Multi-carbazole derivatives: new dyes for highly efficient dye-sensitized solar cells†
Hua
Lai
a,
Jia
Hong
a,
Ping
Liu
b,
Chao
Yuan
a,
Yuxue
Li
*a and
Qiang
Fang
*ac
aKey Laboratory of Organofluorine Chemistry, State Key Laboratory of Organometallic Chemistry and Laboratory for Polymer Materials, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032, PR China. E-mail: qiangfang@mail.sioc.ac.cn; Fax: (+86) 21-54925337)
bCollege of Materials and Engineering, South China University of Technology, Guangzhou, 510640, PR China
cShanghai Advanced Research Institute, Chinese Academy of Sciences, 99 Haike Road, Zhangjiang Hi-Tech Park, Pudong, Shanghai, 201203, PR China
First published on 2nd February 2012
Abstract
Three new multi-carbazole derivatives (2C–4C) with a twisted and zigzag-shape structure were designed, synthesized and used as sensitizers for dye-sensitized solar cells (DSSCs). The results showed that this non-planar structure of 2C–4C, combined with multi alkyl chains, can efficiently inhibit dye aggregation and charge recombination, which gave DSSCs with a high open circuit voltage (Voc) and an overall solar-to-electric conversion efficiency (η) of up to 6.33%.
1. Introduction
Dye-sensitized solar cells (DSSCs) based on organic sensitizers adsorbed on nanocrystalline TiO2 electrodes have attracted a great deal of attention in the past few decades since the breakthrough in photon-to-current conversion efficiency has been achieved by Grätzel and co-workers with ruthenium-based sensitizers.1 In comparison with conventional inorganic photovoltaic devices, DSSCs show high photovoltaic performance and have low production costs.2–4 Accordingly, DSSCs have been regarded as a potential alternative to the conventional silicon-based photovoltaic cells.5 From the point of view of practical applications however, the use of expensive and uneasily purified ruthenium-complexes would hamper the commercialization of DSSCs. Consequently, in recent years, there has been an ever-increasing interest in the development of metal-free dyes, and great progress has been made.6 Compared with ruthenium-based sensitizers, organic dyes have many advantageous features including convenient synthesis and purification at lower cost, as well as easier control of the physical and chemical properties by molecular design because of the wide variety of their structures and facile modification. Nevertheless, the improvement of the power conversion efficiency of the devices still remains a target in this field.There are two main factors that have a negative effect on the power conversion efficiency of DSSCs based organic dyes. One is charge recombination of injected electrons in the TiO2 electrode and I3− ion in the liquid electrolyte. The other is the aggregation of the dyes on TiO2 due to their highly conjugated backbone. Usually, charge recombination can be decreased by introducing alkyl side chains into the backbones of dye molecules,7 and dye aggregation can be restrained via molecular design that changes the molecular structure from planar to non-planar or twisted.8 However, in some cases, the use of the above-mentioned method alone can not simultaneously eliminate both charge recombination and the aggregation of the dyes.9 Hence, carefully designing dyes containing both alkyl side chains and a twisted structure is a preferred strategy for the development of DSSCs with high performance.10
It is noted that the attachment of a carbazole unit to the backbone of the conjugated polymers can efficiently depress π-stacking of the polymers in the solid state,11 and such a unit has also been introduced to the dye molecules used in DSSCs.12 However, few examples involved in 3,6-linked multi-carbazoles have been reported. Hence, three new organic dye molecules, 2C–4C (see Fig. 1), containing a 3,6-linked multi-carbazole donor were designed, and their configurations were calculated by using the density functional theory (DFT). The results indicated that the designed molecules had an interesting twisted structure and a zigzag-shape, in which the dihedral angles between two carbazole units were near 40°, as shown in Fig. 1. Such a calculated result implied that 2C–4C could be considered as good candidates of the dyes for highly efficient DSSCs, in which dye aggregation and charge recombination could be efficiently retarded. This motivated us to synthesize these multi-carbazole derivatives and investigate their photovoltaic properties. Here, we report the detailed results.
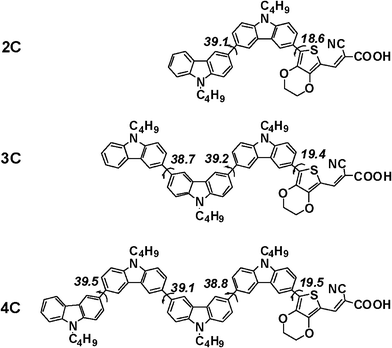 | ||
| Fig. 1 Theoretical estimation of the dihedral angles between the carbazole units of the new dye molecules. Calculated at B3LYP/6-31G** level using the Gaussian03 program. | ||
2. Experimental
2.1 Materials
All solvents were dehydrated and distilled under an inert atmosphere before use. Pd(PPh3)4, cyanoacetic acid, and piperidine were used as received. The supporting electrolyte for the electrochemical measurement, tetrabyutylammonium hexafluorophosphate (TBAPF6) and the materials for the fabrication of DSSCs, 4-tert-butylpyridine (4-TBP), lithium iodide (LiI), iodine (I2) and chenodeoxycholic acid (CDCA), were bought from Aldrich and without further purification. 9-Butyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-carbazole (1)13a, 3,6-dibromo-9-N-butyl-carbazole (2)13b and 7-bromo-2,3-dihydrothieno[3,4-b][1,4]-dioxine-5-carbaldehyde (5)13c were prepared according to the reported procedures.2.2 Measurement
1H{13C} NMR spectra were recorded on Bruker DRX 400 or Varian mercury 300 spectrometer using CDCl3 or DMSO-d6 as solvent and TMS as internal standard. High-resolution mass spectra (HRMS) were obtained by MALDI/DHB. UV-vis and PL spectra were obtained with a Hitachi UV-2810 and a Hitachi F-4500 spectrophotometer, respectively. Both cyclic voltammetry (CV) and EIS measurements were performed with an electrochemical workstation (Autolab PGSTAT30). The redox potentials of the dyes were measured in a CH2Cl2 solution containing 0.1 M TBAPF6 as supporting electrolyte at a scan rate of 100 mV s−1 under argon, using AgCl/Ag and platinum wire as reference and counter electrodes, respectively. The EIS measurements were carried out in a frequency range of 105–10−1 Hz and an amplitude of 10 mv at −0.7 V in the dark.2.3 Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the eluents to afford 6-bromo-9,9′-dibutyl-9H,9′H-3,3′-bicarbazole (3) as a white solid (2.2 g, a yield of 62%). 1H NMR (300 MHz, CDCl3, ppm): δ 8.38 (s, 1H), 8.34 (s, 1H), 8.29 (s, 1H), 8.19–8.16 (d, 1H, J = 7.8 Hz), 7.85–7.78 (m, 2H), 7.55–7.44 (m, 5H), 7.30–7.24 (m, 2H), 4.36–4.27 (m, 4H), 1.92–1.84 (m, 4H), 1.45–1.39 (m, 4H), 0.99–0.93 (m, 6H). 13C NMR (100 MHz, CDCl3, ppm): δ 139.51, 128.26, 126.27, 125.72, 125.43, 123.18, 120.46, 119.00, 118.91, 118.81, 111.54, 110.23, 109.17, 108.95, 108.83, 43.05, 31.22, 20.63, 13.95.
1, v/v) as the eluents to afford 6-bromo-9,9′-dibutyl-9H,9′H-3,3′-bicarbazole (3) as a white solid (2.2 g, a yield of 62%). 1H NMR (300 MHz, CDCl3, ppm): δ 8.38 (s, 1H), 8.34 (s, 1H), 8.29 (s, 1H), 8.19–8.16 (d, 1H, J = 7.8 Hz), 7.85–7.78 (m, 2H), 7.55–7.44 (m, 5H), 7.30–7.24 (m, 2H), 4.36–4.27 (m, 4H), 1.92–1.84 (m, 4H), 1.45–1.39 (m, 4H), 0.99–0.93 (m, 6H). 13C NMR (100 MHz, CDCl3, ppm): δ 139.51, 128.26, 126.27, 125.72, 125.43, 123.18, 120.46, 119.00, 118.91, 118.81, 111.54, 110.23, 109.17, 108.95, 108.83, 43.05, 31.22, 20.63, 13.95.
To a solution of 3 (1.6 g, 3.1 mmol) in dry THF, 2.2 ml of n-BuLi (1.6 M in hexane) was added dropwise under argon at −78 °C. The mixture was warmed to room temperature and recooled to −78 °C, followed by addition of 2-isopropoxy-4,4,5,5-tetramethyl-[1,3,2]dioxaborolane (0.95 ml, 4.5 mmol) using a syringe. After this addition, the mixture was warmed to room temperature, stirred for 12 h, and quenched with water. After the solvent was removed by rotary evaporation, the residue was extracted with CHCl3, washed with saturated aq. NaCl solution, and dried over anhydrous Na2SO4 and filtered. The filtrate was evaporated and the residue was purified by column chromatography on silica gel using a mixture of petroleum ether and CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the eluents to afford a colorless oil that became a white solid after standing for 1 day at room temperature. 4 was thus obtained in a yield of 62% (1.1 g). 1H NMR (300 MHz, CDCl3, ppm): δ 8.69 (s, 1H), 8.49 (s, 1H), 8.42 (s, 1H), 8.22–8.19 (d, 1H, J = 7.5 Hz), 7.95–7.92 (d, 1H, J = 8.1 Hz), 7.86–7.81 (m, 2H), 7.52–7.42 (m, 5H), 7.30–7.25 (t, 1H), 4.39–4.34 (t, 4H), 1.94–1.88 (m, 4H), 1.47–1.41 (m, 16H), 1.01–0.94 (m, 6H). HRMS (MALDI-TOF)): calcd. for C38H43N2O2B+1, 569.3448; found, 569.3435.
1, v/v) as the eluents to afford a colorless oil that became a white solid after standing for 1 day at room temperature. 4 was thus obtained in a yield of 62% (1.1 g). 1H NMR (300 MHz, CDCl3, ppm): δ 8.69 (s, 1H), 8.49 (s, 1H), 8.42 (s, 1H), 8.22–8.19 (d, 1H, J = 7.5 Hz), 7.95–7.92 (d, 1H, J = 8.1 Hz), 7.86–7.81 (m, 2H), 7.52–7.42 (m, 5H), 7.30–7.25 (t, 1H), 4.39–4.34 (t, 4H), 1.94–1.88 (m, 4H), 1.47–1.41 (m, 16H), 1.01–0.94 (m, 6H). HRMS (MALDI-TOF)): calcd. for C38H43N2O2B+1, 569.3448; found, 569.3435.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the eluents to afford 6 as a yellow powder (200 mg, yield of 93%). 1H NMR (300 MHz, CDCl3, ppm): δ 9.93 (s, 1H), 8.64 (s, 1H), 8.42 (s, 2H), 8.21–8.19 (d, 1H), 7.88–7.81 (m, 3H), 7.51–7.40 (m, 5H), 7.28–7.25 (t, 1H), 4.43 (s, 4H), 4.37–4.32 (m, 4H), 1.89 (s, 4H), 1.47–1.38 (m, 4H), 0.99–0.94 (m, 6H). 13C NMR (100 MHz, CDCl3, ppm): δ 179.58, 149.62, 141.20, 141.17, 140.21, 139.88, 136.89, 134.29, 133.28, 131.48, 126.32, 125.97, 125.71, 123.67, 123.61, 123.27, 122.83, 120.69, 119.73, 119.39, 119.19, 119.05, 114.85, 109.51, 109.18, 109.09, 65.47, 64.82, 43.31, 31.44, 14.17. HRMS (MALDI-TOF): calcd. for C39H36N2O3S+1, 612.2441; found, 612.2444.
1, v/v) as the eluents to afford 6 as a yellow powder (200 mg, yield of 93%). 1H NMR (300 MHz, CDCl3, ppm): δ 9.93 (s, 1H), 8.64 (s, 1H), 8.42 (s, 2H), 8.21–8.19 (d, 1H), 7.88–7.81 (m, 3H), 7.51–7.40 (m, 5H), 7.28–7.25 (t, 1H), 4.43 (s, 4H), 4.37–4.32 (m, 4H), 1.89 (s, 4H), 1.47–1.38 (m, 4H), 0.99–0.94 (m, 6H). 13C NMR (100 MHz, CDCl3, ppm): δ 179.58, 149.62, 141.20, 141.17, 140.21, 139.88, 136.89, 134.29, 133.28, 131.48, 126.32, 125.97, 125.71, 123.67, 123.61, 123.27, 122.83, 120.69, 119.73, 119.39, 119.19, 119.05, 114.85, 109.51, 109.18, 109.09, 65.47, 64.82, 43.31, 31.44, 14.17. HRMS (MALDI-TOF): calcd. for C39H36N2O3S+1, 612.2441; found, 612.2444.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the eluents to afford 7 as a yellow solid. (225 mg, yield of 72%). 1H NMR (300 MHz, CDCl3, ppm): δ 9.93 (s, 1H), 8.61 (s, 1H), 8.38 (s, 1H), 8.33 (s, 1H), 8.28 (s, 1H), 7.87–7.79 (m, 3H), 7.56–7.53 (d, 1H), 7.48–7.45 (d, 2H), 7.40–7.37 (d, 1H), 7.29–7.26 (d, 1H), 4.42 (s, 4H), 4.27 (m, 4H), 1.85 (m, 4H), 1.39–1.22 (m, 4H), 0.98–0.84 (m, 6H). 13C NMR (100 MHz, CDCl3, ppm): δ 179.30, 149.34, 140.93, 140.02, 139.84, 139.50, 136.66, 133.47, 131.14, 128.30, 126.18, 125.94, 124.92, 124.72, 123.38, 123.28, 123.15, 122.63, 122.40, 119.44, 119.09, 118.98, 114.62, 111.57, 110.26, 109.30, 109.20, 109.10, 65.22, 64.58, 43.06, 31.17, 20.56, 13.89. HRMS (MALDI-TOF): calcd. for C45H29N3Br+1, 690.1540; found, 690.1543.
1, v/v) as the eluents to afford 7 as a yellow solid. (225 mg, yield of 72%). 1H NMR (300 MHz, CDCl3, ppm): δ 9.93 (s, 1H), 8.61 (s, 1H), 8.38 (s, 1H), 8.33 (s, 1H), 8.28 (s, 1H), 7.87–7.79 (m, 3H), 7.56–7.53 (d, 1H), 7.48–7.45 (d, 2H), 7.40–7.37 (d, 1H), 7.29–7.26 (d, 1H), 4.42 (s, 4H), 4.27 (m, 4H), 1.85 (m, 4H), 1.39–1.22 (m, 4H), 0.98–0.84 (m, 6H). 13C NMR (100 MHz, CDCl3, ppm): δ 179.30, 149.34, 140.93, 140.02, 139.84, 139.50, 136.66, 133.47, 131.14, 128.30, 126.18, 125.94, 124.92, 124.72, 123.38, 123.28, 123.15, 122.63, 122.40, 119.44, 119.09, 118.98, 114.62, 111.57, 110.26, 109.30, 109.20, 109.10, 65.22, 64.58, 43.06, 31.17, 20.56, 13.89. HRMS (MALDI-TOF): calcd. for C45H29N3Br+1, 690.1540; found, 690.1543.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the eluents to afford 2C as a red solid (100 mg, a yield of 90%). 1H NMR (300 MHz, DMSO-d6, ppm): δ 8.70 (s, 1H), 8.65 (s, 1H), 8.59 (s, 1H), 8.30–8.27 (d, 1H), 8.23 (s, 1H), 7.91–7.88 (d, 3H), 7.74–7.66 (m, 3H), 7.61–7.59 (d, 1H), 7.47–7.42 (t, 1H), 7.23–7.18 (t, 1H), 4.54–4.40 (m, 8H), 1.80–1.78 (m, 4H), 1.35–1.28 (m, 4H), 0.91–0.86 (t, 6H). 13C NMR (100 MHz, DMSO-d6, ppm): δ 164.82, 150.16, 141.14, 140.99, 140.56, 140.09, 139.68, 137.63, 133.61, 132.49, 129.97, 126.22, 125.60, 125.32, 123.26, 123.15, 122.87, 122.40, 121.06, 119.60, 119.13, 118.90, 117.75, 110.44, 109.96, 108.44, 93.14, 66.20, 65.15, 42.70, 31.24, 20.30, 14.17. HRMS (MALDI-TOF): calcd. for C42H37N3O4S+1, 679.2499; found, 679.2495.
1, v/v) as the eluents to afford 2C as a red solid (100 mg, a yield of 90%). 1H NMR (300 MHz, DMSO-d6, ppm): δ 8.70 (s, 1H), 8.65 (s, 1H), 8.59 (s, 1H), 8.30–8.27 (d, 1H), 8.23 (s, 1H), 7.91–7.88 (d, 3H), 7.74–7.66 (m, 3H), 7.61–7.59 (d, 1H), 7.47–7.42 (t, 1H), 7.23–7.18 (t, 1H), 4.54–4.40 (m, 8H), 1.80–1.78 (m, 4H), 1.35–1.28 (m, 4H), 0.91–0.86 (t, 6H). 13C NMR (100 MHz, DMSO-d6, ppm): δ 164.82, 150.16, 141.14, 140.99, 140.56, 140.09, 139.68, 137.63, 133.61, 132.49, 129.97, 126.22, 125.60, 125.32, 123.26, 123.15, 122.87, 122.40, 121.06, 119.60, 119.13, 118.90, 117.75, 110.44, 109.96, 108.44, 93.14, 66.20, 65.15, 42.70, 31.24, 20.30, 14.17. HRMS (MALDI-TOF): calcd. for C42H37N3O4S+1, 679.2499; found, 679.2495.
2.4 Fabrication and testing of DSSCs
Nanocrystalline TiO2 (13 nm) films (12 μm) on FTO glass, prepared by a screen printing technique similar to the method reported in ref. 14, were provided from Heptachroma, China. The films were immersed into a solution of a dye in a mixture of THF–CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with a concentration of 1.5 × 10−4 M in the presence of 0.75 mM chenodeoxycholic acid (CDCA) for 12 h at room temperature. The counter electrode, Pt-coated FTO glass, was prepared by casting a drop of a solution of 0.02 M H2PtCl6 in 2-propanol on a FTO glass with a sheet resistance of 15 Ω square−2, followed by drying at ambient temperature and annealing at 450 °C for 30 min. The dye-coated film and Pt-coated FTO glass was sealed with a hot melt adhesive (25 μm, Surlyn, DuPont). The electrolyte, composed of 1.0 M of 1,2-dimethyl-3-propylimidazolium iodide (DMPII), 0.03 M I2, 0.05 LiI, 0.1 M guanidinium thiocyanate (GuSCN) and 0.5 M 4-TBP in a solvent mixture of acetonitrile and valeronitrile (85
1, v/v) with a concentration of 1.5 × 10−4 M in the presence of 0.75 mM chenodeoxycholic acid (CDCA) for 12 h at room temperature. The counter electrode, Pt-coated FTO glass, was prepared by casting a drop of a solution of 0.02 M H2PtCl6 in 2-propanol on a FTO glass with a sheet resistance of 15 Ω square−2, followed by drying at ambient temperature and annealing at 450 °C for 30 min. The dye-coated film and Pt-coated FTO glass was sealed with a hot melt adhesive (25 μm, Surlyn, DuPont). The electrolyte, composed of 1.0 M of 1,2-dimethyl-3-propylimidazolium iodide (DMPII), 0.03 M I2, 0.05 LiI, 0.1 M guanidinium thiocyanate (GuSCN) and 0.5 M 4-TBP in a solvent mixture of acetonitrile and valeronitrile (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 v/v),15 was injected through a hole in the counter electrode by capillary force.
15 v/v),15 was injected through a hole in the counter electrode by capillary force.
The photocurrent–voltage characteristics of the DSSCs were measured using a Keithley 2420 sourcemeter under illumination of AM 1.5 G solar light from 450 W Oriel Class AAA solar simulator (Model 92250A-450). The output power was calibrated by a monocrystalline silicon reference cell (Model 91150a). Incident photo-to-current conversion efficiency (IPCE) of the DSSCs was measured by a direct current (DC) method, using a light source from a 300 W Xenon Lamp (Oriel 6258), a monochromator (Oriel 74125), a Si detector (Oriel 71030NS) and an optical power meter (Oriel 70310).
3. Results and discussion
3.1 Synthesis
The synthetic procedure of 2C, 3C, and 4C is illustrated in Scheme 1. The new dyes were prepared starting from 1 and 5 mainly through Suzuki coupling and the Knoevenagel reaction. The experimental details and the characterization data of the intermediates and the final products 2C–4C are shown in the experimental section and ESI.†![Synthetic procedure of 2C–4C. i) Pd(PPh3)4, 2 M aq. Na2CO3, THF, reflux; ii) n-BuLi, THF, −78 °C, 2-isopropoxy-4,4,5,5-tetramethyl-[1,3,2] dioxaborolane; iii) cyanoacetic acid, piperidine, CH3CN/CHCl3; iv) NBS, DMF, 0 °C.](/image/article/2012/RA/c2ra01002j/c2ra01002j-s1.gif) | ||
| Scheme 1 Synthetic procedure of 2C–4C. i) Pd(PPh3)4, 2 M aq. Na2CO3, THF, reflux; ii) n-BuLi, THF, −78 °C, 2-isopropoxy-4,4,5,5-tetramethyl-[1,3,2] dioxaborolane; iii) cyanoacetic acid, piperidine, CH3CN/CHCl3; iv) NBS, DMF, 0 °C. | ||
3.2 Optical properties
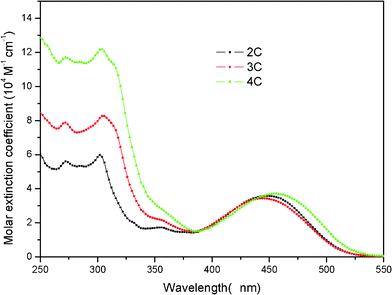
The basic photophysical properties of 2C, 3C and 4C were characterized by UV-Vis spectra. As shown in Fig. 2, all dyes in THF exhibited two major strong absorption bands at 250–325 nm and 375–520 nm. The former was ascribed to a π–π* transition of the carbazole moieties. With increasing the number of the carbazole moieties, the bands possessed a similar shape and showed a slight red-shift, whereas the molar extinction coefficient monotonically increased from 5.98 × 104 M−1 cm−1 for 2C to 12.70 × 104 M−1 cm−1 for 4C. The absorption bands at 375–520 nm arise from the intramolecular charge transition between the electron donators (multi-carbazole and thiophene units) and the electron acceptors (cyanoacrylate moiety). 2C, 3C, and 4C showed maximum absorption peaks at 448, 444 and 456 nm, respectively, with extinction coefficients ranging from 3.44–3.87 × 104 M−1 cm−1. These higher molar extinction coefficients of 2C–4C are higher than that of ruthenium-based sensitizers (< 2.00 × 104 M−1 cm−1), and are highly desirable for the DSSCs with high efficiency.1In comparison with their UV-Vis absorption edges in THF, the dyes adsorbed on TiO2 showed the absorption edges with a red-shift of near 30 nm, as shown in the ESI.† Such a red-shift was attributed to J-aggregation of the dyes on the TiO2 surface because of the presence of carboxyl groups in the dye molecules. However, the maximum absorption peaks of the dyes had little change (< 4 nm), which were much lower than that for the dyes containing alkyl substituted bithiazole and bithiophene moieties,7b,10a indicating the zigzag-shape and twisted structure of molecules can efficiently inhibit aggregation of the molecules on TiO2.
3.3 Electrochemical properties
The electrochemical behaviors of the dyes were measured by cyclic voltammetry (CV), as shown in Fig. 3. The detailed data were listed in Table 1. As can be seen from Table 1, the HOMO levels of the dyes corresponding to their first oxidation potentials in CV were in the range 0.94–0.99 V versusnormal hydrogen electrode (NHE). Such HOMO levels were more positive than I−/I3−oxidation potential value (0.4 V versusNHE), implying that I− ions in the cell system could thermodynamically reduce the oxidized dyes produced when electron injects into the conduction band of TiO2. The LUMO levels of these dyes, obtained from HOMO levels and the zeroth–zeroth energies (E0–0) of the dyes, were in a range from −1.42 V to −1.48 V, which were more negative than that of the conduction band value of TiO2 (−0.5 V versusNHE). Such results suggested that electron injection from the dyes in the excited state into the conduction band of TiO2 was permitted.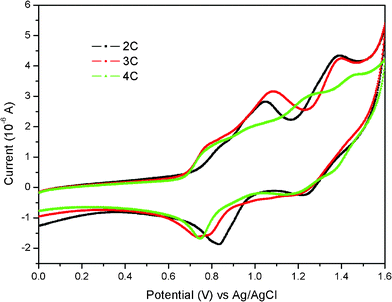 | ||
| Fig. 3 The CV curves of the dyes in dichloromethane containing 0.1 M TBAPF6 as supporting electrolyte at a scan rate of 100 mV s−1. | ||
| Dye | λ max abs(nm) a (ε/104 M−1 cm−1) | HOMOb (V) | E 0–0 c (V) | LUMOd (V) |
|---|---|---|---|---|
| a Absorption spectra were measured in THF solution (10−5 M). b Measured in CH2Cl2 solution using 0.1 M tetrabutylammonium hexafluorofhosphate (TBAPF6) as supporting electrolyte, Pt wire as working and counter electrode, and Ag/AgCl as reference electrode, respectively. All potentials were calibrated with ferrocene/ferrocenium (Fc/Fc+) as an internal standard. c E 0–0 was calculated from the intersection between the absorption and emission spectra. d LUMO levels were obtained as HOMO–E0–0. | ||||
| 2C | 448 (3.57) | 0.99 | 2.41 | −1.42 |
| 3C | 444 (3.44) | 0.94 | 2.42 | −1.48 |
| 4C | 456 (3.87) | 0.94 | 2.41 | −1.47 |
Further calculations for the theoretical HOMO and LUMO levels of the dyes by using the DFT method also revealed that the electron injection from the excited dyes to conduction band of TiO2 could be successfully realized (see ESI†).
3.4 Photovoltaic properties
Incident photon-to-current conversion efficiencies (IPCEs) of the solar cells based on 2C–4C and N719 were measured. Fig. 4 (inset) shows the results. Although the photoresponse range of 2C–4C (300–650 nm) was narrower than that of N719 (300–750 nm), the values of IPCEs for 2C–4C were on a plateau near 80% in the range of 420–550 nm, and were higher than that of N719.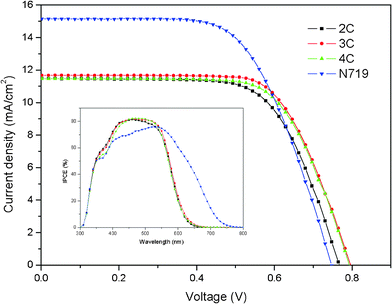 | ||
| Fig. 4 J–V curves and IPCE (inset) of DSSCs based on the dyes and N719. | ||
The photocurrent–voltage (J–V) characteristics of DSSCs were further measured under simulated AM 1.5G irradiation (100 mW cm−2) and the data are depicted in Fig. 4 and Table 2. The cell based on 2C containing two carbazole moieties exhibited an open-circuit voltage (Voc) of 0.767 V, a short-circuit current density (Jsc) of 11.47 mA cm−2 and a fill factor (ff) of 0.67, giving a solar energy-to-electricity conversion (η) of 5.87%. For 3C containing three carbazole moieties, higher Voc (0.796 V), Jsc (11.67 mA cm−2), ff (0.68) and η (6.33%) were achieved. Under the same conditions, a cell based on N719 gave a η of 7.02%. Since 2C and 3C possessed similar light harvesting range and molar extinction coefficient, the reason why 3C showed the better properties was probably that the existence of more twisted structures and alkyl side chains further inhibited dye aggregation and charge recombination. Further increasing the carbazole moieties on donor (4C) did not increase photovoltaic properties, with Voc (0.792 V), Jsc (11.46 mA cm−2), ff (0.68) and η (6.16%).
| Dyes | J sc (mA cm−2) | V oc (V) | ff | η (%) |
|---|---|---|---|---|
a The concentrations of the dyes was 1.5 × 10−4 M in a mixture of THF and CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with 0.75 mM chenodeoxycholic acid (CDCA). Performances of DSSCs were measured with a 0.237 cm2 working area under AM 1.5G irradiation (100 mW cm−2). 1, v/v) with 0.75 mM chenodeoxycholic acid (CDCA). Performances of DSSCs were measured with a 0.237 cm2 working area under AM 1.5G irradiation (100 mW cm−2).
|
||||
| 2C | 11.47 | 0.767 | 0.67 | 5.87 |
| 3C | 11.67 | 0.796 | 0.68 | 6.33 |
| 4C | 11.46 | 0.792 | 0.68 | 6.16 |
| N719 | 15.15 | 0.747 | 0.62 | 7.02 |
It was noteworthy that 2C–4C exhibited relatively high Voc values ranging from 0.767–0.796 V (see Table 2). A high Voc is regarded as a result of low charge recombination.7b,16 In previous reports, electrochemical impedance spectroscopy (EIS) was used to elucidate the charge transfer processes in DSSCs and the charge recombination can be quantified with recombination resistance (Rrec) which was deduced by implementing sophisticated calculations.17 Nevertheless, a simpler and qualitative comparison of the charge transfer processes can be obtained from Nyquist plots.16a,16c,18 As shown in Fig. 5, the first of the three semicircles is ascribed to the charge transfer at Pt/redox (I−/I3−) interface, the second large one is related to charge transfer at dye-sensitized TiO2/redox (I−/I3−) interface and the third one (low frequency, not obvious) is associated with the Nernst diffusion of I3− in the electrolyte. The radius of larger semicircles in Nyquist plot for DSSCs based on 2C–4C increase in the order of 2C < 4C < 3C, indicating less charge recombination took place in cells based on 4C and 3C, which was consistent with the value order of Voc.
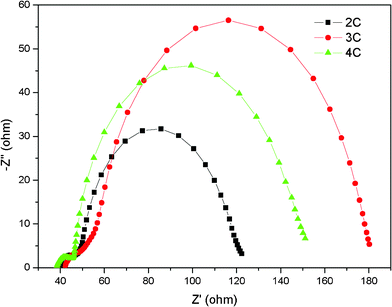 | ||
| Fig. 5 EIS Nyquist plots for DSSCs based on 2C–4C at −0.7 V under dark. | ||
The inferior performance of 4C may be resulted from loose packing by its larger twisted molecular structure and more n-butyl chains compared with 3C, similar to the results that Chen et al. have reported.10a The loose packing probably leads to some voids through which I3− ions in the liquid electrolyte could penetrate the dye layer near TiO2 and then recombine with electron on TiO2. Such a result implies that both molecular structure and the size of dyes should be carefully designed for improving the overall photovoltaic performance of DSSCs.
4. Conclusions
In summary, we have designed and synthesized three new multi-carbazole derivatives with a twisted structure and a zigzag-shape. The DSSCs based on these derivatives showed a high open-circuit voltage and power conversion efficiency, suggesting that the combination of alkyl side chains and twisted linked backbone in a dye molecule could effectively inhibit dye aggregation and charge recombination, which usually have a negative effect on the efficiency of DSSCs.Acknowledgements
Financial support from the National Natural Science Foundation of China (NSFC, No.20872171), Guangdong Provincial Department of Science and Technology (2010A090100001) and the Ministry of Science and Technology of China (2010BAK67B12) are gratefully acknowledged.References
- (a) B. O'Reagen and M. Grätzel, Nature, 1991, 353, 737 CrossRef; (b) M. Grätzel, Acc. Chem. Res., 2009, 42, 1788 CrossRef.
- (a) M. K. Nazeeruddin, F. D. Angelis, S. Fantacci, A. Selloni, G. Viscardi, P. Liska, S. Ito, B. Takeru and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 16835 CrossRef CAS; (b) F. Gao, Y. Wang, D. Shi, J. Zhang, M. Wang, X. Jing, R. Humphry-Baker, P. Wang, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2008, 130, 10720 CrossRef CAS; (c) C. Chen, M. Wang, J. Li, N. Pootrakulchote, C. Alibabaei, J. Decoppet, J. Tsai, C. Grätzel, C. Wu, S. Zakeeruddin and M. Grätzel, ACS Nano, 2009, 3, 3103 CrossRef CAS.
- (a) Q. Yu, S. Liu, M. Zhang, N. Cai, Y. Wang and P. Wang, J. Phys. Chem. C, 2009, 113, 14559 CrossRef CAS; (b) Y. Cao, Y. Bai, Q. Yu, Y. Cheng, S. Liu, D. Shi, F. Gao and P. Wang, J. Phys. Chem. C, 2009, 113, 6290 CrossRef CAS.
- H.-J. Koo, Y. J. Kim, Y. H. Lee, W. I. Lee, K. Kim and N.-G. Park, Adv. Mater., 2008, 20, 195 CrossRef CAS.
- (a) A. Hagfeldt and M. Grätzel, Acc. Chem. Res., 2000, 33, 269 CrossRef CAS; (b) A. Hagfeldt, G. Boschloo, L. C. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595 CrossRef CAS.
- (a) M. Planells, L. Pellej, J. N. Clifford, M. Pastore, F. D. Angelis, N. Lopez, S. R. Marderc and E. Palomares, Energy Environ. Sci., 2011, 4, 1820 RSC; (b) Z. Wang, Y. Cui, Y. Dan-Oh, C. Kasada, A. Shinpo and K. Hara, J. Phys. Chem. C, 2007, 111, 7224 CrossRef CAS; (c) Z. Wang, Y. Cui, Y. Dan-Oh, C. Kasada, A. Shinpo and K. Hara, J. Phys. Chem. C, 2008, 112, 17011 CrossRef CAS; (d) W. Zhu, Y. Wu, S. Wang, W. Li, X. Li, J. Chen, Z. Wang and H. Tian, Adv. Funct. Mater., 2011, 21, 756 CrossRef CAS; (e) G. Li, Y. Zhou, X. Cao, P. Bao, K. Jiang, Y. Lin and L. Yang, Chem. Commun., 2009, 2201 RSC; (f) K. Jiang, K. Manseki, Y. Yu, N. Masaki, K. Suzuki, Y. Song and S. Yanagida, Adv. Funct. Mater., 2009, 19, 2481 CrossRef CAS; (g) D. Cao, J. Peng, Y. Hong, X. Fang, L. Wang and H. Meier, Org. Lett., 2011, 13, 1610 CrossRef CAS.
- (a) J. E. Kroeze, N. Hirata S. Koops, M. K. Nazeeruddin, L. Schmidt-Mende, M. Grätzel and J. R. Durrant, J. Am. Chem. Soc., 2006, 128(50), 16376 CrossRef CAS; (b) J. He, W. Wu, J. Hua, Y. Jiang, S. Qu, J. Li, Y. Long and H. Tian, J. Mater. Chem., 2011, 21, 6054 RSC.
- (a) L.-Y. Lin, C.-H. Tsai, K.-T. Wong, T.-W. Huang, L. Hsieh, S.-H. Liu, H.-W. Lin, C.-C. Wu, S.-H. Chou, S.-H. Chen and A.-I. Tsai, J. Org. Chem., 2010, 75, 4778 CrossRef CAS; (b) N. Cho, H. Choi, D. Kim, K. Song, M.-S. Kang, S.-O. Kang and J. Ko, Tetrahedron, 2009, 65, 6236 CrossRef CAS; (c) D. Heredia, J. Natera, M. Gervaldo, L. Otero, F. Fungo, C.-Y. Lin and K.-T. Wong, Org. Lett., 2010, 12, 12 CrossRef CAS.
- (a) Y. Ooyama and Y. Harima, Eur. J. Org. Chem., 2009, 2903 CrossRef CAS; (b) N. Koumura, Z. Wang, M. Miyashita, Y. Uemura, H. Sekiguchi, Y. Cui, A. Mori, S. Mori and K. Hara, J. Mater. Chem., 2009, 19, 4829 RSC; (c) Z. S. Wang, N. Koumura, Y. Cui, M. Takahashi, H. Sekiguchi, A. Mori, T. Kubo, A. Furube and K. Hara, Chem. Mater., 2008, 20, 3993 CrossRef CAS.
- (a) Y. Liang, B. Peng, J. Liang, Z. Tao and J. Chen, Org. Lett., 2010, 12(6), 1204 CrossRef CAS; (b) X. Zhang, Z. Wang, Y. Cui, N. Koumura, A. Furube and K. Hara, J. Phys. Chem. C, 2009, 113, 13409 CrossRef CAS; (c) J.-I. Nishida, T. Masuko, Y. Cui, K. Hara, H. Shibuya, M. Ihara, T. Hosoyama, R. Goto, S. Mori and Y. Y. Amashita, J. Phys. Chem. C, 2010, 114, 17920 CrossRef CAS.
- (a) C. Xia and R. C. Advincula, Macromolecules, 2001, 34, 5854 CrossRef CAS; (b) J. Fang, Q. Du, D. Bu, S. Ren, A. Cao and X. Chen, Macromol. Rapid Commun., 2005, 26, 1651 CrossRef.
- (a) E. M. Barea, C. Zafer, B. Gultekin, B. Aydin, S. Koyuncu, S. Icli, F. F. Santiago and J. Bisquert, J. Phys. Chem. C, 2010, 114, 19840 CrossRef CAS; (b) Z. J. Ning, Q. Zhang, W. J. Wu, H. C. Pei, B. Liu and H. Tian, J. Org. Chem., 2008, 73, 3791 CrossRef CAS; (c) D. Kim, J. K. Lee, S. O. Kang and J.-J. Ko, Tetrahedron, 2007, 63, 1913 CrossRef CAS; (d) N. Koumura, Z. S. Wang, S. Mori, M. Miyashita, E. Suzuki and K. Hara, J. Am. Chem. Soc., 2006, 128, 14256 CrossRef CAS.
- (a) L. C. Zeng, T. Y.-H. Lee, P. B. Merkel and S. H. Chen, J. Mater. Chem., 2009, 19, 8772 RSC; (b) J. Cabaj, K. Idzik, J. Sołoducho and A. Chyla, Tetrahedron, 2006, 62, 758 CrossRef CAS; (c) M. Jessing, M. Brandt, K. J. Jensen, J. B. Christensen and U. Boas, J. Org. Chem., 2006, 71, 6734 CrossRef CAS.
- S. Ito, P. Liska, P. Comte, R. Charvet, P. Péchy, U. Bach, L. Schmidt-Mende, S. M. Zakeeruddin, M. K. Nazeeruddin and M. Grätzel, Chem. Commun., 2005, 4351 RSC.
- W. Zeng, Y. Cao, Y. Bai, Y. Wang, Y. Shi, M. Zhang, F. Wang, C. Pan and P. Wang, Chem. Mater., 2010, 22, 1915 CrossRef CAS.
- (a) B. Chen, D. Chen, C. Chen, C. Hsu, H. Hsu, K. Wu, S. Liu, P. Chou and Y. Chi, J. Mater. Chem., 2011, 21, 1937 RSC; (b) M. Adachi, M. Sakamoto, J. Jiu, Y. Ogata and S. Isoda, J. Phys. Chem. B, 2006, 110, 13872 CrossRef CAS; (c) Y. J. Chang and T. J. Chow, J. Mater. Chem., 2011, 21, 9523 RSC.
- (a) E. Mª Barea, R. Caballero, F. Fabregat-Santiago, P. De La Cruz, F. Langa and J. Bisquert, ChemPhysChem, 2010, 11, 245 CrossRef; (b) J. Bisquert, F. Fabregat-Santiago, I. Mora-Serό, G. Garcia-Belmonte and S. Giménez, J. Phys. Chem. C, 2009, 113(40), 17278 CrossRef CAS; (c) F. Fabregat-Santiago, G. Garcia-Belmonte, I. Mora-Seró and J. Bisquert, Phys. Chem. Chem. Phys., 2011, 13, 9083 RSC; (d) E. Ma Barea, V. Gόnzalez-Pedro, T. Ripollés-Sanchis, H.-P. Wu, L.-L. Li, C.-Y. Yeh, E. W.-G. Diau and J. Bisquert, J. Phys. Chem. C, 2011, 115(21), 10898 CrossRef.
- (a) C. Teng, X. Yang, C. Yuan, C. Li, R. Chen, H. Tian, S. Li, A. Hagfeldt and L. Sun, Org. Lett., 2009, 11(23), 5542 CrossRef CAS; (b) D. Kuang, S. Uchida, R. H.-Baker, S. M. Zakeeruddin and M. Grätzel, Angew. Chem., Int. Ed., 2008, 47, 1923 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed structure characterization, optical properties and molecular orbital estimation based on the density functional theory (DFT). See DOI: 10.1039/c2ra01002j |
| This journal is © The Royal Society of Chemistry 2012 |