–11425 CrossRef CAS PubMed
.
- D. Gu, H. Bongard, Y.-H. Deng, D. Feng, Z.-X. Wu, Y. Fang, J.-J. Mao, B. Tu, F. Schuth and D. Y. Zhao, An Aqueous Emulsion Route to Synthesize Mesoporous Carbon Vesicles and Their Nanocomposites, Adv. Mater., 2010, 22, 833–837 CrossRef CAS PubMed
.
- J. Liu, T.-Y. Yang, D.-W. Wang, G.-Q. Lu, D.-Y. Zhao and S.-Z. Qiao, A Facile Soft-template Synthesis of Mesoporous Polymeric and Carbonaceous Nanospheres, Nat. Commun., 2013, 4, 2798 CrossRef
.
- H.-L. Xu and W.-Z. Wang, Template Synthesis of Multishelled Cu2O Hollow Spheres with a Single-Crystalline Shell Wall, Angew. Chem., Int. Ed., 2007, 46, 1489–1492 CrossRef CAS PubMed
.
- B.-S. Wang, W.-W. Zhang, Z.-Y. Zhang, R.-Y. Li, Y.-L. Wu, Z.-G. Hu, X.-L. Wu, C.-G. Guo, G.-A. Cheng and R.-T. Zheng, Cu2O Hollow Structures-microstructural Evolution and Photocatalytic Properties, RSC Adv., 2016, 6, 103700 RSC
.
- Z.-G. Teng, X.-D. Su, Y.-Y. Zheng, J.-J. Zhang, Y. Liu, S.-J. Wang, J. Wu, G.-T. Chen, J.-D. Wang, D.-Y. Zhao and G.-M. Lu, A Facile Multi-interface Transformation Approach to Monodisperse Multiple-Shelled Periodic Mesoporous Organosilica Hollow Spheres, J. Am. Chem. Soc., 2015, 137, 7935–7944 CrossRef CAS PubMed
.
- J. Yang, X. Wang, R. Xia, E. Muhire and M.-Z. Gao, Self-assembly in the Synthesis of Shelled ZnO Hollow Spheres and Their UV Sensors Performance, Mater. Lett., 2016, 182, 10–14 CrossRef CAS
.
- Y. Zhang, M.-H. Yu, L. Zhou, X.-F. Zhou, Q.-F. Zhao, H.-X. Li and C.-Z. Yu, Organosilica Multilamellar Vesicles with Tunable Number of Layers and Sponge-Like Walls via One Surfactant Templating, Chem. Mater., 2008, 20, 6238–6243 CrossRef CAS
.
- C.-P. Xu, D.-C. Niu, N. Zheng, H.-N. Yu, J.-P. He and Y.-S. Li, Facile Synthesis of Nitrogen-Doped Double-Shelled Hollow Mesoporous Carbon Nanospheres as High-Performance Anode Materials for Lithium Ion Batteries, ACS Sustainable Chem. Eng., 2018, 6, 5999–6007 CrossRef CAS
.
- J. Liu, S. B. Hartono, Y.-G. Jin, Z. Li, G.-Q. Lu and S.-Z. Qiao, A Facile Vesicle Template Route to Multi-shelled Mesoporous Silica Hollow Nanospheres, J. Mater. Chem., 2010, 20, 4595–4601 RSC
.
- L. Wu, H.-J. Zhang, M.-H. Wu, Y.-F. Zhong, X.-W. Liu and Z. Jiao, Dual-templating Synthesis of Multi-shelled Mesoporous Silica Nanoparticles as Catalyst and Drug Carrier, Microporous Mesoporous Mater., 2016, 228, 318–328 CrossRef CAS
.
- G.-W. Zhou, Y.-J. Chen and J.-H. Yang, and S.-H. Yang, From Cylindrical-channel Mesoporous Silica to Vesicle-like Silica with Well-defined Multilamella Shells and Large Inter-shell Mesopores, J. Mater. Chem., 2007, 17, 2839–2844 RSC
.
- X. Gu, C.-L. Li, X.-H. Liu, J.-W. Ren, Y.-Q. Wang, Y.-L. Guo, Y. Guo and G.-Z. Lu, Synthesis of Nanosized Multilayered Silica Vesicles with High Hydrothermal Stability, J. Phys. Chem. C, 2009, 113, 6472–6479 CrossRef CAS
.
- Y. Zhang, G.-W. Zhou, B. Sun, M.-N. Zhao, J.-Y. Zhang and F. J. Chen, A Cationic-cationic Co-surfactant Templating Route for Synthesizing Well-defined Multilamellar Vesicular Silica with an Adjustable Number of Layers, Chem. Commun., 2014, 50, 2907–2909 RSC
.
- Y.-N. Yang, Y. Lu, P. L. Abbaraju, J. Zhang, M. Zhang, G.-Y. Xiang and C.-Z. Yu, Multi-shelled Dendritic Mesoporous Organosilica Hollow Spheres: Roles of Composition and Architecture in Cancer Immunotherapy, Angew. Chem., Int. Ed., 2017, 56, 8446–8450 CrossRef CAS PubMed
.
- S. Yang, X.-F. Zhou, P. Yuan, M.-H. Yu, S.-H. Xie, J. Zou, G.-Q. Lu and C.-Z. Yu, Siliceous Nanopods from a Compromised Dual-Templating Approach, Angew. Chem., Int. Ed., 2007, 46, 8579–8582 CrossRef CAS PubMed
.
- X. Huang, W. Li, M.-J. Wang, X.-N. Tan, Q. Wang, M.-N. Zhang, C. Wang and H.-F. Zhang, Synthesis of Multiple-shelled Organosilica Hollow Nanospheres via a Dual-template Method by Using Compressed CO2, Microporous Mesoporous Mater., 2017, 247, 66–74 CrossRef CAS
.
- T.-T. Zhou, T. Zhang, R. Zhang, Z. Lou, J.-N. Deng and L.-L. Wang, Hollow ZnSnO3 Cubes with Controllable Shells Enabling Highly Efficient Chemical Sensing Detection of Formaldehyde Vapors, ACS Appl. Mater. Interfaces, 2017, 9, 14525–14533 CrossRef CAS PubMed
.
- S.-L. Xiong and H.-C. Zeng, Serial Ionic Exchange for the Synthesis of Multishelled Copper Sulfide Hollow Spheres, Angew. Chem., Int. Ed., 2012, 51, 949–952 CrossRef CAS
.
- D.-Y. Xue, F.-F. Xue, X.-P. Lin, F.-Y. Zong, J.-M. Zhang and Q.-H. Li, Coordination Polymer Derived General Synthesis of Multi-shelled Hollow Metal Oxides for Lithium-ion Batteries, Nanoscale, 2019, 11, 17478–17484 RSC
.
- L. Liu, J.-J. Shi, R.-Y. Wang, H.-X. Cao and Z.-W. Liu, Fabrication of Double-shelled Fe2O3/CeO2 Boxes from CeO2-modified Prussian Blue and Their Enhanced Performances for CO Removal and Water Treatment, J. Alloys Compd., 2017, 725, 544–556 CrossRef CAS
.
- X.-Z. Song, L. Qiao, K.-M. Sun, Z.-Q. Tan, W. Ma, X.-L. Kan, F.-F. Sun, T. Huang and X.-F. Wang, Triple-shelled ZnO/ZnFe2O4 Heterojunctional Hollow Microspheres Derived from Prussian Blue Analogue as High-performance Acetone Sensors, Sens. Actuators, B, 2018, 256, 374–382 CrossRef CAS
.
- W.-X. Liu, J.-J. Huang, Q. Yang, S.-J. Wang, X.-M. Sun, W.-N. Zhang, J.-F. Liu and F.-W. Huo, Multi-shelled Hollow Metal–Organic Frameworks, Angew. Chem., Int. Ed., 2017, 56, 5512–5516 CrossRef CAS PubMed
.
- D. Y. Hong, Y. K. Hwang, C. Serre, G. Ferey and J. S. Chang, Porous Chromium Terephthalate MIL-101 with Coordinatively Unsaturated Sites: Surface Functionalization, Encapsulation, Sorption and Catalysis, Adv. Funct. Mater., 2009, 19, 1537–1552 CrossRef CAS
.
- G. Ferey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble and I. Margiolaki, A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area, Science, 2005, 309, 2040–2042 CrossRef CAS PubMed
.
- W. Cho, Y. H. Lee, H. J. Lee and M. Oh, Multi Ball-in-ball Hybrid Metal Oxides, Adv. Mater., 2011, 23, 1720–1723 CrossRef CAS PubMed
.
- P. Zhang, B.-Y. Guan, L. Yu and X.-W. Lou, Facile Synthesis of Multi-shelled ZnS–CdS Cages with Enhanced Photoelectrochemical Performance for Solar Energy Conversion, Chem, 2018, 4, 162–173 CAS
.
- B.-Y. Guan, A. Kushima, L. Yu, S. Li, J. Li and X.-W. Lou, Coordination Polymers Derived General Synthesis of Multishelled Mixed Metal–Oxide Particles for Hybrid Supercapacitors, Adv. Mater., 2017, 29, 1605902 CrossRef PubMed
.
- B.-Y. Guan, L. Yu and X.-W. Lou, General Synthesis of Multishell Mixed-Metal Oxyphosphide Particles with Enhanced Electrocatalytic Activity in the Oxygen Evolution Reaction, Angew. Chem., Int. Ed., 2017, 56, 2386–2389 CrossRef CAS PubMed
.
- H. Hu, B.-Y. Guan, B.-Y. Xia and X.-W. Lou, Designed Formation of Co3O4/NiCo2O4 Double-Shelled Nanocages with Enhanced Pseudocapacitive and Electrocatalytic Properties, J. Am. Chem. Soc., 2015, 137, 5590–5595 CrossRef CAS PubMed
.
- H. Hu, L. Han, M.-Z. Yu, Z.-Y. Wang and X.-W. Lou, Metal–organic-framework-engaged Formation of Co Nanoparticle-embedded Carbon@Co9S8 Double-shelled Nanocages for Efficient Oxygen Reduction, Energy Environ. Sci., 2016, 9, 107–111 RSC
.
- L.-F. Shen, L. Yu, X.-Y. Yu, X.-G. Zhang and X.-W. Lou, Self-Templated Formation of Uniform NiCo2O4 Hollow Spheres with Complex Interior Structures for Lithium-ion Batteries and Supercapacitors, Angew. Chem., Int. Ed., 2015, 54, 1868–1872 CrossRef CAS PubMed
.
- G.-Q. Zhang, L. Yu, H.-B. Wu, H. E. Hoster and X.-W. Lou, Formation of ZnMn2O4 Ball-in-Ball Hollow Microspheres as a High-Performance Anode for Lithium-ion Batteries, Adv. Mater., 2012, 24, 4609–4613 CrossRef CAS PubMed
.
- J.-F. Li, J.-Z. Wang, X. Liang, Z.-J. Zhang, H.-K. Liu, Y.-T. Qian and S.-L. Xiong, Hollow MnCo2O4 Submicrospheres with Multilevel Interiors: From Mesoporous Spheres to Yolk-in-double-shell Structures, ACS Appl. Mater. Interfaces, 2014, 6, 24–30 CrossRef CAS PubMed
.
- L.-F. Shen, L. Yu, H.-B. Wu, X.-Y. Yu, X.-G. Zhang and X.-W. Lou, Formation of Nickel Cobalt Sulfide Ball-in-ball Hollow Spheres with Enhanced Electrochemical Pseudocapacitive Properties, Nat. Commun., 2015, 6, 6694 CrossRef CAS PubMed
.
- Y. Lu, J.-W. Nai and X.-W. Lou, Formation of NiCo2V2O8 Yolk-double Shell Spheres with Enhanced Lithium Storage Properties, Angew. Chem., Int. Ed., 2018, 57, 2899–2903 CrossRef CAS PubMed
.
- Y.-W. Wang, L. Yu and X.-W. Lou, Formation of Triple-Shelled Molybdenum-Polydopamine Hollow Spheres and Their Conversion into MoO2/Carbon Composite Hollow Spheres for Lithium-Ion Batteries, Angew. Chem., 2016, 128, 14888–14892 CrossRef
.
- J.-X. Guo, X.-Q. Zhang, Y.-F. Sun, X.-H. Zhang, L. Tang and X. Zhang, Double-shell CuS Nanocages as Advanced Supercapacitor Electrode Materials, J. Power Sources, 2017, 355, 31–35 CrossRef CAS
.
- C.-Z. Wu, X.-D. Zhang, B. Ning, J.-L. Yang and Y. Xie, Shape Evolution of New-phased Lepidocrocite VOOH from Single-Shelled to Double-Shelled Hollow Nanospheres on the Basis of Programmed Reaction-Temperature Strategy, Inorg. Chem., 2009, 48, 6044–6054 CrossRef CAS PubMed
.
- H. B. Lin, H. B. Rong, W. Z. Huang, Y. H. Liao, L. D. Xing, M. Q. Xu, X. P. Li and W. S. Li, Triple-Shelled Mn2O3 Hollow Nanocubes: Forceinduced Synthesis and Excellent Performance as the Anode in Lithium-Ion Batteries, J. Mater. Chem. A, 2014, 2, 14189–14194 RSC
.
- H. Zhao, J.-F. Chen, Y. Zhao, L. Jiang, J.-W. Sun and J. Yun, Hierarchical Assembly of Multilayered Hollow Microspheres from an Amphiphilic Pharmaceutical Molecule of Azithromycin, Adv. Mater., 2008, 20, 3682–3686 CrossRef CAS
.
- A.-Q. Pan, H.-B. Wu, L. Yu and X.-W. Lou, Template-Free Synthesis of VO2 Hollow Microspheres with Various Interiors and Their Conversion into V2O5 for Lithium-Ion Batteries, Angew. Chem., Int. Ed., 2013, 52, 2226–2230 CrossRef CAS PubMed
.
- L. Zhou, H.-Y. Xu, H.-W. Zhang, J. Yang, S. B. Hartono, K. Qian, J. Zou and C.-Z. Yu, Cheap and Scalable Synthesis of α-Fe2O3 Multi-shelled Hollow Spheres as High-performance Anode Materials for Lithium Ion Batteries, Chem. Commun., 2013, 49, 8695–8697 RSC
.
- Z. Padashbarmchi, A. H. Hamidian, H.-W. Zhang, L. Zhou, N. Khorasani, M. Kazemzad and C.-Z. Yu, A Systematic Study on the Synthesis of α-Fe2O3 Multi-shelled Hollow Spheres, RSC Adv., 2015, 5, 10304–10309 RSC
.
- G. D. Park, J. H. Lee, J. K. Lee and Y. C. Kang, Effect of Esterification Reaction of Citric Acid and Ethylene Glycol on the Formation of Multi-shelled Cobalt Oxide Powders with Superior Electrochemical Properties, Nano Res., 2014, 7, 1738–1748 CrossRef CAS
.
- J. S. M. Zanjani, B. S. Okan, I. Letofsky-Papst, M. Yildiz and Y. Z. Menceloglu, Rational Design and Direct Fabrication of Multi-Walled Hollow Electrospun Fibers with Controllable Structure and Surface Properties, Eur. Polym. J., 2015, 62, 66–76 CrossRef
.
- G. Zhang, B. Y. Xia, C. Xiao, L. Yu, X. Wang, Y. Xie and X. W. Lou, General Formation of Complex Tubular Nanostructures of Metal Oxides for the Oxygen Reduction Reaction and Lithium-Ion Batteries, Angew. Chem., Int. Ed., 2013, 52, 8643–8647 CrossRef CAS PubMed
.
- L. Zhang and H. Wang, Interior Structural Tailoring of Cu2O Shell-in-shell Nanostructures through Multistep Ostwald Ripening, J. Phys. Chem. C, 2011, 115, 18479–18485 CrossRef CAS
.
- C. C. Yec and H. C. Zeng, Synthetic Architecture of Multiple Core–Shell and Yolk–shell Structures of (Cu2O@)nCu2O (n = 1–4) with Centricity and Eccentricity, Chem. Mater., 2012, 24, 1917–1929 CrossRef CAS
.
- J. Li and H.-C. Zeng, Size Tuning, Functionalization, and Reactivation of Au in TiO2 Nanoreactors, Angew. Chem., Int. Ed., 2005, 44, 4342–4345 CrossRef CAS PubMed
.
- B. Liao, Z.-G. An and J.-J. Zhang, Highly Efficient Large-scale Preparation and Electromagnetic Property Control of Silica-NiFeP Double Shell Composite Hollow Particles, RSC Adv., 2017, 7, 21721–21732 RSC
.
- A. S. Arico, P. Bruce, B. Scrosati, J.-M. Tarascon and W. V. Schalkwijk, Nanostructured Materials for Advanced Energy Conversion and Storage Devices, Nat. Mater., 2005, 4, 366–377 CrossRef CAS PubMed
.
- J. R. Miller and P. Simon, Electrochemical Capacitors for Energy Management, Science, 2008, 321, 651–652 CrossRef CAS PubMed
.
- J.-X. Zhu, D. Yang, Z.-Y. Yin, Q.-Y. Yan and H. Zhang, Graphene and Graphene-Based Materials for Energy Storage Applications, Small, 2014, 10, 3480–3498 CrossRef CAS PubMed
.
- J. B. Goodenough, Electrochemical Energy Storage in a Sustainable Modern Society, Energy Environ. Sci., 2014, 7, 14–18 RSC
.
- G.-P. Wang, L. Zhang and J.-J. Zhang, A Review of Electrode Materials for Electrochemical Supercapacitors, Chem. Soc. Rev., 2012, 41, 797–828 RSC
.
- Q. Chen, W. Wei, J. Tang, J. Lin, S. Li and M. Zhu, Dopamine-Assisted Preparation of Fe3O4@MnO2 Yolk@Shell Microspheres for Improved Pseudocapacitive Performance, Electrochim. Acta, 2019, 317, 628–637 CrossRef CAS
.
- L. Kong, Q. Ma, Z. Xu, X. Shen, J. Wang and J. Zhu, Three-Dimensional Graphene Network Deposited with Mesoporous Nitrogen-Doped Carbon from Non-Solvent Induced Phase Inversion for High-Performance Supercapacitors, J. Colloid Interface Sci., 2020, 558, 21–31 CrossRef PubMed
.
- S. Li, K. Yang, P. Ye, K. Ma, Z. Zhang and Q. Huang, Three-Dimensional Porous Carbon/Co3O4 Composites Derived from Graphene/Co-MOF for High Performance Supercapacitor Electrodes, Appl. Surf. Sci., 2020, 503, 144090 CrossRef CAS
.
- M. Zhu, Q. Chen, W. Tong, J. Kan and W. Sheng, Preparation and Application of Fe3O4 Nanomaterials, Prog. Chem., 2017, 29, 1366–1394 Search PubMed
.
- M. Sevilla and R. Mokaya, Energy Storage Applications of Activated Carbons: Supercapacitors and Hydrogen Storage, Energy Environ. Sci., 2014, 7, 1250–1280 RSC
.
- W.-F. Wei, X.-W. Cui, W.-X. Chen and D. G. Ivey, Manganese Oxide-based Materials as Electrochemical Supercapacitor Electrodes, Chem. Soc. Rev., 2011, 40, 1697–1721 RSC
.
- C.-Z. Yuan, H.-B. Wu, Y. Xie and X.-W. Lou, Mixed Transition-metal Oxides: Design, Synthesis, and Energy-related Applications, Angew. Chem., Int. Ed., 2014, 53, 1488–1504 CrossRef CAS PubMed
.
- K. Wang, H.-P. Wu, Y.-N. Meng and Z.-X. Wei, Conducting Polymer Nanowire Arrays for High Performance Supercapacitors, Small, 2013, 10, 14–31 CrossRef PubMed
.
- M. Zhu, Q. Chen, J. Tang, W. Wei and S. Li, Core@Shell β-FeOOH@Polypyrolle Derived N, S-codoped Fe3O4@N-Doped Porous Carbon Nanococoons for High Performance Supercapacitors, Appl. Surf. Sci., 2019, 480, 582–592 CrossRef CAS
.
- M. Zhu, Q. Chen, J. Kan, J. Tang, W. Wei, J. Lin and S. Li, Coblat Oxide Nanoparticles Embedded in N-Doped Porous Carbon as Efficient Electrode for Supercapacitor, Energy Technol., 2019, 1800963 CrossRef
.
- M. Zhu, J. Kan, J. Pan, W. Tong, Q. Chen, J. Wang and S. Li, One-Pot Hydrothermal Fabrication of α-Fe2O3@C Nanocomposites for Electrochemical Energy Storage, J. Energy Chem., 2019, 28, 1–8 CrossRef
.
- K. Zhang, Z. Shang, S. Wu, J. Wang, W. Sheng, X. Shen and M. Zhu, Commercialized Benzoxazine Resin-Derived Porous Carbon as high Performance Electrode Materials for Supercapacitor, J. Inorg. Organomet. Polym., 2017, 27, 1423–1429 CrossRef CAS
.
- X. Yuan, X. Yan, C. Zhou, J. Wang, D. Wang, H. Jiang, Y. Zhu, X. Tao and X. Cheng, Decorating Carbon Nanosheets with Copper Oxide Nanoparticles for Boosting the Electrochemical Performance of Symmetric Coin Cell Supercapacitor with Different Electrolytes, Ceram. Int., 2020, 46, 435–443 CrossRef CAS
.
- X. Yang, J. Kan, F. Zhang, M. Zhu and S. Li, Facile Fabrication of Mn2+ Doped Magnetite Microspheres as Efficient Electrode Material for Supercapacitors., J. Inorg. Organomet. Polym., 2017, 27, 542–551 CrossRef CAS
.
- S. Hussain, P. Wan, N. Aslam, G. Qiao, G. Liu and M. Wang, Ag-Doped NiO Porous Network Structure on Ni Foam as Electrode for Supercapacitors, J. Mater. Sci.: Mater. Electron., 2018, 29, 1759–1765 CrossRef CAS
.
- Y. Wang, M. Zhang, Y. Li, T. Ma, H. Liu, D. Pan, X. Wang and A. Wang, Rational Design 3D Nitrogen Doped Graphene Supported Spatial Crosslinked Co3O4@NiCo2O4 on Nickel Foam for Binder-Free Supercapacitor Electrodes, Electrochim. Acta, 2018, 290, 12–20 CrossRef CAS
.
- M. Zhang, Y. Wang, D. Pan, Y. Li, Z. Yan and J. Xie, Nitrogen-Doped 3D Graphene/MWNTs Nanoframework-Embedded Co3O4 for High Electrochemical Performance Supercapacitors, ACS Sustainable Chem. Eng., 2017, 5, 5099–5107 CrossRef CAS
.
- X. Cai, H. Bai, Y. Liu and W. Shi, Facile In Situ Synthesis of Ag and MnO2 Anchored on Carbon Microtubes for High-Performance Asymmetric Supercapacitor Applications. Appl, Mater. Today, 2018, 11, 193–199 Search PubMed
.
- H. Sun, J. Pan, X. Yan, W. Shen, W. Zhong and X. Cheng, MnO2 Nanoneedles Loaded on Silicon Oxycarbide-Derived Hierarchically Porous Carbon for Supercapacitor Electrodes with Enhanced Electrochemical Performance, Ceram. Int., 2019, 45, 24802–24810 CrossRef CAS
.
- Y. Liu, X. Cai, B. Luo, M. Yan, J. Jiang and W. Shi, MnO2 Decorated on Carbon Sphere Intercalated Graphene Film for High-Performance Supercapacitor Electrodes, Carbon, 2016, 107, 426–432 CrossRef CAS
.
- Z.-H. Yang, F.-F. Xu, W.-X. Zhang, Z.-S. Mei, B. Pei and X. Zhu, Controllable Preparation of Multishelled NiO Hollow Nanospheres via Layer-by-layer Self-assembly for Supercapacitor Application, J. Power Sources, 2014, 246, 24–31 CrossRef CAS
.
- H.-L. Wang, Y. Yang, Y.-Y. Liang, G.-Y. Zheng, Y.-G. Li, Y. Cui and H.-J. Dai, Rechargeable Li-O2 Batteries with a Covalently Coupled MnCo2O4-graphene Hybrid as an Oxygen Cathode Catalyst, Energy Environ. Sci., 2012, 5, 7931–7935 RSC
.
- C. Huang, Y. Ding, C. Hao, S. Zhou, X. Wang, H. Gao, L. Zhu and J. Wu, PVP-Assisted Growth of Ni–Co Oxide on N-Doped Reduced Graphene Oxide with Enhanced Pseudocapacitive Behavior, Chem. Eng. J., 2019, 378, 122202 CrossRef CAS
.
- M. Zhu, X. Zhang, Y. Zhou, C. Zhuo, J. Huang and S. Li, Facile Solvothermal Synthesis of Porous ZnFe2O4 Microspheres for Capacitive Pseudocapacitors, RSC Adv., 2015, 5, 39270–39277 RSC
.
- S. E. Moosavifard, S. K. Kaverlavani, J. Shamsi and A. Bakouei, Hierarchical Multi-shelled Nanoporous Mixed Copper Cobalt Phosphide Hollow Microspheres as a Novel Advanced Electrode for High-performance Asymmetric Supercapacitors, J. Mater. Chem. A, 2017, 5, 18429–18433 RSC
.
- A. A. Ensafi, S. E. Moosavifard, B. Rezaei and S. K. Kaverlavani, Engineering Onion-like Nanoporous CuCo2O4 Hollow Spheres Derived from Bimetal-organic Frameworks for High-performance Asymmetric Supercapacitors, J. Mater. Chem. A, 2018, 6, 10497–10506 RSC
.
- H. Niu, Y. Liu, B. Mao, N. Xin, H. Jia and W. Shi, In-Situ Embedding MOFs-Derived Copper Sulfide Polyhedrons in Carbon Nanotube Networks for Hybrid Supercapacitor with Superior Energy Density, Electrochim. Acta, 2020, 329, 135130 CrossRef CAS
.
- X.-F. Lu, D.-J. Wu, R.-Z. Li, Q. Li, S.-H. Ye, Y.-X. Tong and G.-R. Li, Hierarchical NiCo2O4 Nanosheets@Hollow Microrod Arrays for High-performance Symmetric Supercapacitors, J. Mater. Chem. A, 2014, 2, 4706–4713 RSC
.
- D. P. Dubal, G. S. Gund, C. D. Lokhande and R. Holze, Controlled Growth of CoSx Nanostrip Arrays (CoSx-NSA) on Nickel Foam for Asymmetric Supercapacitors, Energy Technol., 2014, 2, 401–408 CrossRef CAS
.
- H.-B. Li, M.-H. Yu, F.-X. Wang, P. Liu, Y. Liang, J. Xiao, C.-X. Wang, Y.-X. Tong and G.-W. Yang, Amorphous Nickel Hydroxide Nanospheres with Ultrahigh Capacitance and Energy Density as Electrochemical Pseudocapacitor Materials, Nat. Commun., 2013, 4, 1894 CrossRef CAS PubMed
.
- X.-Z. Yu, B.-G. Lu and Z. Xu, Super Long-Life Supercapacitors Based on the Construction of Nanohoneycomb-like Strongly Coupled CoMoO4-3D Graphene Hybrid Electrodes, Adv. Mater., 2014, 26, 1044–1051 CrossRef CAS PubMed
.
- C.-Z. Wei, Q.-L. Ru, X.-T. Kang, H.-Y. Hou, C. Cheng and D.-J. Zhang, Self-template Synthesis of Double Shelled ZnS–NiS1.97 Hollow Spheres for Electrochemical Energy Storage, Appl. Surf. Sci., 2018, 435, 993–1001 CrossRef CAS
.
- Y.-P. Wang, T. Zhu, Y.-F. Zhang, X.-Z. Kong, S.-Q. Liang, G.-Z. Cao and A.-Q. Pan, Rational Design of Multi-shelled CoO/Co9S8 Hollow Microspheres for High-performance Hybrid Supercapacitors, J. Mater. Chem. A, 2017, 5, 18448–18456 RSC
.
- Y.-P. Wang, A.-Q. Pan, Y.-F. Zhang, J.-R. Shi, J.-D. Lin, S.-Q. Liang and G.-Z. Cao, Heterogeneous NiS/NiO Multi-shelled Hollow Microspheres with Enhanced Electrochemical Performances for Hybrid-type Asymmetric Supercapacitors, J. Mater. Chem. A, 2018, 6, 9153–9160 RSC
.
- M.-M. Fang, Z.-M. Chen, Y. Liu, W. Liu and Q. Xu, Uniform Discrete Nitrogen-Doped Double-Shelled Cage-like Hollow Carbon Spheres with Direct Large Mesopores for High-Performance Supercapacitors, Energy Technol., 2017, 5, 2198–2204 CrossRef CAS
.
- J. B. Goodenough and Y. Kim, Challenges for Rechargeable Li Batteries, Chem. Mater., 2009, 22, 587–603 CrossRef
.
- X.-M. Ma, Z.-P. Wei, H.-J. Han, X.-B. Wang, K.-Q. Cui and L. Yang, Tunable Construction of Multi-shell Hollow SiO2 Microspheres with Hierarchically Porous Structure as High-performance Anodes for Lithium-ion Batteries, Chem. Eng. J., 2017, 323, 252–259 CrossRef CAS
.
- Y.-F. Yuan, L.-W. Ye, D. Zhang, F. Chen, M. Zhu, L.-N. Wang, S.-M. Yin, G.-S. Cai and S.-Y. Guo, NiCo2S4 Multi-shelled Hollow Polyhedrons as High-performance Anode Materials for Lithium-ion Batteries, Electrochim. Acta, 2019, 299, 289–297 CrossRef CAS
.
- M. V. Reddy, G. V. S. Rao and B. V. R. Chowdari, Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries, Chem. Rev., 2013, 113, 5364–5457 CrossRef CAS PubMed
.
- J.-Y. Wang, N.-L. Yang, H.-J. Tang, Z.-H. Dong, Q. Jin, M. Yang, D. Kisailus, H.-J. Zhao, Z.-Y. Tang and D. Wang, Accurate Control of Multishelled Co3O4 Hollow Microspheres as High-Performance Anode Materials in Lithium-Ion Batteries, Angew. Chem., Int. Ed., 2013, 52, 6417–6420 CrossRef CAS PubMed
.
- Z.-G. Wu, Y.-J. Zhong, J.-T. Li, X.-D. Guo, L. Huang, B.-H. Zhong and S.-G. Sun, l-Histidine-assisted Template-free Hydrothermal Synthesis of α-Fe2O3 Porous Multi-shelled Hollow Spheres with Enhanced Lithium Storage Properties, J. Mater. Chem. A, 2014, 2, 12361–12367 RSC
.
- J.-Z. Yin, Y. Zhang, Q.-Y. Lu, X.-L. Wu, Z.-J. Jiang, L.-Y. Dang, H.-F. Ma, Y.-Y. Guo, F. Gao and Q.-Y. Yan, Unable Co3O4 Hollow Structures (from Yolk–shell to Multi-shell) and their Li Storage Properties, J. Mater. Chem. A, 2017, 5, 12757–12761 RSC
.
- Y. Lu, L. Yu, M.-H. Wu, Y. Wang and X.-W. Lou, Construction of Complex Co3O4@Co3V2O8 Hollow Structures from Metal–Organic Frameworks with Enhanced Lithium Storage Properties, Adv. Mater., 2018, 30, 1702875 CrossRef PubMed
.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J.-M. Tarascon, Nano-sized Transition-metal Oxides as Negative-electrode Materials for Lithium-ion Batteries, Nature, 2000, 407, 496–499 CrossRef CAS PubMed
.
- J. Jiang, Y.-Y. Li, J.-P. Liu, X.-T. Huang, C.-Z. Yuan and X.-W. Lou, Recent Advances in Metal Oxide-based Electrode Architecture Design for Electrochemical Energy Storage, Adv. Mater., 2012, 24, 5166–5180 CrossRef CAS PubMed
.
- H. Li, H.-R. Ma, M. Yang, B. Wang, H. Shao, L. Wang, R.-B. Yu and D. Wang, Highly Controlled Synthesis of Multi-shelled NiO Hollow Microspheres for Enhanced Lithium Storage Properties, Mater. Res. Bull., 2017, 87, 224–229 CrossRef CAS
.
- L. Liu, Q. Liu, W. Zhao, G. Li, L. Wang, W. Shi and L. Chen, Enhanced Electrochemical Performance of Orientated VO2(B) Raft-Like Nanobelt Arrays Through Direct Lithiation for Lithium Ion Batteries, Nanotechnology, 2017, 28, 065404 CrossRef PubMed
.
- Y.-P. Wang, Z.-W. Nie, A.-Q. Pan, Y.-F. Zhang, X.-Z. Kong, T. Zhu, S.-Q. Liang and G.-Z. Cao, Self-templating Synthesis of Double-wall Shelled Vanadium Oxide Hollow Microspheres for High-performance Lithium Ion Batteries, J. Mater. Chem. A, 2018, 6, 6792–6799 RSC
.
- F.-F. Guo, M.-H. Fan, P.-P. Jin, H. Chen, Y.-Y. Wu, G.-D. Li and X.-X. Zou, Precursor-mediated Synthesis of Double-shelled V2O5 Hollow Nanospheres as Cathode Material for Lithium-ion Batteries, CrystEngComm, 2016, 18, 4068–4073 RSC
.
- G.-L. Wu, Z.-R. Jia, Y.-H. Cheng, H.-X. Zhang, X.-F. Zhou and H.-J. Wu, Easy Synthesis of Multi-shelled ZnO Hollow Spheres and their Conversion into Hedgehog-like ZnO Hollow Spheres with Superior Rate Performance for Lithium Ion Batteries, Appl. Surf. Sci., 2019, 464, 472–478 CrossRef CAS
.
- S.-H. Qu, Y.-K. Yu, K.-J. Lin, P.-Y. Liu, C.-H. Zheng, L.-D. Wang, T.-T. Xu, Z.-D. Wang and H.-J. Wu, Easy Hydrothermal Synthesis of Multi-shelled La2O3 Hollow Spheres for Lithium-ion Batteries, J. Mater. Sci.: Mater. Electron., 2018, 29, 1232–1237 CrossRef CAS
.
- P. Dou, Z.-Z. Cao, C. Wang, J. Zheng and X.-H. Xu, Multilayer Zn-doped SnO2 Hollow Nanospheres Encapsulated in Covalently Interconnected Three-dimensional Graphene Foams for High Performance Lithium-ion Batteries, Chem. Eng. J., 2017, 320, 405–415 CrossRef CAS
.
- G.-L. Wu, H.-J. Wu, K.-K. Wang, C.-H. Zheng, Y.-Q. Wang and A.-L. Feng, Facile Synthesis and Application of Multi-shelled SnO2 Hollow Spheres in Lithium Ion Battery, RSC Adv., 2016, 6, 58069–58076 RSC
.
- M.-J. Zhou, Y.-C. Liu, J. Chen and X.-L. Yang, Double Shelled Hollow SnO2/polymer Microsphere as a High-capacity Anode Material for Superior Reversible Lithium Ion Storage, J. Mater. Chem. A, 2015, 3, 1068–1076 RSC
.
- J. Zhang, J.-W. Wan, J.-Y. Wang, H. Ren, R.-B. Yu, L. Gu, Y.-L. Liu, S.-H. Feng and D. Wang, Hollow Multi-shelled Structure with Metal–organic-framework-derived Coatings for Enhanced Lithium Storage, Angew. Chem., Int. Ed., 2019, 58, 5266–5271 CrossRef CAS PubMed
.
- G.-F. Li, S.-Q. Liu, Y. Pan, T.-Y. Zhou, J.-D. Ding, Y.-M. Sun and Y.-Q. Wang, Self-templated Formation of CuCo2O4 Triple-shelled Hollow Microspheres for All-solid-state Asymmetric Supercapacitors, J. Alloys Compd., 2019, 787, 694–699 CrossRef CAS
.
- C.-W. Jiao, Z.-M. Wang, X.-X. Zhao, H. Wang, J. Wang, R.-B. Yu and D. Wang, Triple-Shelled Manganese-cobalt Oxide Hollow Dodecahedra with Highly Enhanced Performance for Rechargeable Alkaline Batteries, Angew. Chem., Int. Ed., 2019, 58, 1008–1013 CrossRef
.
- J.-Y. Xu, H. Zhang, R.-F. Wang, P.-B. Xu, Y.-L. Tong, Q.-Y. Lu and F. Gao, Delicate Control of Multishelled Zn-Mn–O Hollow Microspheres as a High-Performance Anode for Lithium-Ion Batteries, Langmuir, 2018, 34, 1242–1248 CrossRef CAS PubMed
.
- D.-W. Li, X.-X. Zhao, R.-B. Yu, B. Wang, H. Wang and D. Wang, Formation of Multi-shelled Nickel-based Sulfide Hollow Spheres for Rechargeable Alkaline Batteries, Inorg. Chem. Front., 2018, 5, 535–540 RSC
.
- Y. Fang, Y.-Y. Lv, R.-C. Che, H.-Y. Wu, X.-H. Zhang, D. Gu, G.-F. Zheng and D.-Y. Zhao, Two-Dimensional Mesoporous Carbon Nanosheets and Their Derived Graphene Nanosheets: Synthesis and Efficient Lithium Ion Storage, J. Am. Chem. Soc., 2013, 135, 1524–1530 CrossRef CAS PubMed
.
- Z.-S. Wu, W.-C. Ren, L. Xu, F. Li and H.-M. Cheng, Doped Graphene Sheets As Anode Materials with Superhigh Rate and Large Capacity for Lithium Ion Batteries, ACS Nano, 2011, 5, 5463–5471 CrossRef CAS PubMed
.
- D. Zhang, G. Wang, L. Xu, J. Lian, J. Bao, Y. Zhao, J. Qiu and H. Li, Defect-Rich N-Doped Porous Carbon Derived from Soybean for High Rate Lithium-Ion Batteries, Appl. Surf. Sci., 2018, 451, 298–305 CrossRef CAS
.
- Y.-Z. Zhang, X.-L. Zong, L. Zhan, X.-Y. Yu, J. Gao, C.-C. Xun, P.-Y. Li and Y.-L. Wang, Double-shelled Hollow Carbon Sphere with Microporous Outer Shell towards High Performance Lithium-sulfur Battery, Electrochim. Acta, 2018, 284, 89–97 CrossRef CAS
.
- X.-F. Niu, Y.-F. Li, Y.-J. Hu, H. Jiang, X.-Y. Hou, W.-G. Li, S.-J. Qiu and C.-Z. Li, Aerosol Construction of Multi-shelled LiMn2O4 Hollow Microspheres as a Cathode in Lithium Ion Batteries, New J. Chem., 2016, 40, 1839–1844 RSC
.
- F. Wang, J.-Y. Wang, H. Ren, H.-J. Tang, R.-B. Yu and D. Wang, Multi-shelled LiMn2O4 Hollow Microspheres as Superior Cathode Materials for Lithium-ion Batteries, Inorg. Chem. Front., 2016, 3, 365–369 RSC
.
- M. D. Slater, D. H. Kim, E. Lee and C. S. Johnson, Sodium-ion Batteries, Adv. Funct. Mater., 2012, 23, 947–958 CrossRef
.
- V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-González and T. Rojo, Na-ion Batteries, Recent Advances and Present Challenges to Become Low Cost Energy Storage Systems, Energy Environ. Sci., 2012, 5, 5884–5901 RSC
.
- X. Zhang, Y.-P. Zhou, B. Luo, H.-C. Zhu, W. Chu and K. Huang, Microwave-Assisted Synthesis of NiCo2O4 Double-Shelled Hollow Spheres for High-performance Sodium Ion Batteries, Nano-Micro Lett., 2018, 10, 13 CrossRef PubMed
.
- F. Xie, L. Zhang, Q. Gu, D. Chao, M. Jaroniec and S. Z. Qiao, Multi-Shell Hollow Structured Sb2S3 for Sodium-Ion Batteries with Enhanced Energy Density, Nano Energy, 2019, 60, 591–599 CrossRef CAS
.
- S. Huang, C. Meng, M. Xiao, S. Ren, S.-J. Wang, D.-M. Han, Y.-N. Li and Y.-Z. Meng, Multi-shell Tin Phosphide Nanospheres as High Performance Anode Material for a Sodium Ion Battery, Sustain, Energy Fuels, 2017, 1, 1944–1949 CAS
.
- E. M. Salhabi, J.-L. Zhao, J.-Y. Wang, M. Yang, B. Wang and D. Wang, Hollow Multi-shelled Structural TiO2−x with Multiple Spatial Confinement for Long-life Lithium-sulfur Batteries, Angew. Chem., 2019, 58, 9078–9082 CrossRef CAS PubMed
.
- X.-X. Chen, X.-Y. Ding, C.-S. Wang, Z.-Y. Feng, L.-Q. Xu, X. Gao, Y.-J. Zhai and D.-B. Wang, A Multi-shelled CoP Nanosphere Modified Separator for Highly Efficient Li–S Batteries, Nanoscale, 2018, 10, 13694–13701 RSC
.
- S.-Q. Chen, X.-D. Huang, B. Sun, J.-Q. Zhang, H. Liu and G.-X. Wang, Multi-shelled Hollow Carbon Nanospheres for Lithium-sulfur Batteries with Superior Performances, J. Mater. Chem. A, 2014, 2, 16199–16207 RSC
.
- J.-W. Gong, K. Sumathy, Q.-Q. Qiao and Z.-P. Zhou, Review on Dye-sensitized Solar Cells (DSSCs): Advanced Techniques and Research Trends, Renewable Sustainable Energy Rev., 2017, 68, 234–246 CrossRef CAS
.
- W.-Q. Wu, Y.-F. Xu, H.-S. Rao, C.-Y. Su and D.-B. Kuang, Multistack Integration of Three-Dimensional Hyperbranched Anatase Titania Architectures for High-Efficiency Dye-Sensitized Solar Cells, J. Am. Chem. Soc., 2014, 136, 6437–6445 CrossRef CAS PubMed
.
- M. Jafarzadeh, C. S. Sipaut, J. Dayou and R. F. Mansa, Recent Progresses in Solar Cells: Insight into Hollow Micro/nano-Structures, Renewable Sustainable Energy Rev., 2016, 64, 543–568 CrossRef CAS
.
- H.-Q. Wang, M. Miyauchi, Y. Ishikawa, A. Pyatenko, N. Koshizaki, Y. Li, L. Li, X.-Y. Li, Y. Bando and D. Golberg, Single-Crystalline Rutile TiO2 Hollow Spheres: Room-Temperature Synthesis, Tailored Visible-Light-Extinction, and Effective Scattering Layer for Quantum Dot-Sensitized Solar Cells, J. Am. Chem. Soc., 2011, 133, 19102–19109 CrossRef CAS PubMed
.
- L. P. Heiniger, F. Giordano, T. Moehl and M. Graetzel, Mesoporous TiO2 Beads Offer Improved Mass Transport for Cobalt-Based Redox Couples Leading to High Efficiency Dye-Sensitized Solar Cells, Adv. Energy Mater., 2014, 4, 1400168 CrossRef
.
- J. H. Pan, X. Z. Wang, Q.-Z. Huang, C. Shen, Z. Y. Koh, Q. Wang, A. Engel and D. W. Bahnemann, Large-scale Synthesis of Urchin-like Mesoporous TiO2 Hollow Spheres by Targeted Etching and Their Photoelectrochemical Properties, Adv. Funct. Mater., 2014, 24, 95–104 CrossRef CAS
.
- M. S. Ahmad, A. K. Pandey and N. A. Rahim, Advancements in the Development of TiO2 Photoanodes and its Fabrication Methods for Dye Sensitized Solar Cell (DSSC)
Applications, Renewable Sustainable Energy Rev., 2017, 77, 89–108 CrossRef
.
- X.-P. Lin, D.-M. Song, X.-Q. Gu, Y.-L. Zhao and Y. H. Qiang, Synthesis of Hollow Spherical TiO2 for Dye-sensitized Solar Cells with Enhanced Performance, Appl. Surf. Sci., 2012, 263, 816–820 CrossRef CAS
.
- X. Wu, G.-Q. Lu and L.-Z. Wang, Shell-in-Shell TiO2 Hollow Spheres Synthesized by One-pot Hydrothermal Method for Dye-sensitized Solar Cell Application, Energy Environ. Sci., 2011, 4, 3565–3572 RSC
.
- J.-F. Qian, P. Liu, Y. Xiao, Y. Jiang, Y.-L. Cao, X.-P. Ai and H.-X. Yang, TiO2-Coated Multilayered SnO2 Hollow Microspheres for Dye-sensitized Solar Cells, Adv. Mater., 2009, 21, 3663–3667 CrossRef CAS
.
- E. J. Canto-Aguilar, M. Rodríguez-Perez, R. García-Rodríguez, F. I. Lizama-Tzec, A. T. D. Denko, F. E. Osterloh and G. Oskam, ZnO-based Dye-sensitized Solar Cells: Effects of Redox Couple and Dye Aggregation, Electrochim. Acta, 2017, 258, 396–404 CrossRef CAS
.
- R. Ruess, S. Haas, A. Ringleb and D. Schlettwein, Dye-Sensitized Solar Cells with Electrodeposited ZnO and Co(bpy)3 Redox Electrolyte: Investigation of Mass Transport in the Electrolyte andInterfacial Charge Recombination, Electrochim. Acta, 2017, 258, 591–598 CrossRef CAS
.
- S. V. Umale, S. N. Tambat and S. M. Sontakke, Combustion Synthesized CeO2 as an Anodic Material in Dye Sensitized Solar Cells, Mater. Res. Bull., 2017, 94, 483–488 CrossRef CAS
.
- J.-Y. Bai, X.-L. Sun, G. Han and G.-W. Diao, Double-shell CeO2@TiO2 Hollow Spheres Composites with Enhanced Light Harvesting and Electron Transfer in Dye-Sensitized Solar Cells, J. Alloys Compd., 2017, 722, 864–871 CrossRef CAS
.
- R.-F. Zhao, Q.-H. Wu, D.-M. Tang, W.-L. Li, X. Zhang, M. Chen, R. Guo and G.-W. Diao, Double-Shell CeO2:Yb, Er@SiO2@Ag Upconversion Composite Nanofibers as an Assistant Layer Enhanced near-infrared Harvesting for Dye-Sensitized Solar Cells, J. Alloys Compd., 2018, 769, 92–95 CrossRef CAS
.
- G. Han, M. Wang, D.-Y. Li, J.-Y. Bai and G.-W. Diao, Novel Upconversion Er, Yb-CeO2 Hollow Spheres as Scattering Layer Materials for Efficient Dye-sensitized Solar Cells, Sol. Energy Mater. Sol. Cells, 2017, 160, 54–59 CrossRef CAS
.
- R.-F. Zhao, L. Huan, P. Gu, R. Guo, M. Chen and G.-W. Diao, Yb,Er-Doped CeO2 Nanotubes as an Assistant Layer for Photoconversion-enhanced Dye-sensitized Solar Cells, J. Power Sources, 2016, 331, 527–534 CrossRef CAS
.
- M. Waqas, Y.-Z. Wei, D. Mao, J. Qi, Y. Yang, B. Wang and D. Wang, Multi-shelled TiO2/Fe2TiO5 Heterostructured Hollow Microspheres for Enhanced Solar Water Oxidation, Nano Res., 2017, 10, 3920–3928 CrossRef CAS
.
- J. Qi, K. Zhao, G.-D. Li, Y. Gao, H.-J. Zhao, R.-B. Yu and Z.-Y. Tang, Multi-shelled CeO2 Hollow Microspheres as Superior Photocatalysts for Water Oxidation, Nanoscale, 2014, 6, 4072–4077 RSC
.
- Y.-Z. Wei, J.-Y. Wang, R.-B. Yu, J.-W. Wan and D. Wang, Constructing SrTiO3-TiO2 Heterogeneous Hollow Multi-shelled Structures for Enhanced Solar Water Splitting, Angew. Chem., 2019, 131, 1436–1440 Search PubMed
.
- L.-L. Wang, Z. Lou, T. Fei and T. Zhang, Zinc Oxide Core–Shell Hollow Microspheres with Multi-shelled Architecture for Gas Sensor Applications, J. Mater. Chem., 2011, 21, 19331–19336 RSC
.
- F.-D. Qu, W.-N. Shang, D.-T. Wang, S.-Y. Du, T. Thomas, S.-P. Ruan and M.-H. Yang, Coordination Polymer-Derived Multishelled Mixed Ni–Co Oxide Microspheres for Robust and Selective Detection of Xylene, ACS Appl. Mater. Interfaces, 2018, 10, 15314–15321 CrossRef CAS PubMed
.
- J.-S. Kim, J.-W. Yoon, Y. J. Hong, Y. C. Kang, F. Abdel-Hady, A. Wazzan and J.-H. Lee, Highly Sensitive and Selective Detection of Ppb-Level NO2 using Multi-shelled WO3 Yolk–shell Spheres, Sens. Actuators, B, 2016, 229, 561–569 CrossRef CAS
.
- Y.-J. Li, S.-M. Wang, P. Hao, J. Tian, H.-Z. Cui and X.-Z. Wang, Soft-Templated Formation of Double-shelled ZnO Hollow Microspheres for Acetone Gas Sensing at Low Concentration/near Room Temperature, Sens. Actuators, B, 2018, 273, 751–759 CrossRef CAS
.
- J.-Z. Zheng, T.-M. Zhang, H.-J. Zeng, W. Guo, B. Zhao, Y.-H. Sun, Y.-Y. Li and L. Jiang, Multishelled Hollow Structures of Yttrium Oxide for the Highly Selective and Ultrasensitive Detection of Methanol, Small, 2019, 15, 1804688 CrossRef PubMed
.
- Y. J. Hong, J.-W. Yoon, J.-H. Lee and Y. C. Kang, One-Pot Synthesis of Pd-Loaded SnO2 Yolk–shell Nanostructures for Ultraselective Methyl Benzene Sensors, Chem. – Eur. J., 2014, 20, 2737–2741 CrossRef CAS PubMed
.
- S.-J. Kim, I.-S. Hwang, C. W. Na, I.-D. Kim, Y. C. Kang and J.-H. Lee, Ultrasensitive and Selective C2H5OH Sensors using Rh-loaded In2O3 Hollow Spheres, J. Mater. Chem., 2011, 21, 18560–18567 RSC
.
- N. G. Cho, H.-S. Woo, J.-H. Lee and I.-D. Kim, Thin-Walled NiO Tubes Functionalized with Catalytic Pt for Highly Selective C2H5OH Sensors using Electrospun Fibers as a Sacrificial Template, Chem. Commun., 2011, 47, 11300–11302 RSC
.
- J.-W. Yoon, Y. J. Hong, G. D. Park, S.-J. Hwang, F. Abdel-Hady, A. A. Wazzan, Y. C. Kang and J.-H. Lee, Kilogram-scale Synthesis of Pd-Loaded Quintuple-Shelled Co3O4 Microreactors and Their Application to Ultrasensitive and Ultraselective Detection of Methylbenzenes, ACS Appl. Mater. Interfaces, 2015, 7, 7717–7723 CrossRef CAS PubMed
.
- P. Sun, B.-Q. Wang, L.-P. Zhao, H.-Y. Gao, T.-S. Wang, X.-L. Yang, C. Liu and G.-Y. Lu, Enhanced Gas Sensing by Amorphous Double-shell Fe2O3 Hollow Nanospheres Functionalized with PdO Nanoparticles, Sens. Actuators, B, 2017, 252, 322–329 CrossRef CAS
.
- T.-T. Ma, L.-L. Zheng, Y.-Q. Zhao, Y.-S. Xu, J. Zhang and X.-H. Liu, Highly Porous Double-shelled Hollow Hematite Nanoparticles for Gas Sensing, ACS Appl. Nano Mater., 2019, 2, 2347–2357 CrossRef CAS
.
- X.-M. Ma, X.-T. Zhang, L. Yang, K. Wang, K. Jiang, Z.-P. Wei and Y.-M. Guo, An Unusual Temperature Gradient Crystallization Process: Facile Synthesis of Hierarchical ZnO Porous Hollow Spheres with Controllable Shell Numbers, CrystEngComm, 2014, 16, 7933–7941 RSC
.
- D.-F. Zhang, G.-Z. Zhang and L. Zhang, Multi-Shelled FeCo2O4 Hollow Porous Microspheres/CCFs Magnetic Hybrid and its Dual-Functional Catalytic Performance, Chem. Eng. J., 2017, 330, 792–803 CrossRef CAS
.
- L.-B. Zong, P.-Z. Cui, F.-Y. Qin, K. Zhao, Z.-M. Wang and R.-B. Yu, Heterostructured Bismuth Vanadate Multi-shell Hollow Spheres with High Visible-Light-Driven Photocatalytic Activity, Mater. Res. Bull., 2017, 86, 44–50 CrossRef CAS
.
- F.-Y. Qin, P.-Z. Cui, L. Hu, Z.-M. Wang, J. Chen, X.-R. Xing, H. Wang and R.-B. Yu, Construction of Multi-shelled Bi2WO6 Hollow Microspheres with Enhanced Visible Light Photo-Catalytic Performance, Mater. Res. Bull., 2018, 99, 331–335 CrossRef CAS
.
- B.-J. Liu, X.-Y. Li, Q.-D. Zhao, Y. Hou and G.-H. Chen, Self-templated Formation of ZnFe2O4 Double-shelled Hollow Microspheres for Photocatalytic Degradation of Gaseous O-Dichlorobenzene, J. Mater. Chem. A, 2017, 5, 8909–8915 RSC
.
- K.-L. Ma, W.-X. Zou, L. Zhang, L.-L. Li, S.-H. Yu, C.-J. Tang, F. Gao and L. Dong, Construction of Hybrid Multi-shell Hollow Structured CeO2–MnOx Materials for Selective Catalytic Reduction of NO with NH3, RSC Adv., 2017, 7, 5989–5999 RSC
.
- H. Wang, D. Mao, J. Qi, Q.-H. Zhang, X.-H. Ma, S.-Y. Song, L. Gu, R.-B. Yu and D. Wang, Hollow Multishelled Structure of Heterogeneous Co3O4–CeO2−x Nanocomposite for CO Catalytic Oxidation, Adv. Funct. Mater., 2019, 29, 1806588 CrossRef
.
- L. Wang, J.-W. Wan, Y.-S. Zhao, N.-L. Yang and D. Wang, Hollow Multi-shelled Structures of Co3O4 Dodecahedron with Unique Crystal Orientation for Enhanced Photocatalytic CO2 Reduction, J. Am. Chem. Soc., 2019, 141, 2238–2241 CrossRef CAS
.
- X. Wang, Y.-T. Zhong, T.-Y. Zhai, Y.-F. Guo, S.-M. Chen, Y. Ma, J.-N. Yao, Y. Bando and D. Golberg, Multishelled Co3O4–Fe3O4 Hollow Spheres with Even Magnetic Phase Distribution: Synthesis, Magnetic Properties and their Application in Water Treatment, J. Mater. Chem., 2011, 21, 17680–17687 RSC
.
- Y. Suzuki, S. D. Kelly, K. M. Kemner and J. F. Banfield, Nanometre-size Products of Uranium Bioreduction, Nature, 2002, 419, 134 CrossRef CAS PubMed
.
- J. Li, X.-X. Wang, G.-X. Zhao, C.-L. Chen, Z.-F. Chai, A. Alsaedi, T. Hayat and X.-K. Wang, Metal–organic Framework-based Materials: Superior Adsorbents for the Capture of Toxic and Radioactive Metal Ions, Chem. Soc. Rev., 2018, 47, 2322–2356 RSC
.
- Y.-J. Ai, Y. Liu, W.-Y. Lan, J.-R. Jin, J.-L. Xing, Y.-D. Zou, C.-F. Zhao and X.-K. Wang, The Effect of pH on the U(VI) Sorption on Graphene Oxide (GO): A Theoretical Study, Chem. Eng. J., 2018, 343, 460–466 CrossRef CAS
.
- X.-X. Wang, S.-Q. Yu, Y.-H. Wu, H.-W. Pang, S.-J. Yu, Z.-S. Chen, J. Hou, A. Alsaedi, T. Hayat and S.-H. Wang, The Synergistic Elimination of Uranium(VI) Species from Aqueous Solution using Bi-Functional Nanocomposite of Carbon Sphere and Layered Double Hydroxide, Chem. Eng. J., 2018, 342, 321–330 CrossRef CAS
.
- S. Song, S. Zhang, S.-Y. Huang, R. Zhang, L. Yin, Y.-Z. Hu, T. Wen, L. Zhuang, B.-W. Hu and X.-K. Wang, A Novel Multi-Shelled Fe3O4@MnOx Hollow Microspheres for Immobilizing U(VI) and Eu(III), Chem. Eng. J., 2019, 355, 697–709 CrossRef CAS
.
- J.-Q. Feng, H.-X. Guo, S.-P. Wang, Y.-J. Zhao and X.-B. Ma, Fabrication of Multi-shelled Hollow Mg-Modified CaCO3 Microspheres and their Improved CO2 Adsorption Performance, Chem. Eng. J., 2017, 321, 401–411 CrossRef CAS
.
- B.-C. Liu, Q. Wang, S.-L. Yu, T. Zhao, J.-X. Han, P. Jing, W.-T. Hu, L.-X. Liu, J. Zhang, L.-D. Sun and C.-H. Yan, Double Shelled Hollow Nanospheres with Dual Noble Metal Nanoparticle Encapsulation for Enhanced Catalytic Application, Nanoscale, 2013, 5, 9747–9757 RSC
.
- P.-F. Xu, R.-B. Yu, H. Ren, L.-B. Zong, J. Chen and X.-R. Xing, Hierarchical Nanoscale Multi-shell Au/CeO2 Hollow Spheres, Chem. Sci., 2014, 5, 4221–4226 RSC
.
- Y.-Y. Liao, Y. Li, L. Wang, Y.-X. Zhao, D.-Y. Ma, B.-Q. Wang, Y.-X. Wan and S.-L. Zhong, Multi-Shelled Ceria Hollow Spheres with a Tunable Shell Number and Thickness and their Superior Catalytic Activity, Dalton Trans., 2017, 46, 1634–1644 RSC
.
- C. Zhang, Y.-M. Zhou, Y.-W. Zhang, S. Zhao, J.-S. Fang and X.-L. Sheng,
In Situ Doping of Pt Active Sites via Sn in Double-Shelled TiO2 Hollow Nanospheres with Enhanced Photocatalytic H2 Production Efficiency, Chem. – Eur. J., 2017, 41, 11089–11096 CAS
.
- P. K. Bollaa, V. A. Rodrigueza, R. S. Kalhapurea, C. S. Kollib, S. Andrewsa and J. Renukuntla, A Review on pH and Temperature Responsive Gels and Other Less Explored Drug Delivery Systems, J. Drug Delivery Sci. Technol., 2018, 46, 416–435 CrossRef
.
- M. Shahriari, M. Zahiric, K. Abnousa, S. M. Taghdisid, M. Ramezani and M. Alibolandi, Enzyme Responsive Drug Delivery Systems in Cancer Treatment, J. Controlled Release, 2019, 308, 172–189 CrossRef CAS PubMed
.
- C.-C. Huang, W. Huang and C.-S. Yeh, Shell-By-Shell Synthesis of Multi-shelled Mesoporous Silica Nanospheres for Optical Imaging and Drug Delivery, Biomaterials, 2011, 32, 556–564 CrossRef CAS PubMed
.
| This journal is © the Partner Organisations 2020 |




