Molecular recognition of Ca2+ cations by internal and external receptors/interfaces in a spherical porous molybdenum-oxide capsule: unusual coordination scenarios†
Mirta
Rubčić
a,
Vladimir S.
Korenev
b,
Liviu
Toma
a,
Hartmut
Bögge
a,
Vladimir P.
Fedin
b and
Achim
Müller
*a
aFakultät für Chemie, Universität Bielefeld, Postfach 100131, D-33501 Bielefeld, Germany. E-mail: a.mueller@uni-bielefeld.de; Fax: +49 521 106 6003; Tel: +49 521 106 6153
bNikolaev Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, Novosibirsk 630090, Russia
First published on 29th September 2014
Abstract
The unique molybdenum-oxide capsule [{(MoVI)MoVI5O21(H2O)6}12{MoV2O4(SO4)}30]72− shows an unprecedented affinity towards Ca2+ cations which interact in fully or partially hydrated forms with different well-defined internal and external receptor areas via direct coordination and/or hydrogen bonds. The cavity of the capsule comprises an unusual “water assembly” of the Ca20{H2O}60![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ca20{(H2O)5}12 type. Altogether, we can refer to a unique solid-state structure.
Ca20{(H2O)5}12 type. Altogether, we can refer to a unique solid-state structure.
The design of non-natural systems that can mimic some of the properties of their biological analogues has been posed as a fundamental challenge for modern chemistry while it is of interdisciplinary interest, too.1–3 This is the case, e.g. for a capsule having tunable and gateable pores that can function similarly to ion channels,4,5 thus allowing the exchange of cations/anions between the interior and the exterior in a controlled and selective manner. A remarkable example in this context is provided by spherical porous molybdenum-oxide based nanocapsules of the Keplerate type (pentagon)12(linker)30
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) [{(Mo)Mo5}12{Mo2}30]
[{(Mo)Mo5}12{Mo2}30]![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) {Mo132}.6,7 A number of studies on the sulphate-type species [{(MoVI)MoVI5O21(H2O)6}12{MoV2O4(SO4)}30]72−1a8 revealed that in aqueous solution the capsules have the remarkable ability to distinguish also between different cations, depending on the sizes and the labilities of the M–OH2 bonds of the related aqua ions;7a–f this is based on the option for specific binding sites at their internal and/or external interface.7a–f,8b,9 Importantly, the interaction of the capsule with cations can be nicely investigated via NMR studies, which demonstrate that the capsule acts as a semipermeable membrane allowing “trafficking” through its channels/pores especially for water molecules and small cations (and anions) like e.g. Li+.7c–f In attempting to further explore the unusual behaviour of this system, we here refer to the biologically relevant Ca2+ cations4b,10 which react specifically with different well-defined areas of the spherical nanosized capsule 1a while forming coordination polyhedra at the cavity/pore/channel interfaces, above the pores and at the external surface positions.
{Mo132}.6,7 A number of studies on the sulphate-type species [{(MoVI)MoVI5O21(H2O)6}12{MoV2O4(SO4)}30]72−1a8 revealed that in aqueous solution the capsules have the remarkable ability to distinguish also between different cations, depending on the sizes and the labilities of the M–OH2 bonds of the related aqua ions;7a–f this is based on the option for specific binding sites at their internal and/or external interface.7a–f,8b,9 Importantly, the interaction of the capsule with cations can be nicely investigated via NMR studies, which demonstrate that the capsule acts as a semipermeable membrane allowing “trafficking” through its channels/pores especially for water molecules and small cations (and anions) like e.g. Li+.7c–f In attempting to further explore the unusual behaviour of this system, we here refer to the biologically relevant Ca2+ cations4b,10 which react specifically with different well-defined areas of the spherical nanosized capsule 1a while forming coordination polyhedra at the cavity/pore/channel interfaces, above the pores and at the external surface positions.
The reaction of calcium chloride with an aqueous solution of 18 results in the formation of compound 2 which was characterized by elemental analysis,‡ spectroscopic methods (IR, Raman),‡ and single-crystal X-ray structure analysis.§ The compound is highly soluble in water and much less soluble in ethanol.
(NH4)72−n[(H2O)81−n + (NH4)n ⊂ {(MoVI)MoVI5O21(H2O)6}12{MoV2O4(SO4)}30]·ca. 200 H2O![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) (NH4)72−n·1a·ca. 200 H2O
(NH4)72−n·1a·ca. 200 H2O![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) 18
18
Ca16[(Ca20{H2O}60) ⊂ {(MoVI)MoVI5O21(H2O)6}12{MoV2O4(SO4)}30]·ca. [300 H2O + 8 NH4Cl + 7 CaCl2]![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ca16·2a·ca. [300 H2O + 8 NH4Cl + 7 CaCl2]
Ca16·2a·ca. [300 H2O + 8 NH4Cl + 7 CaCl2]![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) 2
2
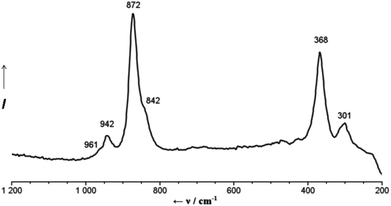
The first easy identification of the {Mo132}-skeleton in 2a is provided by the very characteristic simple Raman spectrum of the aqueous solution of 2, which consists of only a few bands in agreement with the high icosahedral symmetry of the capsule (Fig. 1). The most intense band, observed at 872 cm−1, is associated with the symmetrical breathing vibration (Ag type if considering the icosahedral symmetry of the skeleton) of the μ3-O atoms.11 The IR spectrum of 2 shows – in addition to a characteristic pattern arising from the vibrations of the molybdenum-oxide {Mo132}-skeleton12 and the expected bands due to the presence of NH4+ ions, H2O ligands and lattice water – three bands at 1065, 1150 and 1184 cm−1 which are associated with the SO42− ligands (ESI, Fig. S1†).13 The significant shift of the latter, in comparison with the IR spectrum of 1, clearly supports the involvement of SO42− ligands in the coordination of Ca2+ cations as also established by X-ray crystal structure analysis.
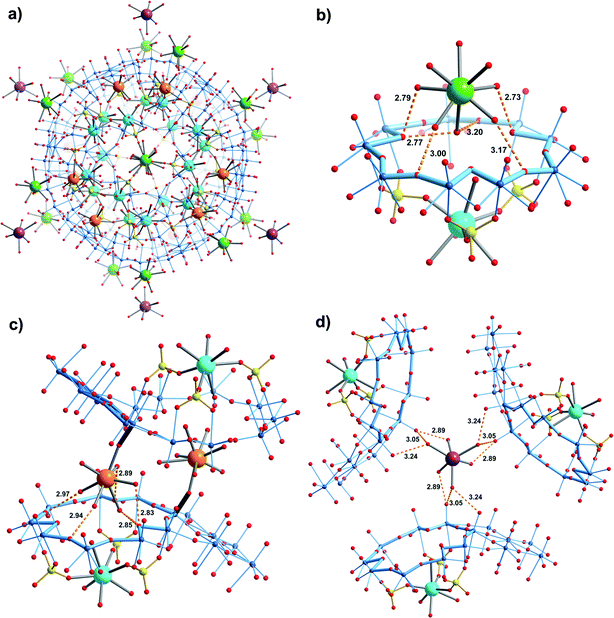
Compound 2 crystallizes in the space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) . The structure is unique regarding the unit cell, which is larger than usually observed for the {Mo132}-type compounds, but especially with respect to the diversity of Ca2+ binding sites (four types) and the related coordination environments (Fig. 2). 20 Ca2+ cations are found inside the capsule, below the 20 {Mo9O9} pores (first type), while they are coordinated to O atoms of three sulphate ligands as well as by three water ligands belonging to the internal water assembly (Fig. 2b). Additionally, the upper part of the pore/channel hosts a water molecule with full or partial occupancy in relation to the disorder of the sulphate ligands (for details see ESI†). Consequently, the coordination number of the confined Ca2+ cations is either six or seven depending on whether a pore/channel water molecule enters their coordination spheres or not (see, e.g. ESI, Fig. S2†).
. The structure is unique regarding the unit cell, which is larger than usually observed for the {Mo132}-type compounds, but especially with respect to the diversity of Ca2+ binding sites (four types) and the related coordination environments (Fig. 2). 20 Ca2+ cations are found inside the capsule, below the 20 {Mo9O9} pores (first type), while they are coordinated to O atoms of three sulphate ligands as well as by three water ligands belonging to the internal water assembly (Fig. 2b). Additionally, the upper part of the pore/channel hosts a water molecule with full or partial occupancy in relation to the disorder of the sulphate ligands (for details see ESI†). Consequently, the coordination number of the confined Ca2+ cations is either six or seven depending on whether a pore/channel water molecule enters their coordination spheres or not (see, e.g. ESI, Fig. S2†).
In contrast to the confined ones, Ca2+ cations of the second type interact in hydrated form via hydrogen bonding with the oxygen atoms of the {Mo9O9} pores while attaining six-, seven- or octa-coordination (Fig. 2b). As they occupy well-defined positions above the pores (this scenario refers to 14 of 20 {Mo9O9} pores per capsule) it allows us to refer to an interesting case of sphere-surface supramolecular chemistry (see also ref. 7a). Important to note is that the Ca2+ cations of the second type lie above those of the first type, at distances of 7.04–7.25 Å (Fig. 2b).
Ca2+ cations of the third type interact on the one hand with terminal skeletal oxo ligands belonging to the pentagonal units of the {Mo132} capsule, while on the other hand their water ligands are involved in hydrogen bonding to the oxygen atoms of a {Mo9O9} pore of a neighbouring capsule (Fig. 2c), similar to the cations of the second type (Fig. 2b). Consequently they lie above the confined Ca2+cations at distances of 6.68–6.89 Å; altogether 6 Ca2+ cations of this type are found per capsule. Interestingly, the X-ray structure analysis revealed a fourth type of hydrated Ca2+ cation in the large voids between the capsules while interacting with the related skeletal O atoms, via hydrogen bonding (Fig. 2d).
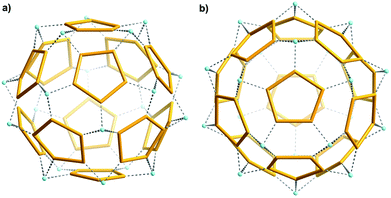
A very interesting result is that Ca2+ cations of the first type serve as “nods”, and mediate the formation of a remarkable new type of “water assembly” of the Ca20{H2O}60![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Ca20{(H2O)5}12 type inside the cluster's cavity (Fig. 3; see also ESI, Fig. S2†).13 Here one finds the relevant numbers 5 and 12 which play an important role in the history of culture and science; in particular twelve pentagons are characteristic for important spherical polyhedral objects (for a mathematical treatment see ref. 14). Spherical-object tilings were important for understanding virus structures in connection with Buckminster Fuller's geodesic-dome constructions,15 the C60 fullerene structure15,16 while they even fascinated the artist M. C. Escher.17
Ca20{(H2O)5}12 type inside the cluster's cavity (Fig. 3; see also ESI, Fig. S2†).13 Here one finds the relevant numbers 5 and 12 which play an important role in the history of culture and science; in particular twelve pentagons are characteristic for important spherical polyhedral objects (for a mathematical treatment see ref. 14). Spherical-object tilings were important for understanding virus structures in connection with Buckminster Fuller's geodesic-dome constructions,15 the C60 fullerene structure15,16 while they even fascinated the artist M. C. Escher.17
It should be noted that the structure of the cluster 2a substantially differs from the previously studied formamidinium and protonated urea “plugged” {Mo132}-sulphate type capsules comprising confined Ca2+ cations (see ref. 9b and f, respectively). In the latter two, the presence of protonated urea/formamidinium cations in the pore/ring area reduces appreciably the affinity of the {Mo132}-sulphate cluster for Ca2+ uptake, presumably due to a decrease of the negative cluster's charge (this also leads to lower solubility and is also the reason why the addition of Ca2+ ions to the solution leads to precipitation, which was not mentioned in ref. 9f) and steric influence, resulting only in Ca2+ confinement/coordination of noticeably smaller numbers of the cations than established here (8 and 6, respectively, vs. 20 in 2a).
Moreover, the protonated urea/formamidinium plugs in the pore/ring area will exclude the presence of pore/channel water (ESI†), consequently influencing the coordination geometry of the confined Ca2+ cations, being exclusively octahedral in the case of “plugged” capsules, while here it is either six- or seven-fold. Interestingly however, encapsulated assemblies of the type Can{H2O}60![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Can{(H2O)5}12 were also observed in the case of the “plugged” analogues with a smaller number of “nods”, i.e. Ca2+ ions (n = 69b or 89f).
Can{(H2O)5}12 were also observed in the case of the “plugged” analogues with a smaller number of “nods”, i.e. Ca2+ ions (n = 69b or 89f).
In summary, the spherical nano-capsule/artificial cell [{(MoVI)MoVI5O21(H2O)6}12{MoV2O4(SO4)}30]72− with well-defined active pores and interior functionalities shows an exceptional affinity towards Ca2+ ions, as demonstrated by the variety of coordination features in the present compound 2. This is based on the specific recognition of Ca2+ ions present in the surrounding environment by well-defined internal and external capsule areas, namely the internal SO42− receptors, the O atoms of the {Mo9O9} pores and the terminal O atoms of the pentagonal units. As the final point, it should be noted that the high solubility of the title compound in water offers new possibilities for studying coordination chemistry under confined conditions.
Acknowledgements
We all thank Prof. P. Gouzerh (Paris) for useful comments. A. M. thanks the ERC (Brussels) for an Advanced Grant. V. S. K. and V. P. F. thank the Alexander von Humboldt Foundation (Germany) for financial assistance during their research stay in the University of Bielefeld.Notes and references
-
(a) J. W. Szostak, D. P. Bartel and P. L. Luisi, Nature, 2001, 409, 387–390 CrossRef CAS PubMed
; (b) A. Pohorille and D. Deamer, Trends Biotechnol., 2002, 20, 123–128 CrossRef CAS PubMed
; (c) K. Kinbara and T. Aida, Science, 2006, 313, 51–52 CrossRef CAS PubMed
; (d) S. Mann, Acc. Chem. Res., 2012, 45, 2131–2141 CrossRef CAS PubMed
.
-
(a) G. J. T. Cooper, P. J. Kitson, R. Winter, M. Zagnoni, D.-L. Long and L. Cronin, Angew. Chem., Int. Ed., 2011, 50, 10373–10376 CrossRef CAS PubMed
; (b) M. Li, R. L. Harbron, J. V. M. Weaver, B. P. Binks and S. Mann, Nat. Chem., 2013, 5, 529–536 CrossRef CAS PubMed
; (c) I. Budin and N. K. Devaraj, J. Am. Chem. Soc., 2012, 134, 751–753 CrossRef CAS PubMed
.
-
J. M. Berg, J. L. Tymoczko and L. Stryer, Biochemistry, W. H. Freeman, New York, 5th edn, 2002 Search PubMed
.
-
(a)
B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts and P. Walter, Molecular Biology of the Cell, Garland Science, New York, 4th edn, 2002 Search PubMed
; (b) Y. Kobuke and S.-I. Kugimiya, in Encyclopedia of Supramolecular Chemistry, ed. J. L. Atwood and J. W. Steed, CRC Press, Taylor & Francis Group, Boca Raton, 2004, vol. 1, pp. 1–11 Search PubMed
; (c) R. MacKinnon, Angew. Chem., Int. Ed., 2004, 43, 4265–4277 CrossRef CAS PubMed
.
- For examples of artificial ion channels see:
(a) I. Tabushi, Y. Kuroda and K. Yokota, Tetrahedron Lett., 1982, 23, 4601–4604 CrossRef CAS
; (b) L. Lien, D. C. J. Jaikaran, Z. Zhang and G. A. Woolley, J. Am. Chem. Soc., 1996, 118, 12222–12223 CrossRef CAS
; (c) V. Percec, A. E. Dulcey, V. S. K. Balagurusamy, Y. Miura, J. Smidrkal, M. Peterca, S. Nummelin, U. Edlund, S. D. Hudson, P. A. Heiney, H. Duan, S. N. Magonov and S. A. Vinogradov, Nature, 2004, 430, 764–768 CrossRef CAS PubMed
; (d) S. Bhosale, A. L. Sisson, P. Talukdar, A. Fürstenberg, N. Banerji, E. Vauthey, G. Bollot, J. Mareda, C. Röger, F. Würthner, N. Sakai and S. Matile, Science, 2006, 313, 84–86 CrossRef CAS PubMed
.
-
(a) A. Müller, E. Krickemeyer, H. Bögge, M. Schmidtmann and F. Peters, Angew. Chem., Int. Ed., 1998, 37, 3360–3363 Search PubMed
; (b) P. Gouzerh and M. Che, L'actual. Chim., 2006, 298, 9–22 CAS
.
-
(a) A. Müller and P. Gouzerh, Chem. Soc. Rev., 2012, 41, 7431–7463 RSC
; (b) A. Müller, H. Bögge and M. Henry, C. R. Chim., 2005, 8, 47–56 CrossRef
; (c) A. Müller, D. Rehder, E. T. K. Haupt, A. Merca, H. Bögge, M. Schmidtmann and G. Heinze-Brückner, Angew. Chem., Int. Ed., 2004, 43, 4466–4470 CrossRef PubMed
; corrigendum: A. Müller, D. Rehder, E. T. K. Haupt, A. Merca, H. Bögge, M. Schmidtmann and G. Heinze-Brückner, Angew. Chem., Int. Ed., 2004, 43, 5115 Search PubMed
; (d) E. T. K. Haupt, C. Wontorra, D. Rehder and A. Müller, Chem. Commun., 2005, 3912–3914 RSC
; (e) D. Rehder, E. T. K. Haupt and A. Müller, Magn. Reson. Chem., 2008, 46, S24–S29 CrossRef PubMed
; (f) D. Rehder, E. T. K. Haupt, H. Bögge and A. Müller, Chem. – Asian J., 2006, 1–2, 76–81 CrossRef PubMed
; (g) R. Carr, I. A. Weinstock, A. Sivaprasadarao, A. Müller and A. Aksimentiev, Nano Lett., 2008, 8, 3916–3921 CrossRef CAS PubMed
; see also (h) D.-L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736–1758 CrossRef CAS PubMed
.
-
(a) A. Müller, E. Krickemeyer, H. Bögge, M. Schmidtmann, B. Botar and M. O. Talismanova, Angew. Chem., Int. Ed., 2003, 42, 2085–2090 CrossRef PubMed
; (b) A. Müller, Y. Zhou, H. Bögge, M. Schmidtmann, T. Mitra, E. T. K. Haupt and A. Berkle, Angew. Chem., Int. Ed., 2006, 45, 460–465 CrossRef PubMed
.
-
(a) A. Müller, S. K. Das, S. Talismanov, S. Roy, E. Beckmann, H. Bögge, M. Schmidtmann, A. Merca, A. Berkle, L. Allouche, Y. Zhou and L. Zhang, Angew. Chem., Int. Ed., 2003, 42, 5039–5044 CrossRef PubMed
; (b) A. Merca, E. T. K. Haupt, T. Mitra, H. Bögge, D. Rehder and A. Müller, Chem. – Eur. J., 2007, 13, 7650–7658 CrossRef CAS PubMed
; (c) A. Merca, H. Bögge, M. Schmidtmann, Y. Zhou, E. T. K. Haupt, M. K. Sarker, C. L. Hill and A. Müller, Chem. Commun., 2008, 948–950 RSC
; (d) A. Müller, Y. Zhou, L. Zhang, H. Bögge, M. Schmidtmann, M. Dressel and J. van Slageren, Chem. Commun., 2004, 2038–2039 RSC
; (e) L. Zhang, Y. Zhou and R. Han, Eur. J. Inorg. Chem., 2010, 2471–2475 CrossRef CAS
; (f) A. Müller, L. Toma, H. Bögge, C. Schäffer and A. Stammler, Angew. Chem., Int. Ed., 2005, 44, 7757–7761 CrossRef PubMed
.
-
(a) Y. Zhou, S. Xue and J. J. Yang, Metallomics, 2013, 5, 29–42 RSC
; (b) R. J. P. Williams, Biochim. Biophys. Acta, 2006, 1763, 1139–1146 CrossRef CAS PubMed
; (c) R. M. Case, D. Eisner, A. Gurney, O. Jones, S. Muallem and A. Verkhratsky, Cell Calcium, 2007, 42, 345–350 CrossRef CAS PubMed
.
-
(a) A. Müller, P. Kögerler and C. Kuhlmann, Chem. Commun., 1999, 1347–1358 RSC
; (b) C. Schäffer, A. M. Todea, P. Gouzerh and A. Müller, Chem. Commun., 2012, 48, 350–352 RSC
; (c) L. Cronin, E. Diemann and A. Müller, in Inorganic Experiments, ed. J. D. Woollins, Wiley-VCH, Weinheim, 2003, pp. 340–346 Search PubMed
.
-
A. Müller, S. K. Das, E. Krickemeyer and C. Kuhlmann, in Inorganic Syntheses, ed. J. R. Shapley, Wiley, New York, 2004, 34, pp. 191–200 Search PubMed
.
-
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley, New York, 4th edn, 1986, Part II, p. 139, Part III, pp. 248–251 Search PubMed
.
-
(a)
O. Delgado, A. Dress and A. Müller, in Polyoxometalate Chemistry: From Topology via Self-Assembly to Applications, ed. M. T. Pope and A. Müller, Kluwer, Dordrecht, 2001, pp. 69–87 Search PubMed
; (b) A. Müller, P. Kögerler and A. W. M. Dress, Coord. Chem. Rev., 2001, 222, 193–218 CrossRef
.
-
(a)
D. Voet and J. G. Voet, Biochemistry, Wiley, New York, 2nd edn, 1995, p. 1082; see also Search PubMed
; (b) J. Baldwin, BuckyWorks: Buckminster Fuller's Ideas for Today, Wiley, New York, 1996 Search PubMed
.
-
(a) H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS
; (b) G. Brinkmann and A. W. M. Dress, J. Algorithms, 1997, 23, 345–358 CrossRef
.
-
D. Schattschneider and W. Walker, M. C. Escher Kaleidocycles, Tarquin, Stradbroke, 1982 Search PubMed
.
-
(a)
G. M. Sheldrick, SADABS, University of Göttingen, 1997/2003 Search PubMed
; (b) G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed
.
-
K. Brandenburg, DIAMOND 2.1, Crystal Impact GbR, 2001 Search PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: IR spectra; supplementary structural information. See DOI: 10.1039/c4qi00131a |
‡ Preparation of 2: to a solution of 1 (1.0 g, 0.035 mmol) dissolved in 50 mL of water, CaCl2·2H2O (5.0 g, 34.01 mmol) was added in small portions under continuous stirring. The mixture was stirred for 2 h at room temperature, filtered and afterwards kept in an open beaker for crystallization. After 5 days the precipitated dark brown crystals were collected by filtration, rinsed with an ice-cold ethanol–water mixture (70/30) and dried in air. Yield: 0.7 g (64% based on Mo). Anal. Calc. for Ca43H796Cl22Mo132N8O874S30: Ca, 5.55; Cl, 2.51; N, 0.36; S, 3.10. Found Ca, 5.6; Cl, 2.6; N, 0.4; S, 3.1%. [The formula given here accounts for the presence of 250 water molecules as the drying/ageing process will lead to the release of some of the crystal water. The formulation of the compound regarding the cations is only formal except for the Ca2+ ions belonging to the Keplerate, which were found by X-ray structure analysis. The large voids between the capsules are highly disordered areas and consequently the positions of the lattice ingredients are difficult to determine by X-ray crystallography.] IR (KBr, ν/cm−1): 1624s δ(H2O), 1402vw δas(NH4+), 1184sh, 1150m and 1065w, all three νas(SO4), 978s 945m, ν(Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 858s, 802vs, 727s ν(Mo–O–Mo), 633m, 571s, 474m. Raman bands (ν/cm−1; water solution; λe = 785 nm): 961sh, 942w ν(Mo O), 858s, 802vs, 727s ν(Mo–O–Mo), 633m, 571s, 474m. Raman bands (ν/cm−1; water solution; λe = 785 nm): 961sh, 942w ν(Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 872s ν(μ3-O-breathing), 842sh, 368m, 301w. O), 872s ν(μ3-O-breathing), 842sh, 368m, 301w. |
§ Crystal structure data for 2: Ca43H896Cl22Mo132N8O924S30 (the maximum number of water molecules which can be present per cluster unit is ca. 300, when considering the unit cell volume, the volume occupied by the clusters, the corresponding ligands and the Ca2+ cations), M = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 928.42 g mol−1, rhombohedral, R 928.42 g mol−1, rhombohedral, R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , a = 32.4275(10), c = 151.811(7) Å, V = 138 , a = 32.4275(10), c = 151.811(7) Å, V = 138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 248(10) Å3, Z = 6, ρ = 2.301 g cm−3, μ = 2.227 mm−1, F(000) = 93 248(10) Å3, Z = 6, ρ = 2.301 g cm−3, μ = 2.227 mm−1, F(000) = 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 612, crystal size = 0.20 × 0.20 × 0.15 mm3. Crystals of 2 were removed from the mother liquor, coated with oil and immediately cooled to 188(2) K on a Bruker AXS SMART diffractometer (a three circle goniometer with a 1 K CCD detector, Mo-Kα radiation, a graphite monochromator; a detector distance of 5.00 cm; ω-scans). A total of 279 612, crystal size = 0.20 × 0.20 × 0.15 mm3. Crystals of 2 were removed from the mother liquor, coated with oil and immediately cooled to 188(2) K on a Bruker AXS SMART diffractometer (a three circle goniometer with a 1 K CCD detector, Mo-Kα radiation, a graphite monochromator; a detector distance of 5.00 cm; ω-scans). A total of 279![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 122 reflections (0.74 < Θ < 27.02°) were collected of which 66 122 reflections (0.74 < Θ < 27.02°) were collected of which 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 909 reflections were unique (R(int) = 0.0931). An empirical absorption correction using equivalent reflections was performed with the program SADABS 2.10.18a The structure was solved with the program SHELXS-9718b and refined using SHELXL-201318b to R = 0.0786 for 41 909 reflections were unique (R(int) = 0.0931). An empirical absorption correction using equivalent reflections was performed with the program SADABS 2.10.18a The structure was solved with the program SHELXS-9718b and refined using SHELXL-201318b to R = 0.0786 for 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 567 reflections with I > 2σ(I), R = 0.1322 for all reflections; max/min residual electron density 2.056 and −1.395 e Å−3 (structure graphics with DIAMOND 2.119). Further details on the crystal structure investigation may be obtained from the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (fax: +(49)7247-808-666; e-mail: crystdata@fiz-karlsruhe.de), on quoting the depository number CSD 428212. 567 reflections with I > 2σ(I), R = 0.1322 for all reflections; max/min residual electron density 2.056 and −1.395 e Å−3 (structure graphics with DIAMOND 2.119). Further details on the crystal structure investigation may be obtained from the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (fax: +(49)7247-808-666; e-mail: crystdata@fiz-karlsruhe.de), on quoting the depository number CSD 428212. |
| This journal is © the Partner Organisations 2014 |