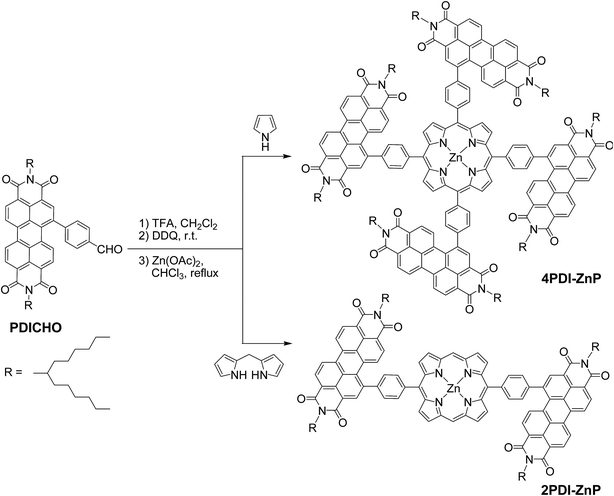
Phenylene-bridged perylenediimide-porphyrin acceptors for non-fullerene organic solar cells†
Quanquan
Zhang‡
ab,
Xiaopeng
Xu‡
c,
Song
Chen‡
b,
Govardhana Babu
Bodedla
b,
Mingzi
Sun
 d,
Qin
Hu
d,
Qin
Hu
 f,
Qiang
Peng
*c,
Bolong
Huang
d,
Hanzhong
Ke
*a,
Feng
Liu
*e,
Thomas P.
Russell
f,
Qiang
Peng
*c,
Bolong
Huang
d,
Hanzhong
Ke
*a,
Feng
Liu
*e,
Thomas P.
Russell
 f and
Xunjin
Zhu
f and
Xunjin
Zhu
 *b
*b
aFaculty of Material Science and Chemistry, China University of Geosciences, Wuhan, Hubei 430074, P. R. China. E-mail: kehanz@aliyun.com
bDepartment of Chemistry and Institute of Molecular Functional Materials, Hong Kong Baptist University, Waterloo Road, Kowloon Tong, Hong Kong, P. R. China. E-mail: xjzhu@hkbu.edu.hk
cDepartment of Chemistry, Sichuan University, Chengdu, Sichuan 610000, P. R. China. E-mail: qiangpeng@scu.edu.cn
dDepartment of Applied Biology & Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, P. R. China
eDepartment of Physics and Astronomy, Collaborative Innovation Center of IFSA, Shanghai Jiao Tong University, Shanghai, 200240, P. R. China. E-mail: fengliu82@sjtu.edu.cn
fDepartment of Polymer Science and Engineering, University of Massachusetts, Amherst, MA 01003, USA
First published on 6th September 2018
Abstract
New perylenediimide-porphyrin acceptors, 4PDI-ZnP and 2PDI-ZnP, have been facilely synthesized by acid-catalyzed condensation of perylenediimide-substituted benzaldehyde with pyrrole and dipyrromethane, respectively, and subsequent Zn(II)-complexation. 4PDI-ZnP with four perylene diimide (PDI) moieties appended onto the zinc(II)-porphyrin core shows higher electron mobility than 2PDI-ZnP with only two PDI units. The π-conjugation between PDI and porphyrin is significantly weakened by the phenylene linkage twisting them due to steric hindrance, which renders the absorption features from porphyrin and PDI undisturbed in the range of 385–600 nm. For spectral absorption properties perfectly complementary to the common polymer donor PTB7-Th, the two acceptors have been evaluated together with PTB7-Th in non-fullerene bulk heterojunction organic solar cells (BHJ OSCs). High power conversion efficiency of 9.64% was achieved using the blend of 4PDI-ZnP:PTB7-Th for optimal visible sunlight harvesting, favorable morphological properties and efficient charge dissociation upon photon absorption. This represents a new benchmark photovoltaic performance for PDI acceptors and PTB7-Th donor systems. It should be noted that the porphyrin core not only acts as a scaffold for PDI moieties, but also contributes the light-harvesting in near-ultraviolet and violet regions, which is unambiguously demonstrated in single-component BHJ OSCs based on the two acceptors.
