Incredible pace of research on mesoporous silica nanoparticles
Sushilkumar A.
Jadhav
Department of Chemistry and Nanostructured Interfaces and Surfaces (NIS) Centre, University of Turin, Via P. Giuria 7, 10125 Turin, Italy. E-mail: sushil.unige@gmail.com; sushilkumar.jadhav@unito.it
First published on 9th October 2014
Abstract
Mesoporous silica nanoparticles and related research has noticed a huge growth in the last few years. Some interesting statistics about reports on mesoporous silica are presented in this highlight. After their arrival on the world stage of research these materials have captured several fields of applications including catalyst supports, drug delivery, water purification and their use in bio-materials. Hydrothermal stability, porous structure stability against hydrolysis and suitable organic or bio-functionalization of them for improved biocompatibility are the main issues to be addressed in the near future. Some recent achievements in the synthesis and surface modification of these materials are discussed briefly.
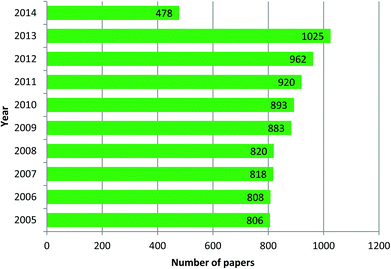
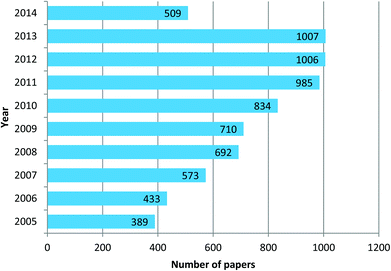
The field of research on mesoporous silica nanoparticles has noticed incredible growth in the last ten years. Mesoporous silica nanoparticles of Mobile Crystal Corporation (MCM-41) and Santa Barbara Amorphous (SBA-15) types are the most investigated and studied materials. According to data obtained from Scifinder, in 2013 around 1025 reports in leading scientific journals were published containing the word “MCM 41” in the title, this means 85.4 research papers per month and approximately 2.8 papers per day. In the case of “SBA 15” type nanoparticles the number is 1007, corresponding to 83.9 papers per month and approximately 2.8 papers per day. These are incredible statistics and it means by the end of the day you finish reading the present highlight three more articles reporting research or updates in the form of papers, news, highlights or reviews will already be published on this topic. These stats are reported in graphical form in Fig. 1 and 2. These enormous number of reports arise because of the fact that mesoporous silica nanoparticles have shown potential applications in almost all technological fields. Silica, silicates and mesoporous silica in general are building blocks of several hybrid materials and such materials are already in use in real applications. Although these statistics appear implausible, these are by no means close to the number of reports published on the topic of gold nanoparticles. The number of reports related to gold nanoparticles in the last three years (2010–2013) is approximately 3000 articles per year, corresponding to 250 papers per month and as many as 8.3 papers per day. Based on this observation, mesoporous silica related research can be then considered as one of the leading hot topics of research in the chemical and material sciences.
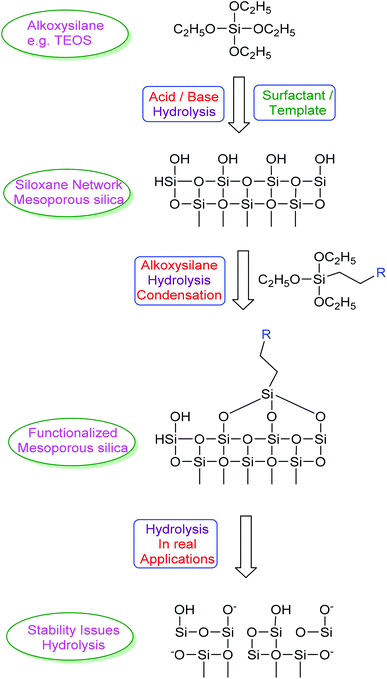
Based on our previous experience of nanoparticles,1 recently we focused our attention on mesoporous silica nanoparticles to explore their applications as drug delivery systems and for their use as water purification materials. Since the first synthesis reports about mesoporous silica nanoparticles2 this material has been studied for all its possible applications in different technological fields.3,4 The most exploited applications include their use as catalysts, catalyst supports and their use as nano drug carriers. Ease of synthesis and surface modification, reasonably good stability, and biocompatibility are the characteristic features of these materials which makes them versatile and increases their suitability in different applications. Mesoporous silica nanoparticles are discussed in detail in some of the recent review articles.5–8 The three main aspects, the synthesis, surface modification and stability, revolve around the hydrolysis cycle as shown in Scheme 1. The synthesis of silica and mesoporous silica is carried out by acid or base hydrolysis of an alkoxysilane precursor. When such hydrolysis is carried out on the micelles formed by amphiphilic compounds in a solvent, the subsequent removal of the template (micelles trapped under silica network) with chemical or thermal treatment leads to the formation of an ordered porous network thus forming mesoporous silica nanoparticles. The pore diameter and length of the pores can be tuned by varying the templating agent used. The main issue recently raised is the breakdown of the silica network upon further hydrolysis of this siloxane network in real applications. Use of mesoporous silica in drastic conditions may lead to collapse of the ordered porous structure. Suitable modification of the surface to consume all surface hydroxyl groups (silanols) generated either after removal of the template or during hydrothermal treatments can be an option to increase the stability of these materials. Looking at the different synthesis methods reported for this type of materials it is evident that all major steps related to their synthesis, functionalization or surface modification are relatively simple and more importantly industrially feasible and reproducible. The time span of technology transfer from laboratory scale to real production of silica or mesoporous silica materials thus can be small which makes this material ideal to replace some of the costly materials presently in use or speculated to be used in real applications.
As stated before the traditional synthesis of mesoporous silica nanoparticles is carried out by a hydrothermal method starting with an alkoxysilane or mixtures of precursor/s. The hydrolysis of the alkoxysilane is carried out on micelles formed by templates (also termed as surfactants). Some alternative green approaches for the synthesis of such nanoparticles were reported. This green approach suggests the replacement of the organic templates with recyclable or eco-friendly amphiphilic compounds which can also be removed with solvent extraction instead of thermal treatments,9 replacing the solvent with ionic liquids which will simplify the synthesis conditions and microwave assisted thermal treatment to save energy10 and by using mild conditions for the synthesis.11 Silica nanoparticles are hydrophilic and their properties can be tuned by changing the composition of the initial alkoxysilane used to carry out the hydrolysis (refer to Scheme 1). Recently the synthesis of mesoporous silica nanoparticles exhibiting a hydrophobic core and hydrophilic shell as shown in Fig. 3 has been reported.12 The bilayer mesoporous particles are synthesized by using a combination of alkoxysilanes including hydrophobic octyltrimethoxysilane and methyl orthosilicate (TMOS) and hydrophilic organosilane 3-((3-(trimethoxysilyl)propyl)ammonio)propane-1-sulfonate. It is an important step towards addressing the hydrothermal stability of such nanoparticles. Hydrophobicity increases the hydrothermal stability of the material in an aqueous environment and the hydrophilicity guarantees biocompatibility. The hydrophilic character comes from the free silanol (Si–OH) groups that are present on the particles which can be further utilized to graft desired functional groups, drug molecules or biomolecules with the help of condensing agents. Further free silanol groups increase interactions of the gust molecules with the mesoporous silica particles through hydrogen bonding depending on the groups present on the guest molecules. This can be of importance when higher loading of drug or other organic molecules is expected inside the mesopores. The report about core-hydrophillic shell mesoporous silica nanoparticles therefore can be described as an important achievement in the modified synthesis of such materials. Several attempts were made to improve the hydrothermal stability of mesoporous silica particles. Use of salts, that is the addition of salts to the synthesis mixture of the surfactant-silica mesostructures, is one of them.13 But such an approach has the major disadvantage of micelle branching which leads to a disordered pore structure. Another approach involves the pre-treatment of mesoporous silicas with ammonium hexafluorosilicate.14 Functionalization of mesoporous silica particles with different hydrophobic organic groups is another strategy to increase the stability.15,16
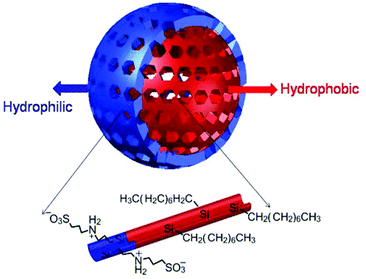 | ||
| Fig. 3 Hydrophobic core-hydrophillic shell mesoporous silica nanoparticle (reproduced with permission from ref. 12 copyright @ 2013 American Chemical Society). | ||
Pre- and post-synthesis surface modification approaches are used to apply organic functionalities to the mesoporous silica nanoparticles.17 Both these methods are widely exploited to synthesize various functionalized reactive silica or mesoporous silica nanoparticles. However it is challenging to further develop in situ functionalization methods to directly functionalize mesoporous silica nanoparticles, functionalized not only with organic groups but also directly with biomolecules: such as DNA, proteins or vitamin molecules, bio-linkers, and sensitive bio-florescent linkers. Direct one step functionalization with thermally or light sensitive polymerizable groups is another challenging task. This approach will directly produce biomolecule functionalized mesoporous silica nanoparticles ready for use as targeted drug delivery systems, in diagnosis and in biosensor construction applications. The main obstacle for the direct functionalization of mesoporous silica nanoparticles with the molecules mentioned is the drastic synthesis conditions which use a high temperature for template removal and strong bases for the hydrolysis of alkoxysilane precursors. The green and mild methods mentioned before however can provide the possibility of direct functionalization of mesoporous silica nanoparticles with less stable or sensitive biomolecules. Synthesis of monodispersed mesoporous silica nanoparticles, reproducibility of the synthetic method and more importantly large scale production of such nanoparticles is another challenge faced. The synthesis of nanoparticles that exhibit a uniform shape and relatively smooth surface with less aggregation tendency has been recently achieved.18 The surfactants used for the formation of micelles were removed by a green method of dialysis (Fig. 4). A common observation about the effect of time on the growth of seeds of nanoparticles or nanocrystals is also reported. More such attempts that combine green synthesis approachs without compromising the quality of the nanoparticles synthesized are expected.
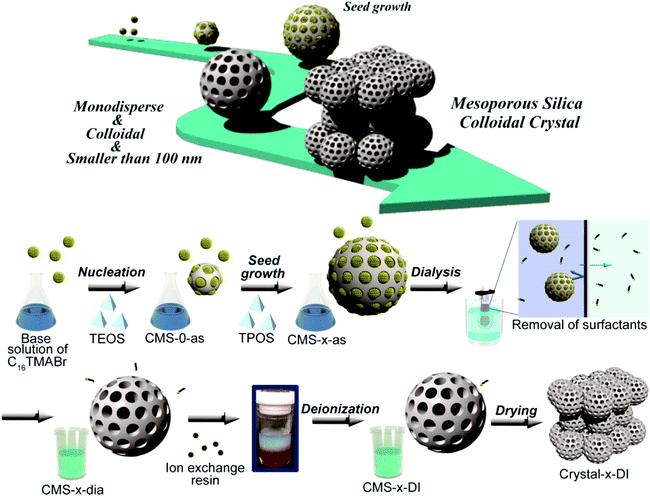 | ||
| Fig. 4 New approach for the synthesis of mesoporous silica nanoparticles (reproduced with permission from ref. 18 copyright @ 2014 American Chemical Society). | ||
One of the main synthesis issues of mesoporous silica particles is the synthesis of nanoparticles with highly ordered mesoporous structures. The pore diameter and the orientation of the pore channels need to be controlled. This is also called ‘pore engineering’. The traditional synthesis of mesoporous silica nanoparticles with cationic and anionic templating agents has undergone a revolution in recent years as several new synthetic techniques were invented after manipulation of the synthetic parameters and reagents used. Such synthetic techniques produced highly ordered pore structures. A recent report by Peng et al.19 demonstrated how the use of micelle core-swelling agents used for the synthesis of bigger pore diameter mesopore channels can give rise to the core–shell structured mesoporous silica nanospheres with dually oriented mesochannels. The secondary mesopore structure (shell) arises in such materials from the release of ethanol molecules (which can form micelles) from TEOS after increasing the rate of hydrolysis with the use of core-swelling agents such as trimethyl benzene. Use of self-assembled micelle forming block co-polymers as templates for the synthesis of mesoporous silica nanoparticles or silica based porous membranes is well-known. Bastakoti et al.20 recently reported a tri-block polymeric micelle assembly for the direct synthesis of functionalized mesoporous silica embedded with platinum nanoparticles. Asymmetric triblock copolymer, poly(styrene-b-2-vinylpyridine-b-ethylene oxide) as the structure directing agent and platinum(II) 2,4-pentanedionate as the platinum nanoparticle precursor were used in the self-assembly. Hydrolysis of TEOS on this hybrid assembly and the subsequent calcination of the material to remove the template (structure directing tri-block co-polymer) not only removed the template but led to the formation and strong attachment of platinum nanoparticles to the mesoporous silica. A similar approach of directly synthesizing silver nanoparticles during the synthesis of MCM-41 nanoparticles was recently reported in the work by Tian et al.21 In the synthesis, co-hydrolysis condensation of TEOS with N-(aminoethyl)-amino-propyl trimethoxysilane (TSD) in the presence of a silver salt was carried out. The amino ligands from TSD trap the silver ions which during hydrolysis and later treatment with formalin are converted to silver nanoparticles (Fig. 5). The process resulted in highly dispersed 2–10 nm silver nanoparticles in the MCM-41 framework. Such in situ nanoparticle functionalization approaches are important technological achievements in this field.
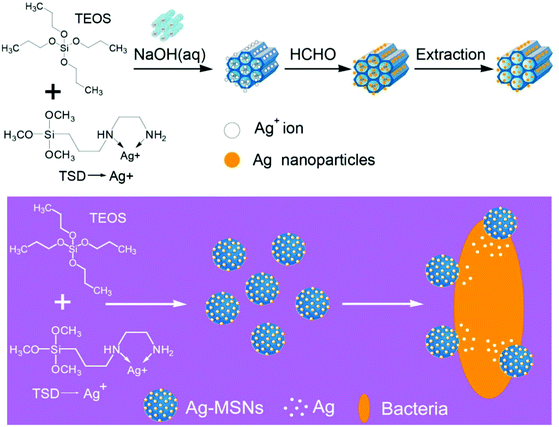 | ||
| Fig. 5 Synthesis of Ag-mesoporous silica nanoparticles (reproduced with permission from ref. 21 copyright @ 2014 American Chemical Society). | ||
The aim of this highlight is to point out that as the number of reports related to mesoporous silica nanoparticles is increasing, they in the near future will help to reveal all the aspects of the material and soon provide a clear understanding of them. Important issues about their green synthesis with industrially feasible synthetic steps, surface modification and stability will be clear and such a material will make its way in real applications. New research will also emerge where they can replace other less stable porous materials. Future research related to mesoporous silica nanoparticles will continue towards their green synthesis, exploring new applications and more importantly towards addressing their stability issues.
Acknowledgements
Funding from Compagnia di San Paolo and University of Turin, Italy for the on-going research project in our laboratory “Bando per il finaziamento di progetti di ricerca di Ateneo-anno 2011” project code ORTO114XNH is acknowledged.Notes and references
- (a) S. A. Jadhav, J. Mater. Chem., 2012, 22, 5894–5899 RSC; (b) S. A. Jadhav and R. Bongiovanni, Adv. Mater. Lett., 2012, 3, 356–362 Search PubMed; (c) S. A. Jadhav, R. Bongiovanni, D. L. Marchisio, D. Fontana and C. Egger, Pigm. Resin Technol., 2014, 43, 219–227 CrossRef; (d) S. A. Jadhav and S. V. patil, Front. Mater. Sci., 2014, 8, 193–198 CrossRef.
- (a) T. Yanagisawa, T. Shimizu, K. Kuroda and C. Kato, Bull. Chem. Soc. Jpn., 1990, 63, 988–992 CrossRef CAS; (b) C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- I. I. Slowing, J. L. Vivero-Escoto, B. G. Trewyn and V. S. Y. Lin, J. Mater. Chem., 2010, 20, 7924–7937 RSC.
- C. Argyo, V. Weiss, C. Brauchle and T. Bein, Chem. Mater., 2014, 26, 435–451 CrossRef CAS.
- D. Tarn, C. E. Ashley, M. Xue, E. C. Carnes, J. I. Zink and C. J. Brinker, Acc. Chem. Res., 2013, 46, 792–801 CrossRef CAS PubMed.
- F. Tang, L. Li and D. Chen, Adv. Mater., 2012, 24, 1504–1534 CrossRef CAS PubMed.
- C. T. Kresge and W. J. Roth, Chem. Soc. Rev., 2013, 42, 3663–3670 RSC.
- A special issue of Chem. Soc. Rev., 07 May 2013, Issue 9, Pages 3649 to 4258 is dedicated to reviews related to synthesis, functionalization and various applications of mesoporous silica nanoparticles.
- H. Dai, J. Yang, J. Ma, F. Chen, Z. Fei and M. Zhong, Microporous Mesoporous Mater., 2012, 147, 281–285 CrossRef PubMed.
- S. E. Lehman and S. C. Larsen, Environ. Sci. Nano, 2014, 1, 200–213 RSC and references there in.
- K. Zhang, L. L. Xu, J. G. Jiang, N. Calin, K. F. Lam, S. J. Zhang, H. H. Wu, G. D. Wu, B. Albela, L. Bonneviot and P. Wu, J. Am. Chem. Soc., 2013, 135, 2427–2430 CrossRef CAS PubMed.
- S. Li, X. Jiao and H. Yang, Langmuir, 2013, 29, 1228–1237 CrossRef CAS PubMed.
- J. M. Kim, S. Jun and R. Ryoo, J. Phys. Chem. B, 1999, 103, 6200–6205 CrossRef CAS.
- S. Mingjuan, Z. Chenglong, N. Guoxing and Z. Dongyuan, Chin. J. Catal., 2012, 33, 140–151 CrossRef.
- D. H. Lee, M. Choi, B. W. Yu and R. Ryoo, Chem. Commun., 2009, 74–76 RSC.
- F. Carniato, C. Bisio, G. Paul, G. Gatti, L. Bertinetti, S. Coluccia and L. Marchese, J. Mater. Chem., 2010, 20, 5504–5509 RSC.
- F. Hoffmann, M. Cornelius, J. Morell and M. Froba, Angew. Chem., Int. Ed., 2006, 45, 3216–3251 CrossRef CAS PubMed.
- E. Yamamoto, M. Kitahara, T. Tsumura and K. Kuroda, Chem. Mater., 2014, 26, 2927–2933 CrossRef CAS.
- J. Peng, J. Liu, J. Liu, Y. yang, C. Li and Q. Yang, J. Mater. Chem. A, 2014, 2, 8118–8125 CAS.
- B. P. Bastakoti, Y. Li, N. Miyamoto, N. M. Sanchez-Ballester, H. Abe, J. Ye, P. Srinivasu and Y. Yamauchi, Chem. Commun., 2014, 50, 9101–9104 RSC.
- Y. Tian, J. Qi, W. Zhang, Q. Cai and X. Jiang, ACS Appl. Mater. Interfaces, 2014, 6, 12038–12045 CAS.
| This journal is © the Partner Organisations 2014 |