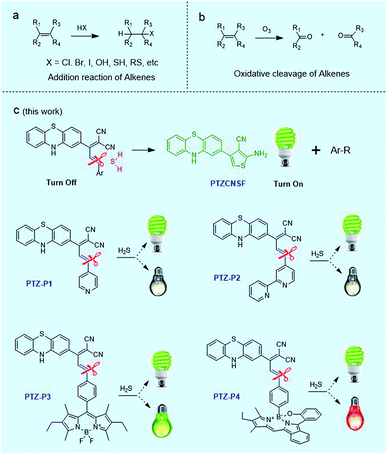
Open Access Article
Open Access ArticleReductive cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) C bonds as a new strategy for turn-on dual fluorescence in effective sensing of H2S†
C bonds as a new strategy for turn-on dual fluorescence in effective sensing of H2S†
Chunfei
Wang
a,
Xiaoxiang
Cheng
a,
Jingyun
Tan
a,
Zhaoyang
Ding
a,
Wenjing
Wang
 b,
Daqiang
Yuan
b,
Daqiang
Yuan
 b,
Gang
Li
a,
Hongjie
Zhang
*a and
Xuanjun
Zhang
b,
Gang
Li
a,
Hongjie
Zhang
*a and
Xuanjun
Zhang
 *a
*a
aFaculty of Health Sciences, University of Macau, Macau SAR, China. E-mail: hjzhang@umac.mo; xuanjunzhang@umac.mo
bState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, China
First published on 11th September 2018
Abstract
Reductive cleavage of alkenes is rarely reported in synthetic chemistry. Here we report a unique H2S-mediated reductive cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds under mild conditions, which is a successful new strategy for the design of probes for effective sensing of H2S with turn-on dual-color fluorescence. A short series of phenothiazine ethylidene malononitrile derivatives were shown to react with H2S, via reductive cleavage of C
C bonds under mild conditions, which is a successful new strategy for the design of probes for effective sensing of H2S with turn-on dual-color fluorescence. A short series of phenothiazine ethylidene malononitrile derivatives were shown to react with H2S, via reductive cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds with intramolecular cyclization reactions to form thiophene rings. Enlightened by this new reaction mechanism, four effective probes with turn-off to turn-on fluorescence switches were successfully applied for sensing H2S, an important gaseous signalling molecule in living systems, among which PTZ-P4 exhibited two fluorescent colors after reductive cleavage. The dual-color probe was applied for imaging endogenous H2S and showed distinct differences in brightness in living C. elegans for wild type N2, glp-1 (e2144) mutants (higher levels of endogenous H2S), and cth-1 (ok3319) mutants (lower levels of endogenous H2S). The discovery of H2S-mediated reductive cleavage of C
C bonds with intramolecular cyclization reactions to form thiophene rings. Enlightened by this new reaction mechanism, four effective probes with turn-off to turn-on fluorescence switches were successfully applied for sensing H2S, an important gaseous signalling molecule in living systems, among which PTZ-P4 exhibited two fluorescent colors after reductive cleavage. The dual-color probe was applied for imaging endogenous H2S and showed distinct differences in brightness in living C. elegans for wild type N2, glp-1 (e2144) mutants (higher levels of endogenous H2S), and cth-1 (ok3319) mutants (lower levels of endogenous H2S). The discovery of H2S-mediated reductive cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds is expected to be valuable for chemical synthesis, theoretical studies, and the design of new fluorescent H2S probes.
C bonds is expected to be valuable for chemical synthesis, theoretical studies, and the design of new fluorescent H2S probes.
Introduction
The discovery of novel reactivities with carbon–carbon double bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) is not only useful for chemical synthesis and theoretical studies, but is also important for applications in biology, because C
C) is not only useful for chemical synthesis and theoretical studies, but is also important for applications in biology, because C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds are abundant in nature. As we all know, C
C bonds are abundant in nature. As we all know, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds are fundamental structures of alkenes, where the C
C bonds are fundamental structures of alkenes, where the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C π bond is localized above and below the C–C σ bond wherein π electrons are relatively far away from the nuclei and are loosely bound, so that they can be easily attacked to construct a new bond.1,2 Indeed, addition reactions are one of the common reactions of C
C π bond is localized above and below the C–C σ bond wherein π electrons are relatively far away from the nuclei and are loosely bound, so that they can be easily attacked to construct a new bond.1,2 Indeed, addition reactions are one of the common reactions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, such as reactions with HX (X = Cl, Br, I, OH, SH, RS, etc.) as described in Scheme 1a.3 Additionally, C
C bonds, such as reactions with HX (X = Cl, Br, I, OH, SH, RS, etc.) as described in Scheme 1a.3 Additionally, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds can also be cleaved by oxidation as in the typical ozonolysis of alkenes (Scheme 1b).4 However, because of their unique structure, reductive cleavage of alkenes (Csp2–Csp2) has rarely been reported so far.
C bonds can also be cleaved by oxidation as in the typical ozonolysis of alkenes (Scheme 1b).4 However, because of their unique structure, reductive cleavage of alkenes (Csp2–Csp2) has rarely been reported so far.
In particular, the cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in styrenes has been activated by a hard Lewis acid and ethanethiol.5,6 In addition, Shi and coworkers reported reductive cleavage of Csp2–Csp3 bonds using rhodium based catalysis.7 In 2014, Bogdanov et al. reported that C
C bonds in styrenes has been activated by a hard Lewis acid and ethanethiol.5,6 In addition, Shi and coworkers reported reductive cleavage of Csp2–Csp3 bonds using rhodium based catalysis.7 In 2014, Bogdanov et al. reported that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in 1,10-disubstituted isoindigos could be reductively cleaved by aqueous hydrazine hydrate.8 In this work, we discovered an interesting H2S-mediated reductive cleavage of C
C bonds in 1,10-disubstituted isoindigos could be reductively cleaved by aqueous hydrazine hydrate.8 In this work, we discovered an interesting H2S-mediated reductive cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds under mild conditions.
C bonds under mild conditions.
As is well known, H2S with sulfur at the lowest valence is an excellent reductant and nucleophile, found predominantly as HS− at physiological pH and therefore displaying higher nucleophilicity compared with many other thiols in cells. Consequently, addition reactions have been a commonly used strategy for developing fluorescent H2S probes in recent years. Highlighting the popularity and influence of this reaction mechanism, many fluorescent probes were well designed with rapid and specific responses to H2S by disrupting the conjugated π-system of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds with addition reactions.9–13 In addition, aryl nitro groups could be cleaved by thiolysis, triggering the fluorescence turn-on for the detection of H2S or HnSn.14–17 In the current work, we report our finding of H2S-mediated reductive cleavage of C
C bonds with addition reactions.9–13 In addition, aryl nitro groups could be cleaved by thiolysis, triggering the fluorescence turn-on for the detection of H2S or HnSn.14–17 In the current work, we report our finding of H2S-mediated reductive cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds under mild conditions (Scheme 1c), anticipated to be a new strategy for devising fluorescent H2S probes. Concomitant with this has been the development of a dual-color fluorescent H2S probe, which was successfully applied to monitor endogenous H2S in vivo.
C bonds under mild conditions (Scheme 1c), anticipated to be a new strategy for devising fluorescent H2S probes. Concomitant with this has been the development of a dual-color fluorescent H2S probe, which was successfully applied to monitor endogenous H2S in vivo.
Results and discussion
Design, synthesis and characterizations
Phenothiazine (PTZ), having a non-planar butterfly conformation, provides strong fluorescence, and has been used as an electron donor in photoelectric materials with a variety of applications.18–22 We found that phenothiazine ethylidene malononitrile derivatives, PTZ-P1, PTZ-P2, PTZ-P3 and PTZ-P4, could effectively react with H2S via reductive cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds to yield a new fluorescent compound PTZCNSF.
C bonds to yield a new fluorescent compound PTZCNSF.
Firstly, we use PTZ-P1 as an example to discuss this novel reaction. PTZ is a strong electron donor whereas the dicyano group is a strong electron acceptor. The strong intramolecular charge transfer (ICT) in PTZ-P1 gave rise to fluorescence quenching. Upon reacting with H2S, PTZ-P1 exhibited turn-on fluorescence with high selectivity and sensitivity.
To dissect the reaction mechanism, PTZ-P1 was used to react with NaHS and the green fluorescent product, PTZCNSF, was successfully isolated. The NMR results of PTZCNSF (Fig. S25 and S26†) demonstrated the disappearance of the pyridine moiety and single crystal X-ray analysis was further used to confirm the structure. Single crystals of PTZ-P1 and PTZCNSF for X-ray diffraction were obtained by slow evaporation of dichloromethane solutions, and ORTEP drawings are depicted in Fig. 1. During the transformation from PTZ-P1 to PTZCNSF, C13, C14, C16 and C17 were connected by S2 from H2S via an intramolecular cyclization reaction to yield PTZCNSF. It is surprising that the C17–C18 double bond in PTZ-P1 was reductively cleaved and the pyridine moiety was cut off from the main structure, which was consistent with the NMR and MS analysis (Fig. 2a and b).
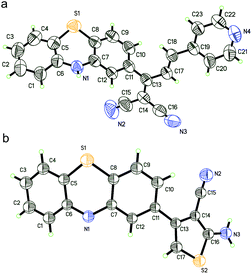 | ||
| Fig. 1 ORTEP diagrams of PTZ-P1 (a) and PTZCNSF (b) with ellipsoids adjusted to 50% probability. Solvent molecules were deleted for clarity. | ||
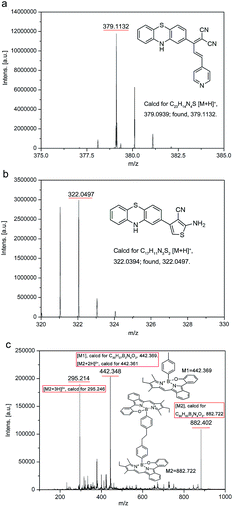 | ||
| Fig. 2 MS data of PTZ-P1, PTZCNSF, and the red emitting products of PTZ-P4 after the reaction with H2S. | ||
Three more derivatives, PTZ-P2, PTZ-P3, and PTZ-P4, were then designed and synthesized. For PTZ-P3, BODIPY was introduced for its good photostability, narrow emission, and high fluorescence quantum yield. In PTZ-P4, a red emitting fluorophore (BOBPY) was chosen to conjugate with phenothiazine ethylidene malononitrile. BOBPY and derivatives were first developed by Jiao and coworkers,23 and they are a kind of N2O-type benzopyrromethene boron complex, wherein axial positions are substituted by corresponding boronic acids. We chose BOBPY here because of its excellent stability and high fluorescence quantum yield in different media. We hypothesized that both green and red emitting fluorophores will be released after reductive cleavage, and these could be used as dual-color fluorescent probes for sensing H2S.
Detailed synthetic procedures are elucidated in the ESI.† Products and intermediates were fully characterized using 1H NMR, 13C NMR, HRMS and MALDI-TOF spectra (Fig. S8–S24, S27–S29†). All four derivatives could effectively react with H2S via reductive cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds to produce PTZCNSF. However, pure single samples of the other part were difficult to isolate, because of the high reactivity of the –CH2 moiety after reductive cleavage. Fortunately, the MALDI-TOF data of PTZ-P4 revealed that the –CH2 moiety could form R–CH3 monomers and dimers (Fig. 2c) under these reductive conditions. Although the exact reaction pathway is still under investigation, MS, NMR, and single-crystal X-ray analysis undoubtedly confirmed this H2S-mediated reductive cleavage of C
C bonds to produce PTZCNSF. However, pure single samples of the other part were difficult to isolate, because of the high reactivity of the –CH2 moiety after reductive cleavage. Fortunately, the MALDI-TOF data of PTZ-P4 revealed that the –CH2 moiety could form R–CH3 monomers and dimers (Fig. 2c) under these reductive conditions. Although the exact reaction pathway is still under investigation, MS, NMR, and single-crystal X-ray analysis undoubtedly confirmed this H2S-mediated reductive cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds.
C bonds.
