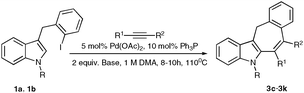
Pd-catalyzed cascade carbopalladation-annulation reaction of 3-(2-iodobenzyl)-indoles into fused 6/5/7/6- and 6/5/5/6- heterocyclic systems†
Natalia
Chernyak
,
David
Tilly
,
Zhou
Li
and
Vladimir
Gevorgyan
*
Department of Chemistry, University of Illinois, 845 West Taylor Street, Chicago, IL 60607, USA. E-mail: vlad@uic.edu; Fax: (+1)-312 996 0431; Tel: (+1)-312-355-3579
First published on 17th November 2009
Abstract
Polycyclic indole structures, possessing fused seven-membered rings were efficiently synthesized via the Pd-catalyzed intramolecular carbopalladation-annulation of 3-(2-iodobenzyl)-indoles and alkynes. A remarkable base effect on the chemoselectivity of this transformation has been found: switching from Et3N to CsOAc completely reverses the reaction path to intramolecular cyclization forming fused five-membered rings.
Polycyclic derivatives of indole have received increasing attention due to their high importance in modern science, particularly in bio-related fields.1 Tricyclic and tetracyclic derivatives of indole, possessing seven-membered rings, are used as direct precursors in synthesis of anti-histaminic and anti-inflammatory compounds.1h–k Although numerous methods have been developed for assembly of these polycyclic frameworks, they are generally lengthy or require employment of stoichiometric amounts of organometallic reagents, which largely limits their scope.2–4 Accordingly, development of alternative catalytic methods for assembly of these important heterocyclic cores is justified. Herein we wish to report the Pd-catalyzed intermolecular cascade annulations reactions of iodobenzyl derivatives of indole 1 with alkynes leading to the formation of polycyclic fused seven-membered heterocycles 2 and five-membered structures 3.
We envisioned that the intermolecular cascade arylation/cyclization reaction of haloaryl heterocycles 1 with alkynes might be an attractive alternative for construction of fused polycyclic compounds possessing seven-membered rings 2 (Scheme 1). The palladium-catalyzed intermolecular reaction of o-halobiaryls with alkynes was developed by Larock.5 This approach represents a powerful approach for construction of fused aromatic systems. The reaction is known to proceed efficiently on polysubstituted electron-rich o-halobiaryls, however, to the best of our knowledge, this annulations strategy has never been used for construction of fused heterocyclic frameworks. Thus, we explored this concept toward assembly of fused derivatives of indole.
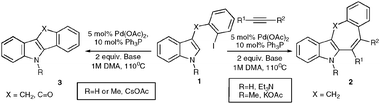 | ||
| Scheme 1 Intramolecular cascade annulation reaction of indole derivatives. | ||
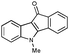
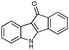
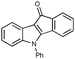
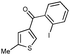
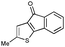
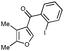
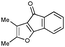
First, 3-benzoyl derivative 4a was examined in arylation/cyclization reaction with 5-decyne (Scheme 2). However, formation of the targeted seven-membered heterocycle 5 was not observed. Instead, tetracyclic indenone 3a, a product of intramolecular arylation, was obtained in 80% yield. The related reaction on indole derivatives is precedented. Thus, it was reported that isomeric 2-benzoyl indole undergoes intramolecular cyclization under palladium catalysis to produce indenoindolone.6 We decided to explored the scope of this novel mode of intramolecular arylation reaction (Table 1). Thus, cyclization of 4a provided slightly better yields of 3a (entry 1) compared to that in the presence of alkyne (Scheme 2). It was found that unprotected indole 4b, in the presence of 5 mol% of PdCl2(Ph3P)2 and CsOAc, produces indeno[1,2]indole-10(5H)-one (3b) in excellent yield (entry 2). N-Phenyl indole derivative 4c cyclized uneventfully, as well. Analogously, carbonyl derivatives of thiophene (entry 4), and furan (entry 5) produced tetracyclic indenones in very good yields.
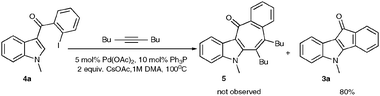 | ||
| Scheme 2 Reaction of 2-benzoyl indole 4a with 5-decyne. | ||
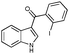
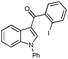
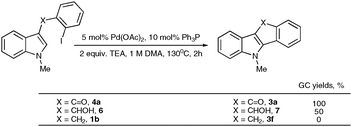
To gain insight into the origins of high propensity of carbonyl derivatives toward intramolecular arylation, we evaluated arylation of differently-tethered indole derivatives 4a, 6, and 1b (Scheme 3). It was found that the carbonyl derivative 4a (X = CO) underwent the fastest intramolecular arylation reaction producing indeno[1,2]indole-10(5H)-one 3a in 100% GC yield. Hydroxymethyl derivative 6 gave 50% yield of 7, whereas no product formation was observed in the case of benzyl derivative 1b (X = CH2) (Scheme 3). These observations imply that the electronic effect of a carbonyl group at the C-3 position accounts for the efficiency of carbonyl derivatives in intramolecular cyclization over the intermolecular arylation/cyclization reaction.7 Based on these results, we reasoned that 3-benzyl indole derivative 1b, unreactive in intramolecular arylation, might be a suitable substrate for intermolecular carbopalladation/annulations cascade toward polycyclic frameworks possessing seven-membered rings.
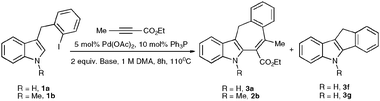
Next, the influence of different bases on the chemoselectivity of this reaction was examined. When 3-benzyl derivative 1a was subjected to the reaction with methyl but-2-yanoate, in the presence of a catalytic amount of PdCl2(Ph3P)2 and 2 equivalents of CsOAc, the desired tetracycle 2a was not formed at all (Table 2). Employment of KOAc resulted in formation of equal amounts of 2a and 3f (entry 2). Finally, use of triethylamine led to exclusive formation of the desired heterocycle2apossessing a seven-membered ring (entry 3).
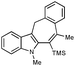
Analogous annulations of NMe indole derivative 1b in the presence of several bases have been examined, as well. Employment of CsOAc led to formation of seven-membered tetracycle 2b accompanied by a comparable amount of its chemoisomer 3f. Surprisingly, switching to KOAc resulted in excellent chemoselectivity of arylation/cyclization of 1b with methylbut-2-yanoate (entry 5).
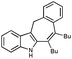
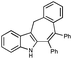
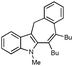
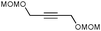
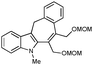
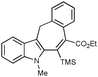
With the optimized conditions in hand, we examined the scope of the cascade transformation of indoles 1a and 1b. To our delight, a variety of alkynes, possessing electron-rich and electron-deficient triple bonds, worked well in this annulations reaction, leading to the formation of fused seven-membered heterocycles in good yields (Table 3). Thus, reaction of 1a with 5-decyne led to the formation of 2c in 98% yield (entry 1). Annulation with diphenylacetylelene produced polyaromatic tetracycle 2d in 78% yield (entry 2). Protected indole derivatives 1b showed somewhat lower reactivity. Thus, reaction of 1b with 5-decyne gave 2e in 70% isolated yield (Table 3, entry 3). Reaction with MOM protected but-2-yne-1,4-diol led to the formation of 2f in 76% yield (entry 4). Ethyl 3-(trimetylsilyl)prop-2-ynoate and trimetylsilylprop-2-yne smoothly reacted with N-methyl indole derivative 1b leading to the formation of tetracycles 2g and 2h, bearing a trimethylsilyl group at the seven-membered ring (entries 5 and 6). On the other hand, reaction of 1b with bistrimethylsilylacetylene produced monodesilylated 2i (entry 7). Analogously, reaction of 1b with aryltrimethylsilyl alkynes resulted in formation of monodesilylated seven-membered tetracycles 2j and 2k (entries 8 and 9).9 In all cases, formation of a single regioisomer was observed.
It is believed that the mechanism of the presented herein intermolecular cascade arylation/cyclization reaction of the indole derivatives follows the path proposed by Larock for the cascade cyclization of o-haloaryls.5 It starts with the oxidative addition of the Pd(0) to iodobenzylindole 1a generating the arylpalladium complex 8 (Scheme 4). Carbopalladation of the alkyne triple bond produces vinylpalladium intermediate 9, which undergoes intermolecular electrophilic carbopalladation at the C-2 position of the indole to form palladacycle 10. The subsequent reductive elimination, produces product 2 and regenerates the Pd(0) catalyst. On the other hand, complex 8, in the presence of CsOAc, may undergo a direct intramolecular cyclization at the C-2 position via a palladacycle 1110 to give 3b.
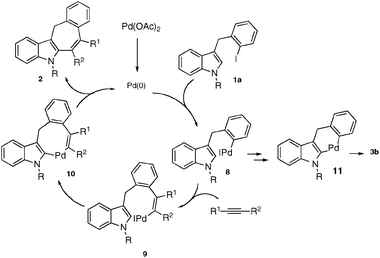 | ||
| Scheme 4 Proposed mechanism. | ||
The kinetic isotope effect studies employing 1b and 1b–d revealed the value of kH/kD = 1.1, thus supporting the suggested above electrophilic nature of the cyclization step (Scheme 5).7
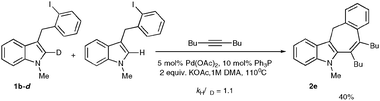 | ||
| Scheme 5 The kinetic isotope effect studies. | ||
In conclusion, we have developed an efficient methodology for construction of fused polycyclic indole heterocycles from easily available starting materials via the palladium-catalyzed cascade annulations/cyclization reaction with both electron rich and electron deficient alkynes. Remarkably, it was found that the base has a dramatic effect on inter- and intramollecular cyclizations leading to seven- or five-membered fused ring systems. In contrast, acyl-tethered substrates, regardless of the base used, undergo intramolecular cyclization to produce five-membered fused structures exclusively.
We are grateful to the National Institutes of Health for funding (Grant GM-64444).
Notes and references
- For reviews on biologically important indole derivatives, see: (a) S. E. Lewis, Tetrahedron, 2006, 62, 8655 CrossRef CAS; (b) T. Higuchi and T. Kawasaki, Nat. Prod. Rep., 2007, 24, 843 RSC.
- I. A. Kashulin and I. E. Nifant’ev, J. Org. Chem., 2004, 69, 5476 CrossRef CAS.
- For radical cyclization reactions in synthesis of polycyclic indole derivatives, see, for example: (a) S. R. Flanagan, D. C. Harrowven and M. Bradley, Tetrahedron Lett., 2003, 44, 1795 CrossRef CAS; (b) M. L. Bennasar, T. Roca, R. Griera and J. Bosch, J. Org. Chem., 2001, 66, 7547 CrossRef CAS.
- For Pd-catalyzed synthesis of fused nitrogen containing heterocycles, see: (a) G. Zeni and R. C. Larock, Chem. Rev., 2004, 104, 2285 CrossRef CAS; (b) C. Xie, Y. Zhang, Z. Huang and P. Xu, J. Org. Chem., 2007, 72, 543; (c) T. Harayama, T. Akiyama, H. Akamutsu, K. Kawano, H. Abe and Y. Takeuchi, Synthesis, 2001, 444 CrossRef CAS; (d) L.-C. Campeau, M. Parisien, A. Jean and K. Fagnou, J. Am. Chem. Soc., 2006, 128, 581 CrossRef CAS; (e) H. Zhang and R. C. Larock, J. Org. Chem., 2003, 68, 5132 CrossRef CAS; (f) M. A. Campo and R. C. Larock, J. Org. Chem., 2002, 67, 5616 CrossRef CAS; (g) C. Bressy, D. Alberico and M. J. Lautens, J. Am. Chem. Soc., 2005, 127, 13148 CrossRef CAS . See also ref. 9.
- R. C. Larock, M. J. Dolty, Q. Tian and J. Zenner, M., J. Org. Chem., 1997, 62, 7536 CrossRef CAS.
- A. P. Kozikowski and D. Ma, Tetrahedron Lett., 1991, 32, 3317 CrossRef CAS.
- For reviews discussing different mechanisms of Pd-catalyzed arylation of heteroaromatic compounds, see: (a) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173 RSC; (b) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS; (c) G. Zeni and R. C. Larock, Chem. Rev., 2006, 106, 4644 CrossRef CAS; See also: (d) S. Chuprakov, N. Chernyak, A. S. Dudnik and V. Gevorgyan, Org. Lett., 2007, 9, 2333 CrossRef CAS; (e) H. A. Chiong and O. Daugulis, Org. Lett., 2007, 9, 1449 CrossRef CAS; (f) N. R. Deprez, D. Kalyani, A. Krause and M. S. Sanford, J. Am. Chem. Soc., 2006, 128, 4972 CrossRef CAS; (g) D. R. Stuart, E. Villemure and K. Fagnou, J. Am. Chem. Soc., 2007, 129, 12072 CrossRef CAS; (h) S. I. Gorelsky, D. Lapointe and K. Fagnou, J. Am. Chem. Soc., 2008, 130, 10848 CrossRef CAS; (i) D. Garcia-Cuadrado, P. de Mendoza, A. A. C. Braga, F. Maseras and A. M. Echavarren, J. Am. Chem. Soc., 2007, 129, 6880 CrossRef CAS; (j) Z. Zhao, A. Jaworski, I. Piel and V. Snieckus, Org. Lett., 2008, 10, 2617 CrossRef CAS; (k) N. Lebrasseur and I. Larrosa, J. Am. Chem. Soc., 2008, 130, 2926 CrossRef CAS; (l) X. Wang, D. V. Gribkov and D. Sames, J. Org. Chem., 2007, 72, 1476 CrossRef CAS.
- General procedure for the palladium-catalyzed annulation-cyclization reaction of 3(2-iodobenzyll)indole derivatives with alkynes: to a solution of 10 mmol 3(2-iodobenzyll)indole derivative in 1 mL of DMA was added PdCl2(Ph3P)2 (5 mol%), 20 mmol of the corresponding base (see Table 3 for details) and 20 mmol of alkyne under inert atmosphere. The reaction was stirred at 110 °C until full consumption of starting indole derivative (8–10 h). When it was completed the resulting solution was diluted with water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic extracts were dried over the sodium sulfate and concentrated under reduced pressure. Resulting crude product was purified via column chromatography on silica gel (Hexanes/EtOAc).
- For examples on desilylation in palladium catalyzed processes, see: (a) R. C. Larock, E. K. Yum and M. D. Refvic, J. Org. Chem., 1998, 63, 7652 CrossRef CAS; (b) M. L. Crawley, I. Goljer, D. J. Jenkin, J. F. Mehlmann, L. Nogle, R. Dooley and P. E. Mahaney, Org. Lett., 2006, 8, 5837 CrossRef CAS.
- It has been proposed that employment of OAc-containing bases facilitates inter- or intramolecular deprotonation leading to palladacycle intermediates. See ref. 4c and 7i.
Footnote |
| † This article is part of a ChemComm ‘Catalysis in Organic Synthesis’ web-theme issue showcasing high quality research in organic chemistry. Please see our website (http://www.rsc.org/chemcomm/organicwebtheme2009) to access the other papers in this issue. |
| This journal is © The Royal Society of Chemistry 2010 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: