Open Access Article
Open Access ArticleCombining DoE and MASE: a winning strategy for the isolation of natural bioactive compounds from plant materials
Valeria
Cavalloro†
 ab,
Giorgio
Marrubini†
ab,
Giorgio
Marrubini†
 *c,
Giacomo
Rossino
*c,
Giacomo
Rossino
 c,
Emanuela
Martino
c,
Emanuela
Martino
 *ab and
Simona
Collina
*ab and
Simona
Collina
 c
c
aDepartment of Earth and Environmental Sciences, University of Pavia, via S. Epifanio 14 Pavia, 27100, Italy. E-mail: valeria.cavalloro01@universitadipavia.it; emanuela.martino@unipv.it
bNBFC, National Biodiversity Future Center, Palermo 90133, Italy
cDepartment of Drug Sciences, University of Pavia, viale Taramelli 12 Pavia, 27100, Italy. E-mail: giorgio.marrubini@unipv.it; giacomo.rossino@unipv.it; simona.collina@unipv.it
First published on 4th December 2023
Abstract
The successes achieved in pursuing a nature-aided drug discovery (NADD) program are many and well-known, but it is still considered a second-order approach. Biomass extraction is a fundamental and critical step in the NADD process and often requires a high volume of usually organic and not eco-compatible solvents and a prolonged time. Optimization of such procedures could drastically decrease the costs required for the NADD process, also considering waste management. For this reason, many extraction techniques have been developed, among which one of the most diffused is microwave assisted solvent extraction (MASE). The MASE procedure is well suited for use in the drug discovery phase from natural sources. Still, there are several factors to consider, and the one-factor-at-a-time (OFAT) approach risks limiting the advantages the technique provides. The way to make it truly green is to couple MASE with DoE, even if this winning combination is limited. Consistently, we analyze the 10-year literature (2013–2022), reporting a critical discussion about DoE applied to set up MASE protocols for the extraction of metabolites (both performed with traditional solvents and with ionic and eutectic solvents) and essential oils.
Introduction
Natural matrices have always represented an invaluable source of active pharmaceutical ingredients.1–3 Over the years, different organisms have been considered as starting points of the Nature-Aided Drug Discovery (NADD) process, e.g., plants, lichens, animals, and microorganisms, either terrestrial or marine.4 The successes achieved pursuing this approach are many and well-known.5 The wide chemical diversity is the strength of natural products (NPs), which allows molecules to be obtained with various mechanisms of action and, therefore, suitable for various fields of application.NADD is still considered a second-order approach concerning molecular modeling- and synthesis-driven drug discovery, although only 33.3% of the in-commerce small molecule drugs are fully synthetic compounds.6 The main reason is the high time and costs of the extraction and fractionation procedures required to obtain pure active ingredients from a natural matrix. Notably, biomass extraction is a fundamental and critical step in the NADD process and often requires a high volume of usually organic and not eco-compatible solvents, and a prolonged time (from 1 to 5 days).7,8 Optimization of such procedures could drastically decrease the costs required for the NADD process, also considering waste management. For this reason, many extraction techniques have been developed over the years, moving from conventional (i.e., maceration, percolation, and steam distillation) to the so-called unconventional methods (i.e., ultrasound- and microwave-assisted extraction, supercritical fluid extraction, and pressurized solvent extraction).9–11
This review is focused on the Microwave-Assisted Solvent Extraction (MASE) methodology that exploits microwave radiation to heat the natural matrix. Microwaves are part of the electromagnetic spectrum, with frequencies from 300 MHz to 300 GHz corresponding to wavelengths ranging from 1 m to 1 mm.12 The heating efficiency depends on the dielectric constant and dielectric loss of the molecules irradiated; the former parameter quantifies the molecule's capability to absorb the microwave energy, while the latter defines the molecule's ability to convert radiation energy into heat.13 The microwaves transfer energy to the molecules by ionic conduction and dipole rotation. Free ions or ionic species inside the cell move under the influence of the force of the oscillating electric field of the microwaves; this induced electrophoretic migration causes friction between the charge carriers and the medium, leading to heat production, thus defining heating by ionic conduction. Concurrently, molecules having non-zero permanent electric dipole moments try to align themselves with the direction of the force of the microwave oscillating electric field, colliding one against the other and producing heat (heating by dipole rotation). In conclusion, due to the molecular electrophoretic migration and rotation, the energy delivered by the electromagnetic waves to the molecules is transformed into thermal energy, and an increase in the temperature of the irradiated system occurs. This highly efficient heating system offers several advantages compared to conventional methods.
MASE offers both economical and practical advantages with respect to conventional methodologies, in line with its intrinsic green and eco-sustainable nature, like a shorter extraction time, less use of solvents and the possibility to substitute hazardous ones with more eco-sustainable alternatives, while maintaining comparable or even higher yields. Another important advantage of MASE is related to energy efficiency. Different from microwave-assisted organic synthesis, where it has been shown that energy consumption can be even higher than traditional heating systems (especially when working on a bench scale), a different picture emerges from literature data for MASE.14 In fact, a recent environmental impact assessment study concluded that MASE shows a better environmental score than conventional techniques and ultrasound assisted extraction, mainly due to the amount and origin of electricity used.
In this work, the authors concluded that its better performance is due to the more efficient extraction with reduced electricity consumption.15 The different results obtained considering microwave-assisted organic synthesis and MASE can be explained considering that extractions of natural matrices are performed on tens of grams to a kilo scale16 As stated in the literature, these scales are associated with an improvement in energy efficiency on a kJ mol−1 basis.17 Moreover, also the already cited significant saving of time plays a pivotal role in reducing energy consumption.
Other advantages of MASE are also related to its high reproducibility and robustness.18 Its high extraction efficiency with solvents such as water and ethanol allows the extraction of non-polar metabolites without the use of other less (or no) eco-friendly solvents and even thermolabile compounds. Today its use on an industrial scale is limited by the high costs associated with the development of ad hoc apparatus, even if steps forward have been taken.
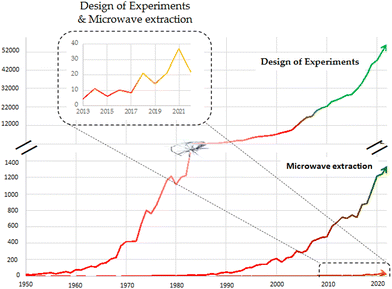
More in detail, the efficiency of MASE is related to many operating factors such as the solvent used, and the liquid/solid ratio, the operating temperature and extraction time, the microwave power, and the rate of stirring, as well as some characteristics depending on the sample, like its water content. Considering this large number of variables, optimization of the procedure is a critical step. A literature search shows that most published research papers address the optimization of a MASE protocol by following a heuristic approach. Nonsystematic, experience-driven approaches often entail many experiments and do not allow the understanding of the factors’ mutual influence. In recent years, Design of Experiment (DoE) is becoming increasingly popular in optimizing the extraction of natural matrices. Therefore, the combination of DoE and MASE may be a winning strategy to set up the extraction of metabolites from natural matrices with a green approach. The DoE approach aims at minimizing the number of experiments required for method/process/product optimization, resulting in saving the amount of solvent, time required, and consequently, energy used. Despite the significant advantages of this combination, DoE is still under-exploited in microwave-assisted extraction method development, as evidenced by the number of papers published (Fig. 1). Still, the number of articles retrieved using both “design of experiments” and “microwave extraction” as keywords on Scopus is very low (a maximum of 37 papers was reached in 2021). In the present paper, after an overview of DoE, we deepened the potential of the combination of these two techniques, reviewing the literature from 2013 to 2022. More in detail, we conducted a bibliographic survey through Scopus and PubMed databases using the keywords “microwave assisted extraction”, “MASE”, “MAE”, “experimental design”, “design of experiments”, “DoE”, “quality by design”, and “QbD”, together with their possible combinations using the Boolean operators “AND”. In addition, we excluded from the survey studies based on heuristic and univariate (OFAT) methodologies and publications that reported extractions with combined techniques, such as combined ultrasound- and microwave-assisted extractions.
This review is proposed to inform the readers about the pros and cons of using DoE for the development and optimization of MASE procedures.
Design of experiments
Design of experiments (DoE) is a rational approach developed by mathematicians for about one century now that can be applied to any process with measurable inputs and outputs. DoE was initially developed in parallel with statistical methods for improving crop yields in the early 1920s.19 It rapidly became an essential tool for studying research methodology, quality improvement, and statistical process control, in several fields of application, like agriculture and botany, psychology, engineering, industrial chemical processes, and many other applied sciences.20The strength of DoE is that it is the most efficient, cost-effective approach to collect information about the best quality (expressed by the experimental variance of the output data), studying virtually any number of factors and requiring the least number of experiments compared to any other approach. Based on mathematical evidence, DoE is superior to heuristic or one-factor-at-a-time (OFAT) approaches in terms of efficiency (expressed by the ratio of the amount of information collected to the number of experiments performed).21 Although this is a long-established fact for mathematicians, the same cannot be said for experimenters in applied sciences. The OFAT method is still widely used in applied experimental research because it requires no specific training and is perceived as “rigorous”.
Out of the many advantages of DoE, it is the only approach that provides knowledge regarding the relevance of the single factors and their interactions, whereas the OFAT approach cannot investigate interactions.22,23
The present trend favoring the diffusion of DoE is related to the availability of powerful computers and information technology tools (e.g., open source software), that allow learning the use of DoE by applying it as a procedure, following a few simple steps.24 The Japanese engineer and statistician Genichi Taguchi first elaborated on this way of proposing DoE to potential users,25 differently to the Western more formal statistical training offered in academia.21,26,27 In the following, we summarize the rational procedure revisited by European research groups for teaching DoE to non-mathematicians.28
The definition of the experimental response, which must be numeric, is the first step in the process. Accordingly, describing the system and analyzing the technical details related to the response measurement is the procedure's fundamental and more difficult phase. Then, a list of all the factors influencing the experimental response and their levels of variation is created. The variation intervals of factors define the space to study the response (design space).28,29 Once these steps are completed, the focus moves to model selection and data interpretation. Two types of experimental designs are available: screening and optimization designs. The first ones are usually used to study the effect of single factors and their two-term interactions on the response. The latter ones are instead used to make predictions (on maxima, minima, or other critical points of the response function) and fully describe the process under examination.
The following list of techniques available in DoE is far from complete since the present section aims to introduce the reader to the topic, showing the main tools available to practitioners. Screening designs include full factorial, fractional factorial, and Plackett–Burman designs.30 Taguchi designs are used to reduce the noise, select the more relevant factors, and optimize the settings of a process.31 Definitive screening designs allow studying single factors, their interactions, and quadratic terms when many factors are involved (i.e., more than 6).32 Response surface designs (RSM), including central composite, Box–Behnken, and Doehlert,33 are canonical quadratic designs used to describe the system fully. The RSM designs are of practical use for a small number of factors (<5) because they involve quadratic polynomials with many terms. In addition to these so-called “canonical” or “optimal” designs, researchers may use D-optimal designs when the system involves both continuous and non-numerical factors varying over more than two levels.34 Mixture designs are instead the designs of choice when dealing with non-independent and constrained numerical factors.35,36
Generally, the first-choice models are two-level full factorials (where the number of experiments is 2k, with k being the number of factors) or fractional factorials (number of experiments 2k−p, where k is the number of factors and p is the size of the fraction).
On the other hand, using the RSM, quadratic models are introduced to describe the response as a function of factors as accurately as possible.37 These designs describe the response dependence on the experimental factors and predict the response values over the entire domain of factor variation.38 Different designs belong to this second group, like (i) central composite designs, which contain a factorial or fractional factorial design with central and other additional points that allows calculating the response surface, (ii) Box–Behnken designs, which is a class of second-order designs based on a three-level incomplete factorial design, and (iii) Doehlert designs, which are obtained from regular k-dimensional simplexes (regular geometric figures with k + 1 vertices) of which the simplest, in 2 dimensions, is the equilateral triangle.39
Once the experimental design is selected, this is one-to-one related to the model equation and matrix (Fig. 2). A simpler experimental matrix is deducible based on the model matrix, and the experimental plan is obtained accordingly. The experimental plan is a table that presents the actual values of the factors for each experiment. The experiments are performed in random order following the fundamental recommendation given since the birth of DoE to obtain a fair estimate of the model coefficients. Regarding the in-depth description of specific experimental designs, models, and details concerning the statistics involved in using these tools, interested readers are referred to the fundamental literature on the subject.5–21,27
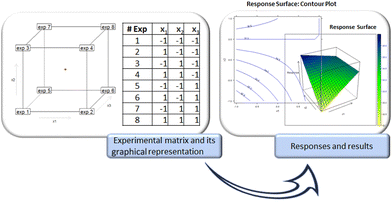 | ||
| Fig. 2 Different steps characterizing the DoE approach. The example refers to a full factorial design. | ||
The last part of the experimental design involves transforming the data obtained into information and their analysis. The model must be examined to determine if it shows good predictability; this means that it must adequately represent the response function in the experimental domain. In other words, the model is judged adequate if the value of the calculated response for every point of the domain matches the value obtained from the outcome of experiments carried out independently from those used to build the model itself.
Design of experiments approach to set up MASE protocols
In this section, articles in which DoE has been applied to set up an appropriate MASE methodology are presented and critically evaluated, dividing them into three groups: the first group includes studies on extraction of metabolites, the second on essential oil extraction, and the third focuses on extraction performed with ionic or eutectic solvents. For the sake of clarity, the main factors affecting the efficiency of microwave extraction are briefly discussed first.Factors affecting microwave extraction efficiency
Solvents are classified into low, medium, or high absorbance based on the ability to absorb microwaves. Typical low-absorbance solvents are hexane, toluene, ethyl acetate, and diethyl ether. Water, dimethylformamide, and acetic acid are classified as medium absorbance solvents, whereas ethanol, methanol, formic acid, and ethylene glycol are high-absorbance solvents.
Moreover, growing interest in eco-friendly solvents like ionic liquids and deep eutectic solvents is developing.
Finally, Solvent-Free Microwave Extraction (SFME) is also possible. The water inside the cells absorbs the microwaves, causing heating of the system and, consequently, the rupture of cell walls. This technique is primarily used to extract essential oils or other volatile compounds.40 Unlike the so-called process parameters (e.g., liquid/solid ratio, extraction time, microwave power, temperature, water content and other quantifiable characteristics), which can take numerical values and are often continuous variables, some factors, like pure solvents, may not be numerical variables. Factors representing the type of solvent used for extraction, for example, “ethanol”, “acetone”, or “water” are qualitative variables. However, the levels of qualitative variables must be referred to as numerical quantities. Therefore, if the choice is between two solvents (e.g., ethanol or water), the selection of the solvent type is usually coded with “−1” indicating the choice of solvent “A” (e.g., ethanol) and “+1” indicating the choice of solvent B (in this case, water) in a specific column of the experimental matrix named, e.g., “solvent”. If the solvents studied are more than two, the simplest approach is to code the levels using a binary coding, with “1” indicating the choice of the given solvent, as shown in Table 1 for three solvents.
| Experiment # | Ethanol | Acetone | Water |
|---|---|---|---|
| 1 | 1 | 0 | 0 |
| 2 | 0 | 1 | 0 |
| 3 | 0 | 0 | 1 |
In Table 1, coding 1 indicates the presence of the type of solvent selected, whereas 0 indicates the absence of the solvent. Therefore, experiment#1 uses 100% ethanol, experiment#2 uses 100% acetone, and experiment#3 uses 100% water. The third column is not necessary since it is given by the values of the first two columns.
The same procedure can be applied for any number of solvents, k, bearing in mind that the number of rows in the experimental matrix will be equal to the number of solvents under investigation, but the number of columns is equal to k − 1 since the last column is implicitly indicating the choice of the k-th solvent. In Table 1, when both ethanol and acetone are at their 0 levels (i.e., not used), it indicates consequently that water is used as the extraction solvent. An example of this kind of coding can be found in reference,41 where the influence of solvents as acetone, ethyl acetate or ethanol in the extraction process is investigated by exploiting a DoE model. The fully coded data of the work taken as an example are reported in Table 2.
| Exp # | X1: Solv1 | X1: Solv2 | X2: Cycles | X3: T |
|---|---|---|---|---|
| 1 | 1 | 0 | −1 | −1 |
| 2 | 1 | 0 | 1 | −1 |
| 3 | 1 | 0 | −1 | 1 |
| 4 | 1 | 0 | 1 | 1 |
| 5 | 0 | 1 | −1 | −1 |
| 6 | 0 | 1 | 1 | −1 |
| 7 | 0 | 1 | −1 | 1 |
| 8 | 0 | 1 | 1 | 1 |
| 9 | 0 | 0 | −1 | −1 |
| 10 | 0 | 0 | 1 | −1 |
| 11 | 0 | 0 | −1 | 1 |
| 12 | 0 | 0 | 1 | 1 |
Discontinuous procedures may be applied to avoid labile compounds’ yield losses or degradation when a longer extraction time is more desirable. Such procedures apply more than one cycle of microwave irradiation in consecutive steps using a fresh aliquot of solvent every time.
Generally, higher power determines higher yields and shorter extraction times. However, increasing the power beyond the optimum can lead to the decomposition of labile molecules. Therefore, the microwave power is set considering the target compounds’ thermal stability, sample amount, type, the volume of the solvent used, and extraction time.18
Microwaves cause the evaporation of the water contained in the sample, developing great pressure inside the cell that promotes its rupture. Moisture also facilitates the transmission of heat through the material.
Moreover, the production of steam in the sample vessel rapidly leads to a build-up of pressure inside the oven that can cause trouble in extraction control. On the other hand, the steam produced and kept under pressure in the sample can facilitate the cells’ rupture.47
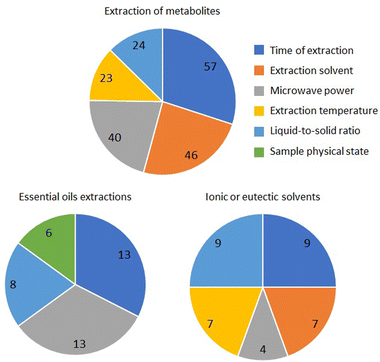
All these factors, except for stirring, have been considered in the three groups of articles considered, even with some differences. Thus, except for the extraction time, all the other factors have been differently considered, e.g., the solvent seems more influential in general solvent extraction, power and liquid-to-solid ratio in essential oils, and temperature in ionic/eutectic solvents (Fig. 3).
Microwave assisted solvent extraction of metabolites
In most cases, microwave assisted extraction is exploited to set up or optimize the extraction of metabolites or a class of metabolites from different natural matrices. Examples in this field are many and can be divided into two main groups: extraction performed with traditional solvents and extraction performed with ionic and eutectic solvents. The strategy and the niceties as the basis of these two groups are different, and they will be discussed separately.Regarding the responses, 22 papers over 63 focused on the total phenol yield, followed by the total extraction yield (14 papers), pure metabolite yield (12 papers), total flavonoids and anthocyanins (8 papers each), while other works report on different classes. Of note, some papers don't focus only on the extraction of a metabolite or a class of metabolites, but also on maximizing the activity of the extract. This is the case of works focusing on the extraction of metabolites with antioxidant, antimicrobial and aldose reductase activity. Table 3 summarizes the responses and measurement methods evaluated to describe MASE results in plant material extractions. In such articles, the authors claimed that DoE was helpful in attaining greener procedures as compared with the original ones, such as in entries #1, 2, 5, and 7 just to cite a few. Thus, optimized procedures usually exploited green solvents like water or ethanol and allowed decreasing time and solvent consumption. Furthermore, a recent work estimated that MASE consumed 59% less energy (expressed in kilowatt-hour per gram of total triterpenoids) than maceration.50
| Responses | Measurement methods | Citing articles | Ref. |
|---|---|---|---|
| Total yield | Dry extract/dry matrix × 100 | 13 | 41, 53 and 61–71 |
| UV method | 1 | 72 | |
| Total phenols | Folin–Ciocalteau method | 21 | 48, 49, 51–53, 56, 57, 60, 67, 69, 70 and 73–81 |
| UV method | 1 | 82 | |
| Total flavonoids | Aluminum chloride colorimetric modified method aluminum nitrate colorimetric method. | 5 | 48, 51, 69, 74 and 83 |
| UV method | 1 | 54 | |
| 2 | 55 and 82 | ||
| Condensed tannins | BuOH/HCl method | 1 | 60 |
| Total tannins | Casein precipitation method | 1 | 74 |
| Folin–Denis method | 1 | 48 | |
| Total saponins | Isolation and weighing | 1 | 84 |
| Total pectins | Isolation and weighing | 4 | 85–88 |
| Total pectins, esterification degree, equivalent weight, anhydrouronic acid and methoxyl content | Isolation, weighing, and titration | 1 | 89 |
| Triterpenoid content | HPLC-UV | 1 | 50 |
| Total anthocyanins | HPLC-UV | 1 | 73 |
| UHPLC-UV | 1 | 75 | |
| pH differential method | 3 | 49, 66 and 90 | |
| UV method | 2 | 59 and 91 | |
| Association of Analytical Communities official method 2005.02 | 1 | 92 | |
| Total polysaccharides | Isolation and weighing | 2 | 93 and 94 |
| Sulphated polysaccharides | 1 | 95 | |
| Single metabolites | HPLC | 10 | 41, 49, 58 and 96–102 |
| HPTLC | 2 | 103 and 104 | |
| Alkaloids | HPLC | 5 | 76, 91, 105–107 |
| Antioxidant activity of the extract | ABTS + FRAP + DPPH | 3 | 48, 53 and 76 |
| DPPH | 4 | 63, 67, 82 and 92 | |
| FRAP + DPPH | 1 | 78 | |
| Not specified | 1 | 59 | |
| Antibacterial compounds | antibacterial diameter (mm) | 1 | 108 |
| Aldose reductase | Enzymatic assay | 1 | 63 |
The most exploited RSM model was the Box–Behnken design (BBD, 30 articles, 48%), followed by the central composite design (CCD, 21 articles, 33%). The success of these two models lies in the fact that they highlight the interactions between parameters and require a limited number of experiments. The other experimental designs that emerged during the literature survey are two-level factorial design,51 Taguchi design,52 incomplete 33 factorial design,53 orthogonal array,54,55 3-level design,56 D-optimal,57 full factorial design,41,58 randomized block design,59 and face-centered central composite design.60,61 All these models emerged a maximum of three times each during the investigations (Table 4).
| # | Plant | DoE model | Factors | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Time (min) | Power (W) | Solvent | L/S ratio | Temp. °C | ||||
| 1 | Acacia mearnsii De Wild. (bark) | FCCD | 1–5 | 150–350 | 20%–80% MeOH | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
— | 60 |
| 2 | Acanthopanax senticosus (Rupr. et Maxim) Harms (stems) | BBD | 15 (fix) | 450–600 | H2O + 0.8% surfactant | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–50 1–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50–70 | 96 |
| 3 | Allium cepa L. (bulbs) | BBD | 5 (fix) | 1800 (fix) | 50%–100% MeOH, pH 2–7 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–100 1–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50–100 °C | 73 |
| 4 | Amomum tsao-ko Crevost et Lemaire (fruits) | BBD | 30–75 | — | 20%–80% EtOH | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–25 1–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
35–65 | 108 |
| 5 | Argania spinosa (L.) Skeels (hulls) | CCD | 15–35 | 400–800 | 10%–30% EtOH | — | — | 74 |
| 6 | Aristotelia chilensis (Mol.) Stuntz | BBD | 2 (fix) | 800 (fix) | 25%–75% MeOH, pH 2–7 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–40 1–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50–100 | 75 |
| 7 | Artocarpus heterophyllus Lamk (wood) | FCCD | 10–50 | 400–800 | Aquadest | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1—1 1—1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
— | 61 |
| 8 | Berberis asiatica Roxb. (leaves) | BBD | 5 (fix) | 100–500 | 30%–80% MeOH | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–45 1–45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 48 |
| 9 | Berberis sp. pl. (roots) | CCD | 2 (fix) | 200–600 | 40%–100% MeOH, pH 2–5 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–70 1–70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 76 |
| 10 | Carica papaya L. (peel) | BBD | 0.3–3 | 320–640 | H2O, pH 1–3 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–25 1–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 85 |
| 11 | Centella asiatica (L.) Urban (tetraploid) | CCD | 5–10 | 100–200 | 40%– 80% EtOH | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
— | 50 |
| 12 | Chromolaena odorata (L.) King & Rob. (leaves) | Two-level factorial design | 1–5 | 400–800 | 20%–60% EtOH | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–14 1–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60–80 | 51 |
| 13 | Chuanminshen violaceum Sheh et Shan (rhizomes) | BBD | 1–15 | 400–600 | H2O | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–40 1–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50–70 | 93 |
| 14 | Cinnamomum burmannii (Nees & T.Nees) Blume (bark) | CCD | 10–30 | 120 (fix) | 80%–90% EtOH | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–30 1–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 65 |
| 15 | Citrullus lanatus (fruit) | BBD | 1–3 | 160–480 | H2O pH 1–2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–30 1–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 86 |
| 16 | Citrus sinensis (L.) Osbeck (peels) | BBD | 3–20 | 100–850 | — | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–25 1–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 97 |
| 17 | Citrus sinensis (L.) Osbeck (peels) | BBD | 1–3 | 160–480 | H2O pH 1–2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–30 1–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 77 |
| 18 | Cladonia foliacea (Whole lichen) | FFD | 10–15 | 100 (fix) | Acetone–AcOEt–EtOH | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
60–80 | 41 |
| 19 | Clitoria ternatea L. (flowers) | CCD | 15–25 | — | 95% EtOH (fix) | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–25 1–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40–60 | 66 |
| 20 | Coffea arabica L. (spent grounds) | CCD | 3–6 | 60–120 | 50%–99% EtOH | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–20 1–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75 °C (fix) | 67 |
| 21 | Coffea liberica L (seeds) | Taguchi design | 5–10 | — | 60%–80% EtOH | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–7.5 1–7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50–90 | 52 |
| 22 | Cola nitida Schott & Endl. (pod) | BBD | 2–10 | 400–500 | EtOH (fix) | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
55–65 | 68 |
| 23 | Coptis chinensis Franch. (rhizomes) | CCD | 3–7 | 120–240 | 25%–75% EtOH | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
— | 91 |
| 24 | Cordyceps militaris L. (fruits) | BBD | 2–6 | 300–700 | H2O (fix) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–50 1–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 98 |
| 25 | Cucumis melo L. (fruits) | BBD | 1–3 | 300–700 | H2O pH 1.5–3 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–30 1–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 87 |
| 26 | Docynia indica (Wall.) Decne. (fruits) | CCD | 15–45 | 240–560 | 40%–80% EtOH, pH 2–6 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
— | 69 |
| 27 | Eucalyptus globulus Labill. (wood) | Incomplete 33factorial design | 5–15 | 150 (fix) | EtOH (fix) | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–10 1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50–70 | 53 |
| 28 | Ficus racemosa L. (leaves) | BBD | 2–6 | 420–490 | MeOH (fix) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–30 1–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 103 |
| 29 | Fragaria ananassa Duchesne (leaves) | BBD | 20–40 s | 300–500 | 40%–60% EtOH | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–70 1–70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 78 |
| 30 | Glycyrrhiza glabra L. (roots) | CCD | 2–6 | — | 80% EtOH–80% MeOH–H2O | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–25 1–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 70 |
| 31 | Grape juice wastes | BBD | 1–5 | 100–600 | H2O | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–50 1–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 90 |
| 32 | Hibiscus sabdariffa L. (calyxes) | RBD | 1–9 | 100–400 | 50%–80% EtOH | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
— | 59 |
| 33 | Hylocereus polyrhizus Britt. & Rose (peels) | Second order CCD | 20–80 s | 300–800 | H2O, pH 1–3 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–110 1–110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 88 |
| 34 | Inula helenium L. (roots) | Orthogonal array | 3.5–4.5 | — | 40%–60% EtOH | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–18 1–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
55–65 | 54 |
| 35 | Juglans regia L. (fruits & seeds) | CCD | 6–30 (1–3 cycles) | 500 (fix) | 50% EtOH (fix) | 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
60–100 | 79 |
| 36 | Lachnum singerianum YM296 (mycelium) | BBD | 1.5–2.5 | — | NaOH 0.5–1.5M | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–20 1–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 72 |
| 37 | Mangifera indica L. (peel) | BBD | 5–8 | 400–800 | H2O, pH 1–3 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
— | 89 |
| 38 | Marrubium vulgare L. (aerial parts) | CCD | 5–15 | 100 (fix) | 20%–80% EtOH | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
40–120 | 71 |
| 39 | Morus alba L. (fruits) | BBD | 5–9 | 150–270 | 20%–60% EtOH | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
— | 62 |
| 40 | Morus alba L. (leaves) | 2-Factor, 3-level design | 7–13 | 480–800 | H2O (fix) | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
— | 99 |
| 41 | Myrmecodia pendens Merr. & Perry (tubers) | CCD | 3–10 | 10–50% | 0%–80% EtOH | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–12 1–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 92 |
| 42 | Nelumbo nucifera Gaertn (plumule) | CCD | 2.9–5.3 | 141–260 | 36%–84% MeOH | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
— | 105 |
| 43 | Nigella glandulifera Freyn (seeds) | BBD | 25–35 | 350 (fix) | 60%–80% EtOH | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–25 1–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60–80 | 63 |
| 44 | Olea europaea L. (leaf) | CCD | 0.5–1.5 | 150–250 | 30% ACN | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–20 1–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 82 |
| 45 | Pachyrhizus sp.pl. (seeds) | CCD | 3–11 | — | 12%–50% MeOH in DCM | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
39–71 | 100 |
| 46 | Peganum harmala L. (seeds) | BBD | 8–12 | 600 (fix) | 65%–95% EtOH | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–40 1–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60–100 | 106 |
| 47 | Phyllostachys edulis Houz. (leaves) | Orthogonal array | 6–8 | — | 60%–80% EtOH | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–33 1–33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 55 |
| 48 | Phyllostachys pubescens (bamboo shoots) | CCD | 3–5 | 35 W (fix) | MeOH (fix) | 6.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–10 1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75–95 | 80 |
| 49 | Physalis alkekengi L. (calyxes + fruits) | BBD | 20–30 | 250–350 | 80% EtOH | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–20 1–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
— | 84 |
| 50 | Porphyra dentata Kjellman (alga) | 3-Level, 4-parameter design | 1–5 | 200–400 | 10%–90% EtOH, pH 4–10 | — | — | 95 |
| 51 | Quercus cerris L. (bark) | D-optimal | 10–30 | 200–1000 | 70% EtOH (fix) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
— | 57 |
| 52 | Rheum australe Don. (rhizomes) | BBD | 5–10 | 245–490 | NaOH 0.01–0.5 M | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–25 1–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 101 |
| 53 | Sedum aizoon (leaves) | BBD | 20 (fix) | — | 70%–90% EtOH | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–25 1–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50–70 | 83 |
| 54 | Selenicereus undatus (Haw.) D.R.Hunt & Selenicereus megalanthus (K.Schum. ex Vaupel) Moran (fruits) | BBD | 5–65 | 600 (fix) | H2O (fix) | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–150 1–150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25–75 | 64 |
| 55 | Spinacia oleracea L. (general waste) | 2-Factor, 3-level design | 5–15 | — | 0%–60% EtOH + HCl | 60–120 | 56 | |
| 56 | Stephania sinica Diels (whole plant) | BBD | 0.5–1.5 | 150 (fix) | 30%–90% EtOH | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–30 1–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 (fix) | 107 |
| 57 | Syzygium nervosum A. Cunn. (seeds) | BBD | 3–4 | 400–500 | 40%–60% EtOH | — | 30 (fix) | 81 |
| 58 | Tarchonanthus camphoratus L. (stems) | BBD | 35–55 | 100–300 | MeOH (fix) | — | 40–60 | 104 |
| 59 | Vaccinium corymbosum L. (fruit) | CCD | 4–24 | 71.05–142.1 | 30% EtOH, citric acid 1.5 M | 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
— | 49 |
| 60 | Vitis vinifera L. (lees) | BBD | 25–44 | 50–60 | 0–100% EtOH | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–60 1–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
85 °C (fix) | 57 |
| 61 | Zea mays L. & Triticum L. (seeds) | FFD | 5–10 | — | MeOH–ACN (alone or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
40–80 | 58 |
| 62 | Zingiber officinale Rosc. (rhizomes) | BBD | 0.3–0.5 | 400–600 | 70%–90% EtOH | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–30 1–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 102 |
| 63 | Zizyphus lotus L. (Pulp and Peel) | CCD | 20–40 | 200–600 | H2O (fix) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–40 1–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 94 |
Going into detail about the factors considered in the DoE, the authors did not always choose the same ones (Table 5). The variability may be due to the natural matrix and the metabolite (or class of metabolites), but it also reflects the different goals of the researchers. Thus, the selection of the factors is related to the desired outcomes, as witnessed in entries #16 and 17. In these cases, the starting biomass is the same (peels of Citrus sinensis), while the responses are different (pure metabolite #16 and class of metabolites, pectin, #17), and the range of the considered factors is entirely different.
It is well known that the extraction time plays a relevant role in extraction efficiency. Only six studies indeed maintain a constant extraction time (9%, entries # 2, 3, 6, 8, 9, and 53). In all the examined papers, the extraction time has been considered and it never exceeded one hour and was often ≤5 minutes (25 times, 40%). The short time required for the metabolite extraction represents one of the major advantages of MASE, since the classical approaches often require at least one day of extraction.
The second most frequently recurring factor (40 mentions out of 63 articles, 63%), as it is closely related to the MASE technique, is microwave power. It strongly depends on the characteristics of the oven and is related to the temperature in the sample vessel. Therefore, varying the microwave power means varying the temperature. For this reason, most researchers vary only one of the two factors while holding the other constant. In line with this consideration, in 35 (56%) articles the microwave power is varied, in 18 (29%) the temperature, and only in 5 (8%, entries #2, 12, 13, 22, 58) are both studied. The remaining articles do not consider either factor. In detail, most researchers judged temperature as a factor of interest, claiming that it significantly influences the extraction yield (i.e., entries # 6, 19, and 35). In general, microwave power is more studied than temperature, suggesting a more relevant role in extraction efficiency.
As is always the case in extraction processes from natural matrices, an important factor that must be considered is the extraction solvent. The most used solvents are EtOH (alone or mixed with water, 35 papers, 56%), water (16 papers, 25%) and MeOH (9 papers, 15%). Only three papers report the use of different solvents: ACN (entry #44), MeOH/DCM (entry #45) and ACN/MeOH (entry #61). The limited types of solvents considered highlighted that not all the solvents are suitable for microwave extraction, since not all the solvents are able to absorb microwaves. Moreover, the prevalence of EtOH and water highlights the intrinsic green nature of MASE. Thus, EtOH was also the best choice compared to others (entries # 18 and 30), and, together with water, it is considered a highly eco-sustainable solvent. Also, investigating the best L/S ratio allows for avoiding solvent excess (the best parameter settings often being in the middle of the considered range).85,96
Extraction with ionic and eutectic solvents
Ionic liquids (ILs) and deep eutectic solvents (DESs) are classes of solvents that have recently attracted increasing interest as MASE solvents.DESs were proposed at the beginning of the century as an alternative to ILs to overcome critical drawbacks such as their toxicity, number of synthetic steps, waste products, the fact that they become persistent pollutants in water, and overall cost.
Some authors consider DESs a subclass of ILs, and sometimes they consider these terms interchangeable. On the other hand, other authors underline that despite many similarities, ILs and DESs are different groups of substances. Briefly, ILs are a combination of heterocyclic cations and organic or inorganic anions, whereas DESs are obtained by hydrogen bonding of two molecules, among which one is a hydrogen bond acceptor (HBA) and the second is a hydrogen bond donor (HBD).109
DESs can be classified as type I (which combines metal chloride and quaternary ammonium salt), type II (which combines metal chloride hydrate and quaternary ammonium salt), type III (which combines a H-bond donor typically carboxylic acid, amide or polyol with quaternary ammonium salt), type IV (which combines metal chloride hydrate and a H-bond donor), and type V (which combines nonionic molecular H-bond acceptors and H-bond donors).110
When the composition of a DES includes chemicals of natural origin, it is defined as a natural deep eutectic solvent (NADES). NADESs are interesting solvents due to their better biodegradability, lower toxicity, and higher solubility properties compared to the organic solvents usually exploited for extracting natural matrices.111,112 A recent review defines NADES as “one of the most promising discoveries in the field of green chemistry”, even though high commercial costs of these solvents still limit their use.113
We refer the readers interested in classifying these peculiar substances to the recent comprehensive review of Justyna Płotka-Wasylka et al. (2020).109
It is impossible to generalize the physical properties of ILs and DESs because of the wide variety of substances that can be obtained by combining the precursors mentioned above. However, some general features are common to all these substances. ILs and DESs have high polarity, low melting points (broadly speaking <100 °C), low vapor pressure, and wide liquid range. They have high density and viscosity, strongly dependent on the temperature. DESs’ viscosity can be reduced by adding water to their solutions. ILs and DESs are all highly tunable solvents. Due to their tunable polarity, ILs, DESs, and especially NADES, can dissolve and even stabilize a variety of analytes, including macromolecules such as enzymes.114,115
In the context of the present review, ILs and DESs since 2013 have been used for MASE with DoE in only ten studies. Table 5 summarizes responses and measurement methods evaluated for describing MASE results. As found in the review of reports regarding general solvent extraction, the most studied response is the total phenol yield. Other responses evaluated include pure metabolite yield, antioxidant activity, and total anthocyanin content.
ILs and DESs are solvents invented to be task-specific, therefore they are selected carefully before performing analyte extractions. In 6 cases (60%), the HBA choline chloride (ChCl) in combination with different HBDs were indicated as the optimal NADES for MASE (entries #64, 65, 66, 67, 70, and 72).
The percentage of water in the mixture prepared for MASE was demonstrated to strongly influence the results, as it affects the heating rate and facilitates the transport of the analytes from the matrix to the extraction solvent. Three reports describe studies on the percentage of water before selecting the extractive solvent (entries #64, 65, and 68).
Regarding the other process factors (see section “Factors affecting microwave extraction efficiency”), time is the most considered factor. Of note, the ranges of time, L/S ratio and temperature studied do not vary significantly among the different experiments.
Conversely, in the reviewed studies both microwave power and temperature varied over different ranges. In 3 papers, the authors varied power (30%), in 6 (60%) temperatures and only in 1 both (10%, entry #67). Temperature plays a more critical role in extractions with ILs and DESs than microwave power. This trend can be explained by considering that higher temperatures are associated with lower viscosity of solvents, resulting in improved diffusion and analyte solubility. However, operating at too high temperatures can cause solvent degradation.126 This significant influence of temperature on the results may explain why more attention is paid to temperature than power in this category of extractions. Table 6 lists all the articles reviewed in this section.
| # | Plant | DoE model | Factors | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Time (min) | Power (W) | Solvent | L/S ratio | Temp °C | ||||
| 64 | Allium cepa L.(bulbs) | BBD | 5–25 | 100–300 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–60 1–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 116 |
| 65 | Cajanus cajan (L.) Millsp (roots) | BBD | 10–30 | 500 (fix) | 1,6-Hexanediol/ChCl 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 30% H2O 1 + 30% H2O |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–15 1–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50–90 | 120 |
| 66 | Eugenia uniflora L. (leaves) | CCD | 12–38 | 800 (fix) | Malic acid, lactic acid or ChCl + sugar | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0261–1 0.0261–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0439 (wt/wt) 0.0439 (wt/wt) |
38–40 | 117 |
| 67 | Hibiscus manihot L. (flower) | TOD | 5–25 | 400–800 | HBDs + ChCl | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–30 1–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40–80 | 118 |
| 68 | Hibiscus sabdariffa L. (calyces) | BBD | 3 (fix) | 250–550 | HBDs + citric acid + 10–50% H2O | — | — | 123 |
| 69 | Larix gmelinii (Rupr.) Kuzen. (different parts) | BBD | 5–15 | 230–540 | 1-Butyl-3-methylimidazolium bromide | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–25 1–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 124 |
| 70 | Morus alba L. (leaves) | BBD | 8–24 | 600 (fix) | ChCl/glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–20 1–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
45–75 | 119 |
| 71 | Peucedanum praeruptorum Dunn (radix) | Ortogonal assay | 5–15 | — | [TMG]CH2CH (OH)COOH 0.4–0.8 M | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–50 1–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40–60 | 125 |
| 72 | Scutellaria baicalensis Georgi (radix) | BBD | 5–15 | — | ChCl lactic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 3 2, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–20 1–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
35–75 | 121 |
| 73 | Toona sinensis (A.Juss.) M.Roem. | Ortogonal assay | 12–20 | — | [Bmim]Br 1–2 M | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–40 1–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60–80 | 122 |
Essential oil extraction
The extraction of essential oils (EOs) assisted by microwaves is considered an emergent new method that may substitute traditional thermal methods like steam distillation and hydrodistillation. Besides the conventional extraction with organic solvents, MASE extraction processes include microwave-assisted hydrodistillation (MAHD) and solvent-free microwave extraction (SFME). Many examples of this kind of extraction are reported in the literature, but only a tiny percentage exploit a DoE approach to optimize the yield of the total extract or of a single metabolite. Table 7 lists the most significant examples.| # | Plant | DoE model | Factors | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Time (min) | Power (W) | Solvent | L/S ratio | Soaking time | Moisture content % | ||||
| 74 | Allium cepa L. (bulb) | PBD | 25–50 | 350–500 | SFME | — | — | 20–50 | 127 |
| 75 | Cannabis sativa L. (inflorescences) | CCD | 60–100 | 1000–1561 | SFME | — | — | 35–55 | 42 |
| 76 | Cannabis sativa L. (inflorescences) | CCD | 80–140 | 700–1500 | SFME | — | — | 13–50 | 43 |
| 77 | Cinnamomum zeylanicum L. (Bark) | Taguchi based design | 40–60 | 450–800 | MAHD | 50/1–15/1 | 5–15 min | — | 128 |
| 78 | Citrus sinensis (L.) Osbeck (leaves) | CCD | 60–120 | 300–600 | MAHD | 2/1–4/1 | — | — | 129 |
| 79 | Glycine max (L.) Merr. (grains) | FFD | 3 (fix) | 480 (fix) | IPA, EtOH ± water | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (fix) 1 (fix) |
0–40 min | — | 130 |
| 80 | Pinus pumila (Pall.) Regel (Needls) | BBD | 20–40 | 385–700 | SFME | — | — | 30–50 | 131 |
| 81 | Rosmarinus officinalis L. (leaves) | CCD | 25–85 | 550–1150 | MAHD | 0–3/1 | — | — | 132 |
| 82 | Syzygium aromaticum (L.) Merr. & L.M.Perry (stems) | CCD | 40–120 | 300–600 | MAHD | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–3 1–3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | 133 |
| 83 | Taxus chinensis (Rehder & E.H. Wilson) Rehder (leaves) | CCD | 10–20 | 400–600 | DCM | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–25 1–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | 134 |
| 84 | Trachyspermum ammi L. (Friuts) | CCD | 50–120 (×1 or 2) | 1000–1560 | MAHD | 24/1–5/1 | 0–4h | — | 135 |
| 85 | Wurfbainia vera (Blackw.) Skornick. & A.D.Poulsen (leaves) | BBD | 40–120 | 140–280 | MAHD | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–15 1–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | 136 |
| 86 | Xanthoceras sorbifolium Bunge (seeds) | BBD | 60–100 | 200–300 | MAHD + NaCl | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–5 1–5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | — | 137 |
| 87 | Zingiber officinale Roscoe (rhizome) | FFD | 10–30 | 288–640 | SFME | — | — | — | 138 |
Almost all the articles reviewed studied the total EO yields as the response. The only exceptions are represented by entries #84 and 85, in which the responses are thymol and 1,8-cineole content, respectively.
As evidenced in Table 7, DoE is mainly applied to optimize the extraction of EOs in MAHD. In this case, the most considered factors are time, power, and L/S ratio, and, less frequently, soaking time. In two reports, authors identify extraction time as the most important parameter (entries #77 and 84), followed by microwave power. Experimental conditions for optimal total yield generally mention the longest extraction time and highest microwave power values, whereas the L/S ratio and soaking time parameters seem case specific. Furthermore, two different works also focused on optimizing the content of specific metabolites in the EOs, e.g., thymol (entry #84) and 1,8-cineole (entry #85). In both cases, the authors recommend mild extraction conditions (lower power in the first case and shorter time in the second), suggesting that an appropriate extraction method should consider the specific metabolites’ stability during setup. Interestingly, the analysis of the process factors studied in the articles reviewed in this section (Table 7) showed that only one report (entry #86) mentioned temperature as an important factor, while raw material particle size appeared three times (entries #74, 83, and 87).
One paper (entry #86) reported that amounts of NaCl in water positively correlate with the extraction yield of seed oil from yellow horn (Xanthoceras sorbifolium Bunge). The presence of salt is an unusual factor, justified by the authors claiming that the salt is a green demulsifying agent of water-in-oil emulsions. The demulsification efficiency was maximized with 24 g L−1 salt in water and the authors observed that the extraction yields abruptly declined at NaCl concentrations higher than 25 g L−1. Such a result is a clear demonstration of the efficacy of the adoption of DoE/RSM in MAHD.
The other extraction method peculiar to EOs is SFME, where the most considered factors are time, microwave power, and sample moisture content. As already observed in MAHD, also in SFME, the best values of power and time are in the upper part of the ranges of variation considered, only the extraction duration in this case is usually much shorter than in MAHD.
Some papers have also reported microwave-assisted extraction of EOs with organic solvents (IPA, EtOH ± water entry #79, DCM entry #83). One report (entry #79) describes a MASE approach using isopropyl alcohol and water, allowing yields comparable to those obtained with hexane, the most widely used solvent for extracting edible oils. Consistently, alcohols represent a viable alternative to replace hexane as an extractive solvent for edible oils in MASE.
Finally, although it is not possible to make comparisons between the results obtained through MAHD and SFME, since the biomasses considered in the reviewed works are different, we propose a general reflection on the improvements shown by microwave-based procedures compared to classical extraction methods. MAHD and SFME are environmentally friendly and sustainable approaches, as they do not use organic solvents.
A survey of the use of DoE in MASE
The survey of recent literature presented in previous paragraphs allowed us to draw general reflections about aims, experimental conditions and outcomes of DoE application to MASE.First, the aim of all the studies reviewed is to set up an extraction method capable of maximizing the outcomes, often keeping in mind the repeatability of the newly set up procedure. To this aim, in 40 articles (45%) the application of a DoE model is performed only after preliminary experiments aimed at screening the influencing factors and their optimal ranges. Therefore, this first step is often overlooked, as less than half of the analyzed papers perform it, suggesting that more attention should be paid to this topic. Thus, this initial step allows the researcher to obtain more significant results, permitting not only to focus on really influencing factors, but also to study their range in a more deepened way. Moreover, among the articles related to extractions with ILs and DES, initial screening often considers only the solvent. Although this factor is crucial, it's also important to underline that in these extractions a deeper preliminary analysis of the other factors should be done.
Another issue associated with MASE is related to the oven. Thus, although in many cases a professional microwave oven is exploited, e.g., MAS-II microwave systems (Shanghai Sineo), Ethos Easy and NEOS systems (Milestone Srl), and MARS /MARSX systems (CEM), the use of adapted domestic oven is still accepted. The use of this kind of apparatus should be limited if not completely avoided, as the temperature and pressure control is often inaccurate. More generally, the most exploited ovens are multimodal apparatuses equipped with two magnetrons, working at a frequency of 2450 MHz.
As mentioned before, microwave heating applied to the extraction of natural matrices can be considered a green approach not only for the reduction of time and use of hazardous solvents, but also for the lower energy required compared to other techniques. This last aspect is guaranteed by both the drastic reduction in time and the gram to kilo scale in which the extractions are usually performed.15,16 Unfortunately, this last aspect is not always considered during the set-up of a new method, since the amount of natural matrix exploited in this phase typically ranges from hundreds of milligrams to a few grams, thus limiting the energy saving usually associated with MASE. Consistently, curtailing the number of experiments required to identify the best extraction conditions and applying a DoE model is mandatory to make the most of MASE potential.
In this context, many different experimental designs have been exploited, but two were the most represented ones, i.e., Box–Behnken (39 times), and central composite (26 times). As mentioned before, both these designs allow computing canonical quadratic models used to describe the system fully by RSM. These models are the most popular because they are implemented in all commercial software and allow studying up to 4–5 factors easily.
Interestingly, the study of the factors considered, particularly the relationship between microwave power and temperature evidenced the need for considering both these factors simultaneously.
In the reports reviewed, only one of these two factors is generally considered in the DoE, and the selection made is mainly dependent on the kind of experiment performed. Microwave power control is judged more critical than temperature control in general solvent extraction and extraction of essential oils, while temperature control is considered of primary relevance in extraction performed with ILs or DESs. This may be due to the already discussed influence of temperature on the solvents’ viscosity and stability of some DESs, however, these problems are not relevant to methods of extraction performed with standard solvents.
A final issue to be highlighted is the DoE model validation. Out of the 87 articles reviewed, only 49 (56%) mentioned model validation. This picture is very common, and already discussed in previous reviews.38 It is worth underlining here that the application of DoE to collect experimental data is always effective (i.e., allows to reduce the amount of work while providing the best information available), and it can be concluded by a mere description of the results after model computation through the ordinary least squares method. In this case, the experimenters provide a picture of their results as obtained under specific conditions applied during the working sessions but cannot provide evidence of the prediction ability of the model computed. On the other hand, when the model is validated, the experimenters may claim that the model describes the process over the entire experimental domain and can appraise quantitative predictions and experimental variance on the outcome of experiments not performed.
Conclusions
The MASE procedure is well suited for use in the drug discovery phase from natural sources because it offers numerous advantages over conventional techniques including a shorter sample processing time, lower amounts of solvent used, wide options of using green solvents, and greater flexibility allowing the users to maximize the extraction efficiency by modulating the factors that regulate the process control. In the set-up of extraction protocols, several factors should be considered simultaneously and for this reason the OFAT approach may severely limit the technique's advantages. As an inherently multifactorial technique, the only way to apply the MASE procedure, fully exploiting its potential, is by using the DoE approach. Such an approach, besides allowing to reduce to a minimum the number of experiments in method development, guarantees to obtain the maximum amount of information of better quality. Regrettably, although DoE has proven its effectiveness in many applied research fields ranging from psychology, agriculture, and engineering to analytical and medicinal chemistry, its application to MASE is still limited. The main advantage of DoE is associated with the ability to make consistent predictions about time, resources, and effort for reaching the research goal. DoE brings with it a deeper knowledge and understanding of the process being studied by modeling the effects of factors and their interactions. The knowledge of the role of the process factors is fundamental for optimizing the extraction and it allows one to minimize the number of experiments required even if a small amount of natural matrix is available.In summary, combining MASE and DoE is not yet widespread, but, as determined by the literature survey and in the opinion of the authors, this could be the winning strategy to speed up the NADD process.
Author contributions
Conceived the literature review. V.C and G.M. conducted the literature review. V.C and G.M. wrote the literature review with input from E.M. and S.C. E.M. and S.C. conceived the analysis; V.C and G.M. wrote the paper with input from all co-authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
G. R. acknowledges MUR (Ministero dell'Università e della Ricerca), PON R&I 2014-2020-Asse IV “Istruzione e Ricerca per il recupero-REACT-EU”, Azione IV.6 “Contratti di Ricerca su tematiche Green”.References
- A. Lakhdari, Z. Merazi, E. H. Hanitet Nour and F. Z. Drir, Bol. Latinoam. Caribe Plant. Med. Aromat., 2019, 18, 392–410 Search PubMed.
- V. Cavalloro, F. Soddu, S. Baroni, F. S. Robustelli della Cuna, E. Tavazzi, E. Martino and S. Collina, Life, 2023, 13, 1913 CrossRef CAS PubMed.
- V. Cavalloro, F. S. Robustelli della Cuna, E. Quai, S. Preda, F. Bracco, E. Martino and S. Collina, Plants, 2022, 11, 2246 CrossRef CAS.
- D. J. Newman, Curr. Pharmacol. Rep., 2023, 9, 67–89 CrossRef CAS.
- A. G. Atanasov, S. B. Zotchev, V. M. Dirsch and C. T. Supuran, Nat. Rev. Drug Discovery, 2021, 20, 200–216 CrossRef CAS.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2020, 83, 770–803 CrossRef CAS PubMed.
- S. Ma, L. Ge, Y. Li, N. Liao, J. Xie and K. Yang, J. Nat. Prod., 2023, 86, 1793–1800 CrossRef CAS.
- I. L. Mouafon, B. Y. G. Mountessou, M. Lateef, J. Tchamgoue, M. Shaiq Ali, J. C. Tchouankeu, I. R. Green, B. T. Ngadjui and S. F. Kouam, Nat. Prod. Res., 2023, 37, 2319–2326 CrossRef CAS.
- G. Rocchetti, F. Blasi, D. Montesano, S. Ghisoni, M. C. Marcotullio, S. Sabatini, L. Cossignani and L. Lucini, Food Res. Int., 2019, 115, 319–327 CrossRef CAS PubMed.
- B. Sik, E. L. Hanczné, V. Kapcsándi and Z. Ajtony, J. Pharm. Biomed. Anal., 2020, 184, 113173 CrossRef CAS.
- C. Bitwell, S. Indra, C. Luke and M. K. Kakoma, Sci. African, 2023, 19, e01585 CAS.
- T. Vats and A. Mishra, Encycl. Inorg. Bioinorg. Chem., 2016, 1–17 Search PubMed.
- N. A. Ibrahim and M. A. Zaini, Chem. Eng. Trans., 2017, 56, 865–870 Search PubMed.
- H. Cho, F. Török and B. Török, Green Chem., 2014, 16, 3623–3634 RSC.
- V. M. Pappas, I. Samanidis, G. Stavropoulos, V. Athanasiadis, T. Chatzimitakos, E. Bozinou, D. P. Makris and S. I. Lalas, Sustainability, 2023, 15, 2328 CrossRef CAS.
- M. Zheng, M. Ma, Y. Yang, Z. Liu, S. Liu, T. Hong, H. Ni and Z. Jiang, Int. J. Biol. Macromol., 2023, 242, 125003 CrossRef CAS.
- J. D. Moseley and C. O. Kappe, Green Chem., 2011, 13, 794 RSC.
- V. Cavalloro, E. Martino, P. Linciano and S. Collina, in Microwave Heating - Electromagnetic Fields Causing Thermal and Non-Thermal Effects, IntechOpen, 2021 Search PubMed.
- J. F. Box, Am. Stat., 1980, 34, 1–7 Search PubMed.
- M. J. Anderson and P. J. Whitcomb, DOE Simplified Practical Tools for Effective Experimentation, CRC Press, 3rd edn, 2017, 2015 Search PubMed.
- A. L. Gareth, M. Didier and R. Phan-Tan-Luu, Pharmaceutical Experimental Design, New York, 1st edn, 1998 Search PubMed.
- A. M. Mood, Ann. Stat., 1946, 17, 432–446 CrossRef.
- V. Czitrom, Am. Stat., 1999, 53, 126 Search PubMed.
- B. Benedetti, V. Caponigro and F. Ardini, Crit. Rev. Anal. Chem., 2022, 52, 1015–1028 CrossRef CAS.
- S. K. Karna and R. Sahai, Int. J. Eng. Math. Sci., 2012, 1, 11–18 Search PubMed.
- G. E. P. Box, J. S. Hunter and W. G. Hunter, Statistics for Experimenters: Design, Innovation, and Discovery, 2nd edn, 2005, 978-0-471-71813-0 Search PubMed.
- D. C. Montgomery, Design and Analysis of the Experiments, New Jersey, 10th edn, 2019 Search PubMed.
- R. Leardi, Anal. Chim. Acta, 2009, 652, 161–172 CrossRef CAS.
- C. Stalikas, Y. Fiamegos, V. Sakkas and T. Albanis, J. Chromatogr. A, 2009, 1216, 175–189 CrossRef CAS PubMed.
- R. L. Plackett and J. P. Burman, Biometrika, 1946, 33, 305 CrossRef.
- A. Freddi and M. Salmon, Design Principles and Methodologies, 2019, pp. 159–180 Search PubMed.
- B. Jones and C. J. Nachtsheim, Technometrics, 2017, 59, 319–329 CrossRef.
- H. Ebrahimi-Najafabadi, R. Leardi and M. Jalali-Heravi, J. AOAC Int., 2014, 97, 3–11 CrossRef CAS PubMed.
- R. Leardi, in Encyclopedia of Analytical Chemistry, Wiley, 2018, pp. 1–11 Search PubMed.
- J. A. Cornell, Experiments with Mixtures, Wiley, 2002 Search PubMed.
- M. A. Bezerra, V. A. Lemos, C. G. Novaes, R. M. de Jesus, H. R. S. Filho, S. A. Araújo and J. P. S. Alves, Microchem. J., 2020, 152, 104336 CrossRef CAS.
- G. E. P. Box and N. R. Draper, Response Surfaces, Mixtures, and Ridge Analyses, Wiley, 2nd edn, 2007 Search PubMed.
- G. Marrubini, S. Dugheri, G. Cappelli, G. Arcangeli, N. Mucci, P. Appelblad, C. Melzi and A. Speltini, Anal. Chim. Acta, 2020, 1119, 77–100 CrossRef CAS PubMed.
- U. M. F. M. Cerqueira, M. A. Bezerra, S. L. C. Ferreira, R. de Jesus Araújo, B. N. da Silva and C. G. Novaes, Food Chem., 2021, 364, 130429 CrossRef CAS.
- T. Lefebvre, E. Destandau and E. Lesellier, J. Chromatogr. A, 2021, 1635, 461770 CrossRef CAS PubMed.
- V. Cavalloro, G. Marrubini, R. Stabile, D. Rossi, P. Linciano, G. Gheza, S. Assini, E. Martino and S. Collina, Molecules, 2021, 26, 455 CrossRef CAS.
- D. Fiorini, S. Scortichini, G. Bonacucina, N. G. Greco, E. Mazzara, R. Petrelli, J. Torresi, F. Maggi and M. Cespi, Ind. Crops Prod., 2020, 154, 112688 CrossRef CAS.
- E. Mazzara, R. Carletti, R. Petrelli, A. M. Mustafa, G. Caprioli, D. Fiorini, S. Scortichini, S. Dall'Acqua, S. Sut, S. Nuñez, V. López, V. D. Zheljazkov, G. Bonacucina, F. Maggi and M. Cespi, J. Sci. Food Agric., 2022, 102, 6220–6235 CrossRef CAS PubMed.
- S. Pimentel-Moral, I. Borrás-Linares, J. Lozano-Sánchez, D. Arráez-Román, A. Martínez-Férez and A. Segura-Carretero, J. Pharm. Biomed. Anal., 2018, 156, 313–322 CrossRef CAS PubMed.
- C. O. Kappe, Chem. Soc. Rev., 2013, 42, 4977 RSC.
- Q. Hu, Y. He, F. Wang, J. Wu, Z. Ci, L. Chen, R. Xu, M. Yang, J. Lin, L. Han and D. Zhang, Chin. Med., 2021, 16, 87 CrossRef CAS.
- Y. Li, A. Hu, X. Wang and J. Zheng, Int. J. Biol. Macromol., 2019, 134, 308–315 CrossRef CAS PubMed.
- T. Belwal, I. D. Bhatt, R. S. Rawal and V. Pande, Ind. Crops Prod., 2017, 95, 393–403 CrossRef CAS.
- W. Routray and V. Orsat, Ind. Crops Prod., 2014, 58, 36–45 CrossRef CAS.
- W. Thong-on, T. Pathomwichaiwat, S. Boonsith, W. Koo-amornpattana and S. Prathanturarug, Sci. Rep., 2021, 11, 22026 CrossRef CAS PubMed.
- O. R. Alara, A. H. Nour and S. Abdul Mudalip, Indones. J. Chem., 2019, 19, 511 CrossRef CAS.
- J. Q. Borja, M. M. Uy, J. S. Lim, M. E. Ong and A. M. Ros, ASEAN J. Chem. Eng., 2015, 14, 58 CrossRef.
- A. Fernández-Agulló, M. S. Freire and J. González-Álvarez, Ind. Crops Prod., 2015, 64, 105–113 CrossRef.
- D.-S. Zhang, C.-Y. Guo, J. Wang, Y. Hou, Y.-M. Zhao and L.-X. Shen, Pharmacogn. Mag., 2013, 9, 192 CrossRef.
- W. Jun, L. Quan-Liang and L. Hang, Adv. J. Food Sci. Technol., 2015, 9, 386–388 CrossRef.
- M. F. Montenegro-Landívar, P. Tapia-Quirós, X. Vecino, M. Reig, C. Valderrama, M. Granados, J. L. Cortina and J. Saurina, J. Environ. Chem. Eng., 2021, 9, 105330 CrossRef.
- A. Nisca, R. Ştefănescu, C. Moldovan, A. Mocan, A. D. Mare, C. N. Ciurea, A. Man, D.-L. Muntean and C. Tanase, Plants, 2022, 11, 240 CrossRef CAS.
- L. Pallaroni, C. von Holst, C. Eskilsson and E. Björklund, Anal. Bioanal. Chem., 2002, 374, 161–166 CrossRef CAS.
- I. S. M. Purbowati and A. Maksum, IOP Conf. Ser. Earth Environ. Sci., 2019, 406, 012005 CrossRef.
- N. Rhazi, H. Hannache, M. Oumam, A. Sesbou, B. Charrier, A. Pizzi and F. Charrier-El Bouhtoury, Arabian J. Chem., 2019, 12, 2668–2684 CrossRef CAS.
- S. Gala, S. Sumarno and M. Mahfud, Eng. Appl. Sci. Res., 2022, 49, 29–35 Search PubMed.
- H. Teng and W. Y. Lee, J. Korean Soc. Appl. Biol. Chem., 2013, 56, 317–324 CrossRef CAS.
- Y. Ma, J. Li, F. Tong, X.-L. Xin and H. A. Aisa, Ind. Crops Prod., 2020, 153, 112592 CrossRef CAS.
- F. Ferreres, C. Grosso, A. Gil-Izquierdo, P. Valentão, A. T. Mota and P. B. Andrade, Food Chem., 2017, 230, 463–474 CrossRef CAS.
- L. Kurniasari and S. Darmanto, Sci. Study Res.: Chem. Chem. Eng., Biotechnol., Food Ind., 2019, 20, 1–10 CAS.
- I. Izirwan, T. D. Munusamy, N. H. Hamidi and S. Z. Sulaiman, Int. J. Mech. Eng. Robot. Res., 2020, 1246–1252 CrossRef.
- J. Coelho, M. Robalo, S. Boyadzhieva and R. Stateva, Molecules, 2021, 26, 7320 CrossRef CAS PubMed.
- O. A. Olalere, C. Gan, O. E. Akintomiwa, O. Adeyi and A. Adeyi, Phytochem. Anal., 2021, 32, 850–858 CrossRef CAS.
- X. Le, M. Nguyen, D. Vu, M. Pham, Q. Pham, Q. Nguyen, T. Nguyen, V. Pham, L. Bach, T. Nguyen and Q. Tran, Processes, 2019, 7, 485 CrossRef CAS.
- Z. Karami, Z. Emam-Djomeh, H. A. Mirzaee, M. Khomeiri, A. S. Mahoonak and E. Aydani, J. Food Sci. Technol., 2015, 52(6), 3242–3253 CAS.
- E. Martino, S. Della Volpe, V. Cavalloro, B. Amri, L. B. B. Kaab, G. Marrubini, D. Rossi and S. Collina, Phytochem. Anal., 2019, 30(4), 377–384 CrossRef CAS.
- Y. Lu, M. Ye, S. Song, L. Li, F. Shaikh and J. Li, Appl. Biochem. Biotechnol., 2014, 174, 762–771 CrossRef CAS PubMed.
- A. V. González-de-Peredo, M. Vázquez-Espinosa, E. Espada-Bellido, M. Ferreiro-González, C. Carrera, G. F. Barbero and M. Palma, Antioxidants, 2022, 11, 846 CrossRef PubMed.
- O. Yassine, B. Fatima and S. M. Faouzi, in 2019 International Conference of Computer Science and Renewable Energies (ICCSRE), IEEE, 2019, pp. 1–6.
- M. Vázquez-Espinosa, E. Espada-Bellido, A. de Peredo, M. Ferreiro-González, C. Carrera, M. Palma, C. G. Barroso and G. F. Barbero, Agronomy, 2018, 8, 240 CrossRef.
- T. Belwal, A. Pandey, I. D. Bhatt and R. S. Rawal, Sci. Rep., 2020, 10, 917 CrossRef CAS.
- J. Prakash Maran, V. Sivakumar, K. Thirugnanasambandham and R. Sridhar, Carbohydr. Polym., 2013, 97, 703–709 CrossRef CAS PubMed.
- D. Lin, Q. Ma, Y. Zhang and Z. Peng, Prep. Biochem. Biotechnol., 2020, 50, 874–882 CrossRef CAS.
- R. Rosa, L. Tassi, G. Orteca, M. Saladini, C. Villa, P. Veronesi, C. Leonelli and E. Ferrari, Food Anal. Methods, 2017, 10, 575–586 CrossRef.
- G. Milani, F. Curci, M. M. Cavalluzzi, P. Crupi, I. Pisano, G. Lentini, M. L. Clodoveo, C. Franchini and F. Corbo, Molecules, 2020, 25, 215 CrossRef CAS PubMed.
- N. Narkprasom, K. Narkprasom and U. Upara, Am. J. Eng. Appl. Sci., 2015, 8, 302–309 CrossRef.
- Ş. İ. Kırbaşlar and S. Şahin, Biomass Convers. Biorefin., 2023, 13, 2849–2861 CrossRef.
- C. Jin, X. Wei, S. Yang, L. Yao and G. Gong, Food Sci. Technol. Res., 2017, 23, 111–118 CrossRef CAS.
- W. Gao, F. Chen, H. Li, X. Wang and Q. Meng, J. Food Meas. Charact., 2019, 13, 2921–2934 CrossRef.
- J. P. Maran and K. A. Prakash, Int. J. Biol. Macromol., 2015, 73, 202–206 CrossRef CAS.
- J. Prakash Maran, V. Sivakumar, K. Thirugnanasambandham and R. Sridhar, Carbohydr. Polym., 2014, 101, 786–791 CrossRef CAS PubMed.
- M. Kazemi, S. Amiri Samani, S. Ezzati, F. Khodaiyan, S. S. Hosseini and M. Jafari, J. Sci. Food Agric., 2021, 101, 6552–6562 CrossRef CAS PubMed.
- S. Rahmati, A. Abdullah and O. L. Kang, Bioact. Carbohydr. Diet. Fibre, 2019, 18, 100186 CrossRef CAS.
- A. Vellaisamy Singaram and N. D. Ganesan, Prep. Biochem. Biotechnol., 2022, 52, 711–723 CrossRef CAS PubMed.
- V. Varadharajan, S. Shanmugam and A. Ramaswamy, J. Food Process Eng., 2017, 40, e12486 CrossRef.
- H. Teng and Y. H. Choi, Food Sci. Biotechnol., 2013, 22, 1–8 CrossRef CAS.
- H. Abuzaid, E. Amin, A. Moawad, U. R. Abdelmohsen, M. Hetta and R. Mohammed, Pharmacogn. Res., 2020, 10, 24–30 Search PubMed.
- H. Dong, Q. Zhang, Y. Li, L. Li, W. Lan, J. He, H. Li, Y. Xiong and W. Qin, Int. J. Biol. Macromol., 2016, 86, 224–232 CrossRef CAS PubMed.
- F. Berkani, F. Dahmoune, S. Achat, S. Dairi, N. Kadri, S. Zeghichi-Hamri, A. Abbou, I. Benzitoune, K. Adel, H. Remini, A. Belbahi and K. Madani, J. Pharm. Innovation, 2021, 16, 630–642 CrossRef.
- Y. P. Lin, S. C. Wu and J. Y. Hwang, J. Mar. Sci. Technol., 2014, 22, 666–671 Search PubMed.
- Z. Wang, H. Pan, J. Xu, Y. Chang, C. Liu, Y. Zhang, H. Yang, C. Duan, J. Huang and Y. Fu, Ind. Crops Prod., 2022, 184, 115043 CrossRef CAS.
- Y. Zhang, H. Li, H. Dou, Z. He, H. Wu, Z. Sun, H. Wang, X. Huang and Y. Ma, Food Sci. Biotechnol., 2013, 22, 153–159 CrossRef CAS.
- L. Deng, T.-Y. Zhou, L. Pi, X.-H. Zhao, T. Han, Y.-K. Li and F. Han, Asian J. Chem., 2013, 25, 8065–8071 CrossRef CAS.
- C. Liu, C.-H. Wang, J. Liu, L. Xu, W. Xiang and Y.-C. Wang, Food Sci. Technol. Res., 2014, 20, 599–605 CrossRef CAS.
- E. Lautié, C. Rasse, E. Rozet, C. Mourgues, J.-P. Vanhelleputte and J. Quetin-Leclercq, J. Sep. Sci., 2013, 36, 758–763 CrossRef.
- A. U. Arvindekar and K. S. Laddha, Ind. Crops Prod., 2016, 83, 587–595 CrossRef CAS.
- W. Liu, C.-L. Zhou, J. Zhao, D. Chen and Q.-H. Li, Acta Sci. Pol., Technol. Aliment., 2014, 13, 155–168 CrossRef CAS.
- A. K. Das, V. Mandal and S. C. Mandal, Phytochem. Anal., 2013, 24, 230–247 CrossRef CAS.
- P. Alam, N. A. Siddiqui, M. T. Rehman, A. Hussain, A. Akhtar, S. R. Mir and M. F. Alajmi, Molecules, 2021, 26, 1876 CrossRef CAS.
- W. Xiong, X. Chen, G. Lv, D. Hu, J. Zhao and S. Li, J. Pharm. Anal., 2016, 6, 382–388 CrossRef PubMed.
- X. Shang, X. Guo, B. Li, H. Pan, J. Zhang, Y. Zhang and X. Miao, J. Ethnopharmacol., 2016, 192, 350–361 CrossRef CAS PubMed.
- D.-T. Xie, Y.-Q. Wang, Y. Kang, Q.-F. Hu, N.-Y. Su, J.-M. Huang, C.-T. Che and J.-X. Guo, Sep. Purif. Technol., 2014, 130, 173–181 CrossRef CAS.
- M. Peng, H. Qiu, W. Chen and D. Wang, Asian J. Chem., 2014, 26, 6549–6552 CrossRef CAS.
- J. Płotka-Wasylka, M. de la Guardia, V. Andruch and M. Vilková, Microchem. J., 2020, 159, 105539 CrossRef.
- M. Meenu, V. Bansal, S. Rana, N. Sharma, V. Kumar, V. Arora and M. Garg, Sustainable Chem. Pharm., 2023, 34, 101168 CrossRef CAS.
- W. Xu, K. Chu, H. Li, Y. Zhang, H. Zheng, R. Chen and L. Chen, Molecules, 2012, 17, 14323–14335 CrossRef CAS.
- K. J. Yong and T. Y. Wu, Bioresour. Technol., 2023, 384, 129238 CrossRef CAS PubMed.
- S. Kaoui, B. Chebli, S. Zaidouni, K. Basaid and Y. Mir, Sustainable Chem. Pharm., 2023, 31, 100937 CrossRef CAS.
- Q.-B. Cheng and L.-W. Zhang, Molecules, 2017, 22, 186 CrossRef PubMed.
- M. H. Zainal-Abidin, M. Hayyan, A. Hayyan and N. S. Jayakumar, Anal. Chim. Acta, 2017, 979, 1–23 CrossRef CAS.
- C. B. T. Pal and G. C. Jadeja, J. Food Sci. Technol., 2019, 56, 4211–4223 CrossRef CAS.
- O. A. Souza, V. G. da S. Ramalhão, L. de M. Trentin, C. S. Funari, R. L. Carneiro, V. da S. Bolzani and D. Rinaldo, Sustainable Chem. Pharm., 2022, 26, 100618 CrossRef CAS.
- J.-Z. Liu, H.-C. Lyu, Y.-J. Fu, J.-C. Jiang and Q. Cui, LWT, 2022, 163, 113533 CrossRef CAS.
- M.-Z. Gao, Q. Cui, L.-T. Wang, Y. Meng, L. Yu, Y.-Y. Li and Y.-J. Fu, Microchem. J., 2020, 154, 104598 CrossRef CAS.
- Q. Cui, X. Peng, X.-H. Yao, Z.-F. Wei, M. Luo, W. Wang, C.-J. Zhao, Y.-J. Fu and Y.-G. Zu, Sep. Purif. Technol., 2015, 150, 63–72 CrossRef CAS.
- Z.-F. Wei, X.-Q. Wang, X. Peng, W. Wang, C.-J. Zhao, Y.-G. Zu and Y.-J. Fu, Ind. Crops Prod., 2015, 63, 175–181 CrossRef CAS.
- X. Liu, X. Huang, Y. Wang, S. Huang and X. Lin, Anal. Methods, 2013, 5, 2591 RSC.
- E. Kurtulbaş, A. G. Pekel, M. Bilgin, D. P. Makris and S. Şahin, Biomass Convers. Biorefin., 2022, 12, 351–360 CrossRef.
- Z. Liu, J. Jia, F. Chen, F. Yang, Y. Zu and L. Yang, Molecules, 2014, 19, 19471–19490 CrossRef.
- X. Ding, L. Li, Y. Wang, J. Chen, Y. Huang and K. Xu, J. Sep. Sci., 2014, 37, 3539–3547 CrossRef CAS.
- J. González-Rivera, C. Pelosi, E. Pulidori, C. Duce, M. R. Tiné, G. Ciancaleoni and L. Bernazzani, Curr. Res. Green Sustainable Chem., 2022, 5, 100333 CrossRef.
- R. Ikram, K. H. Low, N. B. Hashim, W. Ahmad and M. Afiq bin Nasharuddin, Curr. Anal. Chem., 2018, 14, 646–653 CrossRef CAS.
- P. I. Modi, J. K. Parikh and M. A. Desai, Ind. Crops Prod., 2021, 173, 114088 CrossRef CAS.
- T. Dao, D. Nguyen, T. Tran, P. Van Thinh, V. Hieu, D. Vo Nguyen, T. Nguyen and L. Bach, Rasayan J. Chem., 2019, 12, 666–676 CrossRef CAS.
- A. E. Kate, A. Singh, N. C. Shahi, J. P. Pandey, T. P. Singh and O. Prakash, J. Food Meas. Charact., 2017, 11, 272–280 CrossRef.
- X. Peng, X. Yang, H. Gu, L. Yang and H. Gao, Ind. Crops Prod., 2021, 167, 113549 CrossRef CAS.
- M. Akhbari, S. Masoum, F. Aghababaei and S. Hamedi, J. Food Sci. Technol., 2018, 55, 2197–2207 CrossRef CAS PubMed.
- H. Haqqyana, A. Altway and M. Mahfud, Indones. J. Chem., 2021, 21, 1358 CrossRef CAS.
- C. Zhao, X. He, C. Li, L. Yang, Y. Fu, K. Wang, Y. Zhang and Y. Ni, Appl. Sci., 2016, 6, 19 CrossRef.
- E. Mazzara, S. Scortichini, D. Fiorini, F. Maggi, R. Petrelli, L. Cappellacci, G. Morgese, M. R. Morshedloo, G. F. Palmieri and M. Cespi, Pharmaceuticals, 2021, 14, 816 CrossRef CAS PubMed.
- P. Suttiarporn, N. Wongkattiya, K. Buaban, P. Poolprasert and K. Tanruean, Processes, 2020, 8, 449 CrossRef CAS.
- Y. Huang, Z. Yin, J. Guo, F. Wang and J. Zhang, Molecules, 2019, 24, 2598 CrossRef CAS.
- M. Shah and S. K. Garg, J. Eng., 2014, 2014, 1–5 CrossRef.
Footnote |
| † Co-first authors. |
| This journal is © The Royal Society of Chemistry 2024 |