Copper-catalyzed cycloaddition between hydrogen phosphonates and activated alkenes: synthesis of phosphonoisoquinolinediones†
Ju
Wu
a,
Yuzhen
Gao
a,
Xin
Zhao
a,
Liangliang
Zhang
a,
Weizhu
Chen
ab,
Guo
Tang
*a and
Yufen
Zhao
a
aDepartment of Chemistry, College of Chemistry and Chemical Engineering, The Key Laboratory for Chemical Biology of Fujian Province, Xiamen University, Xiamen, Fujian 361005, China. E-mail: t12g21@xmu.edu.cn; Fax: +86-592-2185780
bThe Third Institute of Oceanography of the State Oceanic Administration, Xiamen, Fujian 361005, China
First published on 15th December 2015
Abstract
A new, general method for the synthesis of phosphonoisoquinolinediones has been achieved via copper-catalyzed phosphonation–cyclization of various methacryloylbenzamides with P(O)H compounds. This transformation allows the direct formation of a P–C bond and the construction of an isoquinolinedione ring in one reaction.
As one of the most important heterocyclic compounds, substituted isoquinolinedione derivatives are important scaffolds in a broad array of biologically active compounds. Over the last 20 years, the number of isoquinolinedione-containing compounds as drug candidates has increased greatly.1 Thus, the development of new methods for their synthesis has been a major focus of study.2 Among the synthetic methods to obtain isoquinolinedione derivatives, the difunctionalization reaction of alkenes through a radical process is well known, including direct intramolecular aryltrifluoromethylation2d and arylphosphonylation3 of activated alkenes.
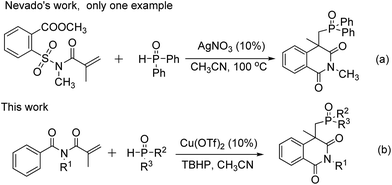
As we know, organophosphorus compounds have broad applications in the fields of organic synthesis, pharmaceuticals and agrochemicals owing to its unique properties.4 Heterocyclic phosphonate compounds are ubiquitous and exhibit interesting biological activities and potential pharmaceutical applications.5 If both phosphonyl group and isoquinolinedione structural motif can be simultaneously introduced into organic compounds, a series of new isoquinolinedione-containing organophosphorus compounds might be expected, and might provide an opportunity to introduce phosphonyl group into the original lead compounds or drugs to adjust their bioactivity. However, the efficient synthesis of molecules bearing both isoquinolinedione motif and phosphonyl group is quite rare. In 2014, Nevado group reported that the reaction of methyl 2-(N-methacryloyl-N-methylsulfamoyl)benzoate with diphenylphosphine oxide in the presence of silver salt led to phosphonoisoquinolinedione.3 Only one example was provided (Scheme 1a). Herein, we report the successful realization of this concept with the introduction of phosphonyl radicals for the flexible synthesis of unprecedented phosphonoisoquinolinediones with a quaternary carbon centre. This transformation allows the direct formation of a P–C bond and the construction of a heterocyclic ring in one reaction.
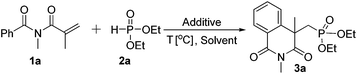
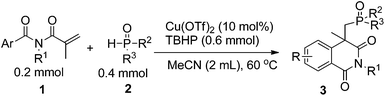
This idea was first examined by using N-methacryloyl-N-methylbenzamide (1a) and diethyl H-phosphonate (2a) as reaction partners (Table 1). It has been found that many salts such as copper,6 silver7 and manganese8 can work with R2P(O)H to form the corresponding phosphonyl radical which promoted phosphonyl radical addition chemistry. In the beginning, various silver and manganese catalysts were tested and all gave moderate yields (entries 1–4, Table 1). In these processes, manganese is used in excess (3 equivalents), which is quite wasteful. For a practical reaction, using readily available and low-cost catalyst for this transformation would be appealing. Gratifyingly, the combined use of Cu(OAc)2 and TBHP (tert-butylhydroperoxide) gave 3a in 40% yield (entry 6). Subsequently, various Cu(II) salts were further checked and the results showed that Cu(OTf)2 was more effective to give the desired product (entries 5–9). However, the attempt to decrease the amount of Cu(OTf)2 was failed (entry 10). Moreover, the yield was reduced to 43% under air (entry 11). Low yield was afforded without copper salt or TBHP (entries 12 and 13). Cu(I) salt and Cu powder couldn't execute this reaction efficiently (entries 14 and 15). After optimization of the reaction conditions, we established an efficient route to the phosphonation–annulation of methacryloylbenzamides (entry 9, Table 1).
| Entry | Additive (equiv.) | Solvent | T (°C) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.4 mmol), additive in solvent (2.0 mL) stirring under nitrogen for 24 h. Oil bath temperature. Yields were determined by 31P NMR based on Ph3PO as internal standard. b Under air. | ||||
| 1 | Mn(OAc)3·2H2O (3) | HOAc | 60 | 63 |
| 2 | Mn(OAc)3·2H2O (3) | HOAc | 70 | 59 |
| 3 | AgNO3 (0.05) + Mg(NO3)2·6H2O (0.5) | CH3CN | 100 | 62 |
| 4 | AgNO3 (0.1) + Mg(NO3)2·6H2O (0.5) | CH3CN | 100 | 65 |
| 5 | Cu(OAc)2 (0.1) + TBHP (3) | CH3CN | 60 | 40 |
| 6 | CuCl2 (0.1) + TBHP (3) | CH3CN | 60 | 15 |
| 7 | CuSO4·5H2O (0.1) + TBHP (3) | CH3CN | 60 | 20 |
| 8 | Cu(NO3)2·3H2O (0.1) + TBHP (3) | CH3CN | 60 | 48 |
| 9 | Cu(OTf) 2 (0.1) + TBHP (3) | CH 3 CN | 60 | 83 |
| 10 | Cu(OTf)2 (0.05) + TBHP (3) | CH3CN | 60 | 70 |
| 11b | Cu(OTf)2 (0.1) + TBHP (3) | CH3CN | 60 | 43 |
| 12 | TBHP (3) | CH3CN | 60 | 34 |
| 13 | Cu(OTf)2 (0.2) | CH3CN | 60 | 0 |
| 14 | CuCl (0.1) + TBHP (3) | CH3CN | 60 | 15 |
| 15 | Cu powder (0.1) + TBHP (3) | CH3CN | 60 | 5 |
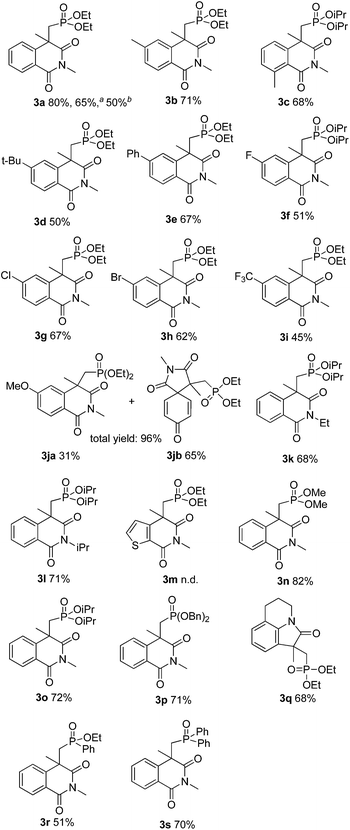
With this preliminary result in hand, the results of phosphonation–annulation for methacryloylbenzamides 1 with different H-phosphonates 2 can be summarized as follows. As shown in Table 2, a variety of functional groups on the phenyl ring of methacryloylbenzamides were compatible under this procedure, affording the desired products in moderate to good yields. The alkyl and phenyl substituted benzamide substrates, such as para-methyl, ortho-methyl, para-tert-butyl and para-phenyl on the aryl ring, reacted with 2a efficiently and gave the desired products 3b–3e in good yields. Halogen atoms such as fluoro, chloro, and bromo on the aromatic ring were unaffected under the present reaction conditions to afford the corresponding products 3f–3h in moderate to good yields, which could allow for further synthetic transformations. Benzamide substrates bearing CF3 group reacted smoothly to give the corresponding product in moderate yield (3i). When N-methacryloyl-4-methoxy-N-methylbenzamide reacted with 2a, we acquired both the normal coupling product 3ja and the dearomatization product 3jb as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture in 96% total yield. The product 3jb was formed by annulation of alkenyl radical with the aryl ring at the carbon atom directly attached to the carbonyl atom to form 1,3,8-trioxo-2-azaspiro compound.9 The reactivity of N-alkyl substituted substrates was further explored. More bulk alkyl groups at the nitrogen atom such as ethyl and isopropyl groups react with diethyl H-phosphonate (2a) furnishing the corresponding isoquinolinedione 3k and 3l in 68% and 71% yields, respectively. N-Methacryloyl-N-methylthiophene-2-carboxamide gave a complicated mixture, no 3m was obtained. Dimethyl, diisopropyl, and dibenzyl H-phosphonates could be also used as substrates, generating the corresponding products (3n–3p) in good yields. 1-Methacryloyl-1,2,3,4-tetrahydroquinoline was also a suitable acceptor to extend the applicability of the current method, and led to the formation of product (3q) in 68% yield. It is worth noting that ethoxyphenylphosphine oxide and diphenylphosphine oxide can be also applied in the preparation of the corresponding phosphonoisoquinolinediones in moderate yields (3r and 3s).
2 mixture in 96% total yield. The product 3jb was formed by annulation of alkenyl radical with the aryl ring at the carbon atom directly attached to the carbonyl atom to form 1,3,8-trioxo-2-azaspiro compound.9 The reactivity of N-alkyl substituted substrates was further explored. More bulk alkyl groups at the nitrogen atom such as ethyl and isopropyl groups react with diethyl H-phosphonate (2a) furnishing the corresponding isoquinolinedione 3k and 3l in 68% and 71% yields, respectively. N-Methacryloyl-N-methylthiophene-2-carboxamide gave a complicated mixture, no 3m was obtained. Dimethyl, diisopropyl, and dibenzyl H-phosphonates could be also used as substrates, generating the corresponding products (3n–3p) in good yields. 1-Methacryloyl-1,2,3,4-tetrahydroquinoline was also a suitable acceptor to extend the applicability of the current method, and led to the formation of product (3q) in 68% yield. It is worth noting that ethoxyphenylphosphine oxide and diphenylphosphine oxide can be also applied in the preparation of the corresponding phosphonoisoquinolinediones in moderate yields (3r and 3s).
In order to demonstrate the practical application of this method, N-methacryloyl-N-methylbenzamide (1a, 10 mmol) was employed in a gram-scale reaction and delivered 3a in 65% yield (Table 2). When the loading of Cu(OTf)2 was reduced to 5 mol%, the reaction also afforded 3a in 50% yield.
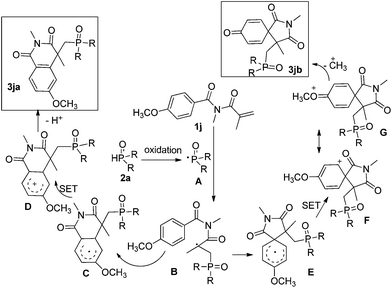
No desired product was obtained when 2.0 equiv. of TEMPO was added in the reaction of 1a with 2a under the optimal conditions. This result indicates that the phosphonyl radicals may be intercepted by TEMPO. According to our previous Cu(II)–TBHP catalytic reaction mechanism study,6a,c a plausible mechanism is proposed as shown in Scheme 2. Initially, the reaction of P(O)H 1 with Cu(II) salt and TBHP generates phosphonyl radical A which then goes through intermolecular addition onto the carbon–carbon double bond of 2 to produce alkyl radical B. Followed by intramolecular attack of the radical B on the pendant aromatic ring subsequently provides radical C. Subsequently, phosphonoisoquinolinedione 3ka is formed through oxidation–deprotonation reaction. Alternatively, alkyl radical B attacks the aryl ring at the carbon atom directly attached to the carbonyl group to form radical E. Cationic intermediate F is formed through single-electron oxidation which could afford resonance structure G. The intermediate G converts into the product 3kbvia demethylation.
In conclusion, we have successfully developed a facile catalytic method for the preparation of phosphonoisoquinolinediones via phosphonation–cyclization of various methacryloylbenzamides with P(O)H compounds. This method provides a rapid access to a broad spectrum of phosphonoisoquinolinediones in moderate to good yields. Moreover, the use of inexpensive Cu(II) catalyst, using readily-prepared methacryloylbenzamides and P(O)H compounds mean that this facile protocol will be attractive for academia and industry.
Acknowledgements
We acknowledge financial support from the Chinese National Natural Science Foundation (21173178, 21232005, 21375113), J1310024, the National Basic Research Program of China (2012CB821600), and the Program for Changjiang Scholars and Innovative Research Team in University.Notes and references
- (a) M. Paige, G. Kosturko, G. Bulut, M. Miessau, S. Rahim, J. A. Toretsky, M. L. Brown and A. Uren, Bioorg. Med. Chem., 2014, 22, 478 CrossRef CAS PubMed; (b) M. Billamboz, V. Suchaud, F. Bailly, C. Lion, J. Demeulemeester, C. Calmels, M. L. Andréola, F. Christ, Z. Debyser and P. Cotelle, ACS Med. Chem. Lett., 2013, 4, 606 CrossRef CAS; (c) L. Chen, M. Conda-Sheridan, P. V. N. Reddy, A. Morrell, E.-J. Park, T. P. Kondratyuk, J. M. Pezzuto, R. B. van Breemen and M. Cushman, J. Med. Chem., 2012, 55, 5965 CrossRef CAS; (d) M. Billamboz, F. Bailly, C. Lion, N. Touati, H. Vezin, C. Calmels, M. L. Andréola, F. Christ, Z. Debyser and P. Cotelle, J. Med. Chem., 2011, 54, 1812 CrossRef CAS; (e) A. Bolognese, G. Correale, M. Manfra, A. Lavecchia, O. Mazzoni, E. Novellino, V. Barone, P. La Colla and R. Loddo, J. Med. Chem., 2002, 45, 5217 CrossRef CAS.
- (a) M. Billamboz, F. Bailly, M. L. Barreca, L. de Luca, J.-F. Mouscadet, C. Calmels, M.-L. Andréola, M. Witvrouw, F. Christ, Z. Debyser and P. Cotelle, J. Med. Chem., 2008, 51, 7717 CrossRef CAS; (b) J. J. Medvedev, M. V. Meleshina, T. L. Panikorovskii, C. Schneider and V. A. Nikolaev, Org. Biomol. Chem., 2015, 13, 9107 RSC; (c) M. C. Garcia-Gonzalez, E. Hernandez-Vazquez, R. E. Gordillo-Cruz and L. D. Miranda, Chem. Commun., 2015, 51, 11669 RSC; (d) L. Zheng, C. Yang, Z. Z. Xu, F. Gao and W. Xia, J. Org. Chem., 2015, 80, 5730 CrossRef CAS; (e) C. Yang, X. Zhang, D. Zhang-Negrerie, Y. Du and K. Zhao, J. Org. Chem., 2015, 80, 5320 CrossRef CAS PubMed; (f) J. Shi, J. Zhou, Y. Yan, J. Jia, X. Liu, H. Song, H. E. Xu and W. Yi, Chem. Commun., 2015, 51, 668 RSC.
- W. Kong, E. Merino and C. Nevado, Angew. Chem., Int. Ed., 2014, 53, 5078 CAS.
- (a) T. Baumgartner and R. Réau, Chem. Rev., 2006, 106, 4681 CrossRef CAS; (b) H. A. McManus and P. J. Guiry, Chem. Rev., 2004, 104, 4151 CrossRef CAS; (c) M. N. Birkholz, Z. Freixa and P. W. N. M. van Leeuwen, Chem. Soc. Rev., 2009, 38, 1099 RSC.
- (a) A. Arnone, P. Bravo, M. Frigerio, F. Viani and C. Zappalà, Synthesis, 1998, 1511 CrossRef CAS; (b) H. Krawczyk, K. Wasek, J. Kedzia, J. Wojciechowski and W. M. Wolf, Org. Biomol. Chem., 2008, 6, 308 RSC; (c) A. Arnone, P. Bravo, M. Frigerio, A. Mele, B. Vergani and F. Viani, Eur. J. Org. Chem., 1999, 2149 CrossRef CAS; (d) M. Frings, I. Thomé, I. Schiffers, F. Pan and C. Bolm, Chem.–Eur. J., 2014, 20, 1691 CrossRef CAS; (e) A. A. Prishchenko, M. V. Livantsov, O. P. Novikova, L. I. Livantsova and V. S. Petrosyan, Heteroat. Chem., 2008, 19, 418 CrossRef CAS; (f) P. Dauban and R. H. Dodd, J. Org. Chem., 1997, 62, 4277 CrossRef CAS; (g) R. Mansueto, V. Mallardo, F. M. Perna, A. Salomone and V. Capriati, Chem. Commun., 2013, 49, 10160 RSC.
- (a) P. Zhang, L. Zhang, Y. Gao, J. Xu, H. Fang, G. Tang and Y. Zhao, Chem. Commun., 2015, 51, 7839 RSC; (b) H. Y. Zhang, L. L. Mao, B. Yang and S. D. Yang, Chem. Commun., 2015, 51, 4101 RSC; (c) Z. Zhao, W. Xue, Y. Gao, G. Tang and Y. Zhao, Chem.–Asian J., 2013, 8, 713 CrossRef CAS PubMed; (d) B. Xiong, X. Feng, L. Zhu, T. Chen, Y. Zhou, C. T. Au and S. F. Yin, ACS Catal., 2015, 5, 537 CrossRef CAS; (e) M. Zhou, M. Chen, Y. Zhou, K. Yang, J. Su, J. Du and Q. Song, Org. Lett., 2015, 17, 1786 CrossRef CAS.
- (a) Y. M. Li, M. Sun, H. L. Wang, Q. P. Tian and S. D. Yang, Angew. Chem., Int. Ed., 2013, 52, 3972 CrossRef CAS; (b) B. Zhang, C. G. Daniliuc and A. Studer, Org. Lett., 2014, 16, 250 CrossRef CAS PubMed; (c) M. C. Lamas and A. Studer, Org. Lett., 2011, 13, 2236 CrossRef CAS; (d) X. Chen, X. Li, X. L. Chen, L. B. Qu, J. Y. Chen, K. Sun, Z. D. Liu, W. Z. Bi, Y. Y. Xia, H. T. Wu and Y. F. Zhao, Chem. Commun., 2015, 51, 3846 RSC; (e) Z. Z. Zhou, D. P. Jin, L. H. Li, Y. T. He, P. X. Zhou, X. B. Yan, X. Y. Liu and Y. M. Liang, Org. Lett., 2014, 16, 5616 CrossRef CAS.
- (a) B. B. Snider, Chem. Rev., 1996, 96, 339 CrossRef CAS; (b) W. Liu, D. Zell, M. John and L. Ackermann, Angew. Chem., Int. Ed., 2015, 54, 13 Search PubMed; (c) X. Li, J. Xu, Y. Gao, H. Fang, G. Tang and Y. Zhao, J. Org. Chem., 2015, 80, 2621 CrossRef CAS; (d) Y. Gao, J. Xu, P. Zhang, H. Fang, G. Tang and Y. Zhao, RSC Adv., 2015, 5, 36167 RSC; (e) H. C. Fisher, O. Berger, F. Gelat and J. L. Montchamp, Adv. Synth. Catal., 2014, 356, 1199 CrossRef CAS; (f) D. P. Li, X. Q. Pan, L. T. An, J. P. Zou and W. Zhang, J. Org. Chem., 2014, 79, 1850 CrossRef CAS; (g) X. H. Cao, X. Pan, P. J. Zhou, J. P. Zou and O. T. Asekun, Chem. Commun., 2014, 50, 3359 RSC; (h) S. F. Zhou, D. P. Li, K. Liu, J. P. Zou and O. T. Asekun, J. Org. Chem., 2015, 80, 1214 CrossRef CAS; (i) Y. Gao, X. Li, J. Xu, Y. Wu, W. Chen, G. Tang and Y. Zhao, Chem. Commun., 2015, 51, 1605 RSC.
- (a) G. Han, Q. Wang, Y. Liu and Q. Wang, Org. Lett., 2014, 16, 5914 CrossRef CAS; (b) G. Han, Y. Liu and Q. Wang, Org. Lett., 2014, 16, 3188 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures for the synthesis, spectral data and NMR spectra of compounds 3a–3s. See DOI: 10.1039/c5ra22570a |
| This journal is © The Royal Society of Chemistry 2016 |