A multifunctional Zr-MOF for the rapid removal of Cr2O72−, efficient gas adsorption/separation, and catalytic performance†
Abstract
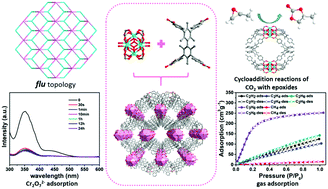
To achieve the selective adsorption of ions and light hydrocarbons by changing the pore structure, Zr-based metal–organic framework [Zr6(μ3-O)4(μ3-OH)4(OH)4(H2O)4(TB)2]·2DMF·2H2O (UPC-50) was synthesized based on 3,3′,5,5′-tetra(4-carboxyphenyl)bimesityl (H4TB) with a steric hindrance effect under hydrothermal conditions. UPC-50 features a three-dimensional frame with two-dimensional channels and high hydrothermal and chemical stability. The Zr–OH bond in the Zr6 cluster endows UPC-50 with excellent removal capacity for trace Cr2O72− in water (0.089 ppm) and efficient adsorption rate (<1 min). Moreover, through the single-component gas sorption and selectivity calculated by the IAST method, the channel of 10.6 × 16.4 Å gives UPC-50 a high adsorption capacity for C3H6 (252.67 cm3 g−1), and also shows effective separation of C3H6/CH4. The unsaturated sites on the Zr6 cluster also impart excellent catalytic performance to UPC-50 in the catalytic cycloaddition reactions of CO2 with epoxides. Therefore, UPC-50 is a multi-functional material with a rapid removal capacity for Cr2O72−, efficient gas adsorption/separation, and catalytic performance.
- This article is part of the themed collection: 2019 Materials Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...