Absorption and fluorescence response of probes to H2S
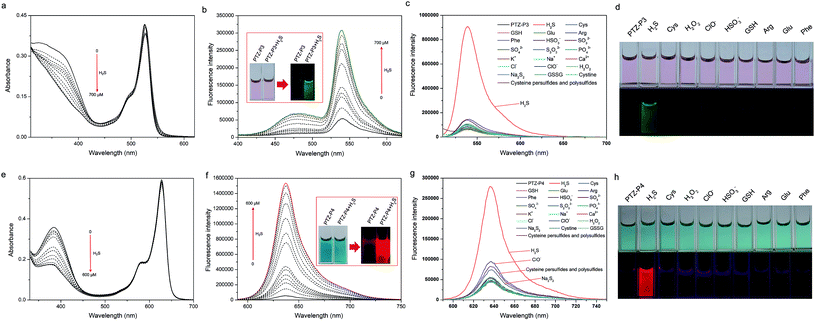
With these probes in hand, their responses to H2S were studied. Initially, the absorption spectra of PTZ-P1 upon addition of H2S were assessed in PBS buffer (pH = 7.4)/DMSO (1/2, 2% v/v PEG 400) using aqueous NaHS as the H2S source. As depicted in Fig. S5a†, PTZ-P1 showed a main absorption peak at 330 nm along with a weak-intensity ICT band at around 500 nm. The absorption intensity decreased upon the gradual addition of H2S (0–800 μM). However, PTZ-P1 showed a turn-on fluorescence response at 488 nm with a 25-fold enhancement (Fig. S5b†). The absorption and fluorescence of PTZ-P2 responding to H2S were similar to those of PTZ-P1 (Fig. S6†). However, the increase of the fluorescence intensity was lower (only a 5-fold increase), which may be attributed to the stronger electron withdrawing strength of the dipyridyl moiety compared with the pyridyl group.Subsequently, spectra titration experiments were performed to further investigate the response of PTZ-P3 towards H2S. The characteristic absorption peaks at 330 nm decreased upon gradual addition of H2S (0–700 μM), whereas the peaks at 525 nm did not change much (Fig. 3a). The decrease of the absorption peak at 330 nm revealed the reductive cleavage of PTZ-P3 induced by H2S, and was consistent with those of PTZ-P1 and PTZ-P2. As shown in Fig. 3b, very weak emission of PTZ-P3 (10 μM) was displayed in PBS buffer (pH = 7.4)/DMSO (1/2, 2% v/v PEG 400) without H2S because of the strong ICT, but the emissions at 480 nm and 540 nm were both remarkably increased upon gradual addition of H2S (0–700 μM). The enhanced emission at 480 nm was caused by PTZCNSF after reductive cleavage, which was consistent with those of PTZ-P1 and PTZ-P2. However, heightening emission at 540 nm was related to the BODIPY moiety being released from PTZ-P3.
In the presence of H2S, the ethylenic bond was reductively cleaved, so that optical properties of PTZ-P4 were observed both from PTZCNSF and BOBPY. Bearing this point in mind, the responses of absorption and fluorescence of PTZ-P4 to H2S were studied by gradual addition of NaHS solution into a PBS buffer (pH = 7.4)/DMSO (1/2, 2% v/v PEG 400) solution containing 10 μM probe. As illustrated in Fig. 3e, the absorption peaks of PTZ-P4 at 384 nm were exhibited as decreasing, while the absorption peaks at 628 nm displayed almost no change after gradually adding H2S (0–600 μM). As expected, the fluorescence spectra were consistent with PTZ-P1 in the green region, but the red fluorescence was so strong that the weak green fluorescence was covered with the insignificant ratiometric change. As shown in Fig. 3f, the emission intensity at 638 nm (λex = 580 nm) increased about 30-fold for PTZ-P4 upon addition of 600 μM H2S.
Selectivity of PTZ-P3 and PTZ-P4 to H2S
The selectivity of the PTZ-P3 and PTZ-P4 towards H2S was further identified. PTZ-P3 and PTZ-P4 (10 μM) were both treated respectively with various biologically relevant analytes in PBS buffer (pH = 7.4)/DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 2% v/v PEG 400) for 10 min. As shown in Fig. 3c, d, g and h, the turn-on fluorescent responses of PTZ-P3 and PTZ-P4 are highly selective for H2S versus biologically relevant thiols, reactive oxygen species (ROS) such as H2O2 and ClO−, ions including K+, Na+, Ca2+, HSO3−, SO32−, SO42− and S2O32−, and so on. Cysteine, cystine, glutathione, Na2S2, and cysteine persulfides and polysulfides24 induced very little increase of fluorescence intensity. Therefore, both PTZ-P3 and PTZ-P4 showed high selectivity for H2S.
2, 2% v/v PEG 400) for 10 min. As shown in Fig. 3c, d, g and h, the turn-on fluorescent responses of PTZ-P3 and PTZ-P4 are highly selective for H2S versus biologically relevant thiols, reactive oxygen species (ROS) such as H2O2 and ClO−, ions including K+, Na+, Ca2+, HSO3−, SO32−, SO42− and S2O32−, and so on. Cysteine, cystine, glutathione, Na2S2, and cysteine persulfides and polysulfides24 induced very little increase of fluorescence intensity. Therefore, both PTZ-P3 and PTZ-P4 showed high selectivity for H2S.
Imaging exogenous H2S in living cells
After developing these probes based on the novel reductive cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, we explored the applications of PTZ-P4 in monitoring H2S under physiological conditions. The cytotoxicity of PTZ-P4 was evaluated in HeLa cells using a MTT assay.
C bonds, we explored the applications of PTZ-P4 in monitoring H2S under physiological conditions. The cytotoxicity of PTZ-P4 was evaluated in HeLa cells using a MTT assay.
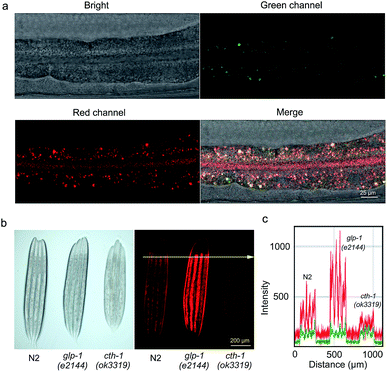
As described in Fig. S7†, PTZ-P4 exhibited relatively low toxicity towards HeLa cells with good viability. Putting PTZ-P4 into practice, exogenous H2S was detected in living HeLa cells. Firstly, HeLa cells were exposed to 20 μM PTZ-P4 for 5 h, then NaHS solution was used as the exogenous H2S source at 100 μM, incubating with cells for another 3 h. Compared with cells treated with only PTZ-P4, there was obvious green and red fluorescence in cells treated with both H2S and PTZ-P4 (Fig. 4).
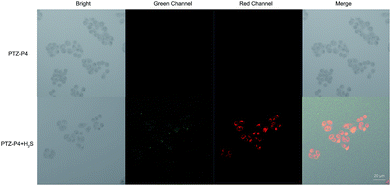 | ||
| Fig. 4 Confocal images of exogenous H2S in HeLa cells without (top) and with (bottom) 100 μM H2S in the presence of 20 μM PTZ-P4. Scale bar: 20 μm. | ||
Imaging endogenous H2S in living C. elegans
Endogenous H2S mainly originates from sulfur-containing amino acids metabolized by at least three enzymes: cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE) and 3-mercaptopyruvate sulfur transferase (3-MST).25C. elegans is an excellent in vivo model system for monitoring physiological H2S with clear molecular mechanisms of H2S action.26,27 Specifically, it has been shown that the germline-deficient glp-1 (e2144) mutants displayed increasing production of endogenous H2S, while the deletion mutation in cth-1, the gene encoding the H2S synthesizing enzyme cystathionine γ-lyase, resulted in down-regulated H2S levels.27–30 To further understand the features of PTZ-P4, in vivo imaging was employed to visualize endogenous H2S in C. elegans. Concentrations of PTZ-P4 and time periods used for feeding were titrated to ensure enough PTZ-P4 absorption and metabolism without significant biotoxicity (data not shown). In living C. elegans, PTZ-P4 should be absorbed, distributed, metabolized and excreted, and so 200 μM PTZ-P4 was used to make sure that there was enough PTZ-P4 in C. elegans for in vivo imaging. As a representative example shown in Fig. 5a, both green and red fluorescence were observed in wild-type N2 worms after being fed with 200 μM PTZ-P4 for 48 h. Additionally, H2S was mainly accumulated in intestinal cells, predominantly in the cytoplasm, and in apical membranes as well (Fig. 5a and data not shown).Consistent with changes of H2S levels in different strains, the red fluorescence intensity of glp-1 (e2144) mutants was obviously stronger than that in wild-type N2 worms, while cth-1 (ok3319) mutants exhibited notably weaker red fluorescence compared with wild-type N2 worms (Fig. 5b and c). This result suggested that red fluorescence signals could be clearly captured with significant changes and successfully reflected different H2S levels under different physiological conditions. Of note, differences in green fluorescence among different strains were also detected, albeit to a less appreciable level (Fig. 5c), due to the weak absorbance of PTZCNSF at 405 nm (the excitation wavelength of the fluorescence microscope). Altogether, these results provided compelling evidence that PTZ-P4 is highly sensitive and selective with dual fluorescent colors to detect endogenous H2S in living systems and can be used as a potential probe for in vivo imaging of endogenous H2S.
A significant bottleneck in detecting H2S is the effective imaging of endogenous H2S in vivo, a problem that restrains the biological applications. In the current study, the dual-color fluorescent probe PTZ-P4 showed high selectivity for H2S versus cysteine or glutathione. It also demonstrated a good response concurrently with the application in different strains of C. elegans, showing distinct differences in brightness for wild type N2, glp-1 (e2144) and cth-1 (ok3319) mutants.
Conclusions
In summary, we have discovered H2S-mediated reductive cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds under mild conditions, which was successful as a new strategy to design fluorescent probes for effective detecting of H2S. Of these probes, PTZ-P4 displayed dual-color turn-on fluorescence upon in vivo sensing of endogenous H2S in different strains of C. elegans, showing distinct differences in brightness for wild type N2, glp-1 (e2144) and cth-1 (ok3319) mutants. As far as we know, this is the first report that H2S can reductively cleave C
C bonds under mild conditions, which was successful as a new strategy to design fluorescent probes for effective detecting of H2S. Of these probes, PTZ-P4 displayed dual-color turn-on fluorescence upon in vivo sensing of endogenous H2S in different strains of C. elegans, showing distinct differences in brightness for wild type N2, glp-1 (e2144) and cth-1 (ok3319) mutants. As far as we know, this is the first report that H2S can reductively cleave C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds under mild conditions. As C
C bonds under mild conditions. As C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds are actively involved in various organic reactions, the discovery of this reductive cleavage of C
C bonds are actively involved in various organic reactions, the discovery of this reductive cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C is anticipated to be valuable not only for the development of C
C is anticipated to be valuable not only for the development of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in synthetic chemistry and theoretical studies, but also for the design of new fluorescent H2S probes for bioimaging and sensing applications.
C bonds in synthetic chemistry and theoretical studies, but also for the design of new fluorescent H2S probes for bioimaging and sensing applications.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Macao Science and Technology Development Fund under Grant No.: 052/2015/A2, 082/2016/A2, and 019/2017/AMJ to X.Z, and 060/2015/A2, 018/2017/AMJ; 050/2018/A2 to H.Z; the Research Grant of University of Macau No.: MYRG2016-00058-FHS and MYRG2017-00066-FHS to X.Z, MYRG2016-00066-FHS and MYRG2017-00082-FHS to H.Z. We thank the C. elegans Genetic Center, funded by NIH Office of Research Infrastructure Programs (P40 OD010440), for strains.Notes and references
- A. Chatupheeraphat, H. H. Liao, W. Srimontree, L. Guo, Y. Minenkov, A. Poater, L. Cavallo and M. Rueping, J. Am. Chem. Soc., 2018, 140, 3724–3735 CrossRef PubMed.
- R. R. Gu, K. Flidrova and J. M. Lehn, J. Am. Chem. Soc., 2018, 140, 5560–5568 CrossRef PubMed.
- M. Daini and M. Suginome, J. Am. Chem. Soc., 2011, 133, 4758–4761 CrossRef PubMed.
- J. Dey, A. C. O’Donoghue and R. A. M. O’Ferrall, J. Am. Chem. Soc., 2002, 124, 8561–8574 CrossRef PubMed.
- K. Fuji, T. Kawabata, M. Node and E. Fujita, Tetrahedron Lett., 1981, 22, 875–878 CrossRef.
- K. Fuji, T. Kawabata, M. Node and E. Fujita, J. Org. Chem., 1984, 49, 3214–3216 CrossRef.
- K. Chen, H. Li, Z. Q. Lei, Y. Li, W. H. Ye, L. S. Zhang, J. Sun and Z. J. Shi, Angew. Chem., Int. Ed., 2012, 51, 1–6 CrossRef.
- A. V. Bogdanov, A. V. Petrova, D. B. Krivolapov and V. F. Mironov, Tetrahedron Lett., 2014, 55, 6615–6618 CrossRef.
- V. S. Lin and C. J. Chang, Curr. Opin. Chem. Biol., 2012, 16, 595–601 CrossRef PubMed.
- Y. C. Chen, C. C. Zhu, Z. H. Yang, J. J. Chen, Y. F. He, Y. Jiao, W. J. He, L. Qiu, J. J. Cen and Z. J. Guo, Angew. Chem., Int. Ed., 2013, 125, 1732–1735 CrossRef.
- J. Liu, Y. Q. Sun, J. Y. Zhang, T. Yang, J. B. Cao, L. S. Zhang and W. Guo, Chem.–Eur. J., 2013, 19, 4717–4722 CrossRef PubMed.
- V. S. Lin, W. Chen, M. Xian and C. J. Chang, Chem. Soc. Rev., 2015, 44, 4596–4618 RSC.
- X. Feng, T. Zhang, J. T. Liu, J. Y. Miao and B. X. Zhao, Chem. Commun., 2016, 52, 3131–3134 RSC.
- R. Wang, F. B. Yu, L. X. Chen, H. Chen, L. J. Wang and W. W. Zhang, Chem. Commun., 2012, 48, 11757–11759 RSC.
- W. Chen, C. R. Liu, B. Peng, Y. Zhao, A. Pacheco and M. Xian, Chem. Sci., 2013, 4, 2892–2896 RSC.
- M. Gao, F. B. Yu, H. Chen and L. X. Chen, Anal. Chem., 2015, 87, 3631–3638 CrossRef PubMed.
- Y. Huang, F. B. Yu, J. C. Wang and L. X. Chen, Anal. Chem., 2016, 88, 4122–4129 CrossRef PubMed.
- R. Y. Lai, X. X. Kong, S. A. Jenekhe and A. J. Bard, J. Am. Chem. Soc., 2003, 125, 12631–12639 CrossRef PubMed.
- W. G. Yang, S. H. Yang, Q. R. Guo, T. Zhang, K. Y. Wu and Y. H. Hu, Sens. Actuators, B, 2015, 213, 404–408 CrossRef.
- K. M. Vengaian, C. D. Britto, K. Sekar, G. Sivaraman and S. Singaravadivel, Sens. Actuators, B, 2016, 235, 232–240 CrossRef.
- Z. S. Huang, H. Meier and D. R. Cao, J. Mater. Chem. C, 2016, 4, 2404–2426 RSC.
- M. Frank, J. Ahrens, I. Bejenke, M. Krick, D. Schwarzer and G. H. Clever, J. Am. Chem. Soc., 2016, 138, 8279–8287 CrossRef PubMed.
- N. Chen, W. J. Zhang, S. Chen, Q. H. Wu, C. J. Yu, Y. Wei, Y. K. Xu, E. H. Hao and L. J. Jiao, Org. Lett., 2017, 19, 2026–2029 CrossRef PubMed.
- S. Koike, S. Nishimoto and Y. Ogasawara, Redox Biol., 2017, 12, 530–539 CrossRef PubMed.
- J. Y. Zhang, Y. P. Ding, Z. Wang, Y. Kong, R. Gao and G. Chen, Med. Gas Res., 2017, 7, 113–119 CrossRef PubMed.
- D. L. Miller and M. B. Roth, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 20618–20622 CrossRef PubMed.
- Y. H. Wei and C. Kenyon, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 2832–2841 CrossRef PubMed.
- N. Arantes-Oliveira, J. Apfeld, A. Dillin and C. Kenyon, Science, 2002, 295, 502–505 CrossRef PubMed.
- J. R. Berman and C. Kenyon, Cell, 2006, 124, 1055–1068 CrossRef PubMed.
- B. Qabazard, L. Li, J. Gruber, M. T. Peh and L. F. Ng, Antioxid. Redox Signaling, 2014, 20, 2621–2630 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1846921 and 1846922. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc03430c |
| This journal is © The Royal Society of Chemistry 2018 |