Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly efficient FRET from aggregation-induced emission to BODIPY emission based on host–guest interaction for mimicking the light-harvesting system†
Shuai
Wang
a,
Jia-Hai
Ye
 *a,
Zhong
Han
b,
Zheng
Fan
a,
Caijiang
Wang
a,
Cancan
Mu
a,
Wenchao
Zhang
*a,
Zhong
Han
b,
Zheng
Fan
a,
Caijiang
Wang
a,
Cancan
Mu
a,
Wenchao
Zhang
 a and
Weijiang
He
a and
Weijiang
He
 *b
*b
aSchool of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, P. R. China. E-mail: yejiahai@njust.edu.cn; Fax: +86 25 8431 5857; Tel: +86 25 8430 3116
bState Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, P. R. China. E-mail: heweij69@nju.edu.cn; Fax: +86 25 8331 4502; Tel: +86 25 8359 7066
First published on 19th July 2017
Abstract
A novel fluorescence resonance energy transfer (FRET) system based on the interaction of an AIE luminogen (tetraphenylethene, TPE) and BODIPY derivative as the host and guest, respectively, was disclosed. This FRET system worked well for mimicking a light harvesting system with highly energy transfer efficiency up to 93% from aggregation-induced emission to BODIPY emission and shows excellent tolerance to acidic and basic environments.
Fluorescence resonance energy transfer (FRET) is a photophysical process through which an electronically excited fluorescent “donor” molecule transfers its excitation energy to an “acceptor” molecule nonradiatively through a long-range dipole–dipole interaction.1,2 FRET is widely studied and proved to be a powerful spectroscopic technique to accurately measure the distance between two fluorescent sources at the nanometer scale,3,4 enabling the study of molecular conformations5 and interaction dynamics.6 Moreover, FRET is a core phenomenon in photosynthesis,7,8 organic photovoltaics,9 lighting sources,10,11 biosensing,12–14 or drug delivery tracing.15 Furthermore, for practical applications, two requirements are necessary for the FRET process to occur: (1) a substantial overlap of the donor emission spectrum with the acceptor absorption; (2) an appropriate linker and distance between the donor and the acceptor.16
The light-harvesting system, which collects sunlight efficiently and funnels the excitation energy to the reaction center, which converting solar to chemical energy, plays a very important role in natural photosynthesis.17 Many artificial light-harvesting molecules are synthesized in succession.18 An ideal light-harvesting system absorbs light efficiently in a relatively broad region covering most of the sunlight spectrum and carries the energy smoothly to the reaction center. In order to achieve this for mimicking the light-harvesting system, the integration of multiple excellent chromophores or fluorophores is necessary based on FRET process. With the broad field of FRET applications at hand, the impressive progress of mimicking the photosynthetic light-harvesting system with synthetic models, which is significantly meaningful not only for our understanding of the function and mechanism of photosynthesis but also in the development of potential applications in terms of organic light-emitting diodes (OLED),19 dye-sensitized solar cells (DSSC),19a,20 and other optoelectronic devices,21 offers appealing opportunities to enhance FRET and extend its versatility and is currently attracting increasing interest from biologists and chemists.
So far, lots of covalent and non-covalent artificial light-harvesting systems based on FRET have been developed, most of which were constructed by fluorescent donor–acceptor models.22 Recently, with the rapid development of supramolecular chemistry, more and more efforts were contributed towards developing the non-covalent artificial light-harvesting systems, and among them crown ethers,23 cyclodextrins,24 pillararenes,25 and host–guest complexes containing multiple hydrogen bonds23,26 were frequently employed as building scaffolds.
It was well-known that most conventional luminophores such as the fluorescent donor and acceptor in host–guest systems with strong luminescence in solution become weakly emissive or nonemissive in the aggregate or solid state due to the notorious aggregation-caused quenching (ACQ),27 which has greatly limited the applications of luminescent materials. To overcome the drawback of ACQ, Tang et al. developed an effective methodology named aggregation-induced emission (AIE) which has drawn increasing research interest as the opposite of ACQ systems.27a,28 Because of their exactly opposite luminescent behaviours, AIE and ACQ materials are not easily coupled to construct new efficient luminescent materials. Recently, some attempts to combine AIE and ACQ luminophores by covalent bonding were made,29 but they failed in realising the advantages of combining both classes of dye. The development of a feasible approach for achieving the effective collaboration of AIE and ACQ luminophores and constructing highly efficient FRET-based light-harvesting materials for practical applications remains an important challenge.
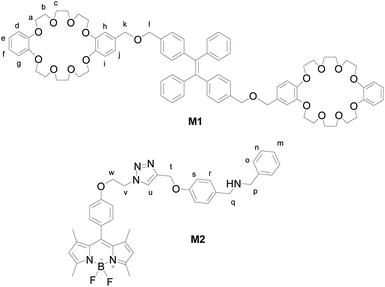
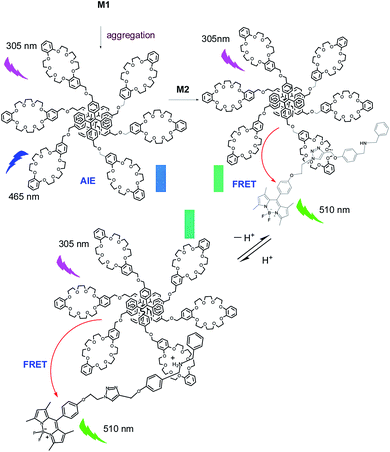
Herein, we introduce an efficient strategy to achieve the coupling of AIE and ACQ chromophores via FRET for the construction of high-efficiency light-harvesting system. As a prototypical AIE luminogen (AIEgen), tetraphenylethene (TPE) was chosen to combine with crown ether moieties as a host and energy donor in FRET system. Meanwhile, due to their outstanding and desirable properties such as high absorption coefficients, sharp emissions, high fluorescence quantum yields, and excellent chemical and photostability, boron-dipyrromethene (BODIPY) dyes were chosen as the chromophores in light-harvesting systems.25,30 We prepared a boron-dipyrromethene (BODIPY) derivative containing benzylamino group as the guest and energy acceptor (Scheme 1). The details of syntheses of the host and guest compounds can be found in the Fig. S1 and S2 (ESI†). The target host compound M1 was prepared from dimethyl TPE and dibenzo[24]-crown-8 (DB24C8). The target guest compound M2 was prepared from BODIPY derivative after several steps. The chemical structures of the target compounds were fully characterized by 1H NMR, 13C NMR, and MS (ESI†).
AIEgen TPE derivatives such as M1 becomes highly emissive in the aggregate state with the gradual increasing of the fraction of the poor solvent (Fig. S3, ESI†). But usually the traditional ACQ luminophores turn to be none or weakly fluorescence emissive under this condition. Numerous endeavors can be made to prevent or alleviate the ACQ problem, including the introduction of bulky substituents,31 enhanced intramolecular charge transfer (ICT) transition,32 cross-dipole packing,33 and J-aggregated formation.34 With the molecular structure of guest M2 in hand, we try to obtain the fluorescence behaviour of M2 in pure organic solvent (THF) and the solution with the increasing addition of the poor solvent. As expected, no remarkable emission intensity of M2 at 510 nm was observed upon the addition of water, even up to 90% fraction of water (Fig. S4(d), ESI†).
As shown in Fig. S4(e) (ESI†), the recording of the absorption and AIE emission spectra for guest M2 and host M1 verified them as the appreciable combinatorial duo to establish the FRET system, where the emission range of donor TPE (400–570 nm) almost coincided with the absorption range of acceptor BODIPY (430–530 nm). Furthermore, the maximum excitation wavelength of M2 was determined as 475 nm, which is consistent with AIE emission wavelength of M1, suggesting the occurrence of FRET.
In order to investigate the complexation of host with guest in solution, the fluorescence titration experiments were first performed to monitor the AIE emission at 465 nm corresponding to M1 and emission at 515 nm for M2 by adding increasing amount of guest M2 into the solution (THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, 2
H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v) of host M1. As shown as in Fig. 1 and S6 (ESI†), with gradual addition of M2, the AIE emission peak of M1 at 465 nm decreased gradually, while the emission peak of M2 at 510 nm appeared and then increased remarkably, indicating the formation of a new complex between M1 and M2. When more than 0.30 equiv. of M2 was added to the M1 solution, the fluorescence intensities at both 480 nm and 510 nm showed almost no change. Moreover, the change in fluorescence emission colour before and after the addition of the guest could be easily distinguished by the naked eye (Fig. S7, ESI†). The above results clearly demonstrated that an efficient FRET effect from donor M1 to acceptor M2 existed in the host–guest complex. From the fluorescence titration result, the FRET efficiency was further calculated to be 93% for the host–guest system. In order to get more evidence for interaction of host and guest, the TEM images were performed. As shown in Fig. 2, the nanoparticle of spherical morphology of host M1 in aggregation state turn to prismatic crystal after the addition of guest M2, which directly representing the host–guest recognition occurred and resulted in the formation of complex system to generate FRET.
8, v/v) of host M1. As shown as in Fig. 1 and S6 (ESI†), with gradual addition of M2, the AIE emission peak of M1 at 465 nm decreased gradually, while the emission peak of M2 at 510 nm appeared and then increased remarkably, indicating the formation of a new complex between M1 and M2. When more than 0.30 equiv. of M2 was added to the M1 solution, the fluorescence intensities at both 480 nm and 510 nm showed almost no change. Moreover, the change in fluorescence emission colour before and after the addition of the guest could be easily distinguished by the naked eye (Fig. S7, ESI†). The above results clearly demonstrated that an efficient FRET effect from donor M1 to acceptor M2 existed in the host–guest complex. From the fluorescence titration result, the FRET efficiency was further calculated to be 93% for the host–guest system. In order to get more evidence for interaction of host and guest, the TEM images were performed. As shown in Fig. 2, the nanoparticle of spherical morphology of host M1 in aggregation state turn to prismatic crystal after the addition of guest M2, which directly representing the host–guest recognition occurred and resulted in the formation of complex system to generate FRET.
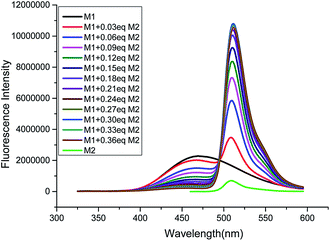 | ||
Fig. 1 Fluorescence titration spectra of compound M1 (10 μM) towards M2 (0, 0.03, 0.06, 0.09, 0.12, 0.15, 0.18, 0.21, 0.24, 0.27, 0.30, 0.33, 0.36 equiv.) in THF/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) medium (λex: 305 nm). 9, v/v) medium (λex: 305 nm). | ||
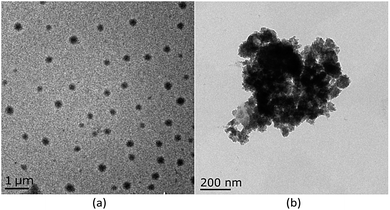 | ||
| Fig. 2 (a) Imagine of M1 (10 μM) in TEM at 1000 nm, (b) imagine of M1 (10 μM) upon addition of M2 (0.3 equiv.) in TEM at 200 nm. | ||
To gain further understanding the pH-responsibility of the observed FRET phenomenon, we monitored the fluorescence changes upon adding acid and base into the THF/water solution containing M1 and M2 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio. The pH-responsive titration was performed by the addition of CF3COOH and DBU to the host/guest complex solution gradually to adjust the pH value of the solution. To our surprise, no obvious changes of the fluorescence emission intensities of the FRET system under acid and base condition were observed. Moreover, the fluorescence emission intensity of the FRET upon the K+ addition also did not change remarkably. Furthermore, the absorbance of M1 and M2 in aggregation state was obtained (Fig. S4(b), ESI†). This FRET system absorbs light efficiently in broad region covering most of the sunlight spectrum (200–550 nm). The above results indicated that this complex of M1 and M2 was a highly efficient and pH stable FRET system for mimicking very stable light-harvesting system. Under acidic condition, the interaction of crown ether in host with dibenzylammonium salt in guest occurred. After the addition of base, the interaction of host and guest was changed to the interaction of crown ether with trizole unit and the distances between donor and acceptor chromophores or fluorophores changed slightly, so this complex of M1 and M2 shows pH stable FRET property.
1 mole ratio. The pH-responsive titration was performed by the addition of CF3COOH and DBU to the host/guest complex solution gradually to adjust the pH value of the solution. To our surprise, no obvious changes of the fluorescence emission intensities of the FRET system under acid and base condition were observed. Moreover, the fluorescence emission intensity of the FRET upon the K+ addition also did not change remarkably. Furthermore, the absorbance of M1 and M2 in aggregation state was obtained (Fig. S4(b), ESI†). This FRET system absorbs light efficiently in broad region covering most of the sunlight spectrum (200–550 nm). The above results indicated that this complex of M1 and M2 was a highly efficient and pH stable FRET system for mimicking very stable light-harvesting system. Under acidic condition, the interaction of crown ether in host with dibenzylammonium salt in guest occurred. After the addition of base, the interaction of host and guest was changed to the interaction of crown ether with trizole unit and the distances between donor and acceptor chromophores or fluorophores changed slightly, so this complex of M1 and M2 shows pH stable FRET property.
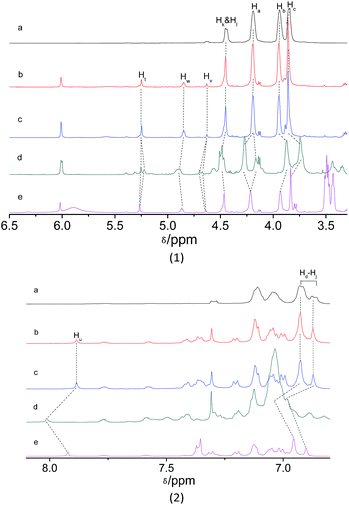
The host–guest interaction between donor and acceptor was investigated by 1H NMR to obtain important insights into the complexation behavior of M1 with M2 in aggregation state (Fig. 3). After the addition of acceptor M2 to donor M1, the slight changes in the chemical shifts of the protons Ha (from 4.190 to 4.192 ppm), Hb (from 3.941 to 3.945 ppm), Hc (from 3.842 to 3.860 ppm), and the benzyl protons Hk and Hl (from 4.452 to 4.453 ppm) in the crown ether units were observed by comparison of the 1H NMR spectra of M1 and the mixture. The multiplet and doublet peaks at 6.877–6.929 ppm corresponds to the aromatic protons Hd–Hj of crown ether units turn into sharp peaks. This result indicated the occurrence of host–guest interactions between donor M1 and acceptor M2, which agreed with the FRET behaviour of host–guest system. Furthermore, the 1H NMR titration under acid and base conditions was also carried out. Upon the addition of two equivalent of CF3COOH to the solution of M1 and M2, obvious changes in the chemical shift were observed. Upfield shifts were observed for cyclohexylmethylene protons Hb (from 3.945 to 3.873 ppm), and Hc (from 3.860 to 3.745 ppm), but the signal peak corresponding to proton Ha splits into two sets of signals, one upshift from 4.192 to 4.165 ppm while another downshift from 4.192 to 4.271. Downfield shifts were also observed for the benzyl protons Hk and Hl (from 4.453 to 4.485 ppm) in host M1. Moreover, downfield shifts was observed for the protons Hv (from 4.626 to 4.686 ppm) and Hw (from 4.848 to 4.893 ppm) in CH2CH2 unit of guest M2. Most important, the remarkable downfield shift was observed for the vinyl proton Hu (from 7.885 to 8.012 ppm) in trizole unit of guest M2. Subsequently, upon the addition of four equivalent of base DBU to the previous solution of M1 and M2, downfield shifts were observed for the protons Hb, Hc and Ht, meanwhile, upshifts were observed for the protons Hb, Hc, Hk, Hl, Hv, Hw, and Huetc. (Fig. S14, ESI†). Based on the combination of the results of FRET titration and 1H NMR titration, the plausible interaction mechanism of the host and guest was proposed as in Scheme 2. Under the acid condition, the recognition of crown ether to occurred. After the enough base addition, the crown ether switch from dibenzylammonium salt to chain unit near BODIPY core.
In conclusion, a new AIE fluorescence donor constructed by TPE with DB24C8 and a new fluorescence acceptor constructed by BODIPY with dibenzylamine linked with trizole were synthesized. The interaction between the donor and acceptor, which act as host and guest respectively, occurred in the poor solution (THF/H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) to generated highly efficient FRET from AIE emission to green emission of BODIPY. The fluorescence titration result shows that this novel FRET system is a pH-stable under acid and base conditions which will undoubtedly shed light on the practical application of FRET system based on host–guest interaction for mimicking the light harvesting system.
9, v/v) to generated highly efficient FRET from AIE emission to green emission of BODIPY. The fluorescence titration result shows that this novel FRET system is a pH-stable under acid and base conditions which will undoubtedly shed light on the practical application of FRET system based on host–guest interaction for mimicking the light harvesting system.
Acknowledgements
We thank the National Natural Science Foundation of China (No. 211571099) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) for financial support.Notes and references
- B. V. Meer, G. Coker and S. Chen, Resonance Energy Transfer: Theory and Data, VCH, New York, 1994 Search PubMed.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Plenum Publishing Corporation, New York, 2nd edn, 1999 Search PubMed.
- A. A. Deniz, M. Dahan, J. R. Grunwell, T. Ha, A. E. Faulhaber, D. S. Chemla, S. Weiss and P. G. Schultz, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 3670 CrossRef CAS.
- S. Weiss, Nat. Struct. Biol., 2000, 7, 724 CrossRef CAS.
- B. Schuler, E. A. Lipman and W. A. Eaton, Nature, 2002, 419, 743 CrossRef CAS.
- B. Schuler and W. A. Eaton, Curr. Opin. Struct. Biol., 2008, 18, 16 CrossRef CAS.
- W. Kühlbrandt and D. N. Wang, Nature, 1991, 350, 130 CrossRef.
- R. Hildner, D. Brinks, J. B. Nieder, R. J. Cogdell and N. F. van Hulst, Science, 2013, 340, 1448 CrossRef CAS.
- D. J. Farrell and N. J. Ekins-Daukes, Nat. Photonics, 2009, 3, 373 CrossRef CAS.
- M. A. Baldo, M. E. Thompson and S. R. Forrest, Nature, 2000, 403, 750 CrossRef CAS.
- A. Gopi, S. Lingamoorthy, S. Soman, K. Yoosaf, R. Haridas and S. Das, J. Phys. Chem. C, 2016, 120, 26569 CrossRef CAS.
- I. L. Medintz, A. R. Clapp, H. Mattoussi, E. R. Goldman, B. Fisher and J. M. Mauro, Nat. Mater., 2003, 2, 630 CrossRef CAS PubMed.
- X. Hu, Y. Li, T. Liu, G. Zhang and S. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 15551 CrossRef CAS.
- L. Yuan, W. Lin, K. Zheng and S. Zhu, Acc. Chem. Res., 2013, 46(7), 1462 CrossRef CAS.
- X. Han, D. Liu, T. Wang, H. Lu, J. Ma, Q. Chen and H. Gao, ACS Appl. Mater. Interfaces, 2015, 7, 23760 CrossRef CAS.
- C. G. dos Remedios and P. D. J. Moens, J. Struct. Biol., 1995, 115, 175 CrossRef CAS.
- X. Hu, A. Damjanovic, T. Ritz and K. Schulten, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 5935 CrossRef CAS.
- (a) S. Prathapan, T. E. Johnson and J. S. Lindsey, J. Am. Chem. Soc., 1993, 115, 7519 CrossRef CAS; (b) L. Flamigni, B. Ventura, C. C. You, C. Hippius and F. Würthner, J. Phys. Chem., 2007, 111, 622 CAS; (c) M. D. Yilmaz, A. Bozdemir and E. U. Akkaya, Org. Lett., 2006, 8, 2871 CrossRef CAS; (d) C. Hippius, F. Schlosser, M. O. Vysotsky, V. Böhmer and F. Würthner, J. Am. Chem. Soc., 2006, 128, 3870 CrossRef CAS.
- (a) S.-C. Lo and P. L. Burn, Chem. Rev., 2007, 107, 1097 CrossRef CAS; (b) G. M. Farinola and R. Ragni, Chem. Soc. Rev., 2011, 40, 3467 RSC; (c) Y. Tao, C. Yang and J. Qin, Chem. Soc. Rev., 2011, 40, 2943 RSC.
- (a) J.-K. Lee and M. Yang, Mater. Sci. Eng., B, 2011, 176, 1142 CrossRef CAS; (b) Y. Koyama, T. Miki, X.-F. Wang and H. Nagae, Int. J. Mol. Sci., 2009, 10, 4575 CrossRef CAS; (c) A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595 CrossRef CAS; (d) H. R. Li, A. Devaux, A. Z. Ruiz and G. Calzaferri, Nanophotonics, 2006, 6195, G1951 Search PubMed; (e) D. Maiti, R. Bhattacharjee, A. Datta and A. Banerjee, J. Phys. Chem. C, 2013, 117, 23178 CrossRef CAS.
- (a) K. Kikuchi, H. Takakusa and T. Nagano, TrAC, Trends Anal. Chem., 2004, 23, 407 CrossRef CAS; (b) D. Holten, D. F. Bocian and J. S. Lindsey, Acc. Chem. Res., 2001, 35, 57 CrossRef; (c) S. Saha and J. F. Stoddart, Chem. Soc. Rev., 2007, 36, 77 RSC; (d) V. Balzani, A. Credi and M. Venturi, Chem.–Eur. J., 2008, 14, 26 CrossRef CAS; (e) C. Chi, M. Chen, D. Liaw, H. Wu, Y. Huang and Y. Tai, ACS Appl. Mater. Interfaces, 2014, 6, 12119 CrossRef CAS.
- (a) F. D'Souza, P. M. Smith, M. E. Zandler, A. L. McCarty, M. Itou, Y. Araki and O. Ito, J. Am. Chem. Soc., 2004, 126, 7898 CrossRef; (b) E. Maligaspe, N. V. Tkachenko, N. K. Subbaiyan, R. Chitta, M. E. Zandler, H. Lemmetyinen and F. D'Souza, J. Phys. Chem. A, 2009, 113, 8478 CrossRef CAS; (c) O. A. Bozdemir, S. Erbas-Cakmak, O. O. Ekiz, A. Dana and E. U. Akkaya, Angew. Chem., Int. Ed., 2011, 50, 10907 CrossRef; (d) J. Y. Liu, Y. Huang, R. Menting, B. Roder, E. A. Ermilov and D. K. Ng, Chem. Commun., 2013, 49, 2998 RSC; (e) B. Rybtchinski, L. E. Sinks and M. R. Wasielewski, J. Am. Chem. Soc., 2004, 126, 12268 CrossRef CAS; (f) X. Zhang, Y. Xiao and X. Qian, Org. Lett., 2008, 10, 29 CrossRef CAS.
- G. Bottari, O. Trukhina, M. Ince and T. Torres, Coord. Chem. Rev., 2012, 256, 2453 CrossRef CAS.
- (a) Z. Y. Gu, D. S. Guo, M. Sun and Y. Liu, J. Org. Chem., 2010, 75, 3600 CrossRef CAS; (b) R. Menting, J. T. Lau, H. Xu, D. K. Ng, B. Roder and E. A. Ermilov, Chem. Commun., 2012, 48, 4597 RSC.
- L. Meng, D. Li, S. Xiong, X. Hu, L. Wang and G. Li, Chem. Commun., 2015, 51, 4643 RSC.
- (a) F. Loiseau, G. Marzanni, S. Quici, M. T. Indelli and S. Campagna, Chem. Commun., 2003, 286 RSC; (b) H. Q. Peng, J. F. Xu, Y. Z. Chen, L. Z. Wu, C. H. Tung and Q. Z. Yang, Chem. Commun., 2014, 50, 1334 RSC.
- (a) J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Tang, Chem. Rev., 2015, 115, 11718 CrossRef CAS; (b) J. B. Birks, Photophysics of Aromatic Molecules, Wiley, New York, 1970 Search PubMed.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740 RSC.
- (a) W. Z. Yuan, P. Lu, S. M. Chen, J. W. Y. Lam, Z. M. Wang, Y. Liu, H. S. Kwok, Y. G. Ma and B. Z. Tang, Adv. Mater., 2010, 22, 2159 CrossRef CAS PubMed; (b) Q. L. Zhao, X. A. Zhang, Q. Wei, J. Wang, X. Y. Shen, A. J. Qin, J. Z. Sun and B. Z. Tang, Chem. Commun., 2012, 48, 11671 RSC; (c) A. Ozawa, A. Shimizu, R. Nishiyabu and Y. Kubo, Chem. Commun., 2015, 51, 118 RSC; (d) G. Chen, W. B. Li, T. R. Zhou, Q. Peng, D. Zhai, H. X. Li, W. Z. Yuan, Y. M. Zhang and B. Z. Tang, Adv. Mater., 2015, 27, 4496 CrossRef CAS; (e) Q. Cui, Y. Yang, C. Yao, R. Liu and L. Li, ACS Appl. Mater. Interfaces, 2016, 8, 35578 CrossRef CAS; (f) A. Ozawa, A. Shimizu, R. Nishiyabu and Y. Kubo, Chem. Commun., 2015, 51, 118 RSC.
- (a) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184 CrossRef CAS; (b) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS.
- (a) A. Iida and S. Yamaguchi, Chem. Commun., 2009, 21, 3002 RSC; (b) H. Langhals, O. Krotz, K. Polborn and P. Mayer, Angew. Chem., Int. Ed., 2005, 44, 2427 CrossRef CAS; (c) T. Qin, G. Zhou, H. Scheiber, R. E. Bauer, M. Baumgarten, C. E. Anson, E. J. W. List and K. Müllen, Angew. Chem., Int. Ed., 2008, 47, 8292 CrossRef CAS; (d) X. Zhu, D. Gindre, N. Mercier, P. Frère and J. M. Nunzi, Adv. Mater., 2003, 15, 906 CrossRef CAS.
- (a) A. Wakamiya, K. Mori and S. Yamaguchi, Angew. Chem., Int. Ed., 2007, 46, 4273 CrossRef CAS; (b) C. H. Zhao, A. Wakamiya, Y. Inukai and S. Yamaguchi, J. Am. Chem. Soc., 2006, 128, 15934 CrossRef CAS.
- Z. Xie, B. Yang, F. Li, G. Cheng, L. Liu, G. Yang, H. Xu, L. Ye, M. Hanif, S. Liu, D. Ma and Y. Ma, J. Am. Chem. Soc., 2005, 127, 14152 CrossRef CAS.
- T. E. Kaiser, H. Wang, V. Stepanenko and F. Wurthner, Angew. Chem., Int. Ed., 2007, 46, 5541 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra05925f |
| This journal is © The Royal Society of Chemistry 2017 |