Hetero-nuclear coordinated compounds for use in high-performance supercapacitor electrode material design†
Liwei
Mi
*a,
Yang
Gao
ab,
Shizhong
Cui
a,
Hongwei
Hou
b and
Weihua
Chen
*b
aCenter for Advanced Functional Materials Research, Zhongyuan University of Technology, Henan 450007, P. R. China. E-mail: mlwzzu@163.com
bCollege of Chemistry and Molecular Engineering, Zhengzhou University, Henan 450001, P. R. China. E-mail: chenweih@zzu.edu.cn
First published on 22nd September 2014
Abstract
Single crystals of cobalt coordination compounds were obtained by one-pot hydrothermal reaction. Nickel ion exchange was used to produce the Ni–Co hetero-metal compound with tunable chemical composition. Using a nickel-exchanged compound as the precursor, we developed a simple chemical reaction to synthesize large-scale 3D hierarchical NixCo3−xS4 (x = 0.15–0.42) microflowers under ambient conditions. As an electrode material for supercapacitors, the discharge capacitance of Ni0.42Co2.58S4 microflowers is approximately one and a half times higher than the discharge capacitance value of Ni0.15Co2.75S4 at a current density of 0.5 A g−1.
Introduction
Single crystals are an essential part of nature and have been the focus of recent research activities because of their distinct physical characteristics; such characteristics are related to polycrystal properties, such as thermal conductivity1a and electron transport properties.1b–d Compared with a single crystal diamond, a polycrystalline diamond exhibits poor thermal conductivity.1 Silicon-based materials also have excellent electron transport properties; single crystal silicon has been widely used in solar energy conversion1b,c and data storage.1d Therefore, using a single crystal as a template to obtain another new single crystal may be an efficient and simple option to achieve the state of co-existence properties of multi-metal coordination complexes.In single-crystal to single-crystal (SCSC) transformation, original lattice parameters are left unchanged or are minimally changed in the single crystal during the phase transition.2–4 Various approaches, such as those involving light, heat, and pressure, have been proposed to achieve SCSC transformation in coordination chemistry.3 Our previous studies focused on cation exchange-induced tunable properties of the nanoporous octanuclear Cu(II) wheel,2a polymeric cadmium ferrocene-1,1-disulfonates,5a and ferrocenyl-based inclusion complexes.5b We believe that the central metal ion exchange can serve as a powerful method to modify properties of crystalline materials by varying the central metal ions. Under this process, we can control the main performance of crystalline materials by adjusting the ratio of central metal ions.
The main challenge in the synthesis of crystalline solid-state materials is altering the chemical composition without changing the underlying topology.6 The tunable composition ratio of central metals is advantageous because controlling the banding gap becomes possible. Such tunability can lead to satisfactory electrochemical properties. However, to the best of our knowledge, cation-exchanged single crystals have not been utilized to prepare multi-metal nanomaterials. Currently, multi-metal sulfides are considered promising electrode materials that are specifically activated in supercapacitors7 and lithium ion batteries;8 these materials have catalytic properties.9 Multi-metal sulfides have high redox activity and electrochemical activity and are inexpensive. However, multiple reactants, i.e. two or more inorganic salts or oxides that are present during preparation, may negatively affect the control of reaction rates and homogeneity. Such negative effects could contribute to the difficulty in large-scale preparation of materials with distinct morphology.
Our strategy for synthesis of large scale regular 3D hierarchical NixCo3−xS4 (x = 0.15–0.42) microflowers utilizes a cobalt coordination complex and its ion exchanged derivatives as a precursor because this method has given high yield and the reaction conditions which are suitable for the small chemical factories. The as-synthesized NixCo3−xS4 microflowers showed high specific capacitance, excellent rate capability, and good cycling stability, and such characteristics contribute to the potential of these microflowers as electrode materials for high performance supercapacitors. We investigated the process of central metal ion exchange to determine the tunable properties caused by cation-exchange-induced SCSC transformation between the cobalt coordination complex and the cation-exchanged products. The perfectly designed electrodes showed higher electrochemical performance than the parent material. The results can help improve product properties as a response to energy crisis.
Experimental section
Materials and methods
All the chemicals and solvents used were of analytic grade purity and used without further purification.The preparation of Nickel-exchanged compound
Single crystals of {[NaCo3(TMA)2(μ3-OH)(μ2-H2O)4(H2O)7]·1.5H2O}n (1) were prepared as described in the literature.10a A series of nickel ion-exchanged crystals were obtained by immersing large crystals of the as-synthesized 1 into an ethanol solution with 40 mg mL−1 nickel nitrate for different replacement durations (1/3, 1, 2, 3, 5, 6, and 8 h) at 160 °C. Finally, the as-synthesized products were separated by filtration and washed sequentially with deionized water and 95% ethanol, and dried at room temperature.Synthesis of 3D hierarchical NixCo3−xS4 microflowers
In a typical preparation procedure, 1 mmol of nickel-exchanged compounds for 1 h, 0.25 mL of glacial acetic acid, and 5 mL of the ethylene glycol solvent were added to a 20 mL beaker under heating and stirring. Subsequently, 1 mmol of thiourea was dissolved in 11 mL ethanediamine to give a homogeneous solution, and then added dropwise to the above mixture solution. After stirring for 8 h, the mixed solution was transferred to a 20 mL Teflon-sealed autoclave, maintained at 160 °C for 24 h and cooled to room temperature naturally. After the solvothermal reaction, NixCo3−xS4 microflowers were formed.To achieve a controllable component, Ni0.24Co2.76S4 and Ni0.42Co2.58S4 were synthesized using nickel-exchanged compounds for 3 h and 6 h, respectively, as precursors with a method similar to above. Finally, the black powder was washed several times with distilled water and 95% ethanol, and dried at 60 °C for 8 h in a vacuum oven.
Characterization
X-ray diffraction (XRD) patterns were obtained with a Bruker D8 Advance X-ray powder diffractometer using Cu-Kα irradiation at a scan rate of 0.1° s−1. XRD measurements of the single crystal 1 and the nickel-exchanged complex were performed within 5° ≤ 2θ ≤ 50°. XRD measurements of NixCo3−xS4 materials were performed within 10° ≤ 2θ ≤ 70°. Crystallographic data were collected on a Bruker SMART APEX CCD diffractometer using graphite-monochromatized Mo Kα radiation (λ = 0.71073 Å) at room temperature. The morphologies and sizes of the as-prepared NixCo3−xS4 microflowers were characterized with a Zeiss EVO LS-15 scanning electron microscope (SEM) equipped with an energy-dispersive X-ray spectroscopy (EDS) system. The microstructures of the resulting Ni0.42Co2.58S4 materials were recorded with a JEOL JEM-2010 transmission electron microscope (TEM). The X-ray photoelectron spectroscopy (XPS) of the as-obtained Ni0.42Co2.58S4 microflower was performed using a Kratos AXIS ULTRA X-ray photoelectron microscope with Al-Kα X-rays as the excitation source.Electrochemical performance test
Cyclic voltammetry (CV) measurements were carried out on a CHI604E electrochemical workstation (Chenhua, Shanghai, China) at voltage scan rates ranging from 1 to 5000 mV s−1. Electrochemical measurements were conducted in a three-electrode mode in 2 M KOH electrolyte. In this case, the 80% of the active material, 10% each of carbon black and polyvinylidene difluoride (PVDF) were uniformly mixed and then cast onto a nickel foam, which was used as a working electrode. A saturated Hg/HgO electrode and a platinum plate were employed as the reference and counter electrode, respectively. The galvanostatic charge–discharge tests were conducted with current densities of 0.5, 1, 2, 5, 10, and 20 A g−1 using a LAND battery test system (CT2001A model). Specific capacitance could be calculated according to the following equation: C = IΔt/mΔV, where I is the charge or discharge current, Δt is the time for a full charge or discharge, m indicates the mass of the active material, and ΔV represents the practical voltage in a full discharge process. The cyclic stabilities were investigated using galvanostatic charge–discharge measurements at constant current densities of 2 A g−1 for 1000 cycles.Results and discussion
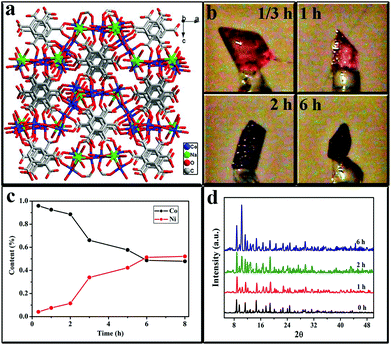
Single crystals of {[NaCo3(TMA)2(μ3-OH)(μ2-H2O)4(H2O)7]·1.5H2O}n (1) were obtained by the reaction of cobalt(II) sulfate with trimesic acid (H3TMA = 1,3,5-benzenetricarboxylic acid) under pH 6.9; pH was adjusted using 0.5 M NaOH.10 A series of nickel ion-exchanged crystals were obtained by immersing large crystals of the as-synthesized 1 into an ethanol solution with 40 mg mL−1 nickel nitrate for different replacement durations (1/3, 1, 2, 3, 5, 6, and 8 h) at 160 °C. Fig. 1a and 1b present the typical crystal packing images of the as-prepared single crystalline 1 and its nickel-exchanged products, respectively. Nickel-exchanged crystals entirely maintained the original shape of 1. However, the color of nickel ion-exchanged crystals turned fuchsia (Fig. 1b), whereas such change was not observed in 1. No further changes in color and in composition were observed after 6 h. Given that the rate of solvent and ligand exchange depended on the nature of each cation, this duration suggests that the ion metathesis reactions reached equilibrium after 6 h. Infrared spectra and powder X-ray diffraction analysis revealed that the single crystal 1 and the Ni ion-exchanged crystals have similar absorbance and diffraction peaks, thereby indicating the presence of SCSC transformations induced by cation exchange and the creation of Ni–Co complexes. Ni–Co complexes were carefully analyzed by using energy dispersive X-ray spectroscopy. The ratios of nickel to cobalt were 95.87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.13, 92.50
4.13, 92.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.50, 88.57
7.50, 88.57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.43, 66.11
11.43, 66.11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.89, 57.72
33.89, 57.72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42.28, 48.72
42.28, 48.72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51.28 and 47.90
51.28 and 47.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52.10 (Fig. 1c). Analysis of single-crystal X-ray diffraction as shown in Fig. 1d revealed that the nickel ion-exchanged crystals have identical lattice parameters with 1, and such similarity further confirmed that SCSC transformation occurred by cation exchange.
52.10 (Fig. 1c). Analysis of single-crystal X-ray diffraction as shown in Fig. 1d revealed that the nickel ion-exchanged crystals have identical lattice parameters with 1, and such similarity further confirmed that SCSC transformation occurred by cation exchange.
We prepared 3D hierarchical NixCo3−xS4 (x = 0.15–0.42) microflowers using Ni–Co precursors with different Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co atomic ratios of 92.50
Co atomic ratios of 92.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.50, 66.11
7.50, 66.11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.89, and 48.72
33.89, and 48.72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
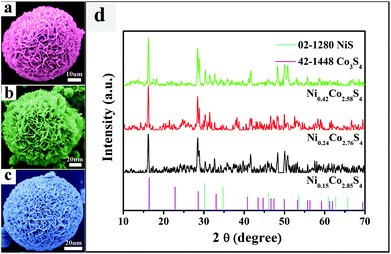
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51.28 under ambient conditions. However, due to metal ion sorption and exchange could be achieved directly by suspending such coordination compounds in a solution containing metal salt.5 The solution concentration of metal ions affects the ion sorption and exchange behavior. When the solution concentration of metal salts is higher, metal ion sorption is mainly occurring, whereas metal ion exchange mainly takes place at a low solution concentration of metal salt. EDX and ICP could not give the amount of metal ions clearly but gave a macro quantity. Therefore, there is only a small range of Ni content in NixCo3−xS4 materials. The panoramic SEM image showed that the NixCo3−xS4 materials were highly uniform large-scale microflowers. The magnified SEM images (Fig. 2a–2c) revealed that each microflower was a hierarchical microsphere assembled by numerous bent nanosheets, which endow this structure with a high surface area, providing sufficient electrolyte–electrode interface for fast diffusion and reaction.10b,c The Ni/Co atomic ratios of Ni0.15Co2.85S4, Ni0.24Co2.76S4, and Ni0.42Co2.58S4 were confirmed by energy-dispersive X-ray spectroscopy. The clear lattice fringes were visible from the high-resolution transmission electron microscopy image, and the distance (2.85 Å) between the adjacent lattice fringes corresponded to the (311) plane of Co3S4 crystals (JCPDS no. 42-1448). Ni0.42Co2.58S4 microflower (220) and (311) crystal planes were clearly observed from the selected area electron diffraction image. The crystal nature is further confirmed by XRD data, as shown in Fig. 2d. Although NixCo3−xS4 microflowers have maintained most of the lattice fringe in Co3S4 and part of lattice fringe of NiS. As a new type of material, it does not consist of individual Co3S4 and NiS. So there are some other peaks in XRD. X-ray photoelectron spectroscopy (XPS) further supports our results. The Co 2p spectrum was fitted in such a way that two spin–orbit doublet characteristics of Co2+ and Co3+ and two shakeup satellites were present. The Co 2p peaks that appeared at 796.9 eV, 786.8 eV, and 781.3 eV are characteristic of Co2+ species,10d,f,g and the Co 2p peaks observed at 779.7 eV and 803.7 eV are characteristic of Co3+ species.10d The Ni 2p spectrum showed the best fit, considering two spin–orbit doublets at 879.2 eV and 873.5 eV, and 861.9 eV and 856.1 eV; the doublets are assigned to Ni2+
51.28 under ambient conditions. However, due to metal ion sorption and exchange could be achieved directly by suspending such coordination compounds in a solution containing metal salt.5 The solution concentration of metal ions affects the ion sorption and exchange behavior. When the solution concentration of metal salts is higher, metal ion sorption is mainly occurring, whereas metal ion exchange mainly takes place at a low solution concentration of metal salt. EDX and ICP could not give the amount of metal ions clearly but gave a macro quantity. Therefore, there is only a small range of Ni content in NixCo3−xS4 materials. The panoramic SEM image showed that the NixCo3−xS4 materials were highly uniform large-scale microflowers. The magnified SEM images (Fig. 2a–2c) revealed that each microflower was a hierarchical microsphere assembled by numerous bent nanosheets, which endow this structure with a high surface area, providing sufficient electrolyte–electrode interface for fast diffusion and reaction.10b,c The Ni/Co atomic ratios of Ni0.15Co2.85S4, Ni0.24Co2.76S4, and Ni0.42Co2.58S4 were confirmed by energy-dispersive X-ray spectroscopy. The clear lattice fringes were visible from the high-resolution transmission electron microscopy image, and the distance (2.85 Å) between the adjacent lattice fringes corresponded to the (311) plane of Co3S4 crystals (JCPDS no. 42-1448). Ni0.42Co2.58S4 microflower (220) and (311) crystal planes were clearly observed from the selected area electron diffraction image. The crystal nature is further confirmed by XRD data, as shown in Fig. 2d. Although NixCo3−xS4 microflowers have maintained most of the lattice fringe in Co3S4 and part of lattice fringe of NiS. As a new type of material, it does not consist of individual Co3S4 and NiS. So there are some other peaks in XRD. X-ray photoelectron spectroscopy (XPS) further supports our results. The Co 2p spectrum was fitted in such a way that two spin–orbit doublet characteristics of Co2+ and Co3+ and two shakeup satellites were present. The Co 2p peaks that appeared at 796.9 eV, 786.8 eV, and 781.3 eV are characteristic of Co2+ species,10d,f,g and the Co 2p peaks observed at 779.7 eV and 803.7 eV are characteristic of Co3+ species.10d The Ni 2p spectrum showed the best fit, considering two spin–orbit doublets at 879.2 eV and 873.5 eV, and 861.9 eV and 856.1 eV; the doublets are assigned to Ni2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10d,e and Ni3+,10d respectively. The high-resolution spectrum for the S 2p region showed sulfide contributions, and the peaks at around 162.0 eV and 163.1 eV can be assigned to S 2p3/27a,10e and S 2p1/2,7a respectively. The S 2p peak observed at 168.1 eV is attributed to surface sulfur with a high oxidation state, such as sulfates.10h These values are also close to the reported values.7a,10d–h
10d,e and Ni3+,10d respectively. The high-resolution spectrum for the S 2p region showed sulfide contributions, and the peaks at around 162.0 eV and 163.1 eV can be assigned to S 2p3/27a,10e and S 2p1/2,7a respectively. The S 2p peak observed at 168.1 eV is attributed to surface sulfur with a high oxidation state, such as sulfates.10h These values are also close to the reported values.7a,10d–h
To obtain information on the electrochemical properties of the as-synthesized NixCo3−xS4, we characterized the electrodes by cyclic voltammetry (CV) and galvanostatic charge/discharge techniques. Fig. 3a, 3c, and 3e show the typical CV curves of Ni0.15Co2.85S4, Ni0.24Co2.76S4, and Ni0.42Co2.58S4 electrodes at a scan rate of 1 mV s−1. The initial curve was different from those in subsequent cycles, and such difference was due to the phase transformation from NixCo3−xS4 to Ni(OH)2 (or Co(OH)2) during the first positive sweep, as described in reaction (1).11 Therefore, the phase transformation reaction occurred first and was followed by the oxidation reaction from M(OH)2 to MOOH during the first positive sweep. Furthermore, only the reversible reduction reaction from MOOH to M(OH)2 (M = Ni and Co species) occurred during the first negative sweep. Only reversible redox reactions [reaction (2)] that occurred in subsequent CV cycle tests were observed. The XPS spectra of Ni0.42Co2.58S4 show that the as-synthesized Ni0.42Co2.58S4 contains three kinds of cations (Ni2+, Co2+, and Co3+). After the first positive sweep and at a scan rate of 0.5 mV s−1, most of the divalent cations in Ni0.42Co2.58S4 are transformed into trivalent cations during the oxidation process. The absence of full oxidation in Ni0.42Co2.58S4 may be due to the inappropriate scan rate. The reaction mechanism of NixCo3−xS4 is given below.11,12
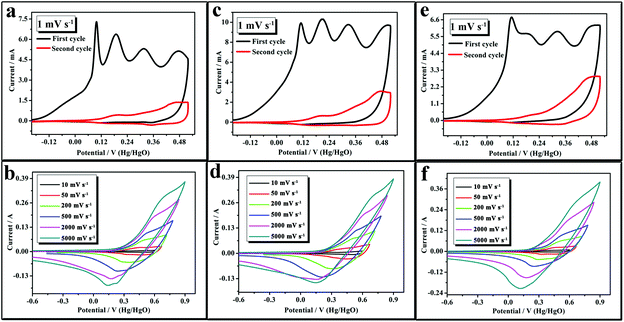 | ||
| Fig. 3 CV curves of the as-synthesized NixCo3−xS4 (x = 0.15–0.42): (a and b) Ni0.15Co2.85S4, (c and d) Ni0.24Co2.76S4, and (e and f) Ni0.42Co2.58S4 at various scan rates. | ||
The phase transformation reaction is as follows:
| NixCo3−xS4 + 3H2O + 3/2O2 → xNi(OH)2 + 3 − xCo(OH)2 + 4S | (1) |
The reversible redox reaction is as follows:
| M(OH)2 + OH− → MOOH + H2O + e− (M = Ni and Co) | (2) |
Fig. 3b, 3d and 3f show the typical CV curves of Ni0.15Co2.85S4, Ni0.24Co2.76S4, and Ni0.42Co2.58S4 electrodes at scan rates of 10, 50, 200, 500, 2000, and 5000 mV s−1. The CV profiles showed the pronounced pseudocapacitive characteristics with a pair of reversible redox peaks within each voltammogram. The peak currents increased with increasing scan rate. Furthermore, the oxidation and reduction peaks of the NixCo3−xS4 microflower electrode gradually shifted to the right and left with increasing scan rate, which is possibly due to the insufficient active material involved in the redox reaction under higher scan rates.12b Moreover, when the electrode has high sweep rates, massive OH− ions are required to intercalate swiftly at the interface of the electrode–electrolyte; however, relatively low concentration of OH− ions could not meet this demand and the processes would be controlled by the diffusion of ions. When the scan rate increased to 5000 mV s−1, the redox peaks of Ni0.15Co2.85S4, Ni0.24Co2.76S4, and Ni0.42Co2.58S4 were still apparent, thereby indicating that Ni0.15Co2.85S4, Ni0.24Co2.76S4, and Ni0.42Co2.58S4 have excellent rate performance.
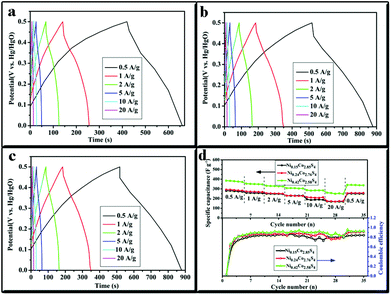
To further evaluate the application potential of various NixCo3−xS4 as electrodes for supercapacitors, galvanostatic charge–discharge measurements were performed between 0 and ∼0.5 V (vs. Hg/HgO) at different current densities (Fig. 4a–4c). The potential plateaus in the charge–discharge curves were consistent with the results of the CV test, indicating the existence of Faradaic progress. The super capacitance and coulombic efficiency of all NixCo3−xS4 electrodes could be calculated based on the charge–discharge curves. The results are plotted in Fig. 4d. Ni0.42Co2.58S4 microflowers exhibited higher average discharge capacitance values of 379.0, 350.4, 329.3, 306.2, 284.64, and 253.9 F g−1 at various current densities of 0.5, 1, 2, 5, 10, and 20 A g−1, respectively, than Ni0.24Co2.76S4 (288.0 F g−1 to 172.7 F g−1 at 0.5 A g−1 to 20 A g−1) and Ni0.15Co2.85S4 (276.7 F g−1 to 169.1 F g−1 at 0.5 A g−1 to 20 A g−1) (Fig. 4d). The increased electrochemical capacitance of the as-obtained NixCo3−xS4 can be due to the presence of the nickel ions. Moreover, the Ni0.15Co2.85S4, Ni0.24Co2.76S4, and Ni0.42Co2.58S4 have excellent rate performance with 67%, 60%, and 61% of the initial capacitance, respectively, when the charge–discharge rate was increased by 40 times from 0.5 A g−1 to 20 A g−1. After continuous cycling for 30 cycles at the increased current densities of 0.5, 1, 2, 5, 10, and 20 A g−1, the current density is reduced back to 0.5 A g−1. The Ni0.15Co2.85S4, Ni0.24Co2.76S4, and Ni0.42Co2.58S4 showed remarkable cycling stability, maintaining 90.8%, 89.3%, and 89.6% of the initial capacitance at 0.5 A g−1. The supercapacitive performance of NixCo3−xS4 microflowers was superior to that of commercial MnO2. The coulombic efficiency of Ni0.15Co2.85S4, Ni0.24Co2.76S4, and Ni0.42Co2.58S4 was relatively high at approximately 95.8%, except in the first cycle because of the phase transformation reactions.
The cycling stability of NixCo3−xS4 electrodes for 1000 cycles was tested by galvanostatic charge–discharge measurements at a current density of 2.0 A g−1. As shown in Fig. 5a, the initial capacitance of Ni0.42Co2.58S4 was approximately 321.4 F g−1 and was subsequently decreased to 311.8 F g−1. Only 3% loss was observed after 1000 repeated cycles. Meanwhile, the initial capacitance of Ni0.24Co2.76S4 was approximately 262.9 F g−1, which slightly decreased to 258.4 F g−1 after 1000 cycles, and the total capacitance loss was 2%. The specific capacitance of the Ni0.15Co2.85S4 microflowers decreased from 222 F g−1 to 214 F g−1 with a capacitance retention of 96% after continuous processing for 1000 cycles. Moreover, the coulombic efficiency curves of Ni0.15Co2.85S4, Ni0.24Co2.76S4, and Ni0.42Co2.58S4 (Fig. 5b) remain 95.03%, 97.0%, and 96.34%, respectively, after 1000 cycles at 2 A g−1, which again demonstrated that NixCo3−xS4 electrodes exhibit a higher degree of reversibility after a long-term cycling test.
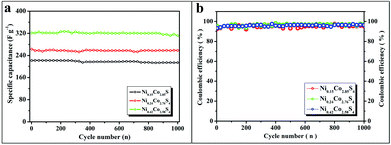 | ||
| Fig. 5 The cycling life (a) and coulombic efficiency curve (b) of Ni0.15Co2.85S4, Ni0.24Co2.76S4, and Ni0.42Co2.58S4 at a current density of 2.0 A g−1. | ||
By contrast, the elevated levels of the nickel ratio could benefit from capacitor performance of these materials. When nickel oxide alone is used as the electrode material, the performance of the supercapacitor was not satisfactory. The capacitance values of NixCo3−xS4 microflowers were higher than those of NiO nanowires (180 F g−1 at 0.5 A g−1),13a NiO nanotubes (47 F g−1 at 0.28 A g−1),13b and NiO with ordered mesoporous structure (120 F g−1).13c Compared with Ni(OH)2 and NiS materials with excellent performance, the capacitance performance of NixCo3−xS4 microflowers was slightly insufficient. MnO2 is the most commonly used supercapacitor electrode material, the SC value for Ni0.42Co2.58S4 microflowers is 379 F g−1 at 0.5 A g−1, which is more than ten times higher than the SC value of commercial MnO2 (∼36 F g−1).14 However, increasing the percentage content of central nickel ions can improve capacitive performance, which means that adjusting the metal center to the proper proportion is important. Thus, an ion-exchanged compound can be considered an appropriate precursor in the synthesis of multiple sulfide nanomaterials.
Conclusions
In summary, Co–Ni complexes were successfully prepared by the Ni exchange method. A cobalt coordination complex was used as a precursor. SCSC transformation was achieved. We also synthesized large-scale regular 3D hierarchical NixCo3−xS4 (x = 0.15–0.42) microflowers because of the different Co/Ni atomic ratios of Co–Ni complexes. The multi-metal sulfides prepared by this method showed better multi-metal ratios than those prepared by the co-precipitating method. The as-synthesized NixCo3−xS4 electrodes for supercapacitors exhibited excellent electrochemical performance and were tuned by adjusting the composition of products. The results of our study provide a novel electroactive material for designing next-generation supercapacitors.Acknowledgements
This work was supported by the National Science Foundation of China (grant no. 21001090, 21443003 and 21103153), and Program for Innovative Research Team (in Science and Technology) in University of Henan Province (grant no. 2012IRTSTHN021) and Education Department of Henan Province (grant no. 13A150648).Notes and references
- (a) S. E. Coe and R. S. Sussmann, Diamond Relat. Mater., 2000, 9, 1726–1729 CrossRef CAS; (b) E. C. Garnett and P. D. Yang, J. Am. Chem. Soc., 2008, 130, 9224–9225 CrossRef CAS PubMed; (c) B. Z. Tian, X. L. Zheng, T. J. Kempa, Y. Fang, N. F. Yu, G. H. Yu, J. L. Huang and C. M. Lieber, Nature, 2007, 449, 885–888 CrossRef CAS PubMed; (d) S. Kim, H. Y. Jeong, S. K. Kim, S. Y. Choi and K. J. Lee, Nano Lett., 2011, 11, 5438–5442 CrossRef CAS PubMed.
- (a) J. A. Zhao, L. W. Mi, J. Y. Hu, H. W. Hou and Y. T. Fan, J. Am. Chem. Soc., 2008, 130, 15222–15223 CrossRef CAS PubMed; (b) H. Aggarwal, P. M. Bhatt, C. X. Bezuidenhout and L. J. Barbour, J. Am. Chem. Soc., 2014, 136, 3776–3779 CrossRef CAS PubMed; (c) T. F. Liu, L. F. Zou, D. W. Feng, Y. P. Chen, S. Fordham, X. Wang, Y. Y. Liu and H. C. Zhou, J. Am. Chem. Soc., 2014, 136, 7813–7816 CrossRef CAS PubMed.
- (a) K. M. Hutchins, T. P. Rupasinghe, L. R. Ditzler, D. C. Swenson, J. R. G. Sander, J. Baltrusaitis, A. V. Tivanski and L. R. MacGillivray, J. Am. Chem. Soc., 2014, 136, 6778–6781 CrossRef CAS PubMed; (b) M. K. Sharma and P. K. Bharadwaj, Inorg. Chem., 2011, 50, 1889–1897 CrossRef CAS PubMed; (c) G. F. Liu, J. Liu, Y. Liu and X. T. Tao, J. Am. Chem. Soc., 2014, 136, 590–593 CrossRef CAS PubMed.
- (a) R. Miyake and M. Shionoya, Inorg. Chem., 2014, 53, 5717–5723 CrossRef CAS PubMed; (b) B. F. Abrahams, A. D. Dharma, M. J. Grannas, T. A. Hudson, H. E. Maynard-Casely, G. R. Oliver, R. Robson and K. F. White, Inorg. Chem., 2014, 53, 4956–4969 CrossRef CAS PubMed; (c) X. N. Cheng, W. X. Zhang and X. M. Chen, J. Am. Chem. Soc., 2007, 129, 15738–15739 CrossRef CAS PubMed.
- (a) L. W. Mi, H. W. Hou, Z. Y. Song, H. Y. Han, H. Xu, Y. T. Fan and S. W. Ng, Cryst. Growth Des., 2007, 7, 2553–2561 CrossRef CAS; (b) L. W. Mi, H. W. Hou, Z. Y. Song, H. Y. Han, H. Xu and Y. T. Fan, Chem. – Eur. J., 2008, 14, 1814–1821 CrossRef CAS PubMed.
- J. L. Atwood, L. J. Barbour, A. Jerga and B. L. Schottel, Science, 2002, 298, 1000–1002 CrossRef CAS PubMed.
- (a) W. T. Wei, L. W. Mi, Y. Gao, Z. Zheng, W. H. Chen and X. X. Guan, Chem. Mater., 2014, 26, 3418–3426 CrossRef CAS; (b) L. Yu, L. Zhang, H. B. Wu and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 3711–3714 CrossRef CAS PubMed.
- (a) R. B. Wu, X. K. Qian, K. Zhou, J. Wei, J. Lou and P. M. Ajayan, ACS Nano, 2014, 8, 6297–6303 CrossRef CAS PubMed; (b) X. H. Cao, B. Zheng, X. H. Rui, W. H. Shi, Q. Y. Yan and H. Zhang, Angew. Chem., Int. Ed., 2014, 53, 1404–1409 CrossRef CAS PubMed; (c) J. C. Bear, N. Hollingsworth, P. D. McNaughter, A. G. Mayes, M. B. Ward, T. Nann, G. Hogarth and I. P. Parkin, Angew. Chem., Int. Ed., 2014, 53, 1598–1601 CrossRef CAS PubMed.
- (a) L. W. Mi, W. T. Wei, Z. Zheng, G. S. Zhu, H. W. Hou, W. H. Chen and X. X. Guan, Nanoscale, 2014, 6, 1124–1133 RSC; (b) D. Merki, H. Vrubel, L. Rovelli, S. Fierro and X. Hu, Chem. Sci., 2012, 3, 2515–2525 RSC; (c) Y. H. Zhang, N. Zhang, Z. R. Tang and Y. J. Xu, Chem. Sci., 2012, 3, 2812–2822 RSC.
- (a) D. P. Cheng, M. A. Khan and R. P. Houser, Cryst. Growth Des., 2004, 4, 599–604 CrossRef CAS; (b) C. Yuan, J. Li, L. Hou, X. Zhang, L. Shen and X. W. Lou, Adv. Funct. Mater., 2012, 22, 4592–4597 CrossRef CAS; (c) X. Y. Liu, Y. Q. Zhang, X. H. Xia, S. J. Shi, Y. Lu, X. L. Wang, C. D. Gu and J. P. Tu, J. Power Sources, 2013, 239, 157–163 CrossRef CAS PubMed; (d) J. F. Li, S. L. Xiong, Y. R. Liu, Z. C. Ju and Y. T. Qian, ACS Appl. Mater. Interfaces, 2013, 5, 981–988 CrossRef CAS PubMed; (e) B. Zhang, X. C. Ye, W. Dai, W. Y. Hou and Y. Xie, Chem. – Eur. J., 2006, 12, 2337–2342 CrossRef CAS PubMed; (f) M. Wirtz and C. R. Martin, Adv. Mater., 2003, 15, 455 CrossRef CAS; (g) C. Q. Shang, S. M. Dong, S. Wang, D. D. Xiao, P. X. Han, X. G. Wang, L. Gu and G. L. Cui, ACS Nano, 2013, 7, 5430–5436 CrossRef CAS PubMed; (h) D. L. Legrand, H. W. Nesbitt and G. M. Bancroft, Am. Mineral., 1998, 83, 1256–1265 CAS.
- J. Yang, X. Duan, Q. Qin and W. Zheng, J. Mater. Chem. A, 2013, 1, 7880 CAS.
- (a) Y. H. Xiao, S. J. Liu, F. Li, A. Q. Zhang, J. H. Zhao, S. M. Fang and D. Z. Jia, Adv. Funct. Mater., 2012, 22, 4052–4059 CrossRef CAS; (b) L. B. Kong, C. L. Mao, C. Liu, Y. C. Luo and L. Kang, J. Solid State Electrochem., 2013, 17, 1463–1471 CrossRef CAS PubMed.
- (a) H. Pang, Q. Y. Lu, Y. Z. Zhang, Y. C. Li and F. Gao, Nanoscale, 2010, 2, 920–922 RSC; (b) H. Pang, Q. Y. Lu, Y. C. Li and F. Gao, Chem. Commun., 2009, 7542–7544 RSC; (c) C. C. Yu, L. X. Zhang, J. L. Shi, J. J. Zhao, J. H. Gao and D. S. Yan, Adv. Funct. Mater., 2008, 18, 1544–1554 CrossRef CAS.
- W. Y. Ko, L. J. Chen, Y. H. Chen, W. H. Chen, K. M. Lu, J. R. Yang, Y. C. Yen and K. J. Lin, J. Phys. Chem. C, 2013, 117, 16290–16296 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S4 (IR spectra, SEM image, HRTEM image, SAED pattern, and XPS spectra) and the galvanostatic charge–discharge curve of the part of obtained samples. See DOI: 10.1039/c4qi00088a |
| This journal is © the Partner Organisations 2014 |