 Open Access Article
Open Access ArticleOptimizing adsorption and photocatalysis for tetracycline and sulfamethoxazole removal: the role of Ag+ and NH4+ in Bi-MOF derivatives
Vy Anh Tran *a,
Thi Tuong Vi Trana,
Tran Thanh Sangb,
Nguyen Anh Thub,
Nguyen Duy Datb,
Hieu Vu_Quangc,
Van Thuan Le*d and
Thu Thao Thi Vo*e
*a,
Thi Tuong Vi Trana,
Tran Thanh Sangb,
Nguyen Anh Thub,
Nguyen Duy Datb,
Hieu Vu_Quangc,
Van Thuan Le*d and
Thu Thao Thi Vo*e
aDepartment of Material Science, Institute of Applied Technology and Sustainable Development, Nguyen Tat Thanh University, Ho Chi Minh City, 700000, Vietnam. E-mail: tavy@ntt.edu.vn
bFaculty of Chemical & Food Technology, University of Technology and Education, Thu Duc, Ho Chi Minh City 700000, Vietnam
cNTT Hi-tech Institute, Nguyen Tat Thanh University, Ho Chi Minh City, 700000, Vietnam
dCenter for Advanced Chemistry, Institute of Research and Development, Duy Tan University, 03 Quang Trung, Da Nang, 550000, Vietnam. E-mail: levanthuan3@duytan.edu.vn
eDepartment of Food Science and Biotechnology, Gachon University, 1342 Seongnamdaero, Sujeong-gu, Seongnam-si, 13120, Republic of Korea. E-mail: vothuthaobd@gmail.com
First published on 23rd January 2025
Abstract
This study focuses on the synthesis, characterization, and evaluation of the photocatalytic efficiency of bismuth-based metal–organic frameworks (Bi-MOFs) and their derivatives, specifically Ag+/Bi-MOF and NH4+/Ag+/Bi-MOF, in the degradation of tetracycline (TC) and sulfamethoxazole (SMX) under visible light irradiation. Bi-MOFs are promising photocatalysts due to their large surface area, tunable porosity, and unique electronic properties that are favorable for visible light absorption. In this study, Bi-MOFs were synthesized using a solvothermal method, with the incorporation of silver (Ag+) and ammonium (NH4+) ions to enhance their photocatalytic performance. The photocatalytic activity of Bi-MOF, Ag/Bi-MOF, and NH4+/Ag+/Bi-MOF was evaluated by measuring the degradation rates of TC and SMX in aqueous solutions under visible light. The experiments demonstrated that Bi-MOFs effectively degraded both antibiotics, with the Ag/Bi-MOF derivative showing superior performance due to improved charge separation and light absorption. NH4+/Ag+/Bi-MOF also exhibited significant photocatalytic activity, influenced by the presence of ammonium ions, which altered the surface chemistry of the catalyst and affected the adsorption and degradation processes. Reusability tests confirmed that these photocatalysts maintained high degradation efficiency over multiple cycles, highlighting their potential for practical environmental applications. This research provides a sustainable and effective solution for the removal of persistent organic pollutants such as TC and SMX.
1. Introduction
The increasing contamination of water sources by organic pollutants, especially pharmaceutical compounds such as antibiotics, has become a critical environmental issue worldwide.1,2 Among these contaminants, tetracycline (TC) and sulfamethoxazole (SMX) are two widely used antibiotics that are frequently detected in various water bodies.3 These antibiotics enter the environment through multiple pathways, including pharmaceutical industry discharge, agricultural runoff, and improper disposal of unused medications.4 The presence of such pollutants in water sources poses a serious threat to aquatic ecosystems and human health, as they can contribute to the development of antibiotic-resistant bacteria, disrupt aquatic life, and even enter the human food chain.5Traditional water treatment methods, including biological processes, chemical oxidation, and filtration, have proven inadequate in completely removing these persistent organic pollutants (POPs) from contaminated water.6,7 This inadequacy has spurred the search for alternative and more effective treatment technologies. In recent years, photocatalysis has emerged as a promising approach for the degradation of POPs in water, utilizing light energy to activate catalysts that can break down organic molecules into less harmful substances.8,9 However, the efficiency of photocatalysis largely depends on the type of catalyst used, its stability, and its ability to absorb light in the visible spectrum.
Metal–organic frameworks (MOFs) have attracted significant attention in the field of photocatalysis due to their unique properties, such as high surface area,10 tunable porosity,11 and the ability to incorporate various metal ions.12–14 MOFs are crystalline materials composed of metal ions or clusters coordinated to organic ligands, forming a porous network.15,16 The versatility in the design and synthesis of MOFs allows for the creation of materials with specific properties tailored to particular applications.17,18 Among the various types of MOFs, bismuth-based MOFs (Bi-MOFs) have shown great potential in photocatalytic applications, particularly for environmental remediation.19,20
Bismuth (Bi) is a heavy metal with unique electronic properties, including a large atomic radius, a high charge-to-size ratio, and the ability to form strong bonds with oxygen and nitrogen-containing ligands.21,22 These properties make bismuth an ideal candidate for photocatalysis, as it can effectively absorb visible light and participate in redox reactions.23,24 The incorporation of bismuth into MOFs results in materials with enhanced photocatalytic properties, capable of degrading organic pollutants under visible light irradiation.25–27 Moreover, the high stability and reusability of Bi-MOFs make them attractive for practical applications in water treatment.28,29
In this study, we focus on the synthesis, characterization, and photocatalytic evaluation of Bi-MOFs and their derivatives, specifically Ag/Bi-MOF and NH4+/Ag+/Bi-MOF. The rationale behind incorporating silver (Ag+) and ammonium (NH4+) into the Bi-MOF structure lies in their potential to further enhance the photocatalytic performance of the material. Silver ions are known for their antimicrobial properties and ability to improve the charge separation efficiency in photocatalysts, which can lead to higher photocatalytic activity. On the other hand, ammonium ions can influence the acidity of the environment and alter the surface chemistry of the photocatalyst, potentially affecting the adsorption and degradation processes of the target pollutants.
2. Methods & experiment
2.1. Chemical materials
Sigma-Aldrich provided the following materials: 1,4-benzoic acid, bismuth(III) nitrate pentahydrate, silver nitrate, sulfamethoxazole, tetracycline, ammonium chloride, N,N-dimethylformamide, sulfuric acid (0.1 N), and sodium hydroxide. Both HPLC-grade water and ethanol were used just as they were, without any further purification. The remaining reagents were all of the best quality that was commercially available, and they were all used exactly as given. After thoroughly cleaning the glassware with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 HNO3 solution, it was rinsed several times with deionized (DI) water.
1 HNO3 solution, it was rinsed several times with deionized (DI) water.
2.2. Synthesis of pristine bismuth-MOF nanomaterial (Bi-MOF)
12 mmol of Bi(NO3)3·5H2O (5.82 g) and 18 mmol of H2BDC (2.99 g) are dissolved in 100 mL of DMF to create a solution. After that, this mixture is agitated for 20 minutes at 600 rpm until it dissolves and turns clear. The resultant mixture is then put into an autoclave lined with Teflon and heated to 120 °C for 60 hours. The mixture is centrifuged three times at 600 rpm for fifteen minutes after it has cooled to room temperature. The catalyst is then separated after the mixture is cleaned with DMF. To make sure all leftover materials are gone, it goes through three further rounds of centrifugation at 600 rpm for ten minutes while using 100 mL of ethanol. To produce Bi-MOF, the mixture is lastly dried for 24 hours at 120 °C.2.3. Synthesis of Ag+/Bi-MOF and NH4+/Ag+/Bi-MOF nanomaterial
For the AgNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi(NO3)3·5H2O molar ratio, weigh 1.02 grams of AgNO3 (6 mmol) and 5.82 grams of Bi(NO3)3·5H2O (12 mmol). Add them to 100 milliliters of DMF and stir until the mixture becomes clear about 30 minutes. Place the transparent solution into an autoclave coated with Teflon and close it firmly. After 60 hours of heating the autoclave to 120 °C, let the mixture cool to ambient temperature. Centrifuge the mixture three times at 3000 rpm for ten minutes to get rid of any leftover reactants. Repeat steps three times: wash mixture with DMF, separate catalyst, and centrifuge again at 300 rpm for 20 minutes using around 100 mL of ethanol. To create the Ag+/Bi-MOF product, dry the final combination that was obtained following the washing operation for a full day at 120 °C.
Bi(NO3)3·5H2O molar ratio, weigh 1.02 grams of AgNO3 (6 mmol) and 5.82 grams of Bi(NO3)3·5H2O (12 mmol). Add them to 100 milliliters of DMF and stir until the mixture becomes clear about 30 minutes. Place the transparent solution into an autoclave coated with Teflon and close it firmly. After 60 hours of heating the autoclave to 120 °C, let the mixture cool to ambient temperature. Centrifuge the mixture three times at 3000 rpm for ten minutes to get rid of any leftover reactants. Repeat steps three times: wash mixture with DMF, separate catalyst, and centrifuge again at 300 rpm for 20 minutes using around 100 mL of ethanol. To create the Ag+/Bi-MOF product, dry the final combination that was obtained following the washing operation for a full day at 120 °C.
2.4. Adsorption experiments
At 25 °C, batch tests were conducted to evaluate the adsorption efficiency of Ag+/Bi-MOF, NH4+/Ag+/Bi-MOF, and Bi-MOF. In the trials, predetermined concentrations of TC and SMX were dissolved in deionized (DI) water. 50 mg of Bi-MOF, Ag+/Bi-MOF, or NH4+ Ag+/Bi-MOF were suspended in 100 mL of a pH 7 solution containing 20 ppm TC or SMX during the first cycle of the adsorption procedure. After one minute of sonication, the mixture was moved to a reactor and agitated for thirty minutes at 25 °C at 400 rpm using a magnetic stirrer. Water flowing in an outer jacket around the reactor maintained its temperature constant. Centrifugation was used to separate the adsorbent from the solution. To eliminate TC or SMX from the Bi-MOF, Ag+/Bi-MOF, or NH4+/Ag+/Bi-MOF, the adsorbent was continuously stirred while being submerged in a large amount of ethanol solvent. The Bi-MOF variants were then dried in a vacuum oven in preparation for more testing.2.5 Photocatalytic experiments
By calculating the photodegradation percentage of TC/SMX in an aqueous solution exposed to visible light, the sample's photocatalytic performance was evaluated. The simulation visible light source was a 250 W high-pressure mercury lamp (Mercury HPL-N 250W). The light source was filtered to primarily emit wavelengths between 400 nm and 700 nm, aligning with the visible spectrum. Generally, 100 mL of TC/SMX solution was placed in a photoreactor together with 50 mg of each kind of nanomaterial photocatalyst. By moving cooling water through an outer jacket around the reactor, the temperature of the photoreactor was kept constant at 25 °C. To attain adsorption–desorption equilibrium, the solution was agitated for 30 minutes in the dark before light exposure. Next, simulation visible light was introduced to the solution. To separate the photocatalysts, 3 mL aliquots were removed from the photoreactor and centrifuged at predetermined intervals. The proportion of TC/SMX photodegradation was tracked using the supernatant. The TC/SMX sample's absorbance was measured, and the solution's absorption wavelengths were noted.The degradation efficiency (DE, %) was calculated based on the initial and final concentrations of the target pollutants, using the formula:
 | (1) |
The reduction in Chemical Oxygen Demand (COD) and Total Organic Carbon (TOC) was evaluated to determine the degree of mineralization. TOC values were measured using a Multi N/S 2100S TOC analyzer (Analytik Jena, Germany). COD measurements were conducted using the dichromate oxidation method with a COD reactor HI839150 (Hanna Instruments, Vietnam). The percentage COD and TOC reductions were calculated by eqn (2) and (3), respectively.
 | (2) |
 | (3) |
2.6. Physicochemical properties of MOF nanomaterials
3. Results & discussion
3.1. Preparation and characterization of as-prepared samples
The formation process of the materials in Fig. 1a reflects the complexity and flexibility in designing photocatalytic materials. Each additional step (Ag+ and NH4+) not only alters the physical structure of the material but also enhances its chemical and catalytic properties. Ag+ plays a crucial role in improving charge separation, while NH4+ can influence adsorption and degradation processes. The final result is the creation of materials with high photocatalytic efficiency, suitable for applications in environmental treatment.
The large pore volume of Bi-MOF can provide more space for the adsorption of antibiotic molecules of suitable size, thereby improving catalytic efficiency. Conversely, NH4+/Ag+/Bi-MOF has a smaller pore volume compared to Bi-MOF. Additionally, pore size affects the diffusion of molecules into and out of the material. In the case of NH4+/Ag+/Bi-MOF, it has smaller pore sizes, this increases the selectivity for antibiotics with smaller molecular sizes like tetracycline or larger molecules like sulfamethoxazole.32,33
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), common in carboxylate ligands used in Bi-MOF. Peaks in the 1524 cm−1 range are associated with the C
O), common in carboxylate ligands used in Bi-MOF. Peaks in the 1524 cm−1 range are associated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of aromatic rings. The 1373 cm−1 range features peaks due to the stretching vibrations of C–O bonds in carboxylate groups and Bi–O bonds, indicating the coordination of bismuth ions with oxygen atoms from the ligands. The region of 737 cm−1, and 500 cm−1, contains a series of peaks corresponding to bending and stretching vibrations of various bonds unique to the specific Bi-MOF structure.36,37
C stretching vibrations of aromatic rings. The 1373 cm−1 range features peaks due to the stretching vibrations of C–O bonds in carboxylate groups and Bi–O bonds, indicating the coordination of bismuth ions with oxygen atoms from the ligands. The region of 737 cm−1, and 500 cm−1, contains a series of peaks corresponding to bending and stretching vibrations of various bonds unique to the specific Bi-MOF structure.36,37When Ag+ is added to Bi-MOF, the FTIR spectrum of Ag+/Bi-MOF may show slight changes in the intensity and position of certain absorption bands. The absorption bands similar to those of Bi-MOF still appear, indicating that the overall MOF structure does not significantly change after the addition of Ag+. However, there is a shift or change in the intensity of absorption bands related to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or C–O groups, suggesting the interaction of Ag+ with the ligands in the MOF. This change may be due to the formation of complex bonds between Ag+ and the functional groups within the MOF structure, which could enhance the photocatalytic efficiency of the material.
O or C–O groups, suggesting the interaction of Ag+ with the ligands in the MOF. This change may be due to the formation of complex bonds between Ag+ and the functional groups within the MOF structure, which could enhance the photocatalytic efficiency of the material.
The FTIR spectrum of NH4+/Ag+/Bi-MOF may display similar characteristics to Ag+/Bi-MOF but with some additional changes. Absorption bands related to the N–H group may appear around 3600 cm−1, representing the presence of ammonium ions (NH4+). Changes in the intensity or position of absorption bands in the region of 1000–1650 cm−1 may indicate the interaction of both NH4+ and Ag+ with the functional groups in the MOF, leading to alterations in the surface chemical structure of the material. These changes could result in improved adsorption capacity and enhanced pollutant degradation efficiency, due to the formation of new bonds or the strengthening of existing bonds between the MOF components and the added ions.
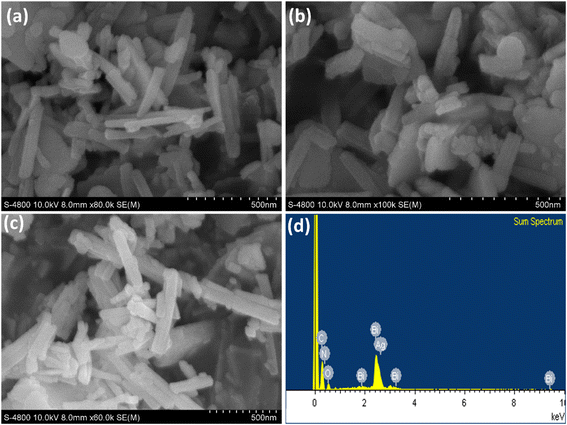 | ||
| Fig. 3 SEM images of (a) Bi-MOF, (b) Ag+/Bi-MOF, (c) NH4+/Ag+/Bi-MOF, and (d) EDX spectrum of NH4+/Ag+/Bi-MOF. | ||
When Ag+ ions are introduced into the Bi-MOF structure, the SEM image shows no change in the nanorod morphology (Fig. 3b). The crystals retain the original morphology of Bi-MOF; however, the appearance of smaller particles on the surface may be due to Ag+ ions creating dispersed metal nanoparticles on the Bi-MOF surface. These particles could play an important role in enhancing photocatalytic efficiency by providing electron traps, which help reduce the recombination rate of electron–hole pairs.
The SEM image of NH4+/Ag+/Bi-MOF shows a more complex structure with the presence of smaller particles and a rougher surface compared to the previous two samples. This could be due to the combination of NH4+ and Ag+ ions causing changes in the surface structure. The small particles uniformly distributed on the surface may result from the aggregation of metal ions, contributing to changes in the surface chemistry of the catalyst, which in turn affects the adsorption and degradation processes of pollutants (Fig. 3c). The EDX spectrum shows the presence of elements including silver, bismuth, carbon, nitrogen, and oxygen, reflecting the chemical composition of NH4+/Ag+/Bi-MOF. The peaks below 1 keV indicate the presence of organic elements from the ligands in the MOF structure. The prominent peak for Ag at 2.6 keV demonstrates that Ag+ ions have been successfully incorporated into the MOF structure. The Bi peaks at 1.9, 2.5, 3.3, and 9.4 keV confirm the strong presence of Bismuth as part of the MOF framework (Fig. 3d).
3.2. Adsorption and photocatalytic activity investigation
Bi3+ has a high affinity for the oxygen and nitrogen atoms in TC's structure, particularly with functional groups such as –OH and –NH2. TC can form strong complexes with Bi3+ through bonding with these functional groups. Additionally, Bi3+, as a heavy metal ion, can form very stable complexes due to its high charge (3+), making the bond between Bi3+ and TC stronger than those with ions of lower charge. Moreover, the porous structure of Bi-MOF is suitably sized to allow the diffusion of small molecules like tetracycline, which facilitates the complex formation between tetracycline and Bi3+.
In contrast, Ag+ has a good affinity for sulfur and nitrogen atoms. However, since TC does not contain sulfur groups, its interaction with Ag+ mainly occurs through the –OH and –NH2 groups, similar to Bi3+. Although Ag+ can form complexes with TC, these complexes are generally less stable than those with Bi3+ because Ag+ only has a 1+ charge, leading to a weaker attraction between the ion and the functional groups.
Bi-MOF is a complex structure where bismuth ions (Bi3+) are incorporated into a network with organic ligands. In this structure, the Bi3+ ions are not free but are tightly coordinated with other molecules within the MOF. Because the Bi3+ ions in bismuth-MOF are “locked” within the MOF structure, they are not readily available to directly form complexes with other molecules like SMX. This limits SMX's ability to interact directly with Bi3+ within the MOF to form a complex (Fig. 5a).
In contrast, when Ag+ is present, it exists as a free ion in solution and can directly interact with SMX. Ag+ has a high affinity for functional groups in SMX (such as the sulfonamide –SO2NH2 and amino –NH2 groups). This is reflected in the peak shift from 263 nm and 201 nm to 240 nm and 203 nm (Fig. 5b). SMX can easily form a complex with Ag+ due to Ag+'s flexible nature and its ability to readily interact with the functional groups in the SMX molecule. This complex can form in solution and is not constrained by a fixed structure like Bi-MOF.
NH4+ can interact with Ag+ ions in the solution, reducing the availability of Ag+ to interact with SMX. Although NH4+ does not strongly bind with Ag+, it can alter the distribution of Ag+ in the solution. In the presence of NH4+, Ag+ may form weak interactions or be surrounded by water molecules and other anions, reducing its likelihood of directly interacting with SMX, thereby diminishing the potential for complex formation.42,43
In a solution containing NH4+, competitive reactions may occur. NH4+ may compete with SMX for binding sites on Ag+ or create weak interactions with Ag+, making Ag+ less likely to form a complex with SMX. Additionally, NH4+ can create a more complex environment by increasing the presence of other ions, altering the chemical equilibrium in the solution, and decreasing the potential for complex formation between SMX and Ag+.
3.3. Photocatalytic efficiency of Bi-MOF derivatives and reusability
The reusability of the Ag+/Bi-MOF material in degrading the antibiotic sulfamethoxazole is illustrated in Fig. 6b. The Ag+/Bi-MOF material also demonstrates strong reusability for difficult-to-degrade antibiotics like SMX, with its degradation efficiency showing minimal reduction after multiple cycles. Specifically, the first cycle achieved a degradation rate of 95.3%, and the efficiency slightly decreased in the subsequent cycles, with 93.9%, 92.2%, and 90.5% after the next three cycles, respectively. This indicates that Ag+/Bi-MOF is a durable photocatalyst, suitable for long-term application in treating hard-to-degrade antibiotics like SMX.
When the Bi-MOF derivatives (such as Ag+/Bi-MOF or NH4+/Ag+/Bi-MOF) are exposed to visible light, they absorb light energy and generate electron–hole pairs within their structure. Ag+ (silver ion) plays a crucial role in preventing the recombination of electron–hole pairs by creating electron traps.44 This allows the free electrons and holes to exist longer, enhancing photocatalytic activity. The excited electrons from the conduction band can participate in reduction reactions, while the holes in the valence band can engage in oxidation reactions. These reactions produce free radicals such as ·OH (hydroxyl radicals) or  (superoxide radicals), which can degrade antibiotic molecules like TC or SMX. These free radicals attack the antibiotic molecules, breaking the chemical bonds in their molecular structure, leading to the degradation of the antibiotics.45,46 After the catalytic process is complete, the catalyst can be reused in subsequent cycles without significant loss in efficiency (Fig. 6c).
(superoxide radicals), which can degrade antibiotic molecules like TC or SMX. These free radicals attack the antibiotic molecules, breaking the chemical bonds in their molecular structure, leading to the degradation of the antibiotics.45,46 After the catalytic process is complete, the catalyst can be reused in subsequent cycles without significant loss in efficiency (Fig. 6c).
Fig. 6d shows that modifying the chemical structure of Bi-MOF by adding Ag+ and NH4+ can alter the material's bandgap, which in turn affects its light absorption and photocatalytic efficiency. A smaller bandgap allows the material to absorb light more effectively and generate more free radicals, enhancing its ability to degrade organic pollutants. This change indicates the potential of adjusting the Bi-MOF structure to improve catalytic performance, especially in environmental treatment and the degradation of persistent pollutants like antibiotics.47,48 The bandgap of Bi-MOF reflects its ability to absorb visible light, but its efficiency is not high due to the rapid recombination of electron–hole pairs. This leads to the generation of fewer free radicals and, consequently, a lower antibiotic degradation efficiency compared to other derivatives.49,50 When Ag+ is added to the Bi-MOF structure, the material's bandgap may change, typically becoming narrower. This narrowing of the bandgap allows Ag+/Bi-MOF to absorb light at longer wavelengths, meaning it can absorb light more effectively in the visible range. Ag+ also helps create electron traps, preventing the rapid recombination of electron–hole pairs, and thereby enhancing photocatalytic efficiency. As a result, Ag+/Bi-MOF can more effectively degrade organic pollutants such as antibiotics. When NH4+ is added along with Ag+, the bandgap may continue to change, usually with a slight adjustment in energy and catalytic efficiency. NH4+ can affect the surface chemistry of the material, influencing the absorption and degradation processes of pollutants. The combination of Ag+ and NH4+ can lead to adjustments in the bandgap and surface properties of the material, making NH4+/Ag+/Bi-MOF a more effective photocatalyst compared to the original Bi-MOF.
Based on the changes in the UV-Vis spectra and previous studies on the photocatalytic degradation mechanism of TC, the potential intermediate products may include deamination and N-demethylation products, ring-opening products, and carboxylic acid products.51–53 In addition, the UV-Vis spectra reveal the appearance and disappearance of intermediate products during the photocatalytic degradation of SMX, with potential intermediates including ring cleavage products, desulfonation products, hydroxylation, and oxidation products (Table 1).54–56
| No | Antibiotic | Intermediate compounds | Nanomaterial | Ref. |
|---|---|---|---|---|
| 01 | Tetracycline | Epimers and hydroxylated tetracyclines | MOF-801/GO composite | 57 |
| 02 | Tetracycline | Dehydroxylation, 6 deamidation, ring opening, oxidation and N-demethylation reactions | MIL-88B on NH2-MIL-125 | 58 |
| 03 | Tetracycline | Ring-opened tetracycline intermediates | ZnIn2S4/MOF-525 (zirconium-based porphyrin metal organic frameworks) | 51 |
| 04 | Tetracycline | Anhydrotetracycline, hydroxytetracycline, 3-amino-5-methylphenol, benzoic acid derivative | 3D Cu-MOFs | 52 |
| 05 | Tetracycline | Deamination and N-demethylation products, ring-opening products, carboxylic acids | NH2-MIL-88B(Fe)@MIL-100(Fe) | 53 |
| 06 | Sulfamethoxazole | N-Hydroxysulfamethoxazole, sulfamethoxazole sulfinic acid derivative, 4-nitrophenol sulfonamide derivative, 3-amino-5-methylisoxazole | Diatomite-supported hydroxyl-modified UIO-66 | 59 |
| 07 | Sulfamethoxazole | Ring cleavage products, desulfonation products, hydroxylation and oxidation products | MIL-101(Fe) supported Mo single-atom | 54 |
| 08 | Sulfamethoxazole | Hydroxy-N-sulfamoyl-benzene derivative, 4-nitrobenzoic acid derivative, hydroxylated sulfamethoxazole, 3-amino-5-methylisoxazole | N-Doped ZnO carbon skeleton@Bi2MoO6 S-scheme heterojunction | 55 |
| 09 | Sulfamethoxazole | Hydroxylated SMX, benzenesulfonamide derivatives, and 3-amino-5-methylisoxazole | MOF derivate Fe@Cs | 56 |
 | ||
| Fig. 7 Photocatalytic efficiency and mineralization performance of NH4+/Ag+/Bi-MOF for TC and SMX under visible light irradiation for 160 min. | ||
The photocatalytic efficiency of Bi-MOFs is primarily associated with their large surface area, tunable porosity, and unique electronic properties that facilitate visible light absorption and promote effective charge separation. These characteristics allow Bi-MOFs to function as efficient photocatalysts in the degradation of organic pollutants, including antibiotics such as tetracycline and sulfamethoxazole. The introduction of silver ions (Ag+) into the Bi-MOF framework has significantly enhanced the photocatalytic efficiency of the material. Ag+ ions are known for their ability to improve charge separation by acting as electron traps, thereby reducing the recombination rate of electron–hole pairs generated during photocatalysis. This leads to a more efficient degradation process of organic pollutants under visible light. Specifically, the Ag+/Bi-MOF derivative exhibited superior photocatalytic efficiency in the degradation of TC and SMX compared to the unmodified Bi-MOF.
Additionally, the incorporation of ammonium ions (NH4+) along with Ag+ into the Bi-MOF structure (forming NH4+/Ag+/Bi-MOF) introduced further modifications to the surface chemistry of the catalyst. NH4+ ions can alter the pH of the surrounding environment and influence the interaction between the catalyst and the target pollutants. This impacts the adsorption and degradation processes, resulting in significant photocatalytic activity observed in NH4+/Ag+/Bi-MOF. Experimental results showed that both Ag+/Bi-MOF and NH4+/Ag+/Bi-MOF derivatives exhibited higher photocatalytic efficiency than the original Bi-MOF, with Ag+/Bi-MOF showing the most promising performance. These findings highlight the potential of modifying the Bi-MOF structure with specific metal ions to enhance their photocatalytic capabilities.
3.3.2.1 Kinetic model. The kinetic model for the photocatalytic activity of Bi-MOF, Ag+/Bi-MOF, and NH4+/Ag+/Bi-MOF in degrading of tetracycline fitted by a power law equation based on the Korsmeyer–Peppas (K–P) model, which was usually used to predict the non-Fickian model:
 | (4) |
The kinetic model for the photocatalytic activity of SMX in Fig. 8b follows the equation:
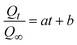 | (5) |
Bi-MOF (black) has the smallest slope, indicating the slowest reaction rate. Meanwhile, Ag+/Bi-MOF (red) shows a steeper slope, demonstrating an improved reaction rate due to the presence of Ag+. Notably, NH4+/Ag+/Bi-MOF (green) exhibits the steepest slope, confirming that the combination of NH4+ and Ag+ significantly enhances the photocatalytic efficiency.
4. Conclusions
In this study, we successfully synthesized and characterized bismuth-based metal–organic frameworks and their derivatives, specifically Ag+/Bi-MOF and NH4+/Ag+/Bi-MOF, using a solvothermal method. The photocatalytic efficiency of these materials was evaluated in the degradation of tetracycline and sulfamethoxazole under visible light. The results showed that Bi-MOFs have significant potential as photocatalysts due to their large surface area, tunable porosity, and favorable electronic properties for visible light absorption. Among the synthesized materials, Ag+/Bi-MOF demonstrated superior photocatalytic efficiency, attributed to enhanced charge separation and improved light absorption due to the incorporation of silver ions. NH4+/Ag+/Bi-MOF also exhibited significant photocatalytic activity, with ammonium ions affecting the surface chemistry of the catalyst and influencing the adsorption and degradation processes. These findings underscore the importance of modifying the MOF structure with metal ions like Ag+ to enhance photocatalytic performance. Reusability tests also confirmed the practical potential of these photocatalysts, as they maintained high degradation efficiency over multiple cycles. This highlights their applicability in real-world environmental treatment technologies, particularly in the removal of persistent organic pollutants such as TC and SMX.Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to restrictions e.g., their containing information that could compromise the privacy of research participants.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge support from Nguyen Tat Thanh University in Ho Chi Minh City, Vietnam.References
- A. Mukhopadhyay, S. Duttagupta and A. Mukherjee, J. Environ. Chem. Eng., 2022, 10, 107560 CrossRef.
- A. Hussain, S. Ashique, M. Zaheen Hassan, O. Afzal, Y. I. Asiri, P. Kumar, K. Dua, T. J. Webster, A. S. A. Altamimi and M. A. Altamimi, J. Mol. Liq., 2023, 389, 122905 CrossRef.
- B. Wang, Z. Xu and B. Dong, J. Hazard. Mater., 2024, 469, 133925 Search PubMed.
- A. E. Gahrouei, S. Vakili, A. Zandifar and S. Pourebrahimi, Environ. Res., 2024, 252, 119029 CrossRef PubMed.
- A. Redwan Haque, M. Sarker, R. Das, M. A. K. Azad and M. M. Hasan, Int. J. Environ. Anal. Chem., 2023, 103, 3704–3721 CrossRef.
- S. Zhang, H. Zheng and P. G. Tratnyek, Nat. Water, 2023, 1, 666–681 CrossRef.
- C. Yu, Z. Xiong, H. Zhou, P. Zhou, H. Zhang, R. Huang, G. Yao and B. Lai, Chem. Eng. J., 2022, 433, 133802 CrossRef.
- A. Intisar, A. Ramzan, S. Hafeez, N. Hussain, M. Irfan, N. Shakeel, K. A. Gill, A. Iqbal, M. Janczarek and T. Jesionowski, Chemosphere, 2023, 336, 139203 CrossRef PubMed.
- M. A. Al-Nuaim, A. A. Alwasiti and Z. Y. Shnain, Chem. Pap., 2023, 77, 677–701 CrossRef PubMed.
- V. A. Tran, G. N. L. Vo, V. D. Doan, N. C. Thanh, T. D. Lam and V. T. Le, Microchem. J., 2024, 197, 109852 CrossRef.
- V. A. Tran, V. Thuan Le, V. D. Doan and G. N. L. Vo, Pharmaceutics, 2023, 15, 931 CrossRef.
- F. Yang, M. Du, K. Yin, Z. Qiu, J. Zhao, C. Liu, G. Zhang, Y. Gao and H. Pang, Small, 2022, 18, 2105715 CrossRef PubMed.
- N. A. Nordin, M. A. Mohamed, M. N. I. Salehmin and S. F. Mohd Yusoff, Coord. Chem. Rev., 2022, 468, 214639 CrossRef.
- P. M. Stanley, J. Haimerl, N. B. Shustova, R. A. Fischer and J. Warnan, Nat. Chem., 2022, 14, 1342–1356 CrossRef PubMed.
- J. Zou, Y. Shao, X. Hu, H. Zhang, S. Ye and J. Li, Chem. Eng. J., 2024, 484, 149337 CrossRef.
- V. Anh Tran, T. Khoa Phung, V. Thuan Le, T. Ky Vo, T. Tai Nguyen, T. Anh Nga Nguyen, D. Quoc Viet, V. Quang Hieu and T.-T. Thi Vo, Mater. Lett., 2021, 284, 128902 CrossRef.
- V. Anh Tran, L. T. Nhu Quynh, T.-T. Thi Vo, P. A. Nguyen, T. N. Don, Y. Vasseghian, H. Phan and S.-W. Lee, Environ. Res., 2022, 204, 112364 CrossRef PubMed.
- V. A. Tran and S.-W. Lee, RSC Adv., 2021, 11, 9222–9234 RSC.
- M. Liu, P. Ye, M. Wang, L. Wang, C. Wu, J. Xu and Y. Chen, J. Environ. Chem. Eng., 2022, 10, 108436 CrossRef.
- D. Pattappan, C.-J. Liao, R. S. Kumar, S. Ramesh, R. T. R. Kumar, W. Yang, Y. Haldorai and Y.-T. Lai, J. Taiwan Inst. Chem. Eng., 2024, 165, 105725 CrossRef.
- S. K. Gopinathan, P. Vishwa, G. D. Nessim, I. Udachyan and S. Kandaiah, ACS Appl. Energy Mater., 2024, 7, 6807–6820 CrossRef.
- M. Kocsis, M. Szabados, S. B. Ötvös, G. F. Samu, Z. Fogarassy, B. Pécz, Á. Kukovecz, Z. Kónya, P. Sipos, I. Pálinkó and G. Varga, J. Catal., 2022, 414, 163–178 CrossRef.
- V. Thakur, S. Singh, P. Kumar, S. Rawat, V. Chandra Srivastava, S.-L. Lo and U. Lavrenčič Štangar, Chem. Eng. J., 2023, 475, 146100 CrossRef.
- R. K. Chava, Y. Im and M. Kang, J. Mater. Chem. A, 2024, 12, 18498–18511 RSC.
- N. K. Sompalli, Y. Li, J. Li and S. Kuppusamy, Environ. Res., 2024, 259, 119532 CrossRef CAS.
- M. B. Chabalala, B. M. Mothudi and B. Ntsendwana, J. Photochem. Photobiol., A, 2024, 447, 115244 CrossRef.
- V. A. Tran, A. N. Kadam and S.-W. Lee, J. Alloys Compd., 2020, 835, 155414 CrossRef.
- S. Dong, L. Wang, W. Lou, Y. Shi, Z. Cao, Y. Zhang and J. Sun, Ultrason. Sonochem., 2022, 91, 106223 Search PubMed.
- L. Ding, Y. Li, Y. Ding, F. Bai, B. Jia, H. Li and X. Wang, Appl. Surf. Sci., 2023, 624, 157100 Search PubMed.
- M.-C. Guo, W.-D. Zhong, T. Wu, W.-D. Han, X.-S. Gao and X.-M. Ren, J. Solid State Chem., 2022, 309, 123005 CrossRef.
- R. Huang, Z. Zhou, X. Lan, F. K. Tang, T. Cheng, H. Sun, K. Cham-Fai Leung, X. Li and L. Jin, Mater. Today Bio, 2023, 18, 100507 CrossRef.
- W. A. Mohamed, J. Chakraborty, L. Bourda, R. Lavendomme, C. Liu, R. Morent, N. De Geyter, K. Van Hecke, A. M. Kaczmarek and P. Van Der Voort, J. Am. Chem. Soc., 2024, 146, 13113–13125 CrossRef.
- J. Jiang, W. Wei, Y. Tang, S. Yang, X. Wang, Y. Xu and L. Ai, Inorg. Chem., 2022, 61, 19847–19856 CrossRef.
- L. Liu, K. Yao, J. Fu, Y. Huang, N. Li and H. Liang, Colloids Surf., A, 2022, 633, 127840 CrossRef.
- J. Jiang, Y. Xu, C. Tang, X. Wang, W. Wei and L. Ai, Desalination, 2023, 560, 116680 CrossRef.
- W. Liu, J. Guan, B. Kong, H. Lu, Y. Wu, X. Qin, H. Jiang and X. Liu, J. Solid State Electrochem., 2023, 27, 3393–3404 CrossRef.
- Z. Gao, M. Hou, Y. Shi, L. Li, Q. Sun, S. Yang, Z. Jiang, W. Yang, Z. Zhang and W. Hu, Chem. Sci., 2023, 14, 6860–6866 RSC.
- H. Liu, Z. Huang, W. Zhang, C. Zhang, S. Wang and W. Wang, Chemosphere, 2024, 359, 142274 CrossRef PubMed.
- Y. Li, D. Han, Z. Wang and F. Gu, ACS Appl. Mater. Interfaces, 2024, 16, 7080–7096 CrossRef.
- M. Chen, S. Wei, J. Wu, J. Li, B. Fu and X. Zhu, Colloids Surf., A, 2023, 664, 131186 CrossRef CAS.
- Z. Tan, C. Shi, Z. Shi, H. Yang, J. yang, C. Wu and D. Wang, J. Alloys Compd., 2024, 1005, 176049 CrossRef CAS.
- T.-H. Ngo, P.-N.-M. Le, C.-H. Truong, N.-D.-T. Huynh, T.-H. Tran, V.-H. Luan, B.-T. Dang, M. Rafie Johan, S. Sagadevan and M.-V. Le, J. Photochem. Photobiol., A, 2024, 446, 115157 CrossRef CAS.
- M. Wang, G. Lu, R. Jiang, T. Dang and J. Liu, J. Colloid Interface Sci., 2022, 622, 995–1007 CrossRef CAS.
- X. Zhang, S. Jiang, L. X. Sun, Y. H. Xing and F. Y. Bai, J. Mol. Struct., 2022, 1270, 133895 CrossRef CAS.
- X. Yue, L. Cheng, F. Li, J. Fan and Q. Xiang, Angew. Chem., Int. Ed., 2022, 61, e202208414 CrossRef CAS.
- B. Zhang, H. Xu, M. Wang, L. Su, S. Zhang, Y. Zhang and Q. Wang, J. Environ. Chem. Eng., 2022, 10, 108469 CrossRef CAS.
- B. Zhang, H. Xu, M. Wang, L. Su, C. Wu and Q. Wang, J. Environ. Chem. Eng., 2023, 11, 110417 CrossRef CAS.
- W. Lou, L. Wang, S. Dong, Z. cao, J. Sun and Y. Zhang, Opt. Mater., 2022, 126, 112168 CrossRef CAS.
- L. Ding, Y. Ding, F. Bai, G. Chen, S. Zhang, X. Yang, H. Li and X. Wang, Inorg. Chem., 2023, 62, 2289–2303 CrossRef CAS.
- G. Huang, Z. Li, K. Liu, Y. Zhang, X. Tang, Q. Peng, J. Huang and G. Zhang, J. Water Process. Eng., 2022, 46, 102636 CrossRef.
- X. Zhang, Z. Liu, B. Shao, T. Wu, Y. Pan, S. Luo, M. He, L. Ge, J. Sun, C. Cheng and J. Huang, Environ. Sci. Pollut. Res. Int., 2023, 30, 67647–67661 CrossRef CAS PubMed.
- R. Zhou, C. Zou, Y. Wang, Z. Zhou, M. Liu and Y. Ji, Water, Air, Soil Pollut., 2024, 235, 295 CrossRef CAS.
- Q. Zhang, J. Xu, X. Ma, J. Xu, Z. Yun, Q. Zuo and L. Wang, J. Water Process. Eng., 2021, 44, 102364 CrossRef.
- W. Kong, Z. Liu, J. Wu, C. Wen, J. Yan, Y. Gao, G. Li, X. Wang and M. Huo, Process Saf. Environ. Prot., 2025, 106778, DOI:10.1016/j.psep.2025.106778.
- A. Wang, J. Ni, W. Wang, X. Wang, D. Liu and Q. Zhu, J. Hazard. Mater., 2022, 426, 128106 CrossRef PubMed.
- M. Pu, J. Wan, F. Zhang, M. L. Brusseau, D. Ye and J. Niu, J. Hazard. Mater., 2021, 414, 125598 CrossRef PubMed.
- Z. Wu, Z. Chen, J. Chen, X. Ning, P. Chen, H. Jiang and H. Qiu, Environ. Sci.: Nano, 2022, 9, 4609–4618 RSC.
- T. Gao, H. Zhang, X. Zhao, S. Xiao, Z. Zhang and S. Yu, Appl. Surf. Sci., 2024, 651, 159227 CrossRef.
- H.-L. Liu, Y. Zhang, X.-X. Lv, M.-S. Cui, K.-P. Cui, Z.-L. Dai, B. Wang, R. Weerasooriya and X. Chen, Nanomaterials, 2023, 13(24), 3116 CrossRef.
- S. Singla, P. Singh, S. Basu and P. Devi, Mater. Chem. Phys., 2023, 295, 127111 CrossRef.
- I. Izquierdo-Barba, E. Sousa, J. C. Doadrio, A. L. Doadrio, J. P. Pariente, A. Martínez, F. Babonneau and M. Vallet-Regí, J. Sol-Gel Sci. Technol., 2009, 50, 421–429 CrossRef.
- P. Costa and J. M. Sousa Lobo, Eur. J. Pharm. Sci., 2001, 13, 123–133 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |






