Soft X-ray activated NaYF4:Gd/Tb scintillating nanorods for in vivo dual-modal X-ray/X-ray-induced optical bioimaging†
Xiaolong
Li
,
Zhenluan
Xue
,
Mingyang
Jiang
,
Youbin
Li
,
Songjun
Zeng
 * and
Hongrong
Liu
*
* and
Hongrong
Liu
*
College of Physics and Information Science, and Key Laboratory of Low-dimensional Quantum Structures and Quantum Control of the Ministry of Education, and Synergetic Innovation Center for Quantum Effects and Applications, Hunan Normal University, Changsha 410081, Hunan, China. E-mail: songjunz@hunnu.edu.cn; hrliu@hunnu.edu.cn
First published on 23rd November 2017
Abstract
Lanthanide (Ln) nanocrystals using soft X-ray as an excitation source have received significant research interest due to the advantages of unlimited penetration depth of X-ray light. In this study, we demonstrated an efficient scintillator based on NaYF4:Gd nanorods (denoted as NRs) doped with different contents of terbium (Tb) ions for optical bioimaging under X-ray irradiation. The experimental results showed that the emission intensity was correlated to the doping contents of Tb3+, and the largest emission intensity was achieved by doping 15% Tb under excitation by soft X-ray light. In addition, the emission intensity of the as-prepared NRs can be significantly improved by increasing the excitation power and irradiation times of the X-ray. Owing to the efficient X-ray-induced emission, these NRs were successfully used as probes for X-ray-induced optical bioimaging with high sensitivity. In addition, the dual-modal X-ray imaging and X-ray induced optical bioimaging were performed on a mouse, which indicated that the NRs were promising dual-modal bioprobes. Therefore, the X-ray activation nature of the designed NRs makes them promising probes for biomedicine and X-ray-induced photodynamic therapy (PDT) applications owing to the unlimited penetration depth of X-ray excitation source and absence of autofluorescence.
1. Introduction
Optical imaging systems detect photon information and have presented potential applications in the biomedical fields.1–5 Significant improvements in the sensitivity and accuracy of these systems towards their applications in molecular and cellular biology or in vivo diagnostic are highly dependent on well-developed optical probes such as organic dyes, fluorescent proteins, and quantum dots.6–13 Although the traditional fluorescent materials for in vivo optical imaging have been successfully achieved, there are still numerous disadvantages. The traditional fluorescent materials using high-energy excitation photons, such as ultra-violet (UV) or blue light, suffer from serious drawbacks: low signal-to-noise ratio, considerable absorption and scattering effects, resulting in short penetration depth in bio-tissues. Moreover, excitation with high energy photon may induce damage to the biological tissues.14–16 To overcome these difficulties, significant efforts have been made towards finding a novel excitation light source with deeper penetration. The application of near infrared (NIR) light cannot only realize the deeper penetration depth but also efficiently suppress autofluorescence and light scattering as compared to the traditional optical sources using high energy light such as UV, blue or visible light.17–20 In recent years, lanthanide (Ln)-doped upconversion nanomaterials as biomarkers based on NIR excitation have attracted wide interest owing to their attractive merits involving low autofluorescence, narrow emission bandwidths, long lifetime, superior photostability, as well as low toxicity.21–35 However, the biological applications of upconversion probes excited by 980 nm laser are still limited by the low luminescence efficiency and overheating phenomenon caused by the large absorption of water in biotissues.36 Therefore, the development of a more complementary and effective optical imaging system activated by a new type of excitation source is urgent. Radioluminescence bioimaging has attracted attention as it utilizes high-energy rays (such as X-rays, gamma rays or beta rays) as the excitation source due to their nature of unlimited penetration depth.37–42 Recently, Ln element-based agents for radioluminescence bioimaging have received special research attention. And, a number of solid-state inorganic materials, such as Gd2O3:Eu3+, Y2O2S:Eu3+/Dy3+, Gd2O2S:Eu3+, and Gd2O2S:Tb3+, with Ln elements were developed as efficient X-ray-to-light convertors.43–46 However, neither the synthesis conditions nor the morphology of these materials were easy to control. As compared to the case of these oxide nanoparticles, not only surface modification but also the control of the size/morphology can be easily achieved using Ln-doped fluoride nanoparticles. However, these Ln-based fluoride nanoparticles have been mainly developed as down-conversion and up-conversion optical nanoprobes, and there are less studies on fluoride scintillating materials for X-ray induced optical bioimaging.47,48In this study, NaYF4:Gd/Tb NRs as X-ray phosphors were fabricated through a hydrothermal route. The crystal phase and structure of the as-synthesized NaYF4:Gd/Tb NRs were characterized by X-ray diffraction (XRD) and transmission electron microscopy (TEM), respectively. The optical spectra of NaYF4:40% Gd NRs doped with different contents of Tb3+ were carefully studied. Moreover, the in vitro and in vivo X-ray-induced optical imaging were demonstrated in detail. Furthermore, the penetration depth-related effectiveness of these X-ray phosphors was further studied. These results reveal that the designed X-ray-activated NRs can be used as ideal contrast agents for biological fields.
2. Experimental
2.1 Chemicals and materials
LnCl3·6H2O (Ln = Y, Gd, and Tb) was purchased from Sinopharm Chemical Reagent Co., Ltd (China). The materials were further dissolved in de-ionized water (DI-water) to form an aqueous solution. All other chemicals, such as anhydrous alcohol, oleic acid (OA), NaOH, and NaF, were used directly without further purification.2.2 Synthesis of hydrophobic Tb3+-doped NaYF4:40% Gd NRs
Monodispersed hydrophobic NaYF4:40% Gd/X% Tb (X = 1, 2, 5, 10, 15, 25, and 35) NRs were synthesized via the following modified hydrothermal method using the stabilizing agent OA.49 At first, 1.2 g of NaOH and 2 mL of DI-water were mixed together to obtain a transparent solution under stirring at room temperature. Further, 10 mL of anhydrous alcohol and 20 mL of OA were added to the aforementioned solution to form a sodium–OA complex under stirring for about 20 minutes. After this, 1 mmol of LnCl3 (Ln = Y, Gd, and Tb) with designed concentrations and molar ratios and 8 mL of NaF aqueous solution (1.0 M) were added slowly. Then, the obtained mixtures were stirred for another 10 minutes. Finally, the mixtures were transferred into a 50 mL stainless Teflon-lined autoclave and maintained at 190 °C for 24 h. After reaction, the system was naturally cooled down to room temperature. The prepared samples were obtained by centrifugation and washed with ethanol. The above procedure was repeated at least three times for removing the residual solvents.2.3 Synthesis of NaYF4:40% Gd/15% Tb LF-NRs
The ligand free NRs (denoted as LF-NRs) were achieved via a HCl treatment method.50 In a typical procedure, 100 mg of the as-prepared NaYF4:40% Gd/15% Tb NRs were mixed with 20 mL of DI-water, and then, a certain amount of dilute HCl solution was slowly added to the system to maintain the pH value approximately at 4. After the mixture was stirred for 2 h, the solution was washed with anhydrous alcohol, and the LF-NRs were obtained by centrifugation.2.4 Characterizations
The crystal phase of the as-prepared NRs was detected by powder XRD using a D/max-γA system X-ray diffractometer at 40 kV and 250 mA with Cu-Kα radiation (λ = 1.5406 Å). The as-prepared samples were characterized by a TEM operating at 200 kV (FEI Tecnai F20) equipped with an energy-dispersive X-ray spectrometer (EDS). The existing elements in the samples were further determined by X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific Escalab 250Xi). The surface ligands of the oleate-capped NRs and LF-NRs were detected via Fourier transform infrared (FTIR) spectroscopy using a Magna 760 spectrometer (Nicolet). The size distribution of LF-NRs was measured in an aqueous solution at 25 °C using a dynamic light scattering (DLS) instrument (Zetasizer Nano ZS, Malvern). Photoluminescence excitation/emission spectra of these powder samples were obtained using a Zolix Analytical Instrument (fluoroSENS 9000 A) at room temperature. The fluorescence imaging of HeLa cells treated with the LF-NRs (200 μg mL−1) was detected in the green emission channel under the excitation of about 488 nm using a fluorescence microscope (Operetta CLS, PerkinElmer).2.5 In vitro cytotoxicity assay
The cytotoxicity of the samples was determined using 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT) proliferation assay.51 HeLa cells were cultured in a 96-well micro-plate at 37 °C under 5% CO2. Then, the cell culture medium in each well was added with different concentrations of LF-NRs (0, 50, 100, 250, 500, and 1000 μg mL−1). Subsequently, the cells were incubated for another 24 h in an incubator at 37 °C under 5% CO2. Thereafter, the cell toxicity was evaluated by the MTT method. The cell viability (%) was calculated according to the following formula: [Abs.490-(treated cells) − background]/[Abs.490-(untreated cells) − background] × 100%.2.6 In vitro phantom X-ray-induced optical imaging
In vitro phantom X-ray-induced optical bioimaging was performed using a multi-modal in vivo imaging system (Bruker In Vivo FX Pro) equipped with a detecting CCD (ML4002, Finger Lakes Instrumentation, USA). Aqueous solutions with the same concentration of NaYF4:40% Gd NRs doped with different contents of Tb3+ were transferred into 96-well tubes for X-ray-induced in vitro optical bioimaging with various excitation powers (25 kVp–45 kVp) and exposure times (1–4 min).2.7 In vivo X-ray-activated optical bioimaging
Kunming mice were anesthetized through intraperitoneal injection of a pentobarbital sodium aqueous solution (10 wt%, 100 μL), and then subcutaneously injected with LF-NRs (150 μL, 2 mg mL−1). After this, X-ray-induced bioimaging was conducted through a multi-modal in vivo imaging system under X-ray (45 kV, 2 min) excitation at room temperature. All animal procedures in this study were performed in accordance with the Guidelines for Care and Use of Laboratory Animal Center of Hunan Normal University and approved by the Animal Ethics Committee of Hunan Province.2.8 In vivo dual-modal X-ray and X-ray-induced optical bioimaging
To validate the feasibility of NRs for dual-modal bioimaging, a Kunming mouse weighing about 20 g was anesthetized with a pentobarbital sodium aqueous solution (100 μL, 10 wt%) and then intraperitoneally injected with LF-NRs (8 mg mL−1, 200 μL). For in vivo imaging, the experimental mouse was detected using a multi-modal in vivo imaging system. The measurements of optical imaging in this part were performed under 2 min irradiation of X-ray at 45 kVp.2.9 Simulation experiment of deep penetration of X-ray and X-ray-induced green emission
To demonstrate the deep-tissue excitation nature of X-ray and X-ray-induced green emission, a series of simulation experiments were performed by placing pork tissue with different thicknesses between the X-ray light sources and CCD detector. Similar to the in vitro phantom X-ray-induced optical imaging, the simulation experiments on deep penetration depth of X-ray were conducted at various excitation voltages and X-ray exposure times. The signal to noise ratio (SNR) of different experimental tests was calculated by the mean intensity of the optical signal and background.52 Moreover, simulation experiments about emission-dependent penetration depth of NRs were performed by placing pork tissue with different thicknesses between samples and the CCD detector. Furthermore, both excitation/emission-related penetration depth properties of NRs were demonstrated by adding pork tissue between the X-ray light sources, samples, and CCD detector.3. Results and discussion
3.1 Phase and microstructure of NRs
Fig. 1 shows the typical XRD results of the as-prepared NRs. As revealed in Fig. 1, all the characteristic diffraction positions of NaYF4:40% Gd/X% Tb (X = 1, 2, 5, 10, 15, 25, and 35) NRs can be indexed as the standard hexagonal phase NaYF4 (JCPDS card no. 16-0334). No other impurity peaks were detected; this indicated that the pure hexagonal phase NRs were obtained from the hydrothermal condition at 190 °C for 24 h. It is noted that the diffraction peak shifts slightly towards the lower angle side owing to the substitution of smaller Y3+ (r = 1.159 Å) by the relatively larger Tb3+ (r = 1.18 Å).53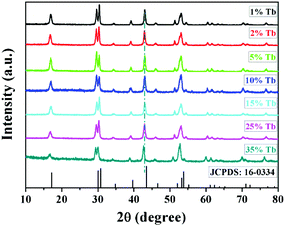 | ||
| Fig. 1 The typical XRD patterns of the as-prepared NaYF4:40% Gd/X% Tb (X = 1, 2, 5, 10, 15, 25, and 35) NRs and the standard pure hexagonal phase NaYF4 (JCPDS no. 16-0334). | ||
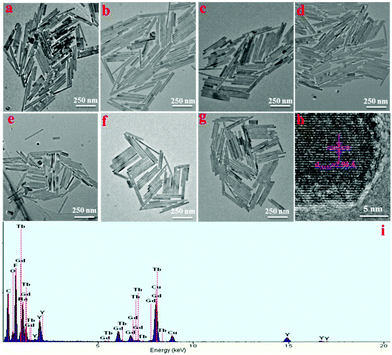
The morphology and structure of NaYF4:Gd/Tb NRs were further characterized by TEM. As shown in Fig. 2, uniform and monodispersed rod-like nanocrystals were observed, and no obvious morphology change was observed upon doping different amounts of Tb3+. The sizes (average lengths and widths) of NaYF4:40% Gd NRs doped with various Tb concentrations were evaluated based on the TEM assay (see Table S1†). Obviously, the Tb-doping has no obvious effect on the size of the NRs when 40% Gd3+ is doped. Fig. 2 h shows the typical high-resolution TEM image of an individual nanorod obtained from NaYF4:40% Gd/15% Tb, and the interplanar distance is measured to be 2.96 Å, corresponding to the d-spacing value of the (110) crystal plane of the hexagonal-phase NaYF4. The main elements detected from EDS (Fig. 2g) were C, Na, Y, Gd, Tb, and F. Notably, the signal of Cu was attributed to the TEM copper grid. According to the XPS test (Fig. S1†), not only all the component elements (Na, Y, Gd, Tb, and F) were observed but also the characteristic Tb and Gd peaks were distinguished, further validating the successful formation of NaYF4:Gd/Tb NRs. The surface functional groups of oleate-capped NRs and HCl-treated NRs (LF-NRs) were measured by FTIR spectra. As presented by the black curve in Fig. S2† (oleate-capped NRs), the broad and strong bands centered at 2933 cm−1 and 2858 cm−1 are ascribed to the symmetric stretching of –CH2. The other two bands at 1564 cm−1 and 1465 cm−1 are attributed to the symmetric (δas) and the asymmetric –COO– (δs) stretching, respectively. On the other hand, after HCl treatment (red curve), the symmetric (δas)/asymmetric –COO– (δs) and symmetric stretching of –CH2 are almost disappeared; this validates the successful removal of the oleate ligands grafted on the surface of the as-synthesized NRs.50 Additionally, DLS analysis was performed to evaluate the size distribution of LF-NRs. As exhibited in Fig. S3,† the average hydrodynamic diameter of the LF-NRs was measured to be about 310 nm, which matched well with the size detected by the TEM.
3.2 Optical properties of the as-synthesized NaYF4 NRs
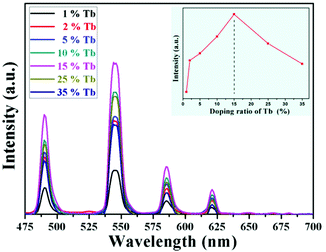
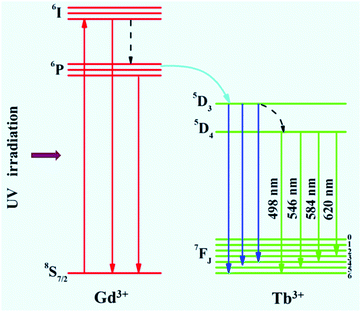
The optical properties of the as-prepared NaYF4:40% Gd/X% Tb were detected using a spectrophotometer (fluoroSENS 9000 A) under UV irradiation at 273 nm at room temperature. Fig. 3 shows the variation in the emission intensity of NaYF4:40% Gd NRs with an increase in the amounts of Tb3+ from 1% to 35%. As shown in Fig. 3, all the emission spectra of NRs presented four typical emission bands centered at 489 nm, 546 nm, 584 nm, and 620 nm of Tb3+. At first, the emission intensity was remarkably enhanced by increasing the doping content of Tb3+ from 1% to 15%. With a further increase in the doping concentration of Tb (from 15% to 35), the emission intensity did not increase, but decreased. The results demonstrated that the sample doped with 15% Tb possessed largest emission intensity (inset of Fig. 3). The remarkably enhanced emission intensity may be attributed to the efficient energy transfer between adjacent Tb3+ due to the decreased distance between Tb3+. However, the excessive doping of Tb may cause concentration quenching, leading to a decrease in emission intensity.54 Therefore, the emission intensity change of NRs can be mainly attributed to the Tb doping because of the same size of NRs doped with different contents of Tb.To reveal the mechanism of UV excitation, the excitation spectrum of the 15% Tb3+-doped NaYF4:40% Gd NRs was obtained by monitoring the 545 nm emission of Tb. As shown in Fig. S4,† the excitation spectrum presents the characteristic peaks of both Gd3+ and Tb3+. The excitation peaks centred at 304, 319, 341, 352, 369, and 378 nm were attributed to the electron transitions from ground state (7F6) to the different excited states 5H6, 5D0, 5G2, 5D2, 5G6, and 5D3 of Tb, respectively.54,55 The sharp excitation bands at 274 nm and 310 nm are attributed to the 8S7/2 → 6I and 8S7/2 → 6P electron transitions of Gd3+, respectively, indicating the efficient energy transfer from Gd3+ to Tb3+, which is analogous to the previous study on Tb3+ in NaGdF4 host.56 As demonstrated in the corresponding energy level diagram (Fig. 4), under the excitation of UV light (273 nm), the incident photons are absorbed by Gd3+, and then, the energy transfer from Gd3+ to Tb3+ occurs, resulting in the promotion of the electrons of Tb3+ from the level of 7J6 (ground state) to the excited states. Then, the corresponding visible light emissions from 489 to 620 nm are observed after irradiative relaxation from 5D4 to the lower energy levels of 7FJ (J = 3, 4, 5, and 6). Therefore, the four emission peaks at 489, 546, 584, and 620 nm were attributed to the 5D4 → 7F6, 5D4 → 7F5, 5D4 → 7F4, and 5D4 → 7F3 transitions of Tb3+, respectively. Under X-ray excitation, we speculated that the mechanism of X-ray-activated optical luminescence in our study was mainly attributed to the X-ray excitation energy absorbed by NaYF4:Gd host and Tb due to the L-edge or K-edge absorption of Gd, Tb, and Y (GdL-edge: 8.343 keV, TbL-edge: 8.679 keV and YL-edge: 2.372 keV; YK-edge: 17.02 keV) to generate electron–hole pairs in the host lattice; this was similar to the absorption of X-ray excitation energy by L-edge of Eu3+ for radioluminescence.46,57–59 Moreover, the energy transfer from electro–hole pairs to Tb3+ luminescent center occurs, subsequently resulting in the electronic transition 5D4 → 7Fj (j = 6–3) of Tb3+ and finally producing X-ray-induced downconversion luminescence of Tb (this process is similar to UV excitation, which differs with excitation source). However, further study is needed to unravel the detailed X-ray-activated luminescence mechanism.
3.3 In vitro cell toxic evaluation
Before their application in bioimaging, the cytotoxicity of NaYF4:40% Gd/15% Tb LF-NRs was evaluated by the MTT assay. As demonstrated in Fig. 5, the cell viability was above 96% when the concentration of the as-synthesized NRs was 50 μg mL−1. Although the concentration was increased from 100 to 500 μg mL−1, the cellular viability was almost unchanged. With a further increase in the concentration of LF-NRs, the cell viability was reduced slightly. However, even when the concentration was at a relatively high dose of 1000 μg mL−1, the cell viability was still maintained as much as 85%, indicating the low cytotoxicity of LF-NRs. Furthermore, the cell imaging was obtained by a fluorescence microscope after the cells were incubated with the LF-NRs for 4 h at 37 °C under 5% CO2. As shown in Fig. S5b,† a green signal was observed on the surface of HeLa cells, indicating the successful uptake of LF-NRs in the HeLa cells. These results demonstrated that the as-prepared LF-NRs were promising candidates for optical bioimaging with low cytotoxicity.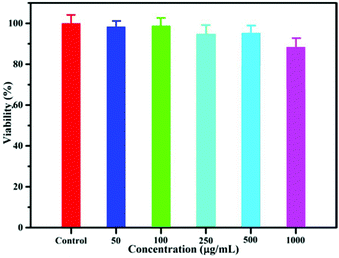 | ||
| Fig. 5 In vitro HeLa cell viability after incubation with various concentrations of LF-NRs for 24 h at 37 °C under 5% CO2. | ||
3.4 Phantom X-ray-induced optical imaging with different excitation powers and exposure times
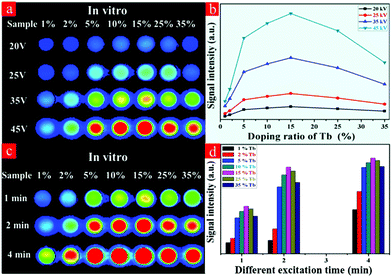
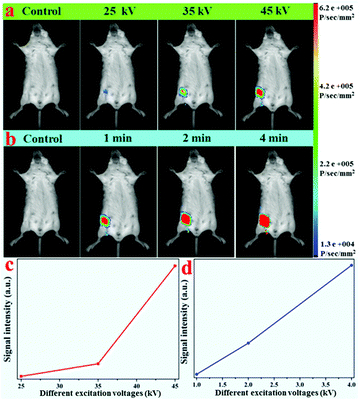
In an attempt to detect the possibility of the as-prepared NRs as optical nanoprobes, the in vitro phantom experiments were carried out using a multi-modal in vivo imaging system. A series of 96-well tubes containing LF-NRs solutions were arrayed in sequence. The green emissions were obtained under the excitation of X-ray light. As demonstrated in Fig. 6a, the X-ray-activated optical signals were gradually increased by increasing the contents of Tb3+ at the same excitation power and exposure time; this was consistent with the aforementioned optical studies. To further reveal the effect of excitation power and exposure time on the emission intensity, these LF-NRs were activated under X-ray radiation by adjusting the excitation voltages (from 20, 25, 35 to 45 kVp) and exposure times (from 1, 2 to 4 min). As demonstrated in Fig. 6a, the emission intensity was significantly enhanced by improving the voltage and exposure time for the same content of Tb3+. As illustrated in Fig. 6b, the average emission intensity was highly dependent on the excitation voltages of X-ray, which further indicated that higher voltage favored the X-ray-induced green emission in the NaY/GdF4 host. Moreover, the NRs doped with 15% Tb possess the largest radioluminescence intensity, which matches well with the result of the spectrum. With an increase of the X-ray exposure time, the intensity of the emitting signal was also significantly increased (Fig. 6c and d); this indicated that the longer the exposure time, the stronger the X-ray-activated green emission signal.3.5 In vivo X-ray-induced optical bioimaging
Due to the efficient X-ray-activated green emissions, these LF-NRs were used as X-ray-irradiated optical bioprobes for in vivo bioimaging. With the purpose of validating the X-ray-induced optical imaging based on these as-synthesized NRs, an aqueous solution containing LF-NRs (0.15 mL, 2 mg mL−1) was injected subcutaneously into a mouse. After injection, optical images of the mouse were obtained at different operating voltages and different X-ray excitation times. Fig. 7a presents the typical in vivo X-ray-induced optical bioimaging of a mouse under different excitation voltages. As demonstrated in Fig. 7a, no signals of the whole body were observed without injection of LF-NRs, whereas significant signals were observed after administration of LF-NRs under X-ray irradiation. In addition, by increasing the X-ray excitation voltages, the signal intensity was also gradually increased. To show the phenomenon more clearly, the average signal intensity under different excitation voltages is demonstrated in Fig. 7c, further validating that the higher voltages facilitate the green emission. To reveal the relationship between the excitation time and signal intensity in vivo, a living mouse with subcutaneous injection of LF-NR solution was imaged using the same voltage (45 kV) with different excitation times. As shown in Fig. 7b and d, by increasing the exposure time, the X-ray-induced green emission intensity was also remarkably improved. These findings revealed that the as-prepared NRs could be successfully used as bio-nanoprobes for X-ray-activated optical bioimaging. Moreover, owing to the unlimited penetration depth in biotissue of X-rays, these X-ray-activated LF-NRs can be used for deep-tissue optical bioimaging.3.6 Dual-modal X-ray/X-ray-induced optical bioimaging
Apart from the excellent X-ray-activated visible light emission properties, the LF-NRs containing Gd and Tb elements with large X-ray absorption coefficients60 can also be used as probes for X-ray imaging. Therefore, based on these advantages of efficient X-ray absorption and X-ray-activated luminescence, the LF-NRs are highly promising candidates to be utilized as a dual-modal imaging agent for X-ray and X-ray-induced optical bioimaging. To further demonstrate the feasibility of the dual-modal bioimaging in one system based on the LF-NR bioprobes, the mouse before and after intraperitoneal injection of LF-NRs were tested by a multi-modal in vivo bioimaging system equipped with an X-ray imaging facility. The whole body dual-modal bioimaging of the as-prepared LF-NRs in the same mouse is presented in Fig. 8. As demonstrated in the middle panel of Fig. 8, compared with the control mouse (the left panel), the intraperitoneally injected mouse showed obvious X-ray contrast signals (the position of the red arrow) at the injection sites. Moreover, significant X-ray-induced optical signals were detected at the same sites (right panel of Fig. 8), indicating the realization of simultaneous in vivo X-ray and X-ray-induced optical bioimaging in the same system. To further assess the contrast efficacy for X-ray imaging, we performed the comparison experiment between the X-ray absorption of LF-NRs and iobitridol (a commonly used X-ray imaging contrast agent) in solution (Fig. S6†). The inset in Fig. S6† shows that both agents have signal enhancement as the concentrations of agents is increased. However, at equivalent concentrations, the X-ray absorption of LF-NRs was higher than that of iobitridol. This finding reveals that our LF-NRs are more superior to the clinically used iobitridol for X-ray imaging.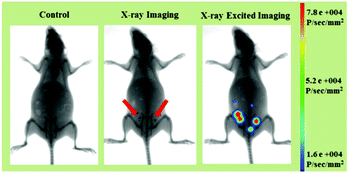 | ||
| Fig. 8 In vivo dual-modal X-ray/X-ray-induced optical bioimaging of a mouse based on intraperitoneal injection of LF-NRs. | ||
3.7 Deep penetration depth of X-ray activation properties
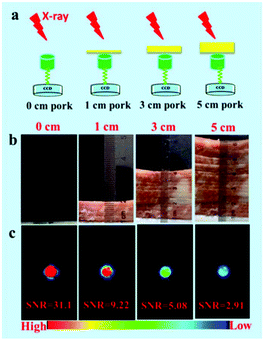
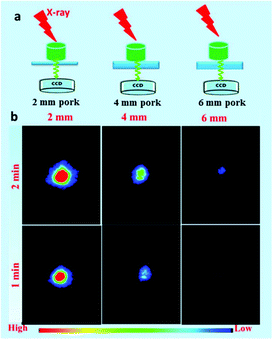
To reveal the deep penetration X-ray-activated nature, a series of simulation experiments were performed. Fig. 9a shows a schematic diagram of the experimental design to reveal the deep penetration of X-rays based on NRs. As shown in Fig. 9c, the experimental results were obtained by placing pork tissue with different thicknesses between the NaYF4:40% Gd/15% Tb NRs and the soft X-ray light source with the operating voltage at 45 kV and the irradiation time at 1 min. Obviously, the intensity of emission decreased when the thickness of the pork tissues increased from 1 cm to 3 cm. However, the luminescence of the NRs was still detected even when the thickness of the pork was as much as 5 cm under the soft X-ray activation. Clearly, as the thickness of the pork tissues increased, the SNR was changed from 31.3, 9.22 to 5.08, and finally to 2.91. To reveal the influence of the excitation voltages and irradiation times of X-ray on the penetration depth, in vitro phantom optical imaging of NRs was carried out at different voltages and irradiation times. As shown in Fig. S7a,† the SNR was decreased and the penetration depth was significantly reduced with a decrease in the excitation voltages of the X-ray; this was also demonstrated by the average emission intensity curves (Fig. S7b†). This finding reveals that the high cube voltage can significantly improve the emission intensity, subsequently increasing the penetration depth. Fig. S8† presents the irradiation time-dependent radioluminescence of the NaYF4:40% Gd/15% Tb NRs covered with a 2 cm thick pork tissue, indicating the SNR can be significantly improved by increasing the irradiation time. The results demonstrate that the radioluminescence intensity can be dramatically improved by increasing the intensity of the X-ray source.To reveal the emission-dependent penetration depth of NRs under the excitation of X-ray, a simulation experiment was performed by placing pork slices of various thicknesses between the samples and CCD. The results (Fig. 10b) showed that the maximum penetration depth was estimated to be about 6 mm under X-ray excitation (45 kVp, 2 min). The findings revealed that the green emission signal was dramatically decreased with the increasing thickness of pork tissue due to the poor penetration of green light. Furthermore, to reveal both the excitation/emission-related penetration depth, in vitro phantom imaging of NRs covered with the same thickness pork tissues on the two sides of samples was performed under X-ray excitation (45 kV, 1 min) (Fig. S9a†). As demonstrated in Fig. S9b,† the signals of NRs were dramatically decreased with an increase in the covered thickness of the pork tissue. Although the emission penetration depth of the Tb-doped NRs is relatively shorter than that of NIR emission light, our experiment results have unambiguously demonstrated that the radioluminescence intensity and penetration depth can be remarkably improved by increasing the voltages/currents and irradiation time of the X-ray. In the clinical diagnosis X-ray apparatus, the intensity (I) of the continuous X-ray can be calculated through the following formula:
| I = K0i Z Un |
4. Conclusions
In conclusion, NaYF4:40% Gd/X% Tb (X = 1, 2, 5, 10, 15, 25, and 35) NRs with a uniform structure and hexagonal phase were successfully synthesized by a facile hydrothermal method. The results of in vitro optical imaging demonstrate that the NaYF4:Gd/Tb NRs can efficiently emit green light under X-ray irradiation. The emission intensity is highly dependent on the doped content of Tb3+, the excitation power, and the X-ray exposure time. Moreover, the sample doped with 15% Tb possesses the largest emission intensity. The dual-modal X-ray/X-ray-induced optical bioimaging based on LF-NRs indicated that these as-prepared NRs were another kind of ideal nanoprobes for in vivo bioimaging with the absence of autofluorescence. Therefore, the designed soft X-ray-activated NaYF4:Gd/Tb scintillating NRs with deep penetration activation nature of X-ray light are ideal probes for bioimaging and potential X-ray-activated deep-tissue PDT applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21671064, and 91530321), Hunan Provincial Natural Science Foundation of China (2017RS3033) and Science and Technology Planning Project of Hunan Province (No. 2017RS3031). We also thank Prof. Rushi Liu, Dr Minjing Liao, and Xiaorui Jin for the help of cell imaging.Notes and references
- J. Zhou, Z. Liu and F. Y. Li, Chem. Soc. Rev., 2012, 41, 1323 RSC.
- J. H. Lee, Y. M. Huh, Y. Jun, J. Seo, J. Jang, H. T. Song, S. J. Kim, E. J. Cho, H. G. Yoon, J. S. Suh and J. W. Cheon, Nat. Med., 2007, 13, 95 CrossRef CAS PubMed.
- J. Shen, L. D. Sun and C. H. Yan, Dalton Trans., 2008, 42, 5687 RSC.
- L. Y. Pan, M. He, J. B. Ma, W. Tang, G. Gao, R. He, H. C. Su and D. X. Cui, Theranostics, 2013, 3, 210 CrossRef CAS PubMed.
- S. A. Hilderbrand and R. Weissleder, Curr. Opin. Chem. Biol., 2010, 14, 71 CrossRef CAS PubMed.
- J. E. H. Buston, J. R. Young and H. L. Anderson, Chem. Commun., 2000, 11, 905 RSC.
- U. Resch-Genger, M. Grabolle, S. Cavaliere-Jaricot, R. Nitschke and T. Nann, Nat. Methods, 2008, 5, 763 CrossRef CAS PubMed.
- R. N. Day and M. W. Davidson, Chem. Soc. Rev., 2009, 38, 2887 RSC.
- K. Nienhaus and G. U. Nienhaus, Chem. Soc. Rev., 2014, 43, 1088 RSC.
- J. H. Gao, K. Chen, R. G. Xie, J. Xie, S. Lee, Z. Cheng, X. G. Peng and X. Y. Chen, Small, 2010, 6, 256 CrossRef CAS PubMed.
- L. A. Bentolila, X. Michalet, F. F. Pinaud, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538 CrossRef PubMed.
- X. H. Gao, Y. Y. Cui, R. M. Levenson, L. W. K. Chung and S. M. Nie, Nat. Biotechnol., 2004, 22, 969 CrossRef CAS PubMed.
- T. Jamieson, R. Bakhshi, D. Petrova, R. Pocock, M. Imani and A. M. Seifalian, Biomaterials, 2007, 28, 4717 CrossRef CAS PubMed.
- D. K. Chatterjee, A. J. Rufaihah and Y. Zhang, Biomaterials, 2008, 29, 937 CrossRef CAS PubMed.
- Q. L. de-Chermont, C. Chaneac, J. Seguin, F. Pelle, S. Maitrejean, J. P. Jolivet, D. Gourier, M. Bessodes and D. Scherman, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 9266 CrossRef PubMed.
- G. S. Yi and G. M. Chow, Adv. Funct. Mater., 2006, 16, 2324 CrossRef CAS.
- G. Y. Chen, J. Shen, T. Y. Ohulchanskyy, N. J. Pstel, A. Kutikov, Z. P. Li, J. Song, R. K. Pandey, H. Agren, P. N. Parasd and G. Han, ACS Nano, 2012, 6, 8280 CrossRef CAS PubMed.
- N. M. Idris, M. K. Gnanasammandhan, J. Zhang, P. C. Ho, R. Mahendran and Y. Zhang, Nat. Med., 2012, 18, 1580 CrossRef CAS PubMed.
- J. Wang, F. Wang, C. Wang, Z. Liu and X. G. Liu, Angew. Chem., Int. Ed., 2011, 50, 10369 CrossRef CAS PubMed.
- W. Li, J. S. Wang, J. S. Ren and X. G. Qu, J. Am. Chem. Soc., 2014, 136, 2248 CrossRef CAS PubMed.
- D. M. Yang, P. A. Ma, Z. Y. Hou, Z. Y. Cheng, C. X. Li and J. Lin, Chem. Soc. Rev., 2015, 44, 1416 RSC.
- X. Liu, C. Yan and J. Capobianco, Chem. Soc. Rev., 2015, 44, 1299 RSC.
- W. Zheng, D. T. Tu, P. Huang, S. Y. Zhou, Z. Chen and X. Y. Chen, Chem. Commun., 2015, 51, 4129 RSC.
- S. J. Zeng, H. B. Wang, W. Lu, Z. G. Yi, L. Rao, H. R. Liu and J. H. Hao, Biomaterials, 2014, 35, 2934 CrossRef CAS PubMed.
- Z. G. Yi, X. L. Li, Z. L. Xue, X. Liang, W. Lu, H. Peng, H. R. Liu, S. J. Zeng and J. H. Hao, Adv. Funct. Mater., 2015, 25, 7119 CrossRef CAS.
- Q. Liu, Y. Sun, T. S. Yang, W. Feng, C. G. Li and F. Y. Li, J. Am. Chem. Soc., 2011, 133, 17122 CrossRef CAS PubMed.
- G. Tian, Z. J. Gu, L. J. Zhou, W. Y. Yin, X. X. Liu, L. Yan, S. Jin, W. L. Ren, G. M. Xing, S. J. Li and Y. L. Zhao, Adv. Mater., 2012, 24, 1226 CrossRef CAS PubMed.
- G. Y. Chen, T. Ohulchanskyy, S. Liu, W. C. Law, F. Wu, M. T. Swihart, H. Agren and P. Prasad, ACS Nano, 2012, 6, 2969 CrossRef CAS PubMed.
- L. J. Zhou, X. P. Zheng, Z. J. Gu, W. Y. Yin, X. Zhang, L. F. Ruan, Y. B. Yang, Z. B. Hu and Y. L. Zhao, Biomaterials, 2014, 35, 7666 CrossRef CAS PubMed.
- H. Dong, L. D. Sun, Y. F. Wang, J. Ke, R. Si, J. W. Xiao, G. M. Lyu, S. Shi and C. H. Yan, J. Am. Chem. Soc., 2015, 137, 6569 CrossRef CAS PubMed.
- Z. Li, T. Liang, S. W. Lv, Q. G. Zhuang and Z. Liu, J. Am. Chem. Soc., 2015, 137, 11179 CrossRef CAS PubMed.
- X. Wu, Y. W. Zhang, K. D. Takle, O. Bilsel, Z. J. Li, H. Lee, Z. J. Zhang, D. S. Li, W. Fan, C. Y. Duan, E. M. Chan, C. Lois, Y. Xiang and G. Han, ACS Nano, 2016, 10, 1060 CrossRef CAS PubMed.
- Y. Sun, W. Feng, P. Y. Yang, C. H. Huang and F. Y. Li, Chem. Soc. Rev., 2015, 44, 1509 RSC.
- G. Y. Chen, H. L. Qiu, P. N. Prasad and X. Y. Chen, Chem. Rev., 2014, 114, 5161 CrossRef CAS PubMed.
- J. N. Liu, W. B. Bu, L. M. Pan and J. L. Shi, Angew. Chem., Int. Ed., 2013, 52, 4375 CrossRef CAS PubMed.
- Q. Q. Zhan, J. Qian, H. J. Liang, G. Somesfalean, D. Wang, S. L. He, Z. G. Zhang and S. Andersson-Engels, ACS Nano, 2011, 5, 3744 CrossRef CAS PubMed.
- H. Chen, D. E. Longfield, V. S. Varahagiri, K. T. Nguyen, A. L. Patrick, H. Qian, D. G. VanDerveer and J. N. Anker, Analyst, 2011, 136, 3438 RSC.
- X. Cao, X. L. Chen, F. Kang, Y. H. Zhan, X. Cao, J. Wang, J. M. Liang and J. Tian, ACS Appl. Mater. Interfaces, 2015, 7, 11775 CAS.
- X. Liu, Q. M. Liao and H. K. Wang, IEEE Trans. Biomed. Eng., 2014, 61, 1621 CrossRef PubMed.
- Z. H. Hu, Y. W. Qu, K. Wang, X. J. Zhang, J. L. Zha, T. M. Song, C. P. Bao, H. X. Liu, Z. L. Wang, J. Wang, Z. Y. Liu, H. F. Liu and J. Tian, Nat. Commun., 2015, 6, 7560 CrossRef PubMed.
- I. N. Stanton, J. A. Ayres and M. J. Therien, Dalton Trans., 2012, 41, 11576 RSC.
- Y. H. Zhan, F. R. Ai, F. Chen, H. F. Valdovinos, H. Orbay, H. Y. Sun, J. M. Liang, T. E. Barnhart, J. Tian and W. B. Cai, Small, 2016, 12, 2782 CrossRef PubMed.
- H. Q. Chen, D. C. Colvin, B. Qi, T. Moore, J. He, O. T. Mefford, F. Alexis, J. C. Gore and J. N. Anker, J. Mater. Chem., 2012, 22, 12802 RSC.
- S. Som, P. Mitra, V. Kumar, V. Kumar, J. J. Terblans, H. C. Swart and S. K. Sharma, Dalton Trans., 2014, 43, 9860 RSC.
- G. Pratx, C. M. Carpenter, C. Sun, R. P. Rao and L. Xing, Opt. Lett., 2010, 35, 3345 CrossRef CAS PubMed.
- H. Y. Chen, T. Moore, B. Qi, D. C. Colvin, E. K. Jelen, D. A. Hitchcock, J. He, O. T. Mefford, J. C. Gore, F. Alexis and J. N. Anker, ACS Nano, 2013, 7, 1178 CrossRef CAS PubMed.
- L. Sudheendra, G. K. Das, C. Q. Li, D. Stark, J. Cena, S. Cherry and I. M. Kennedy, Chem. Mater., 2014, 26, 1881 CrossRef CAS PubMed.
- C. Sun, G. Pratx, C. M. Carpenter, H. G. Liu, Z. Cheng, S. S. Gambhir and L. Xing, Adv. Mater., 2011, 23, H195 CrossRef CAS PubMed.
- S. J. Zeng, Z. G. Yi, W. Lu, C. Qian, H. B. Wang, L. Rao, T. M. Zeng, H. R. Liu, H. J. Liu, B. Fei and J. H. Hao, Adv. Funct. Mater., 2014, 24, 4196 CrossRef.
- N. Bogdan, F. Vetrone, G. A. Ozin and J. A. Capobianco, Nano Lett., 2011, 11, 835 CrossRef CAS PubMed.
- Z. L. Xue, Z. G. Yi, X. L. Li, Y. B. Li, M. Y. Jiang, H. R. Liu and S. J. Zeng, Biomaterials, 2017, 115, 90 CrossRef CAS PubMed.
- T. S. Yang, Y. Sun, Q. Liu, W. Feng, P. Y. Yang and F. Y. Li, Biomaterials, 2012, 33, 3733 CrossRef CAS PubMed.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751 CrossRef.
- Y. A. Tang, J. Hu, A. H. Elmenoufy and X. L. Yang, ACS Appl. Mater. Interfaces, 2015, 7, 12261 CAS.
- F. Wang, R. R. Deng, J. Wang, Q. X. Wang, Y. Han, H. M. Zhu, X. Y. Chen and X. G. Liu, Nat. Mater., 2011, 10, 968 CrossRef CAS PubMed.
- H. X. Guan, G. X. Liu, J. X. Wang, X. T. Dong and W. S. Yu, Dalton Trans., 2014, 43, 10801 RSC.
- L. C. V. Rodrigues, R. Stefani, H. F. Brito, M. C. F. C. Felinto, J. Hlösä, M. Lastusaari, T. Laamanen and M. Malkamäki, J. Solid State Chem., 2010, 183, 2365 CrossRef CAS.
- L. Sudheendra, G. K. Das, C. Q. Li, D. Stark, J. Cena, S. Cherry and I. M. Kennedy, Chem. Mater., 2014, 26, 1881 CrossRef CAS PubMed.
- http://www.kayelaby.npl.co.uk/atomic_and_nuclear_physics/4_2/4_2_1.html .
- Z. Liu, E. G. Ju, J. H. Liu, Y. D. Du, Z. Q. Li, Q. H. Yuan, J. S. Ren and X. G. Qu, Biomaterials, 2013, 34, 7444 CrossRef CAS PubMed.
- D. J. Naczynsk, C. Sun, S. Türkcan, C. Jenkins, A. L. Koh, D. Ikeda, G. Pratx and L. Xing, Nano Lett., 2015, 15, 96 CrossRef PubMed.
- J. M. Boone, T. R. Nelson, K. K. Lindfors and J. A. Seibert, Med. Phys., 2001, 221, 657 CAS.
- C. Wang, H. Q. Tao, L. Cheng and Z. Liu, Biomaterials, 2011, 32, 6145 CrossRef CAS PubMed.
- F. J. Ai, Q. Ju, X. M. Zhang, X. Chen, F. Wang and G. Y. Zhu, Sci. Rep., 2015, 5, 10785 CrossRef CAS PubMed.
- Y. A. Tang, J. Hu, A. H. Elmenoufy and X. L. Yang, ACS Appl. Mater. Interfaces, 2015, 7, 12261 CAS.
- N. M. Idris, M. K. Gnanasammandhan, J. Zhang, P. C. Ho, R. Mahendran and Y. Zhang, Nat. Med., 2012, 18, 1580 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7nr02926h |
| This journal is © The Royal Society of Chemistry 2018 |