Open Access Article
Open Access ArticleNanotechnology in food packaging materials: role and application of nanoparticles
Maria del Rosario Herrera-Riverab,
Sandra P. Torres-Arellanesa,
Carlos Inocencio Cortés-Martíneza,
Diana C. Navarro-Ibarra a,
Laura Hernández-Sáncheza,
Francisco Solis-Pomar
a,
Laura Hernández-Sáncheza,
Francisco Solis-Pomar b,
Eduardo Pérez-Tijerinab and
Ramón Román-Doval
b,
Eduardo Pérez-Tijerinab and
Ramón Román-Doval *a
*a
aTecnológico Nacional de México, Instituto Tecnológico del Valle de Etla, Abasolo S/N, Barrio del Agua Buena, Santiago Suchilquitongo, Oaxaca 68230, Mexico. E-mail: rrdoval.11@gmail.com
bFacultad de Ciencias Físico-Matemáticas, Universidad Autónoma de Nuevo León, San Nicolas de los Garza, Nuevo León 66451, Mexico
First published on 9th July 2024
Abstract
Global concerns about food security, driven by rising demand, have prompted the exploration of nanotechnology as a solution to enhance food supply. This shift comes in response to the limitations of conventional technologies in meeting the ever-increasing demand for food products. Consequently, nanoparticles play a crucial role in enhancing food production, preservation, and extending shelf life by imparting exceptional properties to materials. Nanoparticles and nanostructures with attributes like expansive surface area and antimicrobial efficacy, are versatile in both traditional packaging and integration into biopolymer matrices. These distinctive qualities contribute to their extensive use in various food sector applications. Hence, this review explores the physicochemical properties, functions, and biological aspects of nanoparticles in the context of food packaging. Furthermore, the synergistic effect of nanoparticles with different biopolymers, alongside its different potential applications such as food shelf-life extenders, antimicrobial agents and as nanomaterials for developing smart packaging systems were summarily explored. While the ongoing exploration of this research area is evident, our review highlights the substantial potential of nanomaterials to emerge as a viable choice for food packaging if the challenges regarding toxicity are carefully and effectively modulated.
Introduction
In recent years, nanotechnology has significantly impacted food packaging materials, addressing challenges in both food preservation and environmental considerations.1 Packaging materials are widely acknowledged for their crucial role in protecting food products, preventing degradation from physical, chemical, or biological factors, and ensuring overall quality during storage and handling.2 In this context, the integration of nanotechnology with encapsulation techniques has led to advanced methods for creating innovative food sector devices with potential benefits across various aspects of food science.3Nanomaterials are attracting attention from researchers for their versatile applications in the food industry. Their morphology, size, and distribution contribute to novel and enhanced properties compared to their bulk counterparts from which they originate.4,5 In this sense, a wide variety of nanoparticles (NPs) derived from metal or inorganic metallic oxide-based nanomaterials, including silver, copper, zinc oxide, and titanium dioxide, along with nanostructured materials such as starch, cellulose, chitosan, and montmorillonite (MMT)6,7 have been widely employed in food packaging materials as they demonstrate enhancements in water and gas resistance, mechanical strength and thermal properties.8 However, it should be noted that this will also depend on the concentration in which they are used. Besides, nanomaterials enhance the properties of packaging materials including durability, flexibility, barrier properties, and optical properties.9 For instance, some nanostructures such as cellulose nanocrystal (CNC) and cellulose nanofiber (CNF) were found to greatly improve tensile strength and water vapor permeability of chitosan and whey protein isolate.9,10 Additionally, nanomaterials are extensively employed as antimicrobials to reduce microbial spoilage of packaged foods. In this sense, nanoparticles including copper nanoparticles and silver nanoparticles improved the antibacterial properties, thermal properties, and antioxidant activity when added into agar-based films and CNC, respectively.11,12 Similarly, nanocellulose and nisin based-hydrogel microparticles reduced the growth of L. monocytogenes.13 Nanomaterials are additionally used in intelligent packaging systems to detect microbial contamination, chemical contaminants, or gases indicative of spoilage or quality deterioration in packaged food products.14 In this context, silver nanoparticles (Ag NPs) were reported to effectively detect the lactic acid in fresh milk when incorporated into cysteine and histidine.15 Likewise, titanium dioxide nanoparticles (TiO2 NPs) were employed to check the freshness of meat, showing promising results in the evaluation of the oxidation activity of xanthine oxidase.16
As previously evidenced, nanotechnology possesses a great potential to improve innovative products and processes in the food sector which could offer an excellent opportunity to replace non-degradable plastic packaging materials. However, although there are many review papers aiming to discuss the application of nanotechnology in food packaging context, review papers particularly centered on the utilization of nanoparticles such as silver, zinc oxide, titanium dioxide, copper oxide, graphene oxide, and carbon nanotubes are hard to find. Due to the previously mentioned necessity, the present paper comprehensively reviews the applications of the previously mentioned nanomaterials as antimicrobial packaging systems and sensors to guarantee food safety as well as extend their shelf life. An attempt has also been made to acknowledge the positive impacts of nanomaterials on the properties of biofilms when incorporated into them.
Food packaging roles and contribution of nanomaterials
Packaging is an important component across diverse industries, finding applications in medicine, pharmaceuticals, food, electronics, etc.17 In food-related applications, packaging plays a vital role in slowing down food deterioration, enhancing food quality and safety and extending shelf life, thus reducing food losses and waste.18 In this regard, packaging acts as a barrier against three main types of external influences. Firstly, it addresses chemical influences by minimizing changes caused by environmental factors like gases, moisture, or light. Secondly, it deals with biological contaminants by creating a barrier against microorganisms (pathogens and spoilage agents) and maintaining conditions that control senescence. Lastly, it addresses physical threats by protecting food from mechanical damage.19A wide range of materials are employed in packaging preparation and manufacturing. Commonly, polymer-based composite materials, including films and nanofibers,20,21 incorporate a variety of organic, inorganic, and composite nanomaterials to enhance their fundamental properties (Fig. 1).22 For example, it has been recognized that integrating nanomaterials into biopolymers enhances their mechanical and barrier properties as well as their stability under different temperature and moisture conditions.23 Furthermore, this has led to intelligent packaging that can help identify when the food has been exposed to adverse conditions, such as inadequate temperatures or elevated oxygen levels, this helps with information about food packaged quality products.24 Lastly, owing to their remarkably antimicrobial properties, nanomaterials are well-suited for antimicrobial active packaging, therefore prolonging the shelf life of food products and minimizing food loss.25 Metal-based materials like silver nanoparticles, nanoforms of certain metal oxides such as copper oxide nanoparticles, titanium oxide nanoparticles, zinc oxide nanoparticles, and carbon-based materials including carbon nanotubes and graphene oxide are among the nanomaterials that have been added to food packaging as functional additions.26
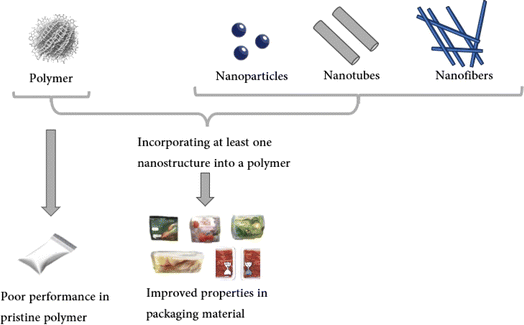 | ||
| Fig. 1 Schematic representation of the overall procedure for the manufacturing of composites containing nanomaterials and enhancement of the functional properties. | ||
Processing techniques
Polymer composites incorporating nanomaterials can be manufactured using processes similar to those employed for conventional polymer composites. This aspect makes them particularly appealing from a production point of view.27 The fabrication method dictates both the concentration and dispersion of the nanofillers and nano reinforcements into the polymer.28Solution mixing or solvent casting implies vigorously stirring or nanoparticle ultrasonication in a polymer solution before casting it into a mold and subsequently evaporating the solvent. This technique allows for obtaining a homogeneous distribution of nanoparticles within the polymer while facilitating the formation of filler-rich layers.29
At the industrial scale, composite materials on nanoscale are commonly produced through extrusion or melt processing, where polymer and nanofillers are compounded in a single or twin-screw extruder. This process involves blending the components into a molten state, aided by shear and elongational stresses within the mixer. The resulting blend can then be utilized to manufacture a wide range of products such as films through processes like profile injection molding, extrusion, and blow molding.30
Electrospinning and electrospraying involve expelling (electrospinning) or spraying (electrospraying) polymer solutions by applying high electric potential at standard temperature and pressure conditions. Electrospinning provides micro and nanostructured fiber-based materials with diverse morphologies and particles in the range of nanometers to a few micrometers for electrospraying31
Sol–gel technique is widely utilized for producing nano-based coatings. A dense colloidal sol is applied to a surface via dipping, spraying, or spin coating methods. The resulting layers possess a thickness ranging from a few nanometres to 0.5 and 3 μm.32
Effect of the most used nanoparticles in food packaging
Food packaging materials are vital for preserving product quality during distribution and storage. They ideally exhibit appropriate mechanical, optical, and thermal characteristics, acting as barriers against contaminants, water vapor, oxygen, carbon dioxide, flavors, and microorganisms.33 Artificial polymers like polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), polyethylene terephthalate (PET), polyvinyl alcohol (PVA), and polyglycolide (PGA) are commonly utilized in food packaging owing to their thermal stability, mechanical properties, lightness, and cost-effectiveness. However, sourced from non-renewable petroleum, these polymers present potential health and environmental hazards.34 Recent research focuses on developing biopolymers for food packaging. Various biodegradable polymers, sourced from plant and animal origins, include polysaccharides (agar, carrageenan, starch, pectin, inulin, chitin, and chitosan), microbial polysaccharides (xanthan, gellan, curdlan, pullulan, dextran, and bacterial cellulose), polyesters, proteins, and synthetic variants (poly(L-lactide) or poly (butylene succinate)). Some are produced through microbial fermentation, such as poly(β-hydroxybutyrate) (PHB).Unfortunately, natural polymers possess challenges due to their high gas and vapor permeability, coupled with poor resistance, limiting their broad applications.34,35 One potential solution to this issue is the enhancement of their properties such as mechanical strength and gas barrier capabilities. This approach enables their effective application in food packaging while addressing environmental concerns.36 Achieving this objective involves integrating nanomaterials, such as nano-fillers, into biodegradable polymers to form biocomposites. These innovative materials can provide enhanced mechanical strength and act as barriers against microorganisms, moisture, and gases.37
On the other hand, nanoparticles are gaining interest due to their microbial and sensor properties. These particles show promise by directly interacting with bacterial cells, offering potential solutions.38 Furthermore, the integration of nanotechnology with appropriate analytical methodologies and detection techniques proves highly valuable for evaluating the quality and safety of food.39 As an example, nanosensors, made from metals and metal oxides, are integrated into ‘smart packaging’ for food. Enabling real-time monitoring of storage conditions, assessing freshness, providing insights into product quality, and enhancing overall safety. These nanosensors can also be used in measurement devices to evaluate factors influencing food quality and safety.40
Silver nanoparticles (Ag NPs)
| Food packaging material | Main finding | Ref. |
|---|---|---|
| Cellulosic paper | The composite film had antimicrobial properties against A. hydrophila. Moreover, the films enhanced shelf life of tomatoes and cabbages | 42 |
| Cellulose-based papers coated with chitosan | The addition of Ag NPs and TiO2 NPs led to an improvement of the water and oxygen barrier properties | 43 |
| Chitosan incorporated with essential oils | The composite film displayed potent antimicrobial activity against E. coli, L. monocytogenes, S. typhimurium and A. niger. Additionally, it extended the shelf life of strawberries by 4 days while maintaining their quality | 44 |
| Polyvinyl alcohol | The silver/PVA composite prolonged the shelf life of salmon fillets up to the 7th day and exhibited antimicrobial properties against S. aureus | 45 |
| Polyvinyl alcohol coated with agar and maltodextrin | The incorporation of Ag NPs improved the antibacterial effectiveness against E. coli and S. aureus while also enhancing the flexibility of the film. Moreover, the composite film extended the shelf life of bananas by 5 days | 46 |
| Pullulan blended with pectin | The composite film exhibited superior physical, mechanical, optical, and barrier characteristics. The incorporation of Ag NPs improved the antimicrobial efficacy against S. typhimurium, E. coli and L. monocytogenes of the film | 47 |
| Polylactic acid | The integration of Ag NPs enhanced the mechanical strength of the film and its ability to act as a barrier against water vapor and oxygen. Additionally, the composite film exhibited potent antibacterial properties, contributing to the extension of the shelf life of strawberries | 48 |
| Polymer matrices | Nanomaterial | Size of nanomaterial (nm) | Active against bacterial species | Possible mechanism of action | Ref. |
|---|---|---|---|---|---|
| Chitosan | (a) Ag NPs | (a) 70 to 100 | (a), (b) S. aureus, P. aureginosa, A. niger, C. albicans | Electrostatic attraction between the negatively charged cell membrane of the microorganisms and the positively charged Ag NPs or Au NPs | 53 |
| (b) Au NPs | (b) 20 to 30 | ||||
| Pullulan reinforced with essential oils | (a) Ag NPs | (a) 100 | (a), (b) S. aureus, L. monocytogenes, E. coli O157:H7 | Interference with vital cellular processes | 54 |
| (b) ZnO NPs | (b) 110 | Disruption of DNA replication | |||
| Oxidative stress through the catalysis of ROS. | |||||
| Pullulan | (a) Ag NPs | (a), (b) 20 to 50 | (a), (b) P. aureginosa, E. coli, S. aureus | — | 55 |
| (b) Au NPs | |||||
| Polyvinyl alcohol reinforced with MMT | Ag NPs | — | S. typhimurium, S. aureus | Interaction between positively charged silver ions (Ag+) and negatively charged bacterial cell wall | 56 |
| Tragacanth blended with hydroxypropyl methylcellulose and beeswax | Ag NPs | — | B. cereus, S. aureus, S. pneumonia, L. monocytogenes, E. coli, S. typhimorum | Interaction between positively charged silver ions (Ag+) and negatively charged phosphorous or sulfur groups of macromolecules in cell walls | 57 |
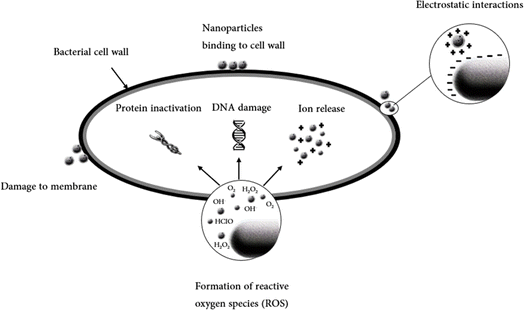
The mechanism of Ag NPs has been well established.50,63–65 It is widely accepted that the positive charge of Ag NPs interacts with the negatively charged phosphorus or sulfur groups found in proteins and nucleic acids through electrostatic forces.66 This interaction has the potential to deform bacterial cell walls and membranes, potentially leading to cell death. On the other hand, Ag NPs can also induce the production of reactive oxygen species (ROS) and free-radical species, such as hydrogen peroxide, superoxide anion, hydroxyl radical, hypochlorous acid, and singlet oxygen. These species can elevate oxidative stress within bacterial cells. Furthermore, they can improve the permeability characteristics of bacterial membranes, leading ultimately to cell death.67 An illustration of reported antimicrobial mechanisms induced by nanoparticles is shown in Fig. 2.
Zinc oxide nanoparticles (ZnO NPs)
| Food packaging material | Main finding | Ref. |
|---|---|---|
| Polyvinyl alcohol blended with gelatin | The addition of ZnO NPs and TiO2 NPs extended the shelf life of shrimp up to 12 days and preserved the sensory characteristics of the films during storage | 79 |
| Polyvinyl alcohol | The incorporation of ZnO NPs improved the antibacterial efficacy against S. aureus and E. coli of the film and extended the shelf life of cherry tomatoes | 80 |
| Starch blended with polyvinyl alcohol | The water and UV barrier and mechanical and antimicrobial properties of starch/PVA film can be improved owing to the incorporation of ZnO NPs | 81 |
| Chitosan and cellulose acetate phthalate | Incorporating ZnO NPs enhanced the thermal stability and barrier properties of the film, extending the shelf life of black grape fruits up to 9 days | 82 |
| Pullulan blended with chitosan | The blend of ZnO NPs and propolis enhanced the mechanical strength of the film by 25%. Additionally, there was a slight increase in the film's water vapor barrier and hydrophobicity | 83 |
| Chitosan | Chitosan film reinforced with ZnO NPs showed an enhancement of 77% and 67% in tensile modulus and tensile strength respectively. Moreover, the enhanced films exhibited a twofold increase in antimicrobial activity against B. subtilis and a 1.5-fold increase against E. coli | 84 |
| Polylactic acid | An improvement in water vapor permeability, elongation at break and elastic modulus of the films was observed. Additionally, the film prolonged the shelf life of fresh-cut apples for 14 days | 85 |
| Polylactic acid | Adding 5 wt% of ZnO NPs enhanced the tensile properties and lowered the permeability to O2 and CO2 of the films. The film extended the shelf life of cherries | 86 |
| Polymer matrices | Nanomaterial | Size of nanomaterial (nm) | Active against bacterial species | Possible mechanism of action | Ref. |
|---|---|---|---|---|---|
| Chitosan blended with PVA | ZnO NPs | 24.5 | S. aureus, E. coli, C. albicans, A. niger | The ROS (OH, O2−, H2O2) and Zn2+ released from ZnO react with bacterial cell wall and intracellular contents of the cell causing damage to nucleic acids and ultimately leading to bacterial death | 89 |
| Polylactic acid | ZnO NPs | 50 to 100 | E. coli, L. monocytogenes | Formation of reactive oxygen species | 87 |
| Gelatin blended with PVA and reinforced with black cumin cake extract | ZnO NPs | <100 | M. luteus, L. monocytogenes, S. aureus | Permeation of Zn2+ particles into the cell membrane, causing oxidative stress ultimately disrupting the cellular functions | 90 |
| Chitosan | ZnO NPs | — | E. coli | ZnO releases ROS species which interact with cell membrane causing severe damage and ultimately kill the bacteria | 92 |
The mechanism of action of ZnO NPs can be explained through the inhibition of bacteria by allowing Zn2+ particles to penetrate the cell membrane, inducing oxidative stress. This stress leads to damage to lipids, carbohydrates, proteins, and DNA, ultimately disrupting cellular functions.93
Titanium dioxide nanoparticles (TiO2 NPs)
| Food packaging material | Main finding | Ref. |
|---|---|---|
| Chitosan | The reinforced film delayed the ripening process of tomatoes and exhibited improved tensile strength, barrier properties and ethylene photodegradation ability | 102 |
| Chitosan | The film with TiO2 and SiO2 NPs improved fruit health, texture firmness, and moisture content while decreasing decay and shrinkage rates | 103 |
| Pullulan blended with carboxymethyl cellulose | Incorporation of TiO2 NPs improved water vapor and UV-visible light barrier properties in the composite films. Furthermore, the films displayed strong activity against E. coli and S. aureus | 104 |
| Polyvinyl alcohol | PVA reinforced with TiO2 NPs exhibited strong antimicrobial activity against Shewanella spp., Pseudomonas putida and Aeromonas hydrophilia and extended the shelf life for 1–2 days of macrobrachium rosenbergii | 105 |
| Polyvinyl alcohol | The incorporation of TiO2 NPs improved the mechanical properties of the film, although elongation at break slightly decreased | 106 |
| Polylactic acid | An improvement in the Young modulus and tensile strength of the films was observed | 107 |
| Zein blended with chitosan | The addition of 0.15%wt TiO2 NPs increased the antibacterial properties of the film. Additionally, composite films showed better mechanical properties, thermal stability and hydrophobic property | 108 |
| Polymer matrices | Nanomaterial | Size of nanomaterial (nm) | Active against bacterial species | Possible mechanism of action | Ref. |
|---|---|---|---|---|---|
| Chitosan | TiO2 NPs | 21 | S. aureus, E. coli, S. Typhimurium, Pseudomonas aeruginosa, Aspergillus, Penicillium | Photocatalytic reaction and generation of ROS under exposure of UV light | 102 |
| Chitosan | TiO2 NPs | 5 | Xanthomonas oryzae strains | Release of toxic metal ions | 113 |
| Generation of hydroxyl radicals and other ROS under radiation with UV light | |||||
| Chitosan | (a) Ag NPs | (a), (b) <100 | E. coli | — | 114 |
| (b) TiO2 NPs | |||||
| Polylactic acid | TiO2 NPs | 10 to 20 | E. coli, S. aureus | Generation of hydrogen peroxide, hydroxyl radicals and superoxide anions under UV light that ultimately contacts and kill the microbes | 115 |
| Polylactic acid | (a) ZnO NPs | (a) 10 to 30 | (a), (b) L. monocytogenes, E. coli | Zn2+ release into the medium leads to direct physical attack on bacterial cell walls via electrostatic interactions | 116 |
| (b) TiO2 NPs | (b) 18 | ||||
| Cellulose blended with PVA | TiO2 NPs | 0.32 to 20 | Bacillus cereus, E. coli | Electromagnetic attraction between microorganism and metal oxides | 117 |
The possible action mode of TiO2 has been established. It mainly includes the release of toxic metal ions causing damage to cell membranes upon direct contact with nanoparticles. This leads to the inactivation and death of bacteria by oxidizing the polyunsaturated phospholipids within the cell membrane. Additionally, it facilitates the degradation of toxic compounds expelled by bacteria through the generation of hydroxyl radicals (OH) and other reactive oxygen species (ROS) when exposed to UV light. The antimicrobial effectiveness of TiO2 NPs is related to their size as well as their high surface-to-volume ratio.121
Copper oxide nanoparticles (CuO NPs)
| Food packaging material | Main finding | Ref. |
|---|---|---|
| Chitosan | Incorporation of CuO and TiO2 NPs improved the antimicrobial potential of composite films. The films exhibited great transparency and better physical characteristics | 127 |
| Chitosan | The incorporation of MMT–CuO NPs increased the tensile strength and elongation at break, but reduced the water vapor permeability and oxygen permeability of the films | 128 |
| Carboxymethyl cellulose blended with polyvinyl alcohol | Incorporating 0.9 wt% CuO NPs improved the water vapor permeability, mechanical properties, and antimicrobial efficacy of the coating material | 129 |
| Polyvinyl alcohol blended with starch and glycerol | The mechanical properties, thermal stability, water resistance, and UV barrier properties of the films were enhanced by incorporating CuO and ZnO nanoparticles | 130 |
| Polylactic acid modified with polyaniline | The antioxidant and antimicrobial characteristics of the film were enhanced due to the addition of CuO and ZnO NPs | 131 |
| Polyvinyl alcohol | The composite film exhibited reinforced mechanical, dielectric properties and antibacterial properties | 132 |
| Polymer matrices | Nanomaterial | Size of nanomaterial (nm) | Active against bacterial species | Possible mechanism of action | Ref. |
|---|---|---|---|---|---|
| Bacterial cellulose and chitosan nanofibers | CuO NPs | 48 | E. coli, P. aeruginosa, L. monocytogenes | Formation of reactive oxygen species | 21 |
| Chitosan and neem | CuO NPs | 22 | S. aureus, E. coli, K. aerogenes, S. pyogenes | Adsorption of Cu2+ ions to the surface of the bacterial and damage the cell membrane | 133 |
| Carboxymethyl chitosan blended with petal anthocyanin | CuO NPs | — | Staphylococcus aureus, E. coli | — | 134 |
The antimicrobial activity of CuO NPs is commonly attributed to various mechanisms. Firstly, CuO NPs attach to the cell membrane, interacting with bacterial lipid membranes. This interaction alters membrane permeability, obstructing nutrient intake and ultimately affecting bacterial viability.135 Also, the release of copper ions and the subsequent formation of copper-peptide complexes disrupt the integrity of the bacterial membrane is another mechanism widely accepted.136 Finally, CuO NPs could catalyze the generation of reactive oxygen species (ROS), significantly enhancing their production, and ultimately resulting in cell death.137
Carbon nanotubes (CNTs)
| Food packaging material | Main finding | Ref. |
|---|---|---|
| Polyvinyl alcohol | PVA reinforced with MWCNTs–ZnO exhibited enhanced thermal stability, water vapor transmission rate, hydrophobicity and antibacterial activity | 141 |
| Sodium alginate and chitosan | Mechanical properties of the composite films were improved by the addition of CNTs and GO. | 142 |
| PHBV | Crystallization temperature, crystallinity, storage modulus and dielectric properties of the films were enhanced by the incorporation of CNTs | 143 |
| Polylactic acid | Crystallization rate, barrier, mechanical, antibacterial and antifungal properties of the films were improved by the addition of MWCNTs | 144 |
| Polylactic acid | PLA reinforced with fMWCNTs exhibited better mechanical properties and Young modulus | 145 |
| Polymer matrices | Nanomaterial | Size of nanomaterial (nm) | Active against bacterial species | Possible mechanism of action | Ref. |
|---|---|---|---|---|---|
| Polylactic acid reinforced with PCL and CIN | CNTs | Diameter: 3–5 | S. aureus, E. coli | — | 146 |
| Outer diameter: 8–15 | |||||
| Length: 0.05 | |||||
| Polylactic acid | (a) Ag NPs | (a), (b) Diameter: 60 to 100, length: 0.015 | S. haemolyticus | — | 147 |
| (b) CNTs | |||||
| Polyvinyl alcohol | CNTs | Diameter: 15 to 5 | P. aureginosa | Membrane lipid disruption or protein binding due to CNT-microorganism contact | 148 |
| Length: 0.005 to 20 | |||||
| Polyvinyl alcohol | MWCNTs | Diameter: 7 to 10 | S. aureus | Harmonious effect of MWCNTs through penetration cell | 149 |
| Length: 0.100 to 0.300 | |||||
| Length: 0.100 to 0.300 |
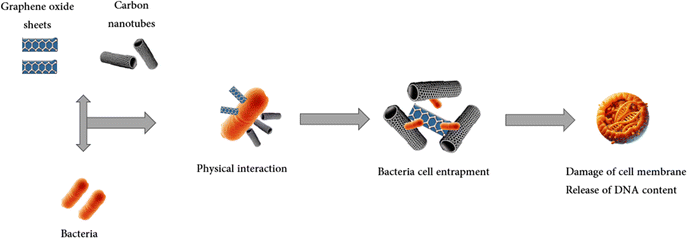
The mechanism of action of CNTs relies on their interaction with microorganisms, which disrupts cellular membranes, metabolic processes, and morphology.152 Research has revealed that the bacteriostatic properties of CNTs arises from their direct contact with microorganisms, causing damage to their cell membranes and subsequent bacterial cell death. Scanning electron microscopy (SEM) observations have revealed morphological alterations in microorganisms following incubation with CNTs, indicative of compromised cellular integrity. The bacteriostatic attributes of CNTs are increasingly acknowledged and attributed to their high surface-to-volume ratio and substantial inner volume.153 Fig. 3 shows the schematic mechanism of antimicrobial activity of some carbon-based nanomaterials such as carbon nanotubes and graphene oxide sheets.
Graphene oxide (GO)
| Food packaging material | Main finding | Ref. |
|---|---|---|
| Chitosan | The resulting film exhibited significantly lower vapor permeability oxygen permeability, water solubility and light transmittance | 157 |
| Chitosan | Antimicrobial effects against B. subtilis and A. niger of the film were enhanced by the incorporation of GO and TiO2 NPs | 160 |
| Chitosan | The packaging material slowed down the ripening process of mangoes, resulting in reduced respiration rate, weight loss, and incidence and severity of diseases | 161 |
| Polyvinyl alcohol | The tensile strength, elastic modulus, and failure stain of the films were markedly enhanced by incorporating 0.3 and 2.0 wt% of graphene oxide | 162 |
| PVA blended with tragacanth gum | The resulting films effectively preserved raw beef samples by inhibiting the growth of natural beef bacteria for a minimum of 7 days | 163 |
| Polylactide doped with clove EO | The addition of GO enhanced the optical and anti-UV properties of the films | 164 |
| Polylactic acid | Polylactic acid reinforced with GO–CNTs exhibited a 75% increase in tensile strength and a 130% increase in Young's modulus | 165 |
| Polymer matrices | Nanomaterial | Size of nanomaterial (nm) | Active against bacterial species | Possible mechanism of action | Ref. |
|---|---|---|---|---|---|
| Chitosan | (a) Graphene oxide | — | S. aureus, E. coli | Active superoxide ions generated on the surface of the oxide reacts with the peptide linkages in the cell wall of bacteria and thus disrupt | 168 |
| (b) ZnO NPs | |||||
| Chitosan | (a) ZnO NPs | — | E. coli, K. pneumonia, S. aureus, S. mutans, Salmonella, P. aureginosa | Ions of nanomaterials deactivate cellular enzymes, DNA and proteins by reacting with electron-donating groups interfering with respiratory sequence and leading to cell death | 169 |
| (b) Graphene oxide | |||||
| Polylactic acid | (a) ZnO NPs | (a), (b) 1–5 nm thickness, 4–8 number of layers | E. coli, B. cereus, S. cerevisiae | — | 170 |
| (b) Graphene oxide | |||||
| Polylactic acid | (a) ZnO NPs | — | S. aureus, E. coli | Inactivation of cell due to direct contact between ZnO and bacteria. Adhesion of GO sheets to the surface cells, covering them and inhibiting cell proliferation | 171 |
| (b) Graphene oxide | |||||
| Polyvinyl alcohol | (a) Cu2O NPs | — | P. aureginosa, S. aureus, S. oralis, E. coli | Generation of reactive oxygen species due to light irradiaton | 172 |
| (b) Reduced graphene oxide | |||||
| (a) TiO2 NPs | |||||
| Polyvinyl alcohol | (a) Ag NPs | — | S. aureus, E. coli | Chemical oxidation based on the oxidation of cellular components by GO sheets | 173 |
| (b) Graphene oxide | |||||
| Polyvinyl alcohol blended with starch | (a) Ag NPs | — | S. aureus, E. coli | — | 174 |
| (b) Graphene oxide | |||||
| (b) Graphene oxide |
The mechanism by which GO nanoparticles inhibit bacterial growth can be explained through (a) physical interactions between the bacterial cell and the nanoparticles. This interaction manifests through direct contact with the basal planes or sharp edges of the graphene material, or through the envelopment of bacteria with nanosheet; (b) physical demolition and chemical oxidation of the cell membrane and cell wall of microorganisms, achieved by the generation of reactive oxygen species (ROS), culminating in microbial death and a subsequent reduction in microbial resistance.173
Cellulose nanocrystals
| Food packaging material | Main finding | Ref. |
|---|---|---|
| Low density poly(ethylene) | The addition of acetic anhydride-modified CNCs increased composites' stiffness and strain at break by 20% and up to 90%, respectively. Further, Young modulus increased by ∼45% | 184 |
| Polyvinyl alcohol blended with chitosan | Composite films increased tensile strength (98.16 MPa) and thermal stability | 185 |
| Polyvinyl alcohol blended with carboxymethyl cellulose | The tensile modulus and strength were markedly enhanced (141% and 83%, respectively) by the addition of 5 wt% CNC. Additionally, water vapor permeability was reduced by 87% while composites maintained the same transparency level | 186 |
| κ-carrageenan | Incorporation of CNCs increased the tensile strength and elongation at break of films (from 38.33 ± 3.79 MPa to 52.73 ± 0.70 MPa and from 21.50 ± 3.72% to 28.27 ± 2.39%, respectively). Additionally, water vapor permeability of the films decreased | 187 |
| Chitosan | Improvement of 26% in films' tensile strength was achieved by the addition of 5% (w/w) CNCs. In addition, water vapor permeability of the composite decreased by 27% | 188 |
| Chitosan | Chitosan films reinforced with functionalized cellulose nanocrystals demonstrated a remarkably improvement in tensile strength (up to 150%), while achieving a considerable decrease in hydrophilicity | 189 |
| Polymer matrices | Nanomaterial | Size of nanomaterial (nm) | Active against bacterial species | Possible mechanism of action | Ref. |
|---|---|---|---|---|---|
| Polyvinyl alcohol | Quaternized cellulose nanocrystals | 150 ± 20 nm length | S. aureus, E. coli | — | 191 |
| 6 ± 1 nm width | |||||
| Polylactic acid | Modified CNCs | — | S. aureus, E. coli | — | 192 |
| Gelatin | Rosing-grafted CNCs | — | S. aureus, E. coli | Increased permeability and leakage of cytoplasm due to the interaction of rosing grafted CNCs with the phospholipid cell membrane | 193 |
| Chitosan | CNCs | ∼75 nm diameter | S. aureus, E. coli, C. albicans | Cell membrane damage due to direct contact with rodlike CNCs particles making microbial cells susceptible to protonated chitosan | 194 |
| Corn starch mixed with chitosan | CNCs | — | L. monocytogenes, S. aureus | Damage to the structural integrity of the cell membrane due to the interaction of nanoparticles and bacteria via electrostatic forces | 195 |
Lipid nanoparticles
| Food packaging material | Main finding | Ref. |
|---|---|---|
| Xanthan gum | Improvement of flexibility and strength of the films was achieved by the addition of 60–75 g L−1 solid lipid nanoparticles | 201 |
| Xanthan gum | Addition of solid lipid nanoparticles improved shelf life of guava fruit by showing lowest range of weight loss and delaying the maturation process | 202 |
| Xanthan gum | Composite films exhibited improved barrier properties | 203 |
| Proteins | Incorporation of solid lipid nanoparticles led to a remarkably decrease in water vapor permeability of the film | 204 |
| Xanthan gum mixed with propylene glycol | The composite films exhibited appropriated properties (decreased decay rates, less fungal growth and weight loss) in order to enhance strawberries' shelf life | 205 |
| Types of nanolipid | Desing of packaging material content | Types of packaging | Food products | Properties | Ref. |
|---|---|---|---|---|---|
| Nanoliposome | Agar/Artemisia annua oil/chitosan | Edible film | Cherry tomato | Cellular leakage and alterations in cell membrane permeability resulting from the release of large molecular substances | 206 |
| Nanoemulsion | Citrus essential oil/chitosan nanoparticles | Edible coating | Silvery pomfret | Inhibited microbial proliferation and decreased oxidation of proteins and lipids | 207 |
| Nanoemuslion | Sodium alginate/lemongrass essential oil/tween 80 | Edible coating | Fresh-cut apple | Extended the shelf life of apples by exhibiting antimicrobial properties, lowering respiration rates, and decreasing ethylene production | 208 |
| Nanoemulsion | Starch/oleic acid/lactic acid/nisin/lauric alginate | Edible coating | Fresh-cut pineapple | Suppresses microbial growth while reducing the respiration rate and mitigating changes in color | 209 |
Regulations of nanomaterials in food packaging
Guidelines from regulatory bodies offer secure routes for manufacturers, importers, and consumers to safeguard food products' safety in the market.213 However, globally, the absence of established regulations for nanotechnology applications persists due to the scarcity of comprehensive and dependable fundamental research concerning the safety assessment and migration properties of nanomaterials from packaging into the food system.24 In certain countries, nanomaterials are permitted for use in Food Contact Materials (FCMs), whereas in others, their usage is prohibited due to concerns regarding potential toxicity as illustrated in Table 17.214 The Food and Drug Administration (FDA) oversees the use of nanomaterials in food packaging within the United States. Manufacturers of nanomaterials must secure pre-market approval from the FDA, either through a Food Additive Petition (FAP) or the Food Contact Notification (FCN) system. However, substances deemed Generally Recognized as Safe (GRAS) are exempt from this premarket authorization requirement. Additionally, if an organization publishes a scientific risk assessment of its product in a peer-reviewed scientific journal, it may market the product without prior FDA approval.219 The regulation of packaging materials including nanomaterials in Europe falls under the review of the European Union, governed by regulation EC 1935/2004. This regulation permits their use in food packaging as long as they do not pose a risk to human health, as outlined in Article 3. Furthermore, according to Article 23 of regulation EC 10/2011, packages containing nanoparticles must undergo evaluation before entering the market, in accordance with the Novel Food Regulation EC 258/97. Additionally, the regulation of nanomaterials, even if previously authorized in bulk form, is necessary under EEC 89/109. In cases where non-authorized substances are utilized, a maximum migration limit of 0.01 mg kg−1 must be ensured through a functional barrier, as specified in Article 14, EC 450/2009.220| Nanomaterial | Applications | Current status | Used in | Commercially available products | Note | Ref. |
|---|---|---|---|---|---|---|
| a FCS: Effective Food Contact Substance (FCS) Notifications.b GRAS: Generally Recognized as Safe. | ||||||
| Ag | Utilized as preservatives, antimicrobials, antibiotics, and antistatic agents | FCS inventorya | Reusable food containers | Nano-silver baby milk bottle | FCN no. 1235. (<4 ppm by weight of silver as an antimicrobial agent blended into polymers) | 214 and 215 |
| Silver-nano noble one-touch mug cup | ||||||
| Fresh food containers (OSO Fresh®) | ||||||
| Nano-silver NS-315 water bottle | ||||||
| Nano-silver salad bowl | ||||||
| FresherLonger™ Miracle Food Storage | ||||||
| FresherLonger™ Plastic Storage Bags | ||||||
| TiO2 | It is utilized as a color additive | Not subject to certification | Less than 1% by weight of the food | 216 | ||
| ZnO | Utilized as an additive for UV filtering, antimicrobial purposes, and as a fungistatic agent | GRAb | Plastic glasses, plastic films | Nano Plastic Wrap (SongSing Nano Technology Co., Ltd. Taiwan) | Authorized under EC regulation 10/2011 (based on conventional particle size) | 217 |
| CuO | Used as a nutritional dietary supplement | Approved for use in animal feed | ||||
| CNT and GO sheets | Food packaging | Films, bottles for packaging | There is no conclusive information available for the U.S at this time | 218 | ||
Limitations and challenges of nanomaterials in food packaging
Numerous challenges must be addressed before nanotechnology can revolutionize product development and processes in food-related applications. The main challenge is the creation of edible delivery systems through cost-effective processing techniques, ensuring formulations that are both effective and safe for human consumption.221 Ensuring food safety involves addressing concerns about nanoparticles leaching and migrating from packaging materials into food products. Nanomaterials, whether added directly or indirectly, can sometimes be isolated due to migration from other sources.222 At the nanoscale, materials exhibit distinct behaviors, and our current understanding of analyzing these phenomena remains limited. A comprehensive understanding of the functional aspects and toxicity of nanomaterials at the nanoscale will significantly enhance the practical applications and safety standards of nanotechnology. The potential risks, toxicity issues, and environmental concerns associated with nanoparticles must be acknowledged. Nanoparticles can pass the biological barriers and permeate various tissues and organs. Moreover, the synthesis of nanoparticles through diverse chemical methods can produce harmful non-eco-friendly by-products that lead to significant environmental pollution.223 Hence, it is relevant to establish a comprehensive risk assessment program, regulatory frameworks, and biosecurity measures, and address public concerns before manufacturing, packaging, and consuming nano-based food products. Additionally, both in vitro and in vivo studies on nanoparticle interactions with living organisms are essential before commercial application, especially in the production of antibacterial nanoparticles that are environmentally friendly.224The trend of research in food packaging
The evolution of food packaging has been driven by continual adaptations to meet the evolving demands of the food industry. Today, the significance of food packaging rivals that of the contents it safeguards, primarily by extending shelf life and reducing waste during transportation, storage, and distribution. However, the growing demand for packaging, predominantly produced from materials that pose potential environmental hazards, underscores a critical opportunity for research into eco-friendly alternatives.Transitioning to environmentally sustainable packaging materials presents challenges, foremost among them being the need to meet current industry standards and demands. Key considerations include economic viability, ecological sustainability, and the ability to uphold essential physical and chemical properties that prolong product freshness and quality. Moreover, modern packaging should incorporate interactive and intelligent features to inform consumers about product freshness and quality, while also being aesthetically pleasing and potentially offering added consumer benefits through active ingredients.
In response to these demands, recent studies in nanotechnology have focused on developing composite materials based on biopolymers. These materials are enhanced with nanoparticles and micro/nanostructures in precise combinations to impart the requisite physicochemical and biological properties demanded by the food industry. Such research represents a fraction of ongoing efforts aimed at transitioning towards advanced, environmentally friendly packaging solutions.
Conclusions
The increasing focus on health, nutrition, and food safety, along with growing global food demand, has fueled interest in exploring nanotechnology for enhancing food quantity and quality. Metal nanoparticles like silver (Ag), zinc oxide (ZnO), titanium dioxide (TiO2), copper oxide (CuO), and nanomaterials such as carbon nanotubes and graphene oxide are widely used in food preservation. Integrating them into various polymer matrices, including biopolymers, enhances properties and functionality, making nanotechnology invaluable in the food industry. Hence, incorporating metal-based nanoparticles into functional food packaging enhances biological properties, including antioxidant and antimicrobial activities. It also improves the mechanical, physical, barrier, and optical characteristics of the material, ensuring food safety, preserving nutritional value, and extending shelf life. Furthermore, nanotechnology enables real-time monitoring during production, allowing for the development of smart packaging systems for continuous monitoring of food quality and safety throughout the supply chain. Indeed, while nanomaterials hold promise in the food sector, it's essential to assess both their benefits and risks. Addressing biosafety concerns and establishing regulatory frameworks present challenges that require thorough research. Ensuring safety and regulatory compliance is important for the successful integration of these materials into the food packaging industry.Data availability
This study constitutes a scientific review and does not include original experimental data or other unpublished information. The images included in this manuscript are original creations, while the tables were compiled from cited references. Consequently, this manuscript does not contain supplementary electronic materials.Author contributions
Conceptualization: R. Herrera-Rivera; S. P. Torres-Arellanes; L. Hernández-Sánchez; R. Román-Doval. Formal analysis: L. Hernández-Sánchez; F. Solis-Pomar; E. Pérez-Tijerina, Diana C. Navarro-Ibarra. Project administration: R. Herrera-Rivera; L. Hernández-Sánchez; F. Solis-Pomar; E. Pérez-Tijerina. Writing – original draft: R. Herrera-Rivera; S. P. Torres-Arellanes; L. Hernández-Sánchez; F. Solis-Pomar; E. Pérez-Tijerina. Writing – review & editing: C. I. Cortés-Martínez; Diana C. Navarro-Ibarra, R. Román-Doval.Conflicts of interest
The authors declare no competing interests.References
- R. Singh, S. Dutt, P. Sharma, A. K. Sundramoorthy, A. Dubey, A. Singh and S. Arya, Future of Nanotechnology in Food Industry: Challenges in Processing, Packaging, and Food Safety, Global chall., 2023, 7, 2200209 CrossRef PubMed.
- A. M. Youssef, S. M. El-Sayed, H. H. Salama, H. S. El-Sayed and A. Dufresne, Evaluation of bionanocomposites as packaging material on properties of soft white cheese during storage period, Carbohydr. Polym., 2015, 132, 274–285 CrossRef CAS PubMed.
- A. V. Singh, Z. Hosseinidoust, B.-W. Park, O. Yasa and M. Sitti, Microemulsion-Based Soft Bacteria-Driven Microswimmers for Active Cargo Delivery, ACS Nano, 2017, 11(10), 9759–9769 CrossRef CAS PubMed.
- M. A. Abdel-Wahhab and F. Márquez, Nanomaterials in Biomedicine, Soft Nanosci. Lett., 2015, 05(03), 53–54 CrossRef.
- S. Hassan and A. V. Singh, Biophysicochemical Perspective of Nanoparticle Compatibility: A Critically Ignored Parameter in Nanomedicine, J. Nanosci. Nanotechnol., 2014, 14(1), 402–414 CrossRef CAS PubMed.
- K. Pathakoti, M. Manubolu and H.-M. Hwang, Nanostructures: Current uses and future applications in food science, J. Food Drug Anal., 2017, 25(2), 245–253 CrossRef CAS PubMed.
- A. Orsuwan and R. Sothornvit, Polysaccharide Nanobased Packaging Materials for Food Application, in Food Packaging and Preservation, Elsevier, 2018, pp. 239–270 Search PubMed.
- S. Shankar and J.-W. Rhim, Bionanocomposite Films for Food Packaging Applications, in Reference Module in Food Science, Elsevier, 2018 Search PubMed.
- Y. Xu, S. Willis, K. Jordan and E. Sismour, Chitosan nanocomposite films incorporating cellulose nanocrystals and grape pomace extracts, Packag. Technol. Sci., 2018, 31(9), 631–638 CrossRef CAS.
- R. A. Carvalho, T. A. Santos, V. M. de Azevedo, P. H. C. Felix, M. V. Dias and S. V. Borges, Bio-nanocomposites for food packaging applications: effect of cellulose nanofibers on morphological, mechanical, optical and barrier properties, Polym. Int., 2018, 67(4), 386–392 CrossRef CAS.
- S. Shankar, X. Teng and J.-W. Rhim, Properties and characterization of agar/CuNP bionanocomposite films prepared with different copper salts and reducing agents, Carbohydr. Polym., 2014, 114, 484–492 CrossRef CAS PubMed.
- M. Salari, M. Sowti Khiabani, R. Rezaei Mokarram, B. Ghanbarzadeh and H. Samadi Kafil, Development and evaluation of chitosan based active nanocomposite films containing bacterial cellulose nanocrystals and silver nanoparticles, Food Hydrocolloids, 2018, 84, 414–423 CrossRef CAS.
- P. Lu, et al., Nanocellulose/Nisin Hydrogel Microparticles as Sustained Antimicrobial Coatings for Paper Packaging, ACS Appl. Polym. Mater., 2022, 4(4), 2664–2673 CrossRef CAS.
- K. D. Grieger, J. Harrington and N. Mortensen, Prioritizing research needs for analytical techniques suited for engineered nanomaterials in food, Trends Food Sci. Technol., 2016, 50, 219–229 CrossRef CAS.
- A. A. Madhavan, R. Qotainy and R. Nair, Synthesis of functionalized silver nanoparticles and its application as chemical sensor, in 2019 Advances in Science and Engineering Technology International Conferences (ASET), IEEE, 2019, pp. 1–4 Search PubMed.
- R. Devi, S. Yadav and C. S. Pundir, Amperometric determination of xanthine in fish meat by zinc oxide nanoparticle/chitosan/multiwalled carbon nanotube/polyaniline composite film bound xanthine oxidase, Analyst, 2012, 137(3), 754–759 RSC.
- B. Tajeddin and M. Arabkhedri, Polymers and food packaging, in Polymer Science and Innovative Applications, Elsevier, 2020, pp. 525–543 Search PubMed.
- J. Han, L. Ruiz-Garcia, J. Qian and X. Yang, Food Packaging: A Comprehensive Review and Future Trends, Compr. Rev. Food Sci. Food Saf., 2018, 17(4), 860–877 CrossRef PubMed.
- K. Marsh and B. Bugusu, Food Packaging—Roles, Materials, and Environmental Issues, J. Food Sci., 2007, 72(3), 39–55 CrossRef PubMed.
- M. Hoseinnejad, S. M. Jafari and I. Katouzian, Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications, Crit. Rev. Microbiol., 2018, 44(2), 161–181 CrossRef CAS PubMed.
- H. Almasi, P. Jafarzadeh and L. Mehryar, Fabrication of novel nanohybrids by impregnation of CuO nanoparticles into bacterial cellulose and chitosan nanofibers: Characterization, antimicrobial and release properties, Carbohydr. Polym., 2018, 186, 273–281 CrossRef CAS PubMed.
- J. O. Adeyemi and O. A. Fawole, Metal-Based Nanoparticles in Food Packaging and Coating Technologies: A Review, Biomolecules, 2023, 13(7), 1092 CrossRef CAS PubMed.
- R. Becerril, C. Nerín and F. Silva, Encapsulation Systems for Antimicrobial Food Packaging Components: An Update, Molecules, 2020, 25(5), 1134 CrossRef CAS PubMed.
- Y. Huang, L. Mei, X. Chen and Q. Wang, Recent Developments in Food Packaging Based on Nanomaterials, Nanomaterials, 2018, 8(10), 830 CrossRef PubMed.
- S. Barage, et al., Nanomaterial in Food Packaging: A Comprehensive Review, J. Nanomater., 2022, 2022, 1–12 CrossRef.
- S. Jafarzadeh, A. Salehabadi and S. M. Jafari, Metal nanoparticles as antimicrobial agents in food packaging, in Handbook of Food Nanotechnology, Elsevier, 2020, pp. 379–414 Search PubMed.
- M. Oliveira and A. V Machado, Preparation of Polymer-Based Nanocomposites by Different Routes, Nova Science Publishers, 2013 Search PubMed.
- S. Lal, U. Jana, P. K. Manna, G. P. Mohanta, R. Manavalan and S. L. Pal, Nanoparticle: An overview of preparation and characterization, J. Appl. Pharm. Sci., 2011,(06), 228–234 Search PubMed.
- P. Wang, Y. Li, C. Zhang, F. Que, J. Weiss and H. Zhang, Characterization and antioxidant activity of trilayer gelatin/dextran-propyl gallate/gelatin films: Electrospinning versus solvent casting, Lebensm. Wiss. Technol., 2020, 128, 109536 CrossRef CAS.
- C. Vasile, Polymeric Nanocomposites and Nanocoatings for Food Packaging: A Review, Materials, 2018, 11(10), 1834 CrossRef PubMed.
- C. Bavatharani, et al., Electrospinning technique for production of polyaniline nanocomposites/nanofibres for multi-functional applications: A review, Synth. Met., 2021, 271, 116609 CrossRef CAS.
- R. A. Caruso and M. Antonietti, Sol−Gel Nanocoating: An Approach to the Preparation of Structured Materials, Chem. Mater., 2001, 13(10), 3272–3282 CrossRef CAS.
- P. Sarkar, R. Choudhary, S. Panigrahi, I. Syed, S. Sivapratha and C. V. Dhumal, Nano-inspired systems in food technology and packaging, Environ. Chem. Lett., 2017, 15(4), 607–622 CrossRef CAS.
- B. Ghanbarzadeh, S. A. Oleyaei and H. Almasi, Nanostructured Materials Utilized in Biopolymer-based Plastics for Food Packaging Applications, Crit. Rev. Food Sci. Nutr., 2015, 55(12), 1699–1723 CrossRef CAS PubMed.
- J.-W. Rhim, H.-M. Park and C.-S. Ha, Bio-nanocomposites for food packaging applications, Prog. Polym. Sci., 2013, 38(10–11), 1629–1652 CrossRef CAS.
- F. Mustafa and S. Andreescu, Nanotechnology-based approaches for food sensing and packaging applications, RSC Adv., 2020, 10(33), 19309–19336 RSC.
- C. Sharma, R. Dhiman, N. Rokana and H. Panwar, Nanotechnology: An Untapped Resource for Food Packaging, Front. Microbiol., 2017, 8, 1735 CrossRef PubMed.
- L. Wang, C. Hu and L. Shao, The antimicrobial activity of nanoparticles: present situation and prospects for the future, Int. J. Nanomed., 2017, 12, 1227–1249 CrossRef CAS PubMed.
- J.-M. Liu, et al., Emerging functional nanomaterials for the detection of food contaminants, Trends Food Sci. Technol., 2018, 71, 94–106 CrossRef CAS.
- G. Fuertes, I. Soto, R. Carrasco, M. Vargas, J. Sabattin and C. Lagos, Intelligent Packaging Systems: Sensors and Nanosensors to Monitor Food Quality and Safety, J. Sens., 2016, 2016, 1–8 Search PubMed.
- M. Carbone, D. T. Donia, G. Sabbatella and R. Antiochia, Silver nanoparticles in polymeric matrices for fresh food packaging, J. King Saud Univ. Sci., 2016, 28(4), 273–279 CrossRef.
- M. Singh and T. Sahareen, Investigation of cellulosic packets impregnated with silver nanoparticles for enhancing shelf-life of vegetables, Lebensm. Wiss. Technol., 2017, 86, 116–122 CrossRef CAS.
- A. Mihaly Cozmuta, et al., Active papers coated with chitosan and containing TiO2 and Ag/TiO2 nanoparticles for increasing the shelf-life of walnut kernels, Cellulose, 2018, 25(9), 5205–5225 CrossRef CAS.
- S. Shankar, D. Khodaei and M. Lacroix, Effect of chitosan/essential oils/silver nanoparticles composite films packaging and gamma irradiation on shelf life of strawberries, Food Hydrocolloids, 2021, 117, 106750 CrossRef CAS.
- E. Kavakebi, A. Anvar, H. Ahari and A. Motalebi, The effect of PVA coated film containing silver nanoparticles synthesized from aqueous Satureja rechingeri extract on shelf life of rainbow trout (Oncorhynchus mykiss) fillet, J. Food BioProcess Eng., 2020, 135–145 Search PubMed.
- T. T. Nguyen, et al., Development of poly (vinyl alcohol)/agar/maltodextrin coating containing silver nanoparticles for banana (Musa acuminate) preservation, Food Packag. Shelf Life, 2021, 29, 100740 CrossRef CAS.
- J. H. Lee, D. Jeong and P. Kanmani, Study on physical and mechanical properties of the biopolymer/silver based active nanocomposite films with antimicrobial activity, Carbohydr. Polym., 2019, 224, 115159 CrossRef CAS PubMed.
- J. Cheng, X. Lin, X. Wu, Q. Liu, S. Wan and Y. Zhang, Preparation of a multifunctional silver nanoparticles polylactic acid food packaging film using mango peel extract, Int. J. Biol. Macromol., 2021, 188, 678–688 CrossRef CAS PubMed.
- S. D. F. Mihindukulasuriya and L.-T. Lim, Nanotechnology development in food packaging: A review, Trends Food Sci. Technol., 2014, 40(2), 149–167 CrossRef CAS.
- E. O. Simbine, L. d C. Rodrigues, J. Lapa-Guimarães, E. S. Kamimura, C. H. Corassin and C. A. F. de Oliveira, Application of silver nanoparticles in food packages: a review, Food Sci. Technol., 2019, 39(4), 793–802 CrossRef.
- Silver Micro-nanoparticles - Properties, Synthesis, Characterization, and Applications, ed. S. Kumar, P. Kumar and C. Shakher Pathak, IntechOpen, 2021 Search PubMed.
- K. Zheng, M. I. Setyawati, D. T. Leong and J. Xie, Antimicrobial silver nanomaterials, Coord. Chem. Rev., 2018, 357, 1–17 CrossRef CAS.
- A. M. Youssef, M. S. Abdel-Aziz and S. M. El-Sayed, Chitosan nanocomposite films based on Ag-NP and Au-NP biosynthesis by Bacillus Subtilis as packaging materials, Int. J. Biol. Macromol., 2014, 69, 185–191 CrossRef CAS PubMed.
- M. K. Morsy, H. H. Khalaf, A. M. Sharoba, H. H. El-Tanahi and C. N. Cutter, Incorporation of Essential Oils and Nanoparticles in Pullulan Films to Control Foodborne Pathogens on Meat and Poultry Products, J. Food Sci., 2014, 79(4) DOI:10.1111/1750-3841.12400.
- M. Ghaffarlou, et al., Green and Facile Synthesis of Pullulan-Stabilized Silver and Gold Nanoparticles for the Inhibition of Quorum Sensing, ACS Appl. Bio Mater., 2022, 5(2), 517–527 CrossRef CAS PubMed.
- S. Mathew, J. Mathew, R. Krishnankutty and S. Babu, Biodegradable and active nanocomposite pouches reinforced with silver nanoparticles for improved packaging of chicken sausages, Food Packag. Shelf Life, 2019, 19, 155–166 CrossRef.
- A. Bahrami, R. Rezaei Mokarram, M. Sowti Khiabani, B. Ghanbarzadeh and R. Salehi, Physico-mechanical and antimicrobial properties of tragacanth/hydroxypropyl methylcellulose/beeswax edible films reinforced with silver nanoparticles, Int. J. Biol. Macromol., 2019, 129, 1103–1112 CrossRef CAS PubMed.
- S. B. Patri, P. S. Adarakatti and P. Malingappa, Silver Nanoparticles-Chitosan Composite Embedded Graphite Screen-Printed Electrodes as a Novel Electrochemical Platform in the Measurement of Trace Level Nitrite: Application to Milk Powder Samples, Curr. Anal. Chem., 2018, 15(1), 56–65 CrossRef.
- A. Sobhan, K. Muthukumarappan, L. Wei, R. Zhou and N. Ghimire, Development of a Biosensor with Electrically Conductive and Biodegradable Composite by Combinatory Use of Silver Nanoparticles, Novel Activated Biochar, and Polylactic Acid, J. Electrochem. Soc., 2021, 168(10), 107501 CrossRef CAS.
- D. Sahu, N. Sarkar, G. Sahoo, P. Mohapatra and S. K. Swain, Nano silver imprinted polyvinyl alcohol nanocomposite thin films for Hg2+ sensor, Sens. Actuators, B, 2017, 246, 96–107 CrossRef CAS.
- A. Orsuwan, S. Shankar, L.-F. Wang, R. Sothornvit and J.-W. Rhim, Preparation of antimicrobial agar/banana powder blend films reinforced with silver nanoparticles, Food Hydrocolloids, 2016, 60, 476–485 CrossRef CAS.
- P. Kanmani and J.-W. Rhim, Physicochemical properties of gelatin/silver nanoparticle antimicrobial composite films, Food Chem., 2014, 148, 162–169 CrossRef CAS PubMed.
- M. Pinaki and G. Samaresh, Green Approach to the Synthesis of Poly(Vinyl Alcohol)-Silver Nanoparticles Hybrid Using Rice Husk Extract and Study of its Antibacterial Activity, Biointerface Res. Appl. Chem., 2020, 10(5), 6474–6480 Search PubMed.
- O. Gherasim, R. A. Puiu, A. C. Bîrcă, A.-C. Burduşel and A. M. Grumezescu, An Updated Review on Silver Nanoparticles in Biomedicine, Nanomaterials, 2020, 10(11), 2318 CrossRef CAS PubMed.
- A. Sharma, A. Sagar, J. Rana and R. Rani, Green synthesis of silver nanoparticles and its antibacterial activity using fungus Talaromyces purpureogenus isolated from Taxus baccata Linn, Micro Nano Syst. Lett., 2022, 10(1), 2 CrossRef.
- B. Chopade, et al., Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents, Int. J. Nanomed., 2012, 483 CrossRef PubMed.
- A. Istiqola and A. Syafiuddin, A review of silver nanoparticles in food packaging technologies: Regulation, methods, properties, migration, and future challenges, J. Chin. Chem. Soc., 2020, 67(11), 1942–1956 CrossRef CAS.
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP), C. Lambré, J. M. Barat Baviera, C. Bolognesi, A. Chesson, P. S. Cocconcelli and G. Rivière, et al., Safety assessment of the substance silver nanoparticles for use in food contact materials, EFSA J., 2021, 19(8), e06790 Search PubMed.
- United States Environmental Protection Agency, Drinking Water Regulations and Contaminants, 2024 Search PubMed.
- B. Panea, G. Ripoll, J. González, Á. Fernández-Cuello and P. Albertí, Effect of nanocomposite packaging containing different proportions of ZnO and Ag on chicken breast meat quality, J. Food Eng., 2014, 123, 104–112 CrossRef CAS.
- F. Bagheri-Abassi, H. Alavi, A. Mohammadipour, F. Motejaded and A. Ebrahimzadeh-Bideskan, The effect of silver nanoparticles on apoptosis and dark neuron production in rat hippocampus, Iran. J. Basic Med. Sci., 2015, 18(7), 644–648 Search PubMed.
- S. S. Ahmad, O. Yousuf, R. U. Islam and K. Younis, Silver nanoparticles as an active packaging ingredient and its toxicity, Packag. Technol. Sci., 2021, 34(11–12), 653–663 CrossRef CAS.
- Y. Echegoyen and C. Nerín, Nanoparticle release from nano-silver antimicrobial food containers, Food Chem. Toxicol., 2013, 62, 16–22 CrossRef CAS PubMed.
- F. Gallocchio, et al., Testing nano-silver food packaging to evaluate silver migration and food spoilage bacteria on chicken meat, Food Addit. Contam.: Part A, 2016, 33(6), 1063–1071 CrossRef CAS PubMed.
- W. Li, L. Li, H. Zhang, M. Yuan and Y. Qin, Evaluation of PLA nanocomposite films on physicochemical and microbiological properties of refrigerated cottage cheese, J. Food Process. Preserv., 2018, 42(1), e13362 CrossRef.
- M. Cushen, J. Kerry, M. Morris, M. Cruz-Romero and E. Cummins, Nanotechnologies in the food industry – Recent developments, risks and regulation, Trends Food Sci. Technol., 2012, 24(1), 30–46 CrossRef CAS.
- H. Sharifan, A. Noori, M. Bagheri and J. M. Moore, Postharvest spraying of zinc oxide nanoparticles enhances shelf life qualities and zinc concentration of tomato fruits, Crop Pasture Sci., 2021, 73(2), 22–31 CrossRef.
- P. J. P. Espitia, C. G. Otoni and N. F. F. Soares, Zinc Oxide Nanoparticles for Food Packaging Applications, in Antimicrobial Food Packaging, Elsevier, 2016, pp. 425–431 Search PubMed.
- M. Azizi-Lalabadi, A. Ehsani, B. Ghanbarzadeh and B. Divband, Polyvinyl alcohol/gelatin nanocomposite containing ZnO, TiO2 or ZnO/TiO2 nanoparticles doped on 4A zeolite: Microbial and sensory qualities of packaged white shrimp during refrigeration, Int. J. Food Microbiol., 2020, 312, 108375 CrossRef CAS PubMed.
- L. Yang, et al., Preparation and characterization of PVA/arginine chitosan/ZnO NPs composite films, Int. J. Biol. Macromol., 2023, 226, 184–193 CrossRef CAS PubMed.
- A. Jayakumar, et al., Starch-PVA composite films with zinc-oxide nanoparticles and phytochemicals as intelligent pH sensing wraps for food packaging application, Int. J. Biol. Macromol., 2019, 136, 395–403 CrossRef CAS PubMed.
- M. P. Indumathi, K. Saral Sarojini and G. R. Rajarajeswari, Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits, Int. J. Biol. Macromol., 2019, 132, 1112–1120 CrossRef CAS PubMed.
- S. Roy, R. Priyadarshi and J.-W. Rhim, Development of Multifunctional Pullulan/Chitosan-Based Composite Films Reinforced with ZnO Nanoparticles and Propolis for Meat Packaging Applications, Foods, 2021, 10(11), 2789 CrossRef CAS PubMed.
- R. Priyadarshi and Y. S. Negi, Effect of Varying Filler Concentration on Zinc Oxide Nanoparticle Embedded Chitosan Films as Potential Food Packaging Material, J. Polym. Environ., 2017, 25(4), 1087–1098 CrossRef CAS.
- W. Li, L. Li, Y. Cao, T. Lan, H. Chen and Y. Qin, Effects of PLA Film Incorporated with ZnO Nanoparticle on the Quality Attributes of Fresh-Cut Apple, Nanomaterials, 2017, 7(8), 207 CrossRef PubMed.
- A. Marra, C. Silvestre, D. Duraccio and S. Cimmino, Polylactic acid/zinc oxide biocomposite films for food packaging application, Int. J. Biol. Macromol., 2016, 88, 254–262 CrossRef CAS PubMed.
- S. Shankar, L.-F. Wang and J.-W. Rhim, Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films, Mater. Sci. Eng. C, 2018, 93, 289–298 CrossRef CAS PubMed.
- V. Hooda and Archita, Enzymes loaded chitosan/coconut fibre/zinc oxide nanoparticles strip for polyamine determination, Food Chem., 2018, 239, 1100–1109 CrossRef CAS PubMed.
- A. M. Hezma, A. Rajeh and M. A. Mannaa, An insight into the effect of zinc oxide nanoparticles on the structural, thermal, mechanical properties and antimicrobial activity of Cs/PVA composite, Colloids Surf., A, 2019, 581, 123821 CrossRef CAS.
- A. Tymczewska, B. U. Furtado, J. Nowaczyk, K. Hrynkiewicz and A. Szydłowska-Czerniak, Functional Properties of Gelatin/Polyvinyl Alcohol Films Containing Black Cumin Cake Extract and Zinc Oxide Nanoparticles Produced via Casting Technique, Int. J. Mol. Sci., 2022, 23(5), 2734 CrossRef CAS PubMed.
- Y. Amaregouda, K. Kamanna and A. Kamath, Multifunctional Bionanocomposite Films Based on Chitosan/Polyvinyl Alcohol with ZnO NPs and Carissa carandas Extract Anthocyanin for Smart Packaging Materials, ACS Food Sci. Technol., 2023, 3(9), 1411–1422 CrossRef CAS.
- S. K. Bajpai, N. Chand and V. Chaurasia, Investigation of water vapor permeability and antimicrobial property of zinc oxide nanoparticles-loaded chitosan-based edible film, J. Appl. Polym. Sci., 2010, 115(2), 674–683 CrossRef CAS.
- B. Lallo da Silva, et al., Relationship Between Structure And Antimicrobial Activity Of Zinc Oxide Nanoparticles: An Overview, Int. J. Nanomed., 2019, 14, 9395–9410 CrossRef PubMed.
- A. Król, P. Pomastowski, K. Rafińska, V. Railean-Plugaru and B. Buszewski, Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism, Adv. Colloid Interface Sci., 2017, 249, 37–52 CrossRef PubMed.
- C. Silvestre, D. Duraccio and S. Cimmino, Food packaging based on polymer nanomaterials, Prog. Polym. Sci., 2011, 36(12), 1766–1782 CrossRef CAS.
- A. Emamifar, M. Kadivar, M. Shahedi and S. Soleimanian-Zad, Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice, Innovat. Food Sci. Emerg. Technol., 2010, 11(4), 742–748 CrossRef CAS.
- W. Zhang and J.-W. Rhim, Titanium dioxide (TiO2) for the manufacture of multifunctional active food packaging films, Food Packag. Shelf Life, 2022, 31, 100806 CrossRef CAS.
- P. N. Navya, A. Kaphle, S. P. Srinivas, S. K. Bhargava, V. M. Rotello and H. K. Daima, Current trends and challenges in cancer management and therapy using designer nanomaterials, Nano Convergence, 2019, 6(1), 23 CrossRef CAS PubMed.
- A. Maldonado, et al., Study of Ethylene-Removing Materials Based on Eco-Friendly Composites with Nano-TiO2, Polymers, 2023, 15(16), 3369 CrossRef CAS PubMed.
- M. Alizadeh Sani, et al., Titanium dioxide nanoparticles as multifunctional surface-active materials for smart/active nanocomposite packaging films, Adv. Colloid Interface Sci., 2022, 300, 102593 CrossRef CAS PubMed.
- M. Alizadeh-Sani, E. Mohammadian and D. J. McClements, Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat, Food Chem., 2020, 322, 126782 CrossRef CAS PubMed.
- U. Siripatrawan and P. Kaewklin, Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging, Food Hydrocolloids, 2018, 84, 125–134 CrossRef CAS.
- F. Tian, et al., Preservation of Ginkgo biloba seeds by coating with chitosan/nano-TiO2 and chitosan/nano-SiO2 films, Int. J. Biol. Macromol., 2019, 126, 917–925 CrossRef CAS PubMed.
- X. Zhang, Z. Li, R. Ji, K. Li and W. Zhang, Preparation and Characterization of Pullulan/Carboxymethyl Cellulose/Nano-TiO2 Composite Films for Strawberry Preservation, Food Biophys., 2021, 16(4), 460–473 CrossRef.
- Z.-P. Tang, C.-W. Chen and J. Xie, Development of antimicrobial active films based on poly(vinyl alcohol) containing nano-TiO 2 and its application in macrobrachium rosenbergii packaging, J. Food Process. Preserv., 2018, 42(8), e13702 CrossRef.
- R. Chougale, D. Kasai, S. Nayak, S. Masti, A. Nasalapure and A. V Raghu, Design of eco-friendly PVA/TiO 2 -based nanocomposites and their antifungal activity study, Green Mater., 2020, 8(1), 40–48 CrossRef.
- J. Alberton, S. M. Martelli, F. M. Fakhouri and V. Soldi, Mechanical and moisture barrier properties of titanium dioxide nanoparticles and halloysite nanotubes reinforced polylactic acid (PLA), IOP Conf. Ser. Mater. Sci. Eng., 2014, 64, 012010 CrossRef CAS.
- L. Qu, et al., Improved mechanical and antimicrobial properties of zein/chitosan films by adding highly dispersed nano-TiO2, Ind. Crops Prod., 2019, 130, 450–458 Search PubMed.
- W. Li, K. Zheng, H. Chen, S. Feng, W. Wang and C. Qin, Influence of Nano Titanium Dioxide and Clove Oil on Chitosan–Starch Film Characteristics, Polymers, 2019, 11(9), 1418 CrossRef CAS PubMed.
- L. Zhang, W. Xia, X. Liu and W. Zhang, Synthesis of titanium cross-linked chitosan composite for efficient adsorption and detoxification of hexavalent chromium from water, J. Mater. Chem. A, 2015, 3(1), 331–340 Search PubMed.
- V. Katiyar and T. Ghosh, Nanotechnology in Edible Food Packaging, Springer Singapore, Singapore, 2021 Search PubMed.
- X. Zhang, Y. Liu, H. Yong, Y. Qin, J. Liu and J. Liu, Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black plum peel extract, Food Hydrocolloids, 2019, 94, 80–92 Search PubMed.
- B. Li, et al., Synthesis, characterization, and antibacterial activity of chitosan/TiO 2 nanocomposite against Xanthomonas oryzae pv. oryzae, Carbohydr. Polym., 2016, 152, 825–831 CrossRef CAS PubMed.
- B. Lin, Y. Luo, Z. Teng, B. Zhang, B. Zhou and Q. Wang, Development of silver/titanium dioxide/chitosan adipate nanocomposite as an antibacterial coating for fruit storage, LWT–Food Sci. Technol., 2015, 63(2), 1206–1213 CrossRef CAS.
- S. Feng, F. Zhang, S. Ahmed and Y. Liu, Physico-Mechanical and Antibacterial Properties of PLA/TiO2 Composite Materials Synthesized via Electrospinning and Solution Casting Processes, Coatings, 2019, 9(8), 525 CrossRef CAS.
- A. Tajdari, A. Babaei, A. Goudarzi and R. Partovi, Preparation and study on the optical, mechanical, and antibacterial properties of polylactic acid/ZnO/TiO 2 shared nanocomposites, J. Plast. Film Sheeting, 2020, 36(3), 285–311 CrossRef CAS.
- S. Ramesh, H. S. Kim and J.-H. Kim, Cellulose–Polyvinyl Alcohol–Nano-TiO 2 Hybrid Nanocomposite: Thermal, Optical, and Antimicrobial Properties against Pathogenic Bacteria, Polym. Plast. Technol. Eng., 2018, 57(7), 669–681 CrossRef CAS.
- J. Luo, G. Xia, L. Liu, A. Ji and Q. Luo, Fabrication of Chitosan/Hydroxyethyl Cellulose/TiO2 Incorporated Mulberry Anthocyanin 3D-Printed Bilayer Films for Quality of Litchis, Foods, 2022, 11(20), 3286 CrossRef CAS PubMed.
- K. Jiang, et al., Smart Indicator Film Based on Sodium Alginate/Polyvinyl Alcohol/TiO2 Containing Purple Garlic Peel Extract for Visual Monitoring of Beef Freshness, Polymers, 2023, 15(21), 4308 CrossRef CAS PubMed.
- S. Pirsa and S. Asadi, Innovative smart and biodegradable packaging for margarine based on a nano composite polylactic acid/lycopene film, Food Addit. Contam.: Part A, 2021, 38(5), 856–869 Search PubMed.
- P. A. Christensen, T. P. Curtis, T. A. Egerton, S. A. M. Kosa and J. R. Tinlin, Photoelectrocatalytic and photocatalytic disinfection of E. coli suspensions by titanium dioxide, Appl. Catal., B, 2003, 41(4), 371–386 Search PubMed.
- Z. Chen, S. Han, S. Zhou, H. Feng, Y. Liu and G. Jia, Review of health safety aspects of titanium dioxide nanoparticles in food application, NanoImpact, 2020, 18, 100224 Search PubMed.
- Q.-B. Lin, H. Li, H.-N. Zhong, Q. Zhao, D.-H. Xiao and Z.-W. Wang, Migration of Ti from nano-TiO 2 -polyethylene composite packaging into food simulants, Food Addit. Contam.: Part A, 2014, 1–7 Search PubMed.
- Z. Lian, Y. Zhang and Y. Zhao, Nano-TiO 2 particles and high hydrostatic pressure treatment for improving functionality of polyvinyl alcohol and chitosan composite films and nano-TiO 2 migration from film matrix in food simulants, Innovat. Food Sci. Emerg. Technol., 2016, 33, 145–153 Search PubMed.
- W. Li, L. Li, H. Zhang, M. Yuan and Y. Qin, Evaluation of PLA nanocomposite films on physicochemical and microbiological properties of refrigerated cottage cheese, J. Food Process. Preserv., 2018, 42(1), e13362 Search PubMed.
- W. Zhang, S. Roy and J. Rhim, Copper-based nanoparticles for biopolymer-based functional films in food packaging applications, Compr. Rev. Food Sci. Food Saf., 2023, 22(3), 1933–1952 Search PubMed.
- A. Kalia, et al., Nettle-Leaf Extract Derived ZnO/CuO Nanoparticle-Biopolymer-Based Antioxidant and Antimicrobial Nanocomposite Packaging Films and Their Impact on Extending the Post-Harvest Shelf Life of Guava Fruit, Biomolecules, 2021, 11(2), 224 Search PubMed.
- A. Nouri, M. T. Yaraki, M. Ghorbanpour, S. Agarwal and V. K. Gupta, Enhanced Antibacterial effect of chitosan film using Montmorillonite/CuO nanocomposite, Int. J. Biol. Macromol., 2018, 109, 1219–1231 Search PubMed.
- A. M. Youssef, F. M. Assem, H. S. El-Sayed, S. M. El-Sayed, M. Elaaser and M. H. Abd El-Salam, Synthesis and evaluation of eco-friendly carboxymethyl cellulose/polyvinyl alcohol/CuO bionanocomposites and their use in coating processed cheese, RSC Adv., 2020, 10(62), 37857–37870 Search PubMed.
- D. V. Francis, S. Thaliyakattil, L. Cherian, N. Sood and T. Gokhale, Metallic Nanoparticle Integrated Ternary Polymer Blend of PVA/Starch/Glycerol: A Promising Antimicrobial Food Packaging Material, Polymers, 2022, 14(7), 1379 CrossRef CAS PubMed.
- P. Abdolsattari, M. Rezazadeh-Bari and S. Pirsa, Smart Film Based on Polylactic Acid, Modified with Polyaniline/ZnO/CuO: Investigation of Physicochemical Properties and Its Use of Intelligent Packaging of Orange Juice, Food Bioproc. Tech., 2022, 15(12), 2803–2825 Search PubMed.
- H. Tang, et al., Effects and Mechanism of Nano-Copper Exposure on Hepatic Cytochrome P450 Enzymes in Rats, Int. J. Mol. Sci., 2018, 19(7), 2140 CrossRef PubMed.
- T. Revathi and S. Thambidurai, Cytotoxic, antioxidant and antibacterial activities of copper oxide incorporated chitosan-neem seed biocomposites, Int. J. Biol. Macromol., 2019, 139, 867–878 CrossRef CAS PubMed.
- M. Fathi, M. Samadi, S. Abbaszadeh and M. R. Nourani, Fabrication and characterization of multifunctional bio-safe films based on Carboxymethyl Chitosan and Saffron Petal Anthocyanin Reinforced with Copper Oxide Nanoparticles for sensing the meat freshness, J. Polym. Environ., 2022, 30(11), 4538–4549 Search PubMed.
- Gh. D. Khalili, S. Alipour, M. R. Akbarpour and S. Moniri Javadhesari, Fabrication, Characterization and Investigating Properties of PVA Based Nanocomposite Films Reinforced with Mechano-chemically Synthesized CuO Nanoparticles, Met. Mater. Int., 2023, 29(8), 2374–2384 CrossRef CAS.
- Y. Haldorai and J.-J. Shim, Novel chitosan-TiO 2 nanohybrid: Preparation, characterization, antibacterial, and photocatalytic properties, Polym. Compos., 2014, 35(2), 327–333 CrossRef CAS.
- A. Errokh, et al., Controlled growth of Cu 2 O nanoparticles bound to cotton fibres, Carbohydr. Polym., 2016, 141, 229–237 Search PubMed.
- C. Gunawan, W. Y. Teoh, C. P. Marquis and R. Amal, Cytotoxic Origin of Copper(II) Oxide Nanoparticles: Comparative Studies with Micron-Sized Particles, Leachate, and Metal Salts, ACS Nano, 2011, 5(9), 7214–7225 CrossRef CAS PubMed.
- P. Asgari, O. Moradi and B. Tajeddin, The effect of nanocomposite packaging carbon nanotube base on organoleptic and fungal growth of Mazafati brand dates, Int. Nano Lett., 2014, 4(1), 98 CrossRef.
- H. Hosseini and S. M. Jafari, Introducing nano/microencapsulated bioactive ingredients for extending the shelf-life of food products, Adv. Colloid Interface Sci., 2020, 282, 102210 Search PubMed.
- Y.-H. Wen, et al., Antibacterial nanocomposite films of poly(vinyl alcohol) modified with zinc oxide-doped multiwalled carbon nanotubes as food packaging, Polym. Bull., 2022, 79(6), 3847–3866 CrossRef CAS.
- G. Khachatryan, K. Khachatryan, J. Szczepankowska, M. Krzan and M. Krystyjan, Design of Carbon Nanocomposites Based on Sodium Alginate/Chitosan Reinforced with Graphene Oxide and Carbon Nanotubes, Polymers, 2023, 15(4), 925, DOI:10.3390/polym15040925.
- J. Luo, W. Sun, H. Zhou, Y. Zhang, B. Wen and C. Xin, Bioderived and Biodegradable Poly(3-hydroxybutyrate- co -3-hydroxyvalerate) Nanocomposites Based on Carbon Nanotubes: Microstructure Observation and EMI Shielding Property Improvement, ACS Sustain. Chem. Eng., 2021, 9(32), 10785–10798 CrossRef CAS.
- F. Z. Yakdoumi, A. S. Hadj-Hamou, N. Rahoui, M. M. Rahman and V. Abetz, Polylactic acid nanocomposites containing functionalized multiwalled carbon nanotubes as antimicrobial packaging materials, Int. J. Biol. Macromol., 2022, 213, 55–69 CrossRef CAS PubMed.
- P. G. Seligra, M. Lamanna and L. Famá, PLA-fMWCNT Bionanofilms with High Modulus and Great Properties to Apply in Packaging and Biomedicine, Procedia Food Sci., 2015, 8, 383–390 CAS.
- R. Cui, et al., Antimicrobial film based on polylactic acid and carbon nanotube for controlled cinnamaldehyde release, J. Mater. Res. Technol., 2020, 9(5), 10130–10138 CrossRef CAS.
- L. Gan, et al., Antibacterial nanocomposite based on carbon nanotubes–silver nanoparticles-co-doped polylactic acid, Polym. Bull., 2020, 77(2), 793–804 CrossRef CAS.
- D. G. Goodwin, et al., Interactions of Microorganisms with Polymer Nanocomposite Surfaces Containing Oxidized Carbon Nanotubes, Environ. Sci. Technol., 2015, 49(9), 5484–5492 CrossRef CAS PubMed.
- E.-S. Lee, et al., Antimicrobial properties of lignin-decorated thin multi-walled carbon nanotubes in poly(vinyl alcohol) nanocomposites, Eur. Polym. J., 2018, 105, 79–84 Search PubMed.
- S. Hui and N. C. Das, Surface Modified Carbon Nanotubes in Food Packaging, 2022, pp. 199–233 Search PubMed.
- B. Rezaei, H. R. Jamei and A. A. Ensafi, An ultrasensitive and selective electrochemical aptasensor based on rGO-MWCNTs/Chitosan/carbon quantum dot for the detection of lysozyme, Biosens. Bioelectron., 2018, 115, 37–44 CrossRef CAS PubMed.
- A. A. P. Khan, A. Khan, M. M. Rahman, A. M. Asiri and M. Oves, Lead sensors development and antimicrobial activities based on graphene oxide/carbon nanotube/poly(O-toluidine) nanocomposite, Int. J. Biol. Macromol., 2016, 89, 198–205 CrossRef CAS PubMed.
- Yu. G. Maksimova, Microorganisms and Carbon Nanotubes: Interaction and Applications (Review), Appl. Biochem. Microbiol., 2019, 55(1), 1–12 CrossRef CAS.
- Composites Materials for Food Packaging, ed. G. Cirillo, M. A. Kozlowski and U. G. Spizzirri, Wiley, 2018 Search PubMed.
- V. Srivastava, D. Gusain and Y. C. Sharma, Critical Review on the Toxicity of Some Widely Used Engineered Nanoparticles, Ind. Eng. Chem. Res., 2015, 54(24), 6209–6233 Search PubMed.
- J. Bott, A. Störmer and R. Franz, Migration of nanoparticles from plastic packaging materials containing carbon black into foodstuffs, Food Addit. Contam.: Part A, 2014, 31(10), 1769–1782 CrossRef CAS PubMed.
- F. Han Lyn, C. P. Tan, R. M. Zawawi and Z. A. Nur Hanani, Physicochemical properties of chitosan/graphene oxide composite films and their effects on storage stability of palm-oil based margarine, Food Hydrocolloids, 2021, 117, 106707 Search PubMed.
- Z. Gou, D. Xu, Q. Dong and X. Wu, Comparison studies on covalently and non-covalently modified MWNTs using chitosan and their starch nanocomposites, Starch/Staerke, 2016, 68(3–4), 220–229 Search PubMed.
- T. Nguyen, et al., Impact of UV irradiation on multiwall carbon nanotubes in nanocomposites: Formation of entangled surface layer and mechanisms of release resistance, Carbon, 2017, 116, 191–200 CrossRef CAS PubMed.
- W. Xu, et al., The graphene oxide and chitosan biopolymer loads TiO2 for antibacterial and preservative research, Food Chem., 2017, 221, 267–277 CrossRef CAS PubMed.
- J. C. Vilvert, et al., Chitosan and graphene oxide-based biodegradable bags: An eco-friendly and effective packaging alternative to maintain postharvest quality of ‘Palmer’ mango, Lebensm. Wiss. Technol., 2022, 154, 112741 CrossRef CAS.
- V. Loryuenyong, C. Saewong, C. Aranchaiya and A. Buasri, The Improvement in Mechanical and Barrier Properties of Poly(Vinyl Alcohol)/Graphene Oxide Packaging Films, Packag. Technol. Sci., 2015, 28(11), 939–947 CrossRef CAS.
- S. Pourmoslemi, S. Shokouhi and R. Mahjub, Investigation of antibacterial activity of polyvinyl alcohol packaging films composed of silver oxide nanoparticles, graphene oxide and tragacanth gum using Box–Behnken design, Packag. Technol. Sci., 2021, 34(10), 613–622 CrossRef CAS.
- Y. A. Arfat, J. Ahmed, M. Ejaz and M. Mullah, Polylactide/graphene oxide nanosheets/clove essential oil composite films for potential food packaging applications, Int. J. Biol. Macromol., 2018, 107, 194–203 CrossRef CAS PubMed.
- Y. Kim, J. S. Kim, S.-Y. Lee, R. L. Mahajan and Y.-T. Kim, Exploration of hybrid nanocarbon composite with polylactic acid for packaging applications, Int. J. Biol. Macromol., 2020, 144, 135–142 CrossRef CAS PubMed.
- W. Chen, H. Weimin, D. Li, S. Chen and Z. Dai, A critical review on the development and performance of polymer/graphene nanocomposites, Sci. Eng. Compos. Mater., 2018, 25(6), 1059–1073 CrossRef CAS.
- M. Azizi-Lalabadi, H. Hashemi, J. Feng and S. M. Jafari, Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites, Adv. Colloid Interface Sci., 2020, 284, 102250 CrossRef CAS PubMed.
- A. Ray Chowdhuri, S. Tripathy, S. Chandra, S. Roy and S. K. Sahu, A ZnO decorated chitosan–graphene oxide nanocomposite shows significantly enhanced antimicrobial activity with ROS generation, RSC Adv., 2015, 5(61), 49420–49428 RSC.
- H. Khawaja, E. Zahir, M. A. Asghar and M. A. Asghar, Graphene oxide, chitosan and silver nanocomposite as a highly effective antibacterial agent against pathogenic strains, Colloids Surf., A, 2018, 555, 246–255 CrossRef CAS.
- H. Moustafa, M. H. Hemida, M. A. Shemis, A. Dufresne and M. Morsy, Functionalized GO nanoplatelets with folic acid as a novel material for boosting humidity sensing of chitosan/PVA nanocomposites for active food packaging, Surface. Interfac., 2023, 41, 103229 CrossRef CAS.
- W. C. Liew, I. I. Muhamad, J. W. Chew and K. J. A. Karim, Synergistic effect of graphene oxide/zinc oxide nanocomposites on polylactic acid-based active packaging film: Properties, release kinetics and antimicrobial efficiency, Int. J. Biol. Macromol., 2023, 253, 127288 CrossRef CAS PubMed.
- Y. Huang, et al., Poly(lactic acid)/graphene oxide-ZnO nanocomposite films with good mechanical, dynamic mechanical, anti-UV and antibacterial properties, J. Chem. Technol. Biotechnol., 2015, 90(9), 1677–1684 CrossRef CAS.
- M. Dhanasekar, et al., Ambient light antimicrobial activity of reduced graphene oxide supported metal doped TiO2 nanoparticles and their PVA based polymer nanocomposite films, Mater. Res. Bull., 2018, 97, 238–243 CrossRef CAS.
- S. Gautam, S. Sharma, B. Sharma and P. Jain, Antibacterial efficacy of poly (vinyl alcohol) nanocomposites reinforced with graphene oxide and silver nanoparticles for packaging applications, Polym. Compos., 2021, 42(6), 2829–2837 CrossRef CAS.
- A. Usman, Z. Hussain, A. Riaz and A. N. Khan, Enhanced mechanical, thermal and antimicrobial properties of poly(vinyl alcohol)/graphene oxide/starch/silver nanocomposites films, Carbohydr. Polym., 2016, 153, 592–599 CrossRef CAS PubMed.
- T. Siripongpreda, K. Siralertmukul and N. Rodthongkum, Colorimetric sensor and LDI-MS detection of biogenic amines in food spoilage based on porous PLA and graphene oxide, Food Chem., 2020, 329, 127165 CrossRef CAS PubMed.
- K. Yang, H. Gong, X. Shi, J. Wan, Y. Zhang and Z. Liu, In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration, Biomaterials, 2013, 34(11), 2787–2795 CrossRef CAS PubMed.
- J.-H. Liu, S.-T. Yang, H. Wang, Y. Chang, A. Cao and Y. Liu, Effect of Size and Dose on The Biodistribution of Graphene Oxide in Mice, Nanomedicine, 2012, 7(12), 1801–1812 CrossRef CAS PubMed.
- T. H. D. Nguyen, M. Lin and A. Mustapha, Toxicity of Graphene Oxide on Intestinal Bacteria and Caco-2 Cells, J. Food Prot., 2015, 78(5), 996–1002 CrossRef CAS PubMed.
- N. A. Manikandan, K. Pakshirajan and G. Pugazhenthi, Preparation and characterization of environmentally safe and highly biodegradable microbial polyhydroxybutyrate (PHB) based graphene nanocomposites for potential food packaging applications, Int. J. Biol. Macromol., 2020, 154, 866–877 CrossRef CAS PubMed.
- Y. Wang, L. Wang, L. Jin, J. Wang and D. Jin, Evolutionary Analysis of Cellulose Gene Family in Grasses, 2011, pp. 178–183 Search PubMed.
- J. I. Morán, V. A. Alvarez, V. P. Cyras and A. Vázquez, Extraction of cellulose and preparation of nanocellulose from sisal fibers, Cellulose, 2008, 15(1), 149–159 CrossRef.
- N. Lin and A. Dufresne, Nanocellulose in biomedicine: Current status and future prospect, Eur. Polym. J., 2014, 59, 302–325 CrossRef CAS.
- A. Anžlovar, M. Kunaver, A. Krajnc and E. Žagar, Nanocomposites of LLDPE and Surface-Modified Cellulose Nanocrystals Prepared by Melt Processing, Molecules, 2018, 23(7), 1782 CrossRef PubMed.
- A. B. Perumal, P. S. Sellamuthu, R. B. Nambiar and E. R. Sadiku, Development of polyvinyl alcohol/chitosan bio-nanocomposite films reinforced with cellulose nanocrystals isolated from rice straw, Appl. Surf. Sci., 2018, 449, 591–602 CrossRef CAS.
- M. El Achaby, et al., Processing and properties of eco-friendly bio-nanocomposite films filled with cellulose nanocrystals from sugarcane bagasse, Int. J. Biol. Macromol., 2017, 96, 340–352 CrossRef CAS PubMed.
- M. Yadav and F.-C. Chiu, Cellulose nanocrystals reinforced κ-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications, Carbohydr. Polym., 2019, 211, 181–194 CrossRef CAS PubMed.
- A. Khan, et al., Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films, Carbohydr. Polym., 2012, 90(4), 1601–1608 CrossRef CAS PubMed.
- J. P. de Mesquita, C. L. Donnici, I. F. Teixeira and F. V. Pereira, Bio-based nanocomposites obtained through covalent linkage between chitosan and cellulose nanocrystals, Carbohydr. Polym., 2012, 90(1), 210–217 CrossRef CAS PubMed.
- R. Mu, et al., Recent trends and applications of cellulose nanocrystals in food industry, Trends Food Sci. Technol., 2019, 93, 136–144 CrossRef CAS.
- L. Meng, et al., Improved mechanical and antibacterial properties of polyvinyl alcohol composite films using quaternized cellulose nanocrystals as nanofillers, Compos. Sci. Technol., 2023, 232, 109885 CrossRef CAS.
- S. Huang, S. Zou and Y. Wang, Construction of compostable packaging with antibacterial property and improved performance using sprayed coatings of modified cellulose nanocrystals, Carbohydr. Polym., 2023, 305, 120539 CrossRef CAS PubMed.
- L. S. F. Leite, et al., Eco-friendly gelatin films with rosin-grafted cellulose nanocrystals for antimicrobial packaging, Int. J. Biol. Macromol., 2020, 165, 2974–2983 CrossRef CAS PubMed.
- S. M. Costa, D. P. Ferreira, P. Teixeira, L. F. Ballesteros, J. A. Teixeira and R. Fangueiro, Active natural-based films for food packaging applications: The combined effect of chitosan and nanocellulose, Int. J. Biol. Macromol., 2021, 177, 241–251 CrossRef CAS PubMed.
- C. A. Díaz-Cruz, C. Caicedo, E. J. Jiménez-Regalado, R. Díaz de León, R. López-González and R. Y. Aguirre-Loredo, Evaluation of the Antimicrobial, Thermal, Mechanical, and Barrier Properties of Corn Starch–Chitosan Biodegradable Films Reinforced with Cellulose Nanocrystals, Polymers, 2022, 14(11), 2166 CrossRef PubMed.
- R. Koshani and A. Madadlou, A viewpoint on the gastrointestinal fate of cellulose nanocrystals, Trends Food Sci. Technol., 2018, 71, 268–273 CrossRef CAS.
- H. Ni, et al., Cellulose nanowhiskers: Preparation, characterization and cytotoxicity evaluation, Bio-Med. Mater. Eng., 2012, 22(1–3), 121–127 Search PubMed.
- S. Dong, A. A. Hirani, K. R. Colacino, Y. W. Lee and M. Roman, Cytotoxicity and cellular uptake of cellulose nanocrystals, Nano LIFE, 2012, 02(03), 1241006 CrossRef.
- EFSA Scientific Committee, A. Hardy, D. B. T. Halldorsson, M. J. Jeger, H. K. Knutsen, S. More, H. Naegeli, H. Noteborn, C. Ockleford, A. Ricci, G. Rychen, J. R. Schlatter, V. Silano, R. Solecki, D. Turck, M. Younes, Q. Chaudhry, F. Cubadda, D. Gott, A. Oomen, S. Weigel, M. Karamitrou, R. Schoonjans and A. Mortensen, Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health, EFSA J., 2018, 16(7), e05327 Search PubMed.
- H. E. Rosa, G. N. T. Gabriela, Z. H. Elvis and A. B. Patricia, Solid Lipid Nanoparticles (SLN), Nanocomposite Materials for Biomedical and Energy Storage Applications, IntechOpen, 2022, DOI:10.5772/intechopen.102536.
- C. I. García-Betanzos, H. Hernández-Sánchez, D. Quintanar-Guerrero, A. Del Real L and M. de la Luz Zambrano-Zaragoza, The Evaluation of Mechanical, Thermal, Optical and Microstructural Properties of Edible Films with Solid Lipid Nanoparticles-Xanthan Gum Stored at Different Temperatures and Relative Humidities, Food Bioproc. Tech., 2016, 9(10), 1756–1768 CrossRef.
- M. L. Zambrano-Zaragoza, E. Mercado-Silva, P. Ramirez-Zamorano, M. A. Cornejo-Villegas, E. Gutiérrez-Cortez and D. Quintanar-Guerrero, Use of solid lipid nanoparticles (SLNs) in edible coatings to increase guava (Psidium guajava L.) shelf-life, Food Res. Int., 2013, 51(2), 946–953 CrossRef CAS.
- V. Miranda-Linares, P. Escamilla-Rendón, A. Del Real-López, R. M. González-Reza and M. L. Zambrano-Zaragoza, Solid lipid nanoparticles based edible coating for saladette tomato preservation, Acta Hortic., 2018, 1194, 305–312 CrossRef.
- V. Wiedenmann, K. Oehlke, U. van der Schaaf, H. M. Koivula, K. S. Mikkonen and H. P. Karbstein, Emulsifier Composition of Solid Lipid Nanoparticles (SLN) Affects Mechanical and Barrier Properties of SLN-Protein Composite Films, J. Food Sci., 2019, 84(12), 3642–3652 CrossRef CAS PubMed.
- M. L. Zambrano-Zaragoza, D. Quintanar-Guerrero, A. Del Real, R. M. González-Reza, M. A. Cornejo-Villegas and E. Gutiérrez-Cortez, Effect of Nano-Edible Coating Based on Beeswax Solid Lipid Nanoparticles on Strawberry's Preservation, Coatings, 2020, 10(3), 253 CrossRef CAS.
- H. Cui, L. Yuan, W. Li and L. Lin, Edible film incorporated with chitosan and Artemisia annua oil nanoliposomes for inactivation of Escherichia coli O157:H7 on cherry tomato, Int. J. Food Sci. Technol., 2017, 52(3), 687–698 CrossRef CAS.
- C. Wu, L. Wang, Y. Hu, S. Chen, D. Liu and X. Ye, Edible coating from citrus essential oil-loaded nanoemulsions: physicochemical characterization and preservation performance, RSC Adv., 2016, 6(25), 20892–20900 RSC.
- L. Salvia-Trujillo, M. A. Rojas-Graü, R. Soliva-Fortuny and O. Martín-Belloso, Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut Fuji apples, Postharvest Biol. Technol., 2015, 105, 8–16 CrossRef CAS.
- I. Sánchez-Ortega, B. E. García-Almendárez, E. M. Santos-López, L. R. Reyes-González and C. Regalado, Characterization and antimicrobial effect of starch-based edible coating suspensions, Food Hydrocolloids, 2016, 52, 906–913 CrossRef.
- D. J. McClements, Edible lipid nanoparticles: Digestion, absorption, and potential toxicity, Prog. Lipid Res., 2013, 52(4), 409–423 CrossRef CAS PubMed.
- Q. Chaudhry, et al., Applications and implications of nanotechnologies for the food sector, Food Addit. Contam.: Part A, 2008, 25(3), 241–258 CrossRef CAS PubMed.
- J. Jiang, G. Oberdörster and P. Biswas, Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies, J. Nanopart. Res., 2009, 11(1), 77–89 CrossRef CAS.
- Ö. Tarhan, Safety and regulatory issues of nanomaterials in foods, in Handbook of Food Nanotechnology, Elsevier, 2020, pp. 655–703 Search PubMed.
- S. A. O. Adeyeye and T. J. Ashaolu, Applications of nano-materials in food packaging: A review, J. Food Process. Eng., 2021, 44(7), 22 Search PubMed.
- J. M. Lourtioz, M. Lahmani, C. Dupas Haeberlin and P. Hesto, Nanosciences and Nanotechnology Evolution or Revolution, 2021 Search PubMed.
- J. C. Hannon, J. P. Kerry, M. Cruz-Romero, S. Azlin-Hasim, M. Morris and E. Cummins, Assessment of the migration potential of nanosilver from nanoparticle-coated low-density polyethylene food packaging into food simulants, Food Addit. Contam.: Part A, 2015, 1–12 Search PubMed.
- G. Singhal, R. Bhavesh, K. Kasariya, A. R. Sharma and R. P. Singh, Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity, J. Nanopart. Res., 2011, 13(7), 2981–2988 CrossRef CAS.
- S. Das, L. Jagan, R. Isiah, B. Rajesh, S. Backianathan and J. Subhashini, Nanotechnology in oncology: Characterization and in vitro release kinetics of cisplatin-loaded albumin nanoparticles: Implications in anticancer drug delivery, Indian J. Pharmacol., 2011, 43(4), 409 CrossRef CAS PubMed.
- U. FDA, Inventory of Effective Food Contact Substance (FCS) Notifications, 2018 Search PubMed.
- Part 676-unified and combined state plans under title i of the workforce innovation and opportunity act contents §676, [online]. available: https://www.ecfr.gov/cgi-bin/text-idx?SID=325e94d7fdb1ce2b1f87c574bccc8ab9%26mc=true%26node=pt20.4.676%26rgn=div5.
- FDA Food Additive Status, “Food additive status list”, 2006.
- E. Jagtiani, Advancements in nanotechnology for food science and industry, Food Front., 2022, 3(1), 56–82 CrossRef.
- V. Amenta, et al., Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries, Regul. Toxicol. Pharmacol., 2015, 73(1), 463–476 CrossRef PubMed.
- M. Cushen, J. Kerry, M. Morris, M. Cruz-Romero and E. Cummins, Migration and exposure assessment of silver from a PVC nanocomposite, Food Chem., 2013, 139(1–4), 389–397 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |