Large scale 2D/3D hybrids based on gallium nitride and transition metal dichalcogenides†
Kehao
Zhang
 ab,
Bhakti
Jariwala
ab,
Bhakti
Jariwala
 a,
Jun
Li
c,
Natalie C.
Briggs
a,
Baoming
Wang
bd,
Dmitry
Ruzmetov
be,
Robert A.
Burke
beg,
Jordan O.
Lerach
f,
Tony G.
Ivanov
be,
Md
Haque
a,
Jun
Li
c,
Natalie C.
Briggs
a,
Baoming
Wang
bd,
Dmitry
Ruzmetov
be,
Robert A.
Burke
beg,
Jordan O.
Lerach
f,
Tony G.
Ivanov
be,
Md
Haque
 bd,
Randall M.
Feenstra
c and
Joshua A.
Robinson
*ab
bd,
Randall M.
Feenstra
c and
Joshua A.
Robinson
*ab
aDepartment of Materials Science and Engineering & Center for Two-Dimensional and Layered Materials, The Pennsylvania State University, University Park, Pennsylvania 16802, USA. E-mail: jrobinson@psu.edu
bCenter for Atomically Thin Multifunctional Coating, University Park, Pennsylvania 16802, USA
cDepartment of Physics, Carnegie Mellon University, Pittsburgh, Pennsylvania 15213, USA
dDepartment of Mechanical and Nuclear Engineering, The Pennsylvania State University, University Park 16802, USA
eSensors and Electron Devices Directorate, U.S. Army Research Laboratory, Adelphi, Maryland 20783, USA
fMaterials Characterization Laboratory, The Pennsylvania State University, University Park 16802, USA
gGeneral Technical Services, LLC, Wall, New Jersey 07727, United States
First published on 1st December 2017
Abstract
Two and three-dimensional (2D/3D) hybrid materials have the potential to advance communication and sensing technologies by enabling new or improved device functionality. To date, most 2D/3D hybrid devices utilize mechanical exfoliation or post-synthesis transfer, which can be fundamentally different from directly synthesized layers that are compatible with large scale industrial needs. Therefore, understanding the process/property relationship of synthetic heterostructures is priority for industrially relevant material architectures. Here we demonstrate the scalable synthesis of molybdenum disulfide (MoS2) and tungsten diselenide (WSe2) via metal organic chemical vapor deposition (MOCVD) on gallium nitride (GaN), and elucidate the structure, chemistry, and vertical transport properties of the 2D/3D hybrid. We find that the 2D layer thickness and transition metal dichalcogenide (TMD) choice plays an important role in the transport properties of the hybrid structure, where monolayer TMDs exhibit direct tunneling through the layer, while transport in few layer TMDs on GaN is dominated by p–n diode behavior and varies with the 2D/3D hybrid structure. Kelvin probe force microscopy (KPFM), low energy electron microscopy (LEEM) and X-ray photoelectron spectroscopy (XPS) reveal a strong intrinsic dipole and charge transfer between n-MoS2 and p-GaN, leading to a degraded interface and high p-type leakage current. Finally, we demonstrate integration of heterogeneous 2D layer stacks of MoS2/WSe2 on GaN with atomically sharp interface. Monolayer MoS2/WSe2/n-GaN stacks lead to near Ohmic transport due to the tunneling and non-degenerated doping, while few layer stacking is Schottky barrier dominated.
Introduction
Beyond two dimensional (2D) heterostructures,1–4 2D/3D hybrids enable mixed dimensional van der Waals heterostructures5 and provide unique opportunities towards steep slope transistors,6 highly efficient p–n junction solar cells,7 light emitters8 and photovoltaics.9 Transition metal dichalcogenide (TMD) and nitride semiconducting heterostructures are especially interesting because of the wide variety of band alignments, and the possibility to convert the nitride to a 2D form.10 Recently, p–n diodes11 and room temperature Esaki diodes12 were demonstrated with transferred 2D layers. However, transfer-induced contamination and damage often degrades the performance of the devices.13 Additionally, the device size is limited by the exfoliated sample size (typically < 10μm), which is not compatible to the industrial process. Our previous work using powder vaporization14 demonstrates that monolayer MoS2 can be epitaxially grown on GaN and is electrically active in the vertical direction. However, the non-uniformity still limits its applicability to large area synthetic 2D/3D heterostructures.15,16In this work, we utilize metal organic chemical vapor deposition (MOCVD) to synthesize mono to few-layer MoS2 and WSe2 on p-type and n-type GaN, respectively, to probe the 2D/3D electrical properties. MOCVD enables large area, uniform TMDs films via layer-by-layer growth as identified by atomic force microscopy (AFM) and Raman spectroscopy, where the layer number is tuned via growth time. X-ray photoelectron spectroscopy (XPS) characterization confirms nearly stoichiometric MoS2 (Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1
S = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.95) and WSe2 (W
1.95) and WSe2 (W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se = 1
Se = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.03) layers are grown with negligible metal–oxide bonding compared to powder vaporized TMDs.14,17 Furthermore, there is no structural change of the GaN substrate. Importantly, we demonstrate that the vertical transport in the 2D/3D hybrids vary with 2D thickness and the choice of the heterostructure. Monolayer TMDs on GaN exhibit clear direct tunneling, while few layer (FL) (>2 layers) TMDs lead to p–n junction behavior. Although p–n diodes based on FL MoS2/p-GaN and WSe2/n-GaN exhibit similar turn-on voltages under forward bias, FL MoS2/p-GaN exhibits 100× higher current under reversed bias due to strong charge transfer and an intrinsic dipole between the MoS2/p-GaN interface. As a result, we hypothesize that few layer WSe2/n-GaN is the most appropriate choice for high quality synthetic 2D/3D p–n heterostructures. Finally, we demonstrate MoS2/WSe2/n-GaN hybrids with atomically sharp interfaces. Unlike the resonant tunneling observed on MoS2/WSe2/epitaxial graphene (EG) heterostructures,18 single layer MoS2/WSe2 on n-GaN exhibits Ohmic behavior, while FL MoS2/WSe2 on n-GaN is dominated by Schottky barrier transport.
2.03) layers are grown with negligible metal–oxide bonding compared to powder vaporized TMDs.14,17 Furthermore, there is no structural change of the GaN substrate. Importantly, we demonstrate that the vertical transport in the 2D/3D hybrids vary with 2D thickness and the choice of the heterostructure. Monolayer TMDs on GaN exhibit clear direct tunneling, while few layer (FL) (>2 layers) TMDs lead to p–n junction behavior. Although p–n diodes based on FL MoS2/p-GaN and WSe2/n-GaN exhibit similar turn-on voltages under forward bias, FL MoS2/p-GaN exhibits 100× higher current under reversed bias due to strong charge transfer and an intrinsic dipole between the MoS2/p-GaN interface. As a result, we hypothesize that few layer WSe2/n-GaN is the most appropriate choice for high quality synthetic 2D/3D p–n heterostructures. Finally, we demonstrate MoS2/WSe2/n-GaN hybrids with atomically sharp interfaces. Unlike the resonant tunneling observed on MoS2/WSe2/epitaxial graphene (EG) heterostructures,18 single layer MoS2/WSe2 on n-GaN exhibits Ohmic behavior, while FL MoS2/WSe2 on n-GaN is dominated by Schottky barrier transport.
Results and discussion
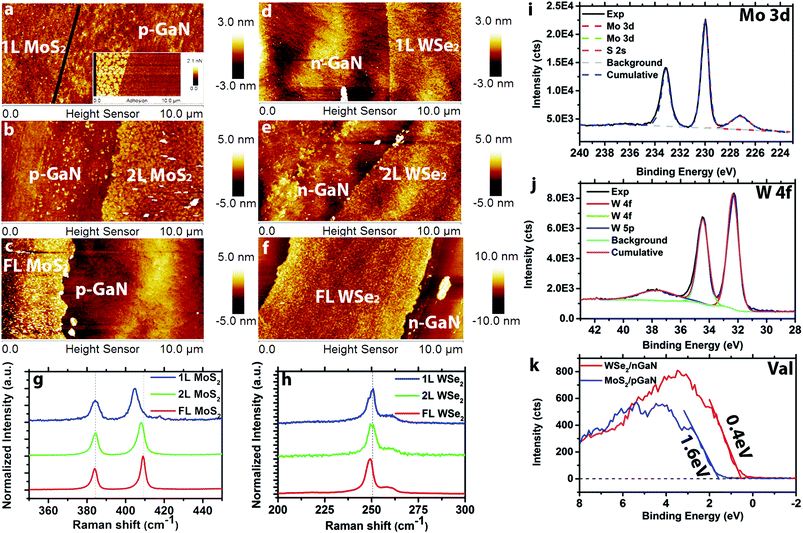
Transition metal dichalcogenide films are directly deposited on (p- and n-)GaN/c-sapphire substrates via MOCVD. The hole concentration for p-type GaN is 1017 cm−3 and the electron concentration for n-type GaN is and 1018 cm−3. The MoS2 films are grown in a hot wall reactor at 650 °C using molybdenum hexacarbonyl (Mo(CO)6) and diethyl sulfide (DES) precursors with NaCl powder16 placed upstream from the reactor hot zone for nucleation control and carbon sequestration (Fig. S1†). The WSe2 films are prepared using a cold wall reactor at 650 °C with tungsten hexacarbonyl (W(CO)6) and hydrogen selenide (H2Se) (Fig. S2†). Layer-by-layer growth has previously been demonstrated via MOCVD,15,16 and here we tune the layer thickness via growth time. Atomic force microscopy (Fig. 1a–c) demonstrates controlled formation of 1L, 2L, and FL (3.5 nm) MoS2 with increasing growth time from 30 min to 60 min to 120 min, and the topography of monolayer MoS2 (Fig. 1a) closely matches the roughness of the GaN substrate.14 An additional adhesion map (inset of Fig. 1a) is provided to visualize the distinction between 1L MoS2 and p-GaN. Monolayer, 2L and FL (4.7 nm) WSe2 (Fig. 1d–f, respectively) is realized using growth times of 5 min, 10 min and 30 min. Raman spectra (see ESI† for measurement details) of MoS2 (Fig. 1g) exhibits the typical 384 cm−1 (E2g) and 403 cm−1 (A1g) vibration modes for 1L MoS2, 384 cm−1 (E2g) and 405 cm−1 (A1g) for 2L MoS2, and 383 cm−1 (E2g) and 407 cm−1 (A1g) for FL MoS2, confirming the thickness difference.19 A similar Raman shift trend is also observed on WSe2 (Fig. 1h) for E2g peak (250 cm−1 for 1L and 249 cm−1 for 6L), agreeing with previous reports.20Synthetic MoS2 and WSe2 is observed to be nearly stoichiometric (Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 1
S = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.95; W
1.95; W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se = 1
Se = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.03) with negligible metal–oxygen bonding and no structural degradation. In the case of MoS2, the molybdenum 3d doublet peaks are observed at 230.0 eV and 233.1 eV together with a sulfur 2s peak at 227.2 eV (Fig. 1i). Following peak fitting, the only detected bonding is between Mo and S. Importantly, Mo–O bonding is significantly reduced for MoS2 grown by MOCVD compared to PV.14 This is likely the result of the elimination of the oxygen precursor, MoO3, often used as the Mo source in the PV method. X-ray photoelectron spectroscopy of WSe2 (Fig. 1j) exhibits three W peaks at 32.3 eV (W 4f7/2), 34.4 eV (W 4f5/2) and 37.7 eV (W 5p3/2), indicating no W–O bonding or other impurities within the detection limits of XPS.21 Finally, valence band edge measurements (Fig. 1k) of FL MoS2 and FL WSe2 indicate the valence band maximum (VBM) of MoS2 is 1.6 eV below the Fermi level, suggesting that MoS2 is electron doped (n-type). In contrast, the VBM of WSe2 is 0.4 eV below the Fermi level, indicating hole doping (p-type) behavior of WSe2.22 This is typical of MoS2 and WSe2, and is attributed to native defects in the TMD layer.16,23,24 Further XPS characterization of the GaN before and after growth also indicates no detectable structural degradation of the GaN after the TMD growth (Fig. S3†).
2.03) with negligible metal–oxygen bonding and no structural degradation. In the case of MoS2, the molybdenum 3d doublet peaks are observed at 230.0 eV and 233.1 eV together with a sulfur 2s peak at 227.2 eV (Fig. 1i). Following peak fitting, the only detected bonding is between Mo and S. Importantly, Mo–O bonding is significantly reduced for MoS2 grown by MOCVD compared to PV.14 This is likely the result of the elimination of the oxygen precursor, MoO3, often used as the Mo source in the PV method. X-ray photoelectron spectroscopy of WSe2 (Fig. 1j) exhibits three W peaks at 32.3 eV (W 4f7/2), 34.4 eV (W 4f5/2) and 37.7 eV (W 5p3/2), indicating no W–O bonding or other impurities within the detection limits of XPS.21 Finally, valence band edge measurements (Fig. 1k) of FL MoS2 and FL WSe2 indicate the valence band maximum (VBM) of MoS2 is 1.6 eV below the Fermi level, suggesting that MoS2 is electron doped (n-type). In contrast, the VBM of WSe2 is 0.4 eV below the Fermi level, indicating hole doping (p-type) behavior of WSe2.22 This is typical of MoS2 and WSe2, and is attributed to native defects in the TMD layer.16,23,24 Further XPS characterization of the GaN before and after growth also indicates no detectable structural degradation of the GaN after the TMD growth (Fig. S3†).
Vertical charge transport through the 2D/3D hybrid is highly sensitive to the 2D layer properties. Here, we use conductive AFM (CAFM) with a Pt/Ir tip to probe the nanoscale vertical transport of p–n junction TMD/GaN heterostructures. Based on fitting the local current–voltage (I–V) characteristics with the Fowler–Nordheim tunneling equations,25,26 the carrier transport can be described to be dominated by direct tunneling through the TMDs for 1L MoS2/p-GaN and 1L WSe2/n-GaN (Fig. S4†). Thus, monolayer TMDs are considered nearly electrically transparent due to the full depletion region overlap27 (see ESI† for depletion width calculation). Additionally, the TMD layer between the Pt/Ir metal tip and GaN surface leads to a reduction in the Schottky barrier due to Fermi level pinning at the Tip/TMD interface, thereby reducing the contact resistance.14,27,28 As the 2D layer thickness increases, the carrier transport becomes dominated by the barrier at the 2D/GaN interface (Fig. S4†). This is evident when analyzing the I–V characteristics of FL n-MoS2/p-GaN and p-WSe2/n-GaN (Fig. 2a). For simplicity, positive bias is applied on the p-type semiconductor (p-GaN or WSe2). The two heterostructures show slightly different turn-on voltage at approximately 1 V for WSe2/n-GaN and 1.4 V for MoS2/p-GaN under forward bias, with an ideality factor (n) of 30 for MoS2/p-GaN and 15 for WSe2/n-GaN. The high ideality factor is likely due to the Schottky barrier between the tip and TMDs,14,29 leading to a combination of p–n and Schottky barrier transport. Improved Ohmic contacts to the TMDs will enable lower ideality factors. We note that under reverse bias, the MoS2/p-GaN structure exhibits high current, even at low voltages. To understand this behavior, Kelvin Probe Force Microscopy (KPFM) and Low Energy Electron Microscopy/Reflectivity (LEEM/LEER) measurements are employed (see Fig. S5† for KPFM calibration). KPFM measurements of FL MoS2 (Fig. 2b) and FL WSe2 (Fig. 2c) reveal distinct work function differences between the TMDs and (p-, n-)GaN. The work function of WSe2 is measured to be ∼300 meV higher than n-GaN, and the work function of MoS2 is ∼150 meV higher than p-GaN. This work function difference is confirmed by LEEM/LEER measurements, where multiple spots are evaluated on FL MoS2/p-GaN (LEEM, Fig. 2d) and FL WSe2/n-GaN (LEEM, Fig. 2e). The corresponding LEER spectra (Fig. 2f) suggest that the work function difference between FL MoS2 and p-GaN is ∼220 meV and ∼340 meV between FL WSe2 and n-GaN. Based on the measured work function (ΦWSe2 = 4.8 eV; ΦGaN = 4.5 eV), XPS measurements30 (see ESI† for details) and reported electron affinity (χWSe2 = 3.9 eV (ref. 31); χGaN = 4.1 eV (ref. 14)), the band diagram of WSe2/n-GaN can be established (Fig. 2g), which agrees with literature reported staggered band alignment for WSe2/GaN.30 However, counter to the reported values,13,32 KPFM and LEER in this work both demonstrate that the work function of MoS2 is higher than that of p-GaN (Fig. 2b and d). Note that the GaN surface is exposed by scratching the continuous TMD, and it results in some roughness on the GaN. We hypothesize that this could be due to modification of the GaN Mg dopant properties. First, hydrogen present during the growth process may passivate the Mg dopant, leading to a reduced hole concentration of p-GaN.33–36 Second, the strong intrinsic dipole and charge transfer at the semiconductor/semiconductor interface may be responsible for the observation.37,38 In either case, this would shift the Fermi level (and hence work function) toward higher values in the GaN bandgap, leading to a modification of the MoS2/p-GaN behavior. Additional XPS measurements (Fig. S6†) reveal the Type I band alignment between MoS2 and p-GaN, agreeing with the recent study (Fig. 2h).37 Combining this information with the aforementioned KPFM and LEEM/LEER measurement, the band bending under different biases is shown in Fig. 2h. Conventional electron conduction is responsible for the forward biased current, but unlike WSe2, the hole conduction due to the tunneling can introduce reversed bias current.39 Furthermore, material modifications (below the detection limits of XPS) in the GaN due to high temperature synthesis of the MoS2 could also be the source of high leakage currents in reverse bias observed in Fig. 2a,40 however, additional theoretical work is needed to understand the aforementioned scenario in depth. This suggests that the utilization of n-MoS2/p-GaN could suffer from performance and reliability challenges due to the required elevated temperature of the MoS2 growth, thereby pointing to WSe2/n-GaN the preferred choice for 2D/3D hybrids for p–n junction applications.
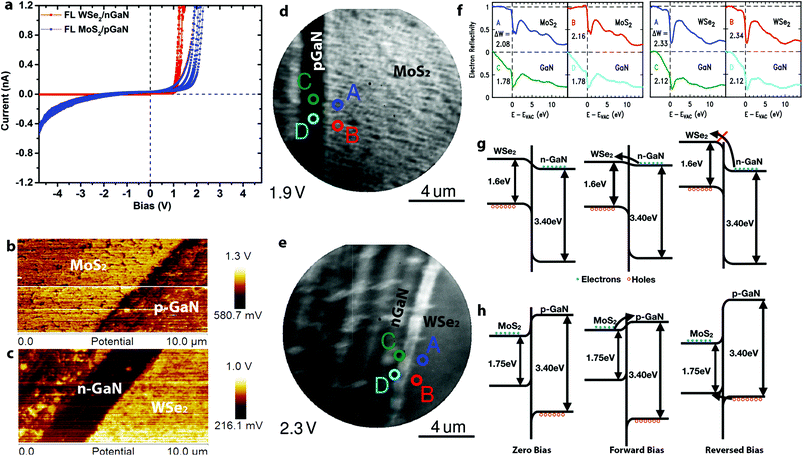 | ||
| Fig. 2 The electronic properties of TMD/GaN heterostructures: a. I–V measurement of FL MoS2/p-GaN and WSe2/n-GaN. The turn on voltage for FL MoS2/p-GaN and FL WSe2 is 1.4 V and 1.0 V under forward bias, respectively. However, FL MoS2/p-GaN exhibits a higher leakage current; b–c. KPFM characterization of FL MoS2/p-GaN (b) and FL WSe2/n-GaN (c), the work function of WSe2 and MoS2 is 300 mV and 150 mV higher than n-GaN and p-GaN respectively; d–e. LEEM images of FL MoS2/p-GaN acquired at 1.9 eV (d) and FL WSe2/n-GaN acquired at 2.3 eV (e). The scratch of GaN is clearly visible by LEE. The LEER analysis points are labeled with different colors in Fig. 3d and e; f. the LEER spectra of FL MoS2/p-GaN (left) and FL WSe2/n-GaN (right) corresponding to points labeled in (d) and (e), which quantifies the electrostatic surface variation and hence the variation of vacuum level; g. the band alignment of WSe2/n-GaN; h. the band alignment of MoS2/p-GaN. It is clear that hole conduction can be responsible for the reversed bias current. | ||
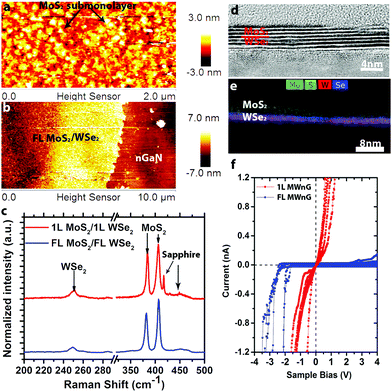
Utilizing the same synthesis techniques, it is possible to realize more complex 2D/3D hybrids such as MoS2/WSe2/n-GaN (MWnG). Here, WSe2 films are deposited on n-GaN, and subsequently MoS2 films are deposited on WSe2/n-GaN to form the MWnG hybrid. The MoS2 is grown at 650 °C, resulting in nanocrystalline sub-monolayers on 1L WSe2 (Fig. 3a) and uniform FL MoS2 on FL WSe2 (Fig. 3b). Raman spectroscopy (Fig. 3c) confirms the presence of MoS2 and WSe2, with no evidence of intermediated phases (WS2, MoSe2, etc.). Cross-sectional high resolution transmission electron microscopy (HRTEM) and energy dispersive spectroscopy (EDS) (Fig. 3d and e) reveal that the FL hybrid structure is 6–7 layers, evenly split between MoS2 and WSe2. The EDS mapping (Fig. 3e) indicates that the layers of MoS2 and WSe2 are well-defined with a sharp interface, and no evidence of alloying. The electrical properties (characterized by CAFM, Fig. 3f) are shown to vary greatly between monolayer heterostructures and FL heterostructures. When the film contains FL MoS2 and FL WSe2, a rectifying conductivity is observed. This rectification is attributed to the forward biased Schottky barrier between the Pt/Ir tip and MoS2.14,41 Interestingly, unlike the reported resonant tunneling of 1L MoS2/1L WSe2 heterostructures on graphene, we find nearly Ohmic behavior of 1L MoS2/1L WSe2 on n-GaN, with no negative differential resistance (NDR) at room temperature. Such behavior is likely due to the thermionic emission at room temperature and non-degenerate doping concentration of our MoS2 and WSe2 films.18,42
Conclusions
This work demonstrates the scalable growth of TMD/GaN hybrid structures, where film thickness is controlled by tuning the growth time. The process results in high quality MoS2 and WSe2, and enables a route to 2D heterostructures on GaN for next generation electronics. Transport vertically through the hybrid structures is dominated by the 2D layer thickness and materials choice, where monolayer films exhibit strong direct tunneling characteristics and few layer 2D enables p–n junction formation at the 2D/3D interface. Compared to FL WSe2, FL MoS2 films exhibit high leakage current in reversed bias. Our KPFM and LEEM/LEER characterization reveals a counter-intuitive work function difference between the MoS2 and p-GaN, potentially due to strong interface charge transfer and possible non-structural degradation (Mg passivation) in p-GaN. Furthermore, FL MoS2/FL WSe2 heterostructures on n-GaN exhibits rectifying behavior due to the presence of the metal/MoS2 Schottky barrier. In contrast, we find Ohmic behavior when the thickness of the film is reduced to one atomic layer. This study elucidates that the thickness and materials choice is critical towards developing high quality 2D/3D heterostructures.Author contributions
J. A. R and K. Z conceived the idea, and J. A. R., T. G. I., R. M. F., and M. H directed the research; K. Z. and B. J synthesized the 2D/3D hybrids; K. Z. carried out AFM, Raman, and electrical measurements; N. C. B. conducted XPS characterization and K. Z. analyzed XPS data and proposed the band alignment; J. L. performed LEEM/LEER measurements; R. A. B. and D. R. prepared GaN sample and analyzed electrical data; B. W. carried out TEM sample preparation and characterization; J. O. L. performed TOF-SIMS characterization. All authors discussed the results. K. Z. and J. A. R., wrote the paper with significant input from J. L., R. M. F. and R. A. B. All authors have read and approved the manuscript.Conflicts of interest
Authors declare no conflicts of interest.Acknowledgements
Research was conducted as part of the Center for Atomically Thin Multifunctional Coatings (ATOMIC), sponsored by the National Science Foundation (NSF) division of Industrial, Innovation & Partnership (IIP) under award #1540018. The work at Carnegie Mellon University is supported by the center for Low Energy Systems Technology (LEAST), one of the six STARnet centers, sponsored by MARCO and DARPA.References
- L. Britnell, R. V. Gorbachev, R. Jalil, B. D. Belle, F. Schedin, A. Mishchenko, T. Georgiou, M. I. Katsnelson, L. Eaves, S. V. Morozov, N. M. R. Peres, J. Leist, A. K. Geim, K. S. Novoselov and L. A. Ponomarenko, Science, 2012, 335, 947–950 CrossRef CAS PubMed.
- L. Britnell, R. M. Ribeiro, A. Eckmann, R. Jalil, B. D. Belle, A. Mishchenko, Y.-J. Kim, R. V. Gorbachev, T. Georgiou, S. V. Morozov, A. N. Grigorenko, A. K. Geim, C. Casiraghi, A. H. Castro Neto and K. S. Novoselov, Science, 2013, 340, 1311–1314 CrossRef CAS PubMed.
- T. Georgiou, R. Jalil, B. D. Belle, L. Britnell, R. V. Gorbachev, S. V. Morozov, Y.-J. Kim, A. Gholinia, S. J. Haigh, O. Makarovsky, L. Eaves, L. A. Ponomarenko, A. K. Geim, K. S. Novoselov and A. Mishchenko, Nat. Nanotechnol., 2013, 8, 100–103 CrossRef CAS PubMed.
- W. J. Yu, Z. Li, H. Zhou, Y. Chen, Y. Wang, Y. Huang and X. Duan, Nat. Mater., 2013, 12, 246–252 CrossRef CAS PubMed.
- D. Jariwala, T. J. Marks and M. C. Hersam, Nat. Mater., 2016, 16, 170–181 CrossRef PubMed.
- D. Sarkar, X. Xie, W. Liu, W. Cao, J. Kang, Y. Gong, S. Kraemer, P. M. Ajayan and K. Banerjee, Nature, 2015, 526, 91–95 CrossRef CAS PubMed.
- X. Liu, T. Galfsky, Z. Sun, F. Xia, E.-C. Lin, Y.-H. Lee, S. Kéna-Cohen and V. M. Menon, Nat. Photonics, 2014, 9, 30–34 CrossRef.
- D. Li, R. Cheng, H. Zhou, C. Wang, A. Yin, Y. Chen, N. O. Weiss, Y. Huang and X. Duan, Nat. Commun., 2015, 6, 7509 CrossRef PubMed.
- X. Li, W. Chen, S. Zhang, Z. Wu, P. Wang, Z. Xu, H. Chen, W. Yin, H. Zhong and S. Lin, Nano Energy, 2015, 16, 310–319 CrossRef CAS.
- Z. Y. Al Balushi, K. Wang, R. K. Ghosh, R. A. Vilá, S. M. Eichfeld, J. D. Caldwell, X. Qin, Y.-C. Lin, P. A. DeSario, G. Stone, S. Subramanian, D. F. Paul, R. M. Wallace, S. Datta, J. M. Redwing and J. A. Robinson, Nat. Mater., 2016, 15, 1166–1171 CrossRef CAS PubMed.
- E. W. Lee, C. H. Lee, P. K. Paul, L. Ma, W. D. McCulloch, S. Krishnamoorthy, Y. Wu, A. R. Arehart and S. Rajan, Appl. Phys. Lett., 2015, 107, 103505 CrossRef.
- S. Krishnamoorthy, E. W. Lee, C. H. Lee, Y. Zhang, W. D. McCulloch, J. M. Johnson, J. Hwang, Y. Wu and S. Rajan, Appl. Phys. Lett., 2016, 109, 183505 CrossRef.
- H. Jeong, S. Bang, H. M. Oh, H. J. Jeong, S. An and G. H. Han, ACS Nano, 2015, 9, 10032–10038 CrossRef CAS PubMed.
- D. Ruzmetov, K. Zhang, G. Stan, B. Kalanyan, G. R. Bhimanapati, S. M. Eichfeld, R. A. Burke, P. B. Shah, T. P. O'Regan, F. J. Crowne, A. G. Birdwell, J. A. Robinson, A. V. Davydov and T. G. Ivanov, ACS Nano, 2016, 10, 3580–3588 CrossRef CAS PubMed.
- S. M. Eichfeld, L. Hossain, Y.-C. Lin, A. F. Piasecki, B. Kupp, A. G. G. Birdwell, R. A. Burke, N. Lu, X. Peng, J. Li, A. Azcatl, S. McDonnell, R. M. Wallace, M. J. Kim, T. S. Mayer, J. M. Redwing and J. A. Robinson, ACS Nano, 2015, 9, 2080–2087 CrossRef CAS PubMed.
- K. Kang, S. Xie, L. Huang, Y. Han, P. Y. Huang, K. F. Mak, C.-J. Kim, D. Muller and J. Park, Nature, 2015, 520, 656–660 CrossRef CAS PubMed.
- K. Zhang, S. Feng, J. Wang, A. Azcatl, N. Lu, R. Addou, N. Wang, C. Zhou, J. Lerach, V. Bojan, M. J. Kim, L. Q. Chen, R. M. Wallace, M. Terrones, J. Zhu and J. A. Robinson, Nano Lett., 2015, 15, 6586–6591 CrossRef CAS PubMed.
- Y.-C. Lin, R. K. Ghosh, R. Addou, N. Lu, S. M. Eichfeld, H. Zhu, M.-Y. Li, X. Peng, M. J. Kim, L.-J. Li, R. M. Wallace, S. Datta and J. a. Robinson, Nat. Commun., 2015, 6, 7311 CrossRef CAS PubMed.
- H. Li, Q. Zhang, C. C. R. Yap, B. K. Tay, T. H. T. Edwin, A. Olivier and D. Baillargeat, Adv. Funct. Mater., 2012, 22, 1385–1390 CrossRef CAS.
- H. Zeng, G.-B. Liu, J. Dai, Y. Yan, B. Zhu, R. He, L. Xie, S. Xu, X. Chen, W. Yao and X. Cui, Sci. Rep., 2013, 3, 1608 CrossRef PubMed.
- A. Azcatl, S. Kc, X. Peng, N. Lu, S. McDonnell, X. Qin, F. de Dios, R. Addou, J. Kim, M. J. Kim, K. Cho and R. M. Wallace, 2D Mater., 2015, 2, 14004 CrossRef.
- R. Addou, S. McDonnell, D. Barrera, Z. Guo, A. Azcatl, J. Wang, H. Zhu, C. L. Hinkle, M. Quevedo-Lopez, H. N. Alshareef, L. Colombo, J. W. P. Hsu and R. M. Wallace, ACS Nano, 2015, 9, 9124–9133 CrossRef CAS PubMed.
- Y.-H. Lee, X.-Q. Zhang, W. Zhang, M.-T. Chang, C.-T. Lin, K.-D. Chang, Y.-C. Yu, J. T.-W. Wang, C.-S. Chang, L.-J. Li and T.-W. Lin, Adv. Mater., 2012, 24, 2320–2325 CrossRef CAS PubMed.
- J.-K. Huang, J. Pu, C.-L. Hsu, M.-H. Chiu, Z.-Y. Juang, Y.-H. Chang, W.-H. Chang, Y. Iwasa, T. Takenobu and L.-J. Li, ACS Nano, 2014, 8, 923–930 CrossRef CAS PubMed.
- R. H. Fowler and L. Nordheim, Proc. R. Soc. A, 1928, 119, 173–181 CrossRef CAS.
- G.-H. Lee, Y.-J. Yu, C. Lee, C. Dean, K. L. Shepard, P. Kim and J. Hone, Appl. Phys. Lett., 2011, 99, 243114 CrossRef.
- T. P. O'Regan, D. Ruzmetov, M. R. Neupane, R. A. Burke, A. A. Herzing, K. Zhang, A. G. Birdwell, D. E. Taylor, E. F. C. Byrd, S. D. Walck, A. V. Davydov, J. A. Robinson and T. G. Ivanov, Appl. Phys. Lett., 2017, 111, 51602 CrossRef.
- S. Das, H.-Y. Chen, A. V. Penumatcha and J. Appenzeller, Nano Lett., 2013, 13, 100–105 CrossRef CAS PubMed.
- F. Giannazzo, G. Fisichella, a. Piazza, S. Agnello and F. Roccaforte, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 1–4 CrossRef.
- M. Tangi, P. Mishra, C. C. Tseng, T. K. Ng, M. N. Hedhili, D. H. Anjum, M. S. Alias, N. Wei, L. J. Li and B. S. Ooi, ACS Appl. Mater. Interfaces, 2017, 9, 9110–9117 CAS.
- W. Liu, W. Cao, J. Kang and K. Banerjee, ECS Trans., 2013, 58, 281–285 CrossRef.
- M. Tangi, P. Mishra, T. K. Ng, M. N. Hedhili, B. Janjua, M. S. Alias, D. H. Anjum, C. C. Tseng, Y. Shi, H. J. Joyce, L. J. Li and B. S. Ooi, Appl. Phys. Lett., 2016, 109, 032104 CrossRef.
- M. E. Zvanut, Y. Uprety, J. Dashdorj, M. Moseley and W. Alan Doolittle, J. Appl. Phys., 2011, 110, 44508 CrossRef.
- H.-K. Kim, I. Adesida and T.-Y. Seong, J. Vac. Sci. Technol., A, 2004, 22, 1101–1104 CAS.
- O. Gelhausen, M. R. Phillips, E. M. Goldys, T. Paskova, B. Monemar, M. Strassburg and A. Hoffmann, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 125210 CrossRef.
- Y. Nakagawa, M. Haraguchi, M. Fukui, S. Tanaka, A. Sakaki, K. Kususe, N. Hosokawa, T. Takehara, Y. Morioka, H. Iijima, M. Kubota, M. Abe, T. Mukai, H. Takagi and G. Shinomiya, Jpn. J. Appl. Phys., 2004, 43, 23–29 CrossRef CAS.
- H. Henck, Z. Ben Aziza, O. Zill, D. Pierucci, C. H. Naylor, M. G. Silly, N. Gogneau, F. Oehler, S. Collin, J. Brault, F. Sirotti, F. Bertran, P. Le Fèvre, S. Berciaud, A. T. C. Johnson, E. Lhuillier, J. E. Rault and A. Ouerghi, Phys. Rev. B, 2017, 96, 115312 CrossRef.
- Y.-C. Lin, J. Li, S. C. de la Barrera, S. M. Eichfeld, Y. Nie, R. Addou, P. C. Mende, R. M. Wallace, K. Cho, R. M. Feenstra and J. A. Robinson, Nanoscale, 2016, 8, 8947–8954 RSC.
- H. Jeong, S. Bang, H. M. Oh, H. J. Jeong, S.-J. An, G. H. Han, H. Kim, K. K. Kim, J. C. Park, Y. H. Lee, G. Lerondel and M. S. Jeong, ACS Nano, 2015, 9, 10032–10038 CrossRef CAS PubMed.
- H. Li, Z. Yin, Q. He, H. Li, X. Huang, G. Lu, D. W. H. Fam, A. I. Y. Tok, Q. Zhang and H. Zhang, Small, 2012, 8, 63–67 CrossRef CAS PubMed.
- F. Giannazzo, G. Fisichella, A. Piazza, S. Agnello and F. Roccaforte, Phys. Rev. B: Condens. Matter Mater. Phys, 2015, 92, 81307 CrossRef.
- T. Roy, M. Tosun, X. Cao, H. Fang, D.-H. Lien, P. Zhao, Y.-Z. Chen, Y.-L. Chueh, J. Guo and A. Javey, ACS Nano, 2015, 9, 2071–2079 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7nr07586c |
| This journal is © The Royal Society of Chemistry 2018 |