Introduction
Bulk heterojunction organic solar cells (BHJ OSCs) with active layers comprised of an electron donor and electron acceptor have been extensively studied due to their solution processability, materials tunability, and appealing lightweight and flexible device characteristics. Solution processability enables high-throughput roll-to-roll fabrication, leading to potentially low-cost manufacturing, while materials tunability permits modulation of molecular energetics and spectral properties for optimal donor–acceptor energetic matching and efficient solar energy harvesting.1 Most efforts in the past decade have been devoted to studies of donor materials, culminating in development of many electron donors with optimal energy levels, light absorption capacity, etc. for BHJ OSCs.2 Among these donor materials, porphyrin-based molecular compounds have been widely investigated in view of their extended molecular π-system and structural tunability through peripheral substitution.3 With respect to electron acceptor materials, fullerene derivatives (e.g., PC61BM and PC71BM) are the classical electron acceptor used in BHJ OSCs with power conversion efficiencies (PCEs) over 11%. It is known that PC71BM can efficiently transport mobile photogenerated electrons by percolated pathways to respective electrodes and also contributes to the photocurrent of OSCs at a wavelength of approximately 340 nm.4 However, it suffers from limitations, including low spectral absorptivity in the visible region, difficulty in tuning energy levels, and propensity to aggregate in undesirably large domains. Accordingly, there has been intense activity directed to develop non-fullerene acceptors with enhanced absorption in the visible and even near-infrared (NIR) regions and adjustable energy levels, resulting in commendable photovoltaic performances.5 Zhan et al. reported a series of high-performance non-fullerene acceptors, ITIC-Th, with broad and strong visible light absorption and tunable energy levels, which showed PCEs up to 12.1% when blended with wide band gap polymers.6 Hou et al. achieved a record efficiency of 14.4% using IT-4F as non-fullerene acceptor.7 Another class of non-fullerene molecular acceptors incorporating perylene diimide (PDI) have also been investigated, as it is an excellent electron transport system.8 Peng et al. likewise showed a tris-PDI-functionalized 1,3,5-triazine derivative with an efficiency of 9.15%.9 To maximize charge transport property, Henry Yan et al. reported two PDI-tetramers with fused and non-fused structures based on based on a tetrathienylbenzene (TTB) core, leading to dramatically improved efficiencies up to 10.58%.10 Porphyrins are a group of naturally occurring, intensely colored heterocyclic compounds with 4-meso and 8-β positions which can readily be appended with multiple PDI units for high electron mobility. Moreover, porphyrins' characteristic UV-visible spectra consist of two distinct regions: near ultraviolet and visible regions, which might contribute to the photocurrent in this region. Zhang et al. reported an electron acceptor with four peripheral PDI conjugated to porphyrin core by alkynyl group, affording a PCE of 7.4% in polymer-based OSC.11 Although it shows high electron mobility with PBDB-T polymer, the porphyrin core does not contribute to photocurrent much in the violet region because its absorption is red-shifted due to the strong π-conjugation of porphyrin core with four PDIs. Nonetheless, these results have given strong credence to the potential of porphyrin derivatives as acceptors for non-fullerene-based OSCs.To explore new PDI-based acceptors with high electron mobility and optimal light harvesting in OSCs, we designed perylenediimide-porphyrin acceptor, 4PDI-ZnP (Scheme 1), in which four PDI units were appended to the porphyrin core via p-phenylene linkages. For comparison, 2PDI-ZnP with two PDI units appended was similarly prepared. The porphyrin core with four meso-positions acts as a perfect scaffold for four PDI units; excessive steric interference between the protons of phenylene and those of porphyrin twists the phenylene bridge out of plane with the porphyrin core, essentially disrupting the π-conjugation of PDI with porphyrin. This is important as the electron-rich porphyrin core would adversely affect electron accepting ability if the PDI moiety and porphyrin core were in full π-conjugation. The situation would be quite different if the connecting linkage were an acetylene bond11 where the electron accepting ability of PDI moiety might be suppressed due to the full π-conjugation of porphyrin core to PDI unit. At the same time, the disrupted π-conjugation of PDI with porphyrin gives the new acceptors an undisturbed strong near-ultraviolet and violet light response from the porphyrin core. In addition, the twisting effect reduces intermolecular π–π stacking, thus leading to bulk heterojunction with small domain sizes. Accordingly, the acceptor, 4PDI-ZnP with four peripheral PDI moieties, would be expected to be an excellent acceptor with optimal visible light response in BHJ OSCs. Moreover, the two acceptors with separated PDI and porphyrin moieties have potential applications in single-component BHJ OSCs.
Results and discussion
Synthesis
The syntheses of 4PDI-ZnP and 2PDI-ZnP were straightforward, involving a trifluoroacetic acid (TFA)-catalyzed condensation of 4-formylphenyl-PDI (PDICHO) with pyrrole and dipyrromethane, respectively, followed by reaction with Zn(OAc)2, as illustrated in Scheme 1. The crude products were purified by column chromatography on silica gel. All compounds were fully characterized by 1H NMR and matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS). By virtue of severely twisted molecular structures and presence of solubilizing long branched alkyl side-chains, these compounds displayed good solubility in common organic solvents such as toluene, dichloromethane, chloroform, etc. In addition, both compounds exhibited good thermal stability as their thermogravimetric analyses showed only 5% weight loss at over 400 °CTheoretical simulation studies
Simulation studies on conformational structures of 2PDI-ZnP and 4PDI-ZnP were carried out using density functional theory (DFT). Results revealed a substantial structural twisting of p-phenylene linkage from the relatively planar porphyrin core in these compounds, as expected given steric interference considerations (Fig. 1a and Fig. S1†). Structural twisting was more severe in 4PDI-ZnP, with dihedral angles between phenylene and porphyrin core ranging from about 51° to about 61°, than in 2PDI-ZnP, which showed less twisted dihedral angles of about 30°. This was due to the more congested molecular structure of 4PDI-ZnP with four PDI substitutions. Accordingly, significant disruption of π-conjugation of PDI moieties with porphyrin core in these two compounds, particularly in 4PDI-ZnP, was expected. Consequences of structural twisting are four-fold: (i) the lowest unoccupied molecular orbitals (LUMOs) and the highest occupied molecular orbitals (HOMOs) are located separately on different parts of the molecules (Fig. 1b); (ii) LUMOs of both compounds are primarily dominated by contributions from their PDI moieties, thus preserving electron accepting ability and excellent electron transporting capability of PDI moieties in the compounds; (ii) structural twisting of porphyrin core avoids the red-shift of its absorption spectrum and renders the acceptor specific ultraviolet and violet light response; and (iv) the twisting of PDI moieties to a large extent restrains the otherwise uncontrolled aggregation of these compounds in their dispersions in polymer donor matrices through intermolecular PDI π–π stacking, as for example in the active layers of BHJ OPV devices.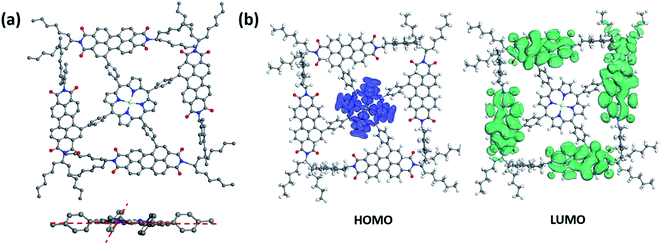 | ||
| Fig. 1 (a) Simulated conformational side-view and partial front-view structures and (b) HOMO/LUMO electron distributions of 4PDI-ZnP. | ||
Absorption spectral properties
UV-visible absorption spectrum of 4PDI-ZnP in dilute chloroform (CHCl3) solution exhibited three absorption bands, featuring porphyrin's Soret band at 425 nm and PDI absorptions from 450 to 600 nm, with Q bands of the porphyrin core concealed in the PDI absorption bands. 2PDI-ZnP exhibited a blue-shifted peak at 412 nm due to potential H-aggregation and similar PDI absorption peaks at 500 nm and 536 nm (Fig. 2, Table 1). In contrast, the previously reported acceptor with acetylene linkage between porphyrin and PDI moieties showed greatly red-shifted Soret (450–550 nm) and Q bands.11 Maximum extinction coefficients (ε) for Soret bands of 2PDI-ZnP and 4PDI-ZnP were similar, about 2.9 × 105 M−1 cm−1 and 3.2 × 105 M−1 cm−1, respectively. In contrast, thin-film spectra of 2PDI-ZnP and 4PDI-ZnP showed broader absorption bands, together with approximately 15 nm red-shifts for their Soret bands. These were indicative of relatively weak intermolecular interactions and molecular aggregation in the thin films due to severely twisted molecular structures.3a The relatively broad absorptions of these acceptors with high extinction coefficients in both blue and red regions complemented those of donor polymer PTB7-Th (Fig. 2b)12 to provide reasonably wide spectral absorption coverage for efficient solar energy harvesting.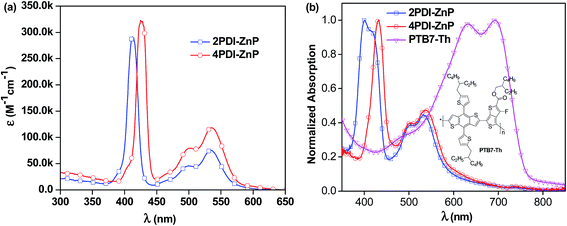 | ||
| Fig. 2 (a) Solution absorption spectra (CHCl3) of 2PDI-ZnP and 4PDI-ZnP and (b) normalized thin-film absorption spectra of 2PDI-ZnP, 4PDI-ZnP and PTB7-Th. | ||
| Compound | λ abs (nm) | (eV) | E 0–0 (eV) | E HOMO/ELUMOc(eV) |
|---|---|---|---|---|
a Oxidation potentials of PDI-porphyrin acceptors were recorded in CHCl3 (1 × 10−4 M) with 0.1 M TBAPF6 as electrolyte.
b
E
0–0 was determined from the edge of absorption spectra by 1240/λ.
c
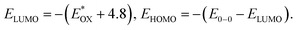
|
||||
| 2PDI-ZnP | 412, 497, 533 | −1.05 | 2.16 | −5.94/−3.78 |
| 4PDI-ZnP | 425, 500, 536 | −1.07 | 2.10 | −5.87/−3.77 |
Electrochemical properties
Lowest unoccupied molecular orbital (LUMO) energy level was calculated to be −3.78 eV for 2PDI-ZnP and −3.77 eV for 4PDI-ZnP from their respective cyclic voltammetric (CV) half-wave reduction potentials in solution (Fig. S2,†Table 1). Accordingly, highest occupied molecular orbital (HOMO) energy levels for 2PDI-ZnP and 4PDI-ZnP were estimated to be −5.94 eV and −5.87 eV, respectively, from their LUMOs and optical bandgaps.13 Similarly, the CV of PBT7-Th was also recorded in chloroform solution and its HOMO/LUMO energy levels were calculated to be −5.32/−3.69 eV (Fig. S3†), which are similar to those reported in literature (HOMO/LUMO = −5.24/−3.66 eV).12 The large HOMO energy offset between 4PDI-ZnP (or 2PDI-ZnP) and PBT7-Th ensures efficient hole transfer from electron donor to electron acceptor. Although the LUMO energy offsets are relatively low, the photoluminescence quenching experiment (Fig. S4†) and high performances in BHJ OSCs (Table 2) suggested efficient electron transfer from PBT7-Th donor to 4PDI-ZnP (PBT7-Th) acceptor. In addition, a slighter higher LUMO for 4PDI-ZnP than 2PDI-ZnP might contribute to a higher VOC in OSCs with PBT7-Th as donor (Table 2).| Compound | J SC (mA cm−2) | V OC (V) | FF (%) | PCE (%) | μ h/μe (cm2 V−1 s−1) | R s (Ω cm2) | R sh (Ω cm2) |
|---|---|---|---|---|---|---|---|
| a Each value is averaged from 10 devices with standard deviation after ±. b Device based on ITO/ZnO/ZrAcac/PTB7-Th:acceptor/MoO3/Ag. c Device based on ITO/ZnO/PNDIT-F3N-Br/PTB7-Th:acceptor/MoO3/Ag. | |||||||
| 4PDI-ZnPb | 14.80 ± 0.05 | 0.87 ± 0.01 | 62.9 ± 1.4 | 8.14 ± 0.26 | 3.43 × 10−4/2.18 × 10−4 | 9.3 | 944.1 |
| 4PDI-ZnPc | 15.35 ± 0.08 | 0.89 ± 0.01 | 68.8 ± 1.6 | 9.40 ± 0.24 | — | 7.9 | 1476.9 |
| 2PDI-ZnPb | 12.66 ± 0.06 | 0.84 ± 0.01 | 49.3 ± 2.1 | 5.22 ± 0.31 | 6.37 × 10−5/1.34 × 10−4 | 14.7 | 316.3 |
| 2PDI-ZnPc | 13.00 ± 0.01 | 0.85 ± 0.01 | 54.7 ± 1.9 | 6.04 ± 0.34 | — | 12.4 | 586.4 |
Charge transport and photovoltaic properties
Electron mobility of 2PDI-ZnP and 4PDI-ZnP in BHJ films with PTB7-Th was measured by space charge limited current (SCLC) technique using spin-cast thin films from chlorobenzene solutions with 1,8-diiodooctane (DIO) additive. Results showed electron mobility of 1.34 × 10−4 cm2 V−1 s−1 and 2.18 × 10−4 cm2 V−1 s−1 for BHJ films with 2PDI-ZnP and 4PDI-ZnP acceptors, respectively, demonstrating higher electron mobility with higher number of PDI moieties (Fig. 3, Table 2). Unlike 2PDI-ZnP:PTB7-Th BHJ film with substantially imbalanced hole (6.37 × 10−5 cm2 V−1 s−1) and electron (1.34 × 10−4 cm2 V−1 s−1) mobilities, 4PDI-ZnP:PTB7-Th BHJ film exhibited reasonably balanced hole (3.43 × 10−4 cm2 V−1 s−1) and electron (2.18 × 10−4 cm2 V−1 s−1) mobilities, an attribute particularly beneficial for exciton dissociation.Experimental BHJ OSC devices with an active layer of PTB7-Th and 2PDI-ZnP or 4PDI-ZnP using a device configuration of ITO/ZnO/Zirconium acetylacetonate (ZrAcac)/PTB7-Th:acceptor/MoO3/Ag were characterized under AM 1.5 illumination (detailed device fabrication in ESI†). J–V curves and external quantum efficiency (EQE) spectra for optimized devices are presented in Fig. S5† (device parameters in Table 2). Optimized BHJ OSC devices with an active layer of PTB7-Th:2PDI-ZnP exhibited a moderate PCE of 5.53%, with VOC of 0.85 V, JSC of 12.72 mA cm−2 and FF of 51.4%. In sharp contrast, devices with PTB7-Th:4PDI-ZnP as active layer afforded higher PCE of 8.40%, with an improved VOC of 0.88 V, JSC of 14.85 mA cm−2 and FF of 64.3%. Measured EQEs of optimized devices showed broad spectral responses at 300–800 nm, consistent with the complementary absorption spectral properties of donor and acceptor in the active layer films (Fig. 2b). Furthermore, a high EQE of over 80% was observed for 4PDI-ZnP devices, as compared to an EQE value of about 78% for 2PDI-ZnP devices. Integration of the EQE spectra corresponds to JSC values of 12.22 and 14.51 mA cm−2 for the optimal devices of PTB7-Th:2PDI-ZnP and PTB7-Th:4PDI-ZnP, respectively, comparable to the JSC values measured in the J–V curves. Performance of the PBT7-Th:4PDI-ZnP device was also consistent with its higher shunt resistance (Rsh) of 944.1 Ω cm2 and lower series resistance (Rs) of 9.3 Ω cm2 (Table 2), which were closely related to JSC and FF and led to better device performance without significant charge recombination under both AM 1.5 illumination and weak light irradiation.
We further used PNDIT-F3N-Br instead of ZrAcac as electron transport layer (ETL) in BHJ OSCs device.14J–V curves for the optimal devices are presented in Fig. 4a (device parameters in Table 2). The optimal BHJ OSC devices with an active layer of PTB7-Th:2PDI-ZnP exhibited enhanced PCE of 6.38%, with VOC of 0.86 V, JSC of 13.10 mA cm−2 and FF of 56.6%. Devices with PTB7-Th:4PDI-ZnP as active layer also afforded an enhanced PCE of 9.64%, with an improved VOC of 0.90 V, JSC of 15.43 mA cm−2 and FF of 69.4%. Measured EQEs of optimized devices showed similar broad spectral responses in the 300–800 nm range (Fig. 4b). Integration of EQE spectra corresponds to JSC values of 12.87 and 15.12 mA cm−2 for optimal devices of PTB7-Th:2PDI-ZnP and PTB7-Th:4PDI-ZnP, respectively, comparable to the JSC values measured in J–V curves. Enhancement of the devices is mainly due to highly conductive PNDIT-F3N-Br as ETL with reduced ohmic loss for electron transport and extraction. Results are consistent with its increased shunt resistance (Rsh) of 1476.9 Ω cm2 and lower series resistance (Rs) of 7.9 Ω cm2 (Table 2). In addition, device stability was tested under heating at 100 °C. After 24 hours, the PCE retained around 75% and 51% of the initial values for the PTB7-Th:2PDI-ZnP and PTB7-Th:4PDI-ZnP devices (Fig. S6†), respectively.
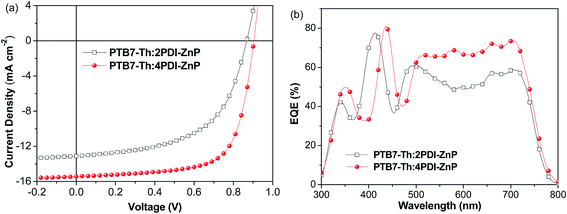 | ||
| Fig. 4 (a) J–V and (b) EQE of BHJ OSCs based on ITO/ZnO/PNDIT-F3N-Br/PTB7-Th:acceptor/MoO3/Ag device under AM 1.5 G illumination at 100 mW cm−2. | ||
Recently, Li and co-workers reported a series of “double-cable” conjugated polymers, which contain electron-donating poly(benzodithiophene) (BDT) as linear conjugated backbone for hole transport and pendant electron-deficient perylene bisimide (PBI) units for electron transport, and applied them in efficient single-component polymer solar cells with PCEs up to 4.18%.15 Herein, the two acceptors with disrupted conjugation between PDI moieties and porphyrin core were tested in single-component organic solar cells based on the inverted (ITO/ZnO/PNDIT-F3N-Br/acceptor/MoO3/Ag) or conventional configuration (ITO/PEDOT:PSS/acceptor/PFN/Al). J–V and EQE curves of optimized devices are presented in Fig. S7† (device parameters in Table S2†). Due to difficulty in controlling morphology of the small molecules, the devices showed poor performances with PCEs of 0.27% and 0.43% in inverted devices and 0.25% and 0.39% in conventional devices for 2PDI-ZnP and 4PDI-ZnP, respectively. Measured EQEs of devices clearly indicated significant light-harvesting contribution of the porphyrin core, typically in a range of 300–450 nm. We measured the CV of single N,N′-1-(tridecan-7-yl)-perylenebis(dicarboximide) (PDI) molecule and calculated its HOMO/LUMO energy levels to be −6.08/−3.80 eV, well aligned with the HOMO/LUMO (−5.06/−2.10 eV) reported for zinc(II) tetraphenylporphyrin (ZnTPP).16 Therefore, electron transfer from porphyrin core to PDI moieties is reasonable due to decoupling of PDI moieties from the porphyrin core. We believe by rationally designing the electron-withdrawing and electron-donating moieties, as well as the linkage between them, an optimized nanophase separation in single-component small molecules can be realized and therefore high-performance single-component BHJ OSCs can be achieved.
To study exciton dissociation and carrier collection processes, charge dissociation probability P(E, T), P(E, T) = Jph/Jsat, was investigated according to reported methods.17 Photocurrent density Jph was measured as follows: Jph = JL − JD, where JL and JD are current densities of the devices under illumination and dark, respectively, as a function of effective voltage Veff [(Veff = V0 − V), where V0 is the voltage at Jph = 0]. If Jph = Jsat at high reverse voltage (Veff > 2 V), all photogenerated excitons would be dissociated to free charge carriers and collected by electrodes, as recombination would be suppressed by high internal electric field.18 As shown in Fig. S8,† the Jph values of PTB7-Th:2PDI-ZnP and PTB7-Th:4PDI-ZnP devices showed substantial differences. PTB7-Th:4PDI-ZnP devices displayed a flat plateau at a region of relatively low Veff and did not saturate at high Veff region, even up to 2.5 V. The calculated charge dissociation probability P(E, T), estimated from the ratio Jph/Jsat, for the PTB7-Th:4PDI-ZnP devices was 91%, indicating efficient exciton dissociation. In contrast, PTB7-Th:2PDI-ZnP devices afforded a lower P(E, T) value of 82%, suggesting less effective exciton dissociation and serious charge recombination. Accordingly, a higher number of PDI units in the acceptor had the positive effects of promoting exciton dissociation and reducing charge recombination, thus contributing to enhanced photovoltaic performance.
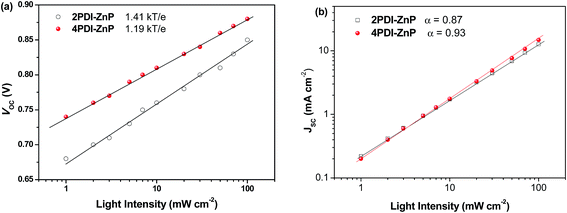
We investigated JSC as a function of illumination intensity in logarithmic scale to gain further insight into exciton recombination (Fig. 5). If the slope of the curve reaches 1, it implies that bimolecular recombination is not a crucial loss mechanism at the short-circuit point. In the case of PTB7-Th:2PDI-ZnP and PTB7-Th:4PDI-ZnP devices, the α values were estimated to be 0.87 and 0.93, respectively, indicating that PTB7-Th:4PDI-ZnP devices had weaker bimolecular recombination than PTB7-Th:2PDI-ZnP devices. The dependence of VOC on light intensity (Plight) can be used to describe the order of recombination processes in BHJ film. The semi-logarithmic plot of VOC depends linearly on light intensity with a slope of kT/q for bimolecular recombination. A slope greater than kT/q points toward trap-assisted recombination, where k, T, and q are the Boltzmann constant, temperature in kelvin, and the elementary charge.19 For our evaluation, the optimal devices of PTB7-Th:2PDI-ZnP and PTB7-Th:4PDI-ZnP were measured with slopes of 1.41kT/q and 1.19kT/q, respectively, demonstrating that reduced trap-assisted recombination was realized in PTB7-Th:4PDI-ZnP devices (Fig. 5a).
Morphological properties
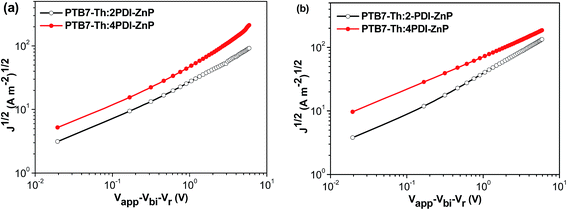
Structural order of PTB7-Th:acceptor BHJ films was investigated using grazing incidence wide-angle X-ray diffraction (GIWAXS) (Fig. 6a and b). PTB7-Th is a well-studied polymer that showed face-on orientation in both neat and BHJ thin films with well-recorded packing features. Scattering features of PTB7-Th:2PDI-ZnP BHJ film were dominated by the acceptor component. A sharp (100) peak was seen in out-of-plane direction at 0.27 Å−1 with crystal coherence length (CCL) of 21.6 nm. The packing orientation showed high edge-on preference, but a broad π–π stacking peak was also noted. In the in-plane direction, a sharp π–π stacking peak at 1.56 Å−1, with a CCL of 12.1 nm, and a relatively weak (100) peak were noted. The broad out-of-plane π–π stacking peak was attributed to both PTB7-Th and 2PDI-ZnP, which could not be fully disentangled. These results showed poor ordering of 2PDI-ZnP acceptor in the BHJ film, which led to poor charge carrier transport and photovoltaic power conversion efficiency.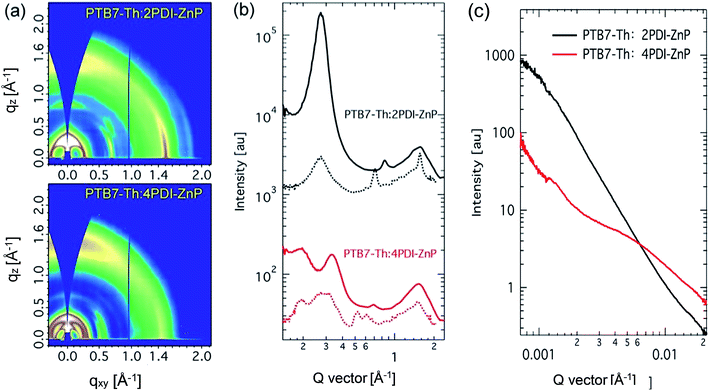 | ||
| Fig. 6 (a) Grazing incidence X-ray diffraction patterns, (b) line-cut profiles (solid line: out-of-plane, dotted line: in-plane) and (c) resonant soft X-ray scattering of BHJ films. | ||
PTB7-Th:4PDI-ZnP BHJ films showed quite different scattering features. The low q region showed multiple scattering peaks at 0.19 and 0.33 Å−1 in the in-plane direction coming from 4PDI-ZnP and (100) scattering at 0.27 Å−1 attributable to PTB7-Th, agreeing well with previous report. The out-of-plane direction showed packing features of 4PDI-ZnP, similar to those in the in-plane directions, with much lower scattering intensities compared to the 2PDI-ZnP:PTB7-Th composite film. No sharp π–π stacking was observed from 4PDI-ZnP in the in-plane direction, but a quite broad and intense peak in the out-of-plane direction at 1.53 Å−1 was noted. These results were in strong agreement with the suggestion that the twisted structure of 4PDI-ZnP effectively suppressed uncontrolled aggregation, which adversely impacted exciton dissociation and charge carrier transport efficiencies. Phase separation of BHJ films was further studied by resonant soft X-ray scattering (RSoXS) at 287 eV. As seen from scattering profiles (Fig. 6c), 2PDI-ZnP:PTB7-Th showed high scattering intensity in low q region with a steep decay line as a result of large phase separation or surface roughness, which deteriorated charge separation and collection, as well as device performance. This feature was seen in both off-edge and in-edge photon energies. 4PDI-ZnP:PTB7-Th showed low intensity low q scattering but a broad hump from 0.003–0.02 Å−1, indicating a broad length scale of phase separation at 30–200 nm. The small length-scale phase separation would be critical in driving charge separation and collection as well as promoting large internal surface area, leading to improved device current. Though less ordered, 4PDI-ZnP:PTB7-Th film with better π–π stacking orientation helped improve charge transport and collection and thus yielded a high FF.
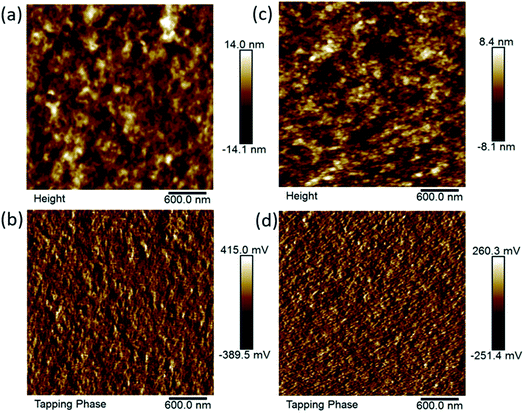
4PDI-ZnP/2PDI-ZnP:PTB7-Th BHJ films were also characterized using atomic force microscopy (AFM) (Fig. 7). Results showed that the number of PDI units in the acceptors had a definite effect on film morphology. Specifically, 4PDI-ZnP:PTB7-Th film exhibited more homogeneous morphology with a root-mean-squared (RMS) surface roughness of 2.4 versus 3.5 nm for 2PDI-ZnP:PTB7-Th film. In addition, topographic patterns for 2PDI-ZnP:PTB7-Th film displayed a rough nodular character, indicative of a significantly large extent of phase separation unfavorable for exciton diffusion20 and leading to lower JSC. In contrast, a lesser extent of phase separation and smoother surface morphology were observed for 4PDI-ZnP:PTB7-Th film, which explains the observed higher JSC and PCE arising from enhanced exciton diffusion/dissociation and charge carrier transport. Optimal crystalline domains and phase separation in the active layer are beneficial to charge carrier transport and for achieving high JSC, while uncontrolled aggregation lowers the JSC and PCE.21
Conclusions
We demonstrated that PDI-functionalized porphyrin derivatives are potentially good electron acceptor materials in combination with an appropriate polymer donor in BHJ OSCs for their high electron mobility and optimal light-harvesting. The number of PDI moieties in the acceptors has a profound effect on their photovoltaic performances as it impacts spectral absorptivity, molecular structural twisting, active layer morphology, exciton dissociation, carrier transport, etc., and thus PCE as a whole. Accordingly, 4PDI-ZnP acceptor, in conjunction with PBT7-Th donor, afforded an optimal PCE of 9.64%, which is a benchmark photovoltaic performance for PDI-porphyrin acceptor/PBT7-Th system. It should be noted that the porphyrin core not only acted as scaffold for PDI moieties, but also contributed to light-harvesting in the near-ultraviolet and violet regions, as was unambiguously demonstrated in single-component BHJ OSCs based on the two acceptors. We believe that with further process and solar cell structural optimization, higher PCEs could be achieved with these acceptors in combination with a more appropriate donor.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Hong Kong Research Grants Council (HKBU 22304115-ECS and C5015-15GF), Areas of Excellence Scheme ([AoE/P-03/08]), Hong Kong Baptist University (FRG1/15-16/052, FRG2/16-17/024) and The Science, Technology and Innovation Committee of Shenzhen Municipality (JCYJ20150630164505504) for support. TPR was supported by the U.S. Office of Naval Research under contract N00014-15-1-2244. Portions of this research were carried out at beamline 7.3.3 and 11.0.1.2 at the Advanced Light Source, and Molecular Foundry, Lawrence Berkeley National Laboratory, which was supported by the DOE, Office of Science, and Office of Basic Energy Sciences.References
- H. Kang, G. Kim, J. Kim, S. Kwon, H. Kim and K. Lee, Adv. Mater., 2016, 28, 7821–7861 CrossRef PubMed.
- X. X. Liu, H. J. Chen and S. T. Tan, Renewable Sustainable Energy Rev., 2015, 52, 1527–1538 CrossRef.
- (a) L. G. Xiao, S. Chen, K. Gao, X. B. Peng, F. Liu, Y. Cao, W. Y. Wong, W. K. Wong and X. J. Zhu, ACS Appl. Mater. Interfaces, 2016, 8, 30176–30183 CrossRef PubMed; (b) H. D. Wang, L. G. Xiao, L. Yan, S. Chen, X. J. Zhu, X. B. Peng, X. Z. Wang, W. K. Wong and W. Y. Wong, Chem. Sci., 2016, 7, 4301–4307 RSC; (c) J. Kesters, P. Verstappen, M. Kelchtermans, L. Lutsen, D. Vanderzande and W. Maes, Adv. Energy Mater., 2015, 5(13), 1500218 CrossRef; (d) L. Bucher, N. Desbois, P. D. Harvey, G. D. Sharma and C. P. Gros, Sol. RRL, 2017, 1(12), 1700127 CrossRef; (e) H. M. Qin, L. S. Li, F. Q. Guo, S. J. Su, J. B. Peng, Y. Cao and X. B. Peng, Energy Environ. Sci., 2014, 7, 1397–1401 RSC; (f) L. Bucher, L. Tanguy, D. Fortin, N. Desbois, P. D. Harvey, G. D. Sharma and C. P. Gros, ChemPlusChem, 2017, 82, 625–630 CrossRef; (g) S. Chen, L. Yan, L. G. Xiao, K. Gao, W. Tang, C. Wang, C. H. Zhu, X. Z. Wang, F. Liu, X. B. Peng, W. K. Wong and X. J. Zhu, J. Mater. Chem. A, 2017, 5, 25460–25468 RSC; (h) N. F. Montcada, S. Arrechea, A. Molina-Ontoria, A. I. Aljarilla, P. de la Cruz, L. Echegoyen, E. Palomares and F. Langa, Org. Electron., 2016, 38, 330–336 CrossRef.
- N. C. Nicolaidis, B. S. Routley, J. L. Holdsworth, W. J. Belcher, X. Zhou and P. C. Dastoor, J. Phys. Chem. C, 2011, 115, 7801–7805 CrossRef.
- (a) C. Q. Yan, S. Barlow, Z. H. Wang, H. Yan, A. K. Y. Jen, S. R. Marder and X. W. Zhan, Nat. Rev. Mater., 2018, 3, 18003 CrossRef; (b) C. B. Nielsen, E. Voroshazi, S. Holliday, K. Cnops, B. P. Rand and I. McCulloch, J. Mater. Chem. A, 2013, 1, 73–76 RSC.
- P. Cheng, G. Li, X. W. Zhan and Y. Yang, Nat. Photonics, 2018, 12, 131–142 CrossRef.
- S. Q. Zhang, Y. P. Qin, J. Zhu and J. H. Hou, Adv. Mater., 2018, 30, 1703973 CrossRef PubMed.
- (a) D. Sun, D. Meng, Y. H. Cai, B. B. Fan, Y. Li, W. Jiang, L. J. Huo, Y. M. Sun and Z. H. Wang, J. Am. Chem. Soc., 2015, 137, 11156–11162 CrossRef PubMed; (b) D. Meng, D. Sun, C. M. Zhong, T. Liu, B. B. Fan, L. J. Huo, Y. Li, W. S. Jiang, H. S. Choi, T. Kim, J. Y. Kim, Y. M. Sun, Z. H. Wang and A. J. Heeger, J. Am. Chem. Soc., 2016, 138, 375–380 CrossRef PubMed.
- Y. W. Duan, X. P. Xu, H. Yan, W. L. Wu, Z. J. Li and Q. Peng, Adv. Mater., 2017, 29(7), 1605115 CrossRef PubMed.
- J. Q. Zhang, Y. K. Li, J. C. Huang, H. W. Hu, G. Y. Zhang, T. X. Ma, P. C. Y. Chow, H. Ade, D. Pan and H. Yan, J. Am. Chem. Soc., 2017, 139, 16092–16095 CrossRef PubMed.
- A. D. Zhang, C. Li, F. Yang, J. Q. Zhang, Z. H. Wang, Z. X. Wei and W. W. Li, Angew. Chem., Int. Ed., 2017, 56, 2694–2698 CrossRef PubMed.
- Z. G. Wang, X. P. Xu, Z. J. Li, K. Feng, K. Li, Y. Li and Q. Peng, Adv. Electron. Mater., 2016, 2(6), 1600061 CrossRef.
- H. R. Lin, S. S. Chen, H. W. Hu, L. Zhang, T. X. Ma, J. Y. L. Lai, Z. K. Li, A. J. Qin, X. H. Huang, B. Z. Tang and H. Yan, Adv. Mater., 2016, 28, 8546–8551 CrossRef PubMed.
- Z. H. Wu, C. Sun, S. Dong, X. F. Jiang, S. P. Wu, H. B. Wu, H. L. Yip, F. Huang and Y. Cao, J. Am. Chem. Soc., 2016, 138, 2004–2013 CrossRef PubMed.
- G. T. Feng, J. Y. Li, F. J. M. Colberts, M. M. Li, J. Q. Zhang, F. Yang, Y. Z. Jin, F. L. Zhang, R. A. J. Janssen, C. Li and W. W. Li, J. Am. Chem. Soc., 2017, 139, 18647–18656 CrossRef PubMed.
- J. N. Clifford, E. Martinez-Ferrero, A. Viterisi and E. Palomares, Chem. Soc. Rev., 2011, 40, 1635–1646 RSC.
- Z. Li, J. D. A. Lin, H. Phan, A. Sharenko, C. M. Proctor, P. Zalar, Z. H. Chen, A. Facchetti and T. Q. Nguyen, Adv. Funct. Mater., 2014, 24, 6989–6998 CrossRef.
- Y. Z. Lin, Q. He, F. W. Zhao, L. J. Huo, J. Q. Mai, X. H. Lu, C. J. Su, T. F. Li, J. Y. Wang, J. S. Zhu, Y. M. Sun, C. R. Wang and X. W. Zhan, J. Am. Chem. Soc., 2016, 138, 2973–2976 CrossRef PubMed.
- (a) A. K. K. Kyaw, D. H. Wang, C. Luo, Y. Cao, T. Q. Nguyen, G. C. Bazan and A. J. Heeger, Adv. Energy Mater., 2014, 4(7), 1301469 CrossRef; (b) H. Azimi, A. Senes, M. C. Scharber, K. Hingerl and C. J. Brabec, Adv. Energy Mater., 2011, 1, 1162–1168 CrossRef.
- P. Cheng, L. Ye, X. G. Zhao, J. H. Hou, Y. F. Li and X. W. Zhan, Energy Environ. Sci., 2014, 7, 1351–1356 RSC.
- X. Zhang, Z. H. Lu, L. Ye, C. L. Zhan, J. H. Hou, S. Q. Zhang, B. Jiang, Y. Zhao, J. H. Huang, S. L. Zhang, Y. Liu, Q. Shi, Y. Q. Liu and J. N. Yao, Adv. Mater., 2013, 25(40), 5791–5797 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Method of device fabrication, synthesis and characterizations of porphyrin acceptors 2PDI-ZnP and 4PDI-ZnP. See DOI: 10.1039/c8se00427g |
| ‡ Q. Zhang, X. Xu and S. Chen contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |