Open Access Article
Open Access ArticleNi-catalyzed 1,2-benzylboration of 1,2-disubstituted unactivated alkenes†
Seewon
Joung‡
 ,
Allison M.
Bergmann
and
M. Kevin
Brown
,
Allison M.
Bergmann
and
M. Kevin
Brown
 *
*
Indiana University, Department of Chemistry, 800 E. Kirkwood Ave, Bloomington, IN 47405, USA. E-mail: brownmkb@indiana.edu
First published on 22nd October 2019
Abstract
Nickel-catalyzed 1,2-carboboration of alkenes is emerging as a useful method for chemical synthesis. Prior studies have been limited to only the incorporation of aryl groups. In this manuscript, a method for the 1,2-benzylboration of unactivated alkenes is presented. The reaction combines readily available alkenes, diboron reagents and benzylchlorides to generate synthetically versatile products with control of stereochemistry. The utility of the products as well as the mechanistic details of the process are also presented.
Introduction
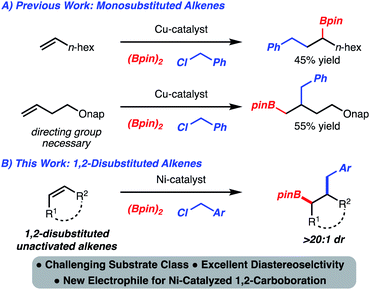
Development of alkene functionalization reactions has garnered intense interest for decades. This is largely due to the availability of alkenes either from commercial sources or from a plethora of established synthetic strategies. In contrast to homodifunctionalization reactions (epoxidation, dihydroxylation, bromination, etc.), heterodifunctionalization is less established.1 Among known processes, 1,2-carboboration has emerged as a useful reaction because in addition to the formation of a C–C bond, a synthetically versatile C–B bond is generated.2The incorporation of benzyl groups through 1,2-carboboration is significant as these motifs are common to numerous intermediates used in synthesis and molecules of interest (e.g., bioactive compounds and/or natural products). While 1,2-benzylboration reactions of activated alkenes have been developed,3a,b,4 variants that function with unactivated alkenes are rare. Yoshida reported a Cu-catalyzed 1,2-benzylboration of monosubstituted alkenes to generate the internal boronic ester (Scheme 1A).3b Subsequently, Fu disclosed a related process that employed homo-allylic ethers and led to the formation of the terminal boronic ester (Scheme 1A).3c Despite these advances, the 1,2-benzylboration of unactivated internal alkenes is an unmet challenge. In this manuscript, we address this problem and report a stereoselective Ni-catalyzed 1,2-benzylboration of unactivated 1,2-disubstituted alkenes, as well as demonstrate the synthetic versatility of the products (Scheme 1B).
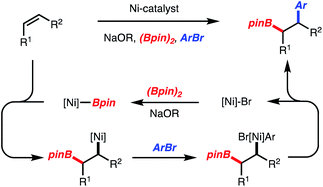
Recently, our lab introduced a Ni-catalyzed 1,2-arylboration of unactivated alkenes with arylbromides and diboron reagents (Scheme 2).5,6 The reactions likely operate by addition of [Ni]-Bpin across an alkene followed by capture of the generated alkyl-[Ni]-complex with an arylbromide (Scheme 2). The resulting alkyl–aryl-[Ni]-complex undergoes reductive elimination to generate a Csp3–Csp2 bond. Since Ni-catalysis has been utilized for Csp3–Csp3 bond formation by reductive elimination of dialkyl-[Ni]-complexes,7 we envisioned that if the alkyl-[Ni]-complex could be captured by a benzyl electrophile, a benzylboration reaction could be developed.
Results and discussion
Under conditions for arylboration described in our initial study,5a substitution of PhBr for BnCl resulted in formation of benzylboration product 3 in 5% yield but in >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr.8 Standard optimization of this reaction resulted in the conditions shown in Table 1, in which 3 was formed in 72% yield and >20
1 dr.8 Standard optimization of this reaction resulted in the conditions shown in Table 1, in which 3 was formed in 72% yield and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
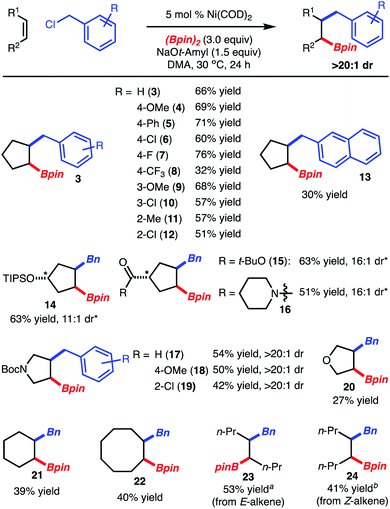
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr.9 Crucial to attaining good yield was the use of dimethylacetamide (DMA) instead of THF as the reaction solvent, which suppressed the side product derived from homocoupling of BnCl.5b It is also important to note that while use of Ni(COD)2 was deemed the optimal pre-catalyst, the more user-friendly NiCl2(DME) could be substituted with a modest decrease in yield (Table 1, entries 1 and 2). The use of BnBr or BnOCO2t-Bu did not lead to product formation (Table 1, entries 6–7). Finally, attempted reaction with other primary and secondary alkyl halides did not lead to product formation.8
1 dr.9 Crucial to attaining good yield was the use of dimethylacetamide (DMA) instead of THF as the reaction solvent, which suppressed the side product derived from homocoupling of BnCl.5b It is also important to note that while use of Ni(COD)2 was deemed the optimal pre-catalyst, the more user-friendly NiCl2(DME) could be substituted with a modest decrease in yield (Table 1, entries 1 and 2). The use of BnBr or BnOCO2t-Bu did not lead to product formation (Table 1, entries 6–7). Finally, attempted reaction with other primary and secondary alkyl halides did not lead to product formation.8
| Entry | Change from standard conditions | Yieldb (%) |
|---|---|---|
| a Reaction run on 0.5 mmol scale. b Yield determined by GC analysis with a, calibrated internal standard. | ||
| 1 | No change | 72 |
| 2 | 5 mol% NiCl2(DME) instead of Ni(COD)2 | 53 |
| 3 | NaOt-Bu instead of NaOt-amyl | 65 |
| 4 | THE instead of DMA | 19 |
| 5 | DMF instead of DMA | 54 |
| 6 | BnBr instead of BnCl | <2 |
| 7 | BnOCO2t-Bu instead of BnCl | <2 |
Under the optimized conditions, the scope of the reaction was explored. It was found that reaction of cyclopentene with various benzylchlorides modified with electron-donating (products 4, 9), electron-withdrawing (products 6–8, 10, 12) and sterically demanding groups (products 11, 12) led to product formation. In the case of substituted cyclopentenes, benzylboration occurs on the opposite face of the alkene with respect to the substituent (products 14–16). Pyrrolidine and furan heterocycles could also be used (products 17–20). Finally, reaction of larger ring sizes or alkenes within aliphatic carbon chains allowed for product formation, but in reduced yield (products 21–24). In these cases, the lower yield resulted from incomplete conversion. However, it should be noted that reaction of E-4-octene and Z-4-octene resulted in formation of product of two different diastereomers confirming that the reactions are stereospecific (products 23 and 24, respectively).5 With respect to the known limitations, reaction of 1,1-disubtituted alkenes, terminal alkenes, and alkenyl arenes were poorly reactive and resulted in low yield.8 In addition, reaction of 1-phenylethyl chloride led to only homo-dimerization of the electrophile.8
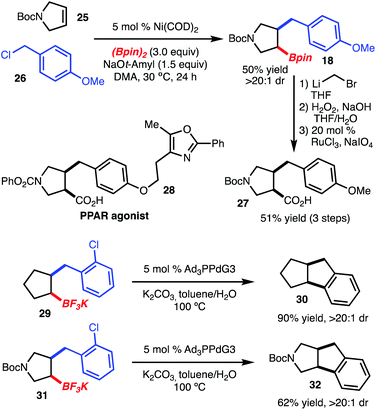
Benzylboration of cyclopentenes and related heterocycles represents a useful method for chemical synthesis, as related structures are common in natural products and drug-like molecules (Scheme 4). For example, homologation and oxidation of 18 to the carboxylic acid provided 27, which maps onto the structure of PPAR α/γ agonist 28, disclosed by Bristol-Myers Squibb.10 In addition, potassium trifluoroborates 29 and 31 could be prepared and subjected to intramolecular cross coupling catalyzed by [PdPAd3]11 (Ad = 1-adamantyl) to provide tricyclic structures 30 and 32, respectively.12 The intramolecular cross coupling shown here deserves further comment. As previously reported, cross coupling of chiral, non-racemic secondary alkyl boronic acids under the conditions shown in Scheme 4 resulted in inversion of stereochemistry. The reaction shown here proceeded with retention of stereochemistry, likely a result of the constraints imposed through formation of a 5/5-ring system.
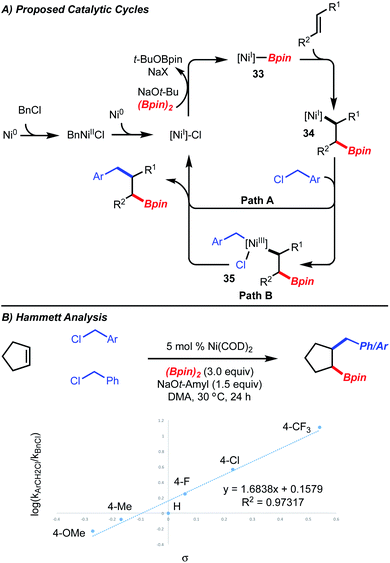
In accordance with detailed mechanistic studies for the related Ni-catalyzed arylboration reaction,5 the catalytic cycle shown in Scheme 5A is proposed, in which alkyl-[Ni]-complex 34 undergoes reaction with benzyl chloride. Long-lived radical intermediates are unlikely to be involved, as the reaction is stereospecific (Scheme 3, products 23 and 24). The reaction of the putative alkyl-[Ni]-complex 34 with the benzyl chloride could proceed either by a substitution (Path A) or an oxidative addition/reductive elimination pathway (Path B). With respect to Path B, the oxidative addition could proceed via a two-electron or one electron process.
To interrogate Path A and Path B, Hammett analysis of the reaction was undertaken. If direct substitution was operative, a “U-shaped” curve would be expected, where both benzyl chlorides substituted with electron-donating and electron-withdrawing groups undergo reaction faster than the unsubstituted benzyl chloride.13 In the case of an oxidative addition, a Hammett analysis is not available for comparison for either the two-electron or one-electron process. For the Ni-catalyzed 1,2-benzylboration, a linear correlation with a ρ-value of 1.68 (R2 = 0.973) was observed, suggesting that substitution might not be operative (Scheme 5B). However, we caution that this analysis does not rule out a substitution pathway as the present system is undoubtedly different than the reactions used for comparison.13
Conclusions
In summary, a 1,2-benzylboration of unactivated alkenes is presented. The reaction efficiently transforms several classes of alkenes and benzyl chlorides into interesting products that can be elaborated to scaffolds related to biologically active molecules. This study also serves to broaden the scope of Ni-catalyzed carboboration reactions to now include Csp3-based electrophiles.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Indiana University and the NIH (R01GM114443 and R35GM128779) for financial support. This project was partially funded by the Vice Provost for Research through the Research Equipment Fund and the NSF (CHE1726633).Notes and references
- For reviews, regarding alkene functionalization, see: (a) K. H. Jensen and M. S. Sigman, Org. Biomol. Chem., 2008, 6, 4083 RSC; (b) R. Giri and S. Kc, J. Org. Chem., 2018, 83, 3013–3022 CrossRef CAS PubMed.
- For reviews, see: (a) Y. Shimizu and M. Kanai, Tetrahedron Lett., 2014, 55, 3727–3737 CrossRef CAS; (b) K. Semba, T. Fujihara, J. Terao and Y. Tsuji, Tetrahedron, 2015, 71, 2183–2197 CrossRef CAS; (c) F. Lazreg, F. Nahra and C. S. J. Cazin, Coord. Chem. Rev., 2015, 293–294, 48–79 CrossRef CAS; (d) E. C. Neeve, S. J. Geier, I. A. I. Mkhalid, S. A. Westcott and T. B. Marder, Chem. Rev., 2016, 116, 9091–9161 CrossRef CAS PubMed; (e) D. Hemming, R. Fritzemeier, S. A. Westcott, W. L. Santos and P. G. Steel, Chem. Soc. Rev., 2018, 47, 7477–7494 RSC.
- (a) H. Yoshida, I. Kageyuki and K. Takaki, Org. Lett., 2013, 15, 952–955 CrossRef CAS PubMed; (b) I. Kageyuki, H. Yoshida and K. Takaki, Synthesis, 2014, 46, 1924–1932 CrossRef; (c) W. Su, T.-J. Gong, X. Lu, M.-Y. Xu, C.-G. Yu, Z.-Y. Xu, H.-Z. Yu, B. Xiao and Y. Fu, Angew. Chem., Int. Ed., 2015, 54, 12957–12961 CrossRef CAS PubMed.
- For a Ni-catalyzed 1,1-benzylboration unactivated alkenes and 1,2-benzylboration of vinylarenes, see: Y. Li, H. Pang, D. Wu, Z. Li, W. Wang, H. Wei, Y. Fu and G. Yin, Angew. Chem., Int. Ed., 2019, 58, 8872–8876 CrossRef CAS PubMed.
- (a) K. M. Logan, S. R. Sardini, S. D. White and M. K. Brown, J. Am. Chem. Soc., 2018, 140, 159–162 CrossRef CAS PubMed; (b) S. R. Sardini, A. L. Lambright, G. L. Trammel, H. M. Omer, P. Liu and M. K. Brown, J. Am. Chem. Soc., 2019, 141, 9391–9400 CrossRef PubMed; (c) L.-A. Chen, A. R. Lear, P. Gao and M. K. Brown, Angew. Chem., Int. Ed., 2019, 38, 10956 CrossRef PubMed.
- (a) W. Wang, C. Ding, Y. Li, Z. Li, Y. Li, L. Peng and G. Yin, Angew. Chem., Int. Ed., 2019, 58, 4612–4616 CrossRef CAS PubMed; (b) W. Wang, C. Ding, H. Pang and G. Yin, Org. Lett., 2019, 21, 3968–3971 CrossRef CAS PubMed.
- (a) J. Zhou and G. C. Fu, J. Am. Chem. Soc., 2003, 125, 14726–14727 CrossRef CAS PubMed; (b) B. Saito and G. C. Fu, J. Am. Chem. Soc., 2007, 129, 9602–9603 CrossRef CAS PubMed.
- See the ESI for details.†.
- The only detectable byproducts from the reaction are dibenzyl (∼20% yield) and benzylBpin (∼10% yield).
- H. Zhang, C. Z. Ding and Z. Lai, et al. , Bioorg. Med. Chem. Lett., 2015, 25, 1196–1205 CrossRef CAS PubMed.
- L. Chen, P. Ren and B. P. Carrow, J. Am. Chem. Soc., 2016, 138, 6392–6395 CrossRef CAS PubMed.
- S. Zhao, T. Gensch, B. Murray, Z. L. Niemeyer, M. S. Sigman and M. R. Biscoe, Science, 2018, 362, 670 CrossRef CAS PubMed.
- P. R. Young and W. P. Jencks, J. Am. Chem. Soc., 1979, 101, 3288–3294 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Procedure, full characterization data, and copies of spectra. See DOI: 10.1039/c9sc04199k |
| ‡ Current address: Department of Chemistry, Mokpo National University, 58554, Muan, South Korea. |
| This journal is © The Royal Society of Chemistry 2019